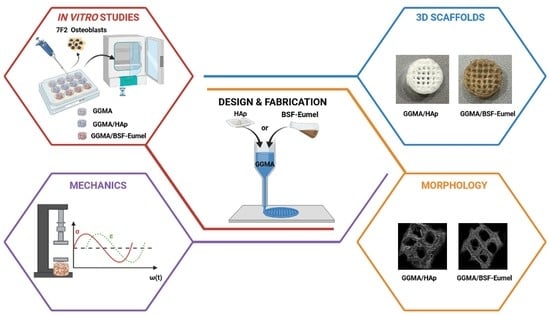
Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
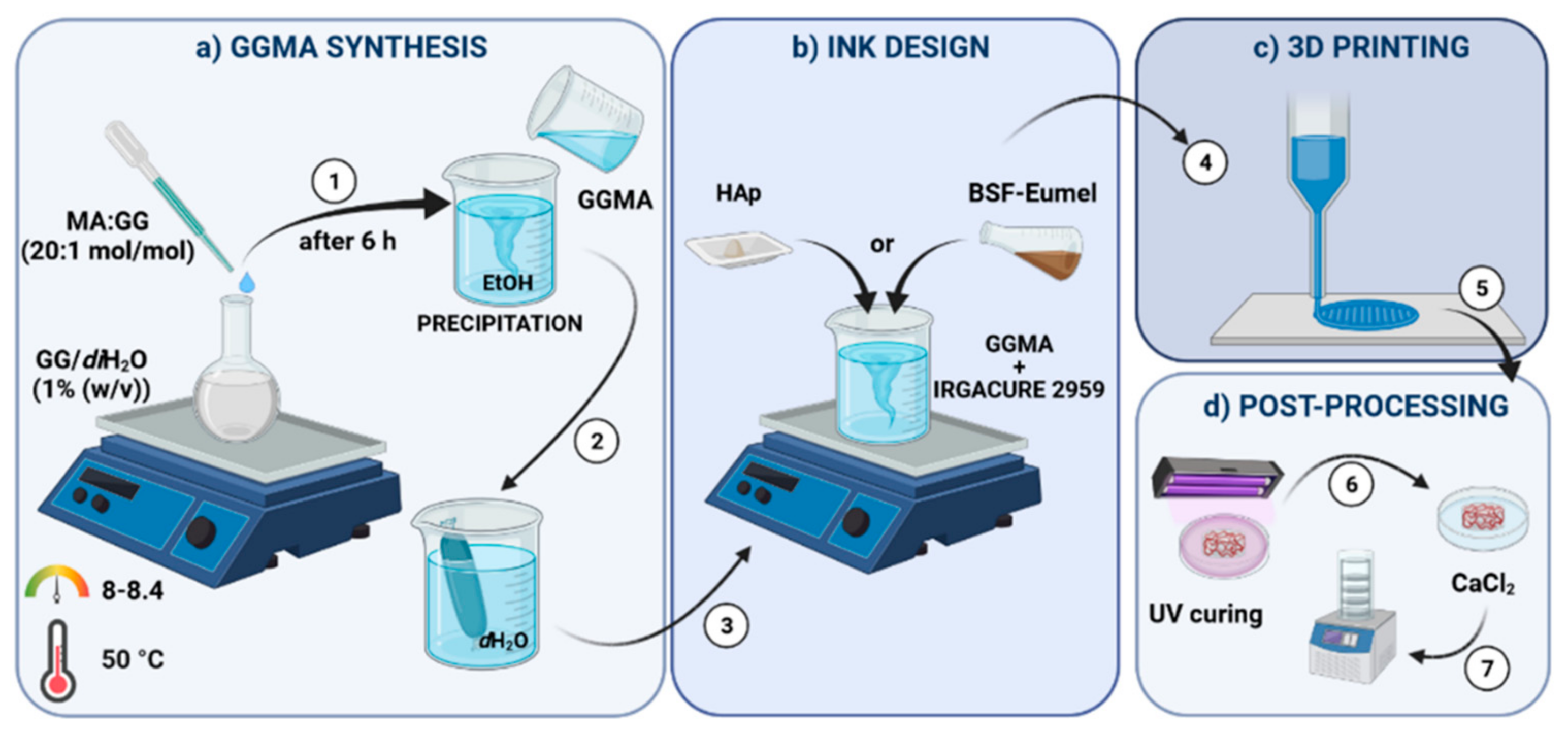
2.2. Synthesis and Characterization of Photocrosslinkable GGMA
2.3. Preparation and Characterization of GGMA-Based Biomaterial Inks
2.4. Preparation and Characterization of GGMA-Based Scaffolds
2.5. Statistics and Data Analysis
3. Results
3.1. GG modification and Characterization
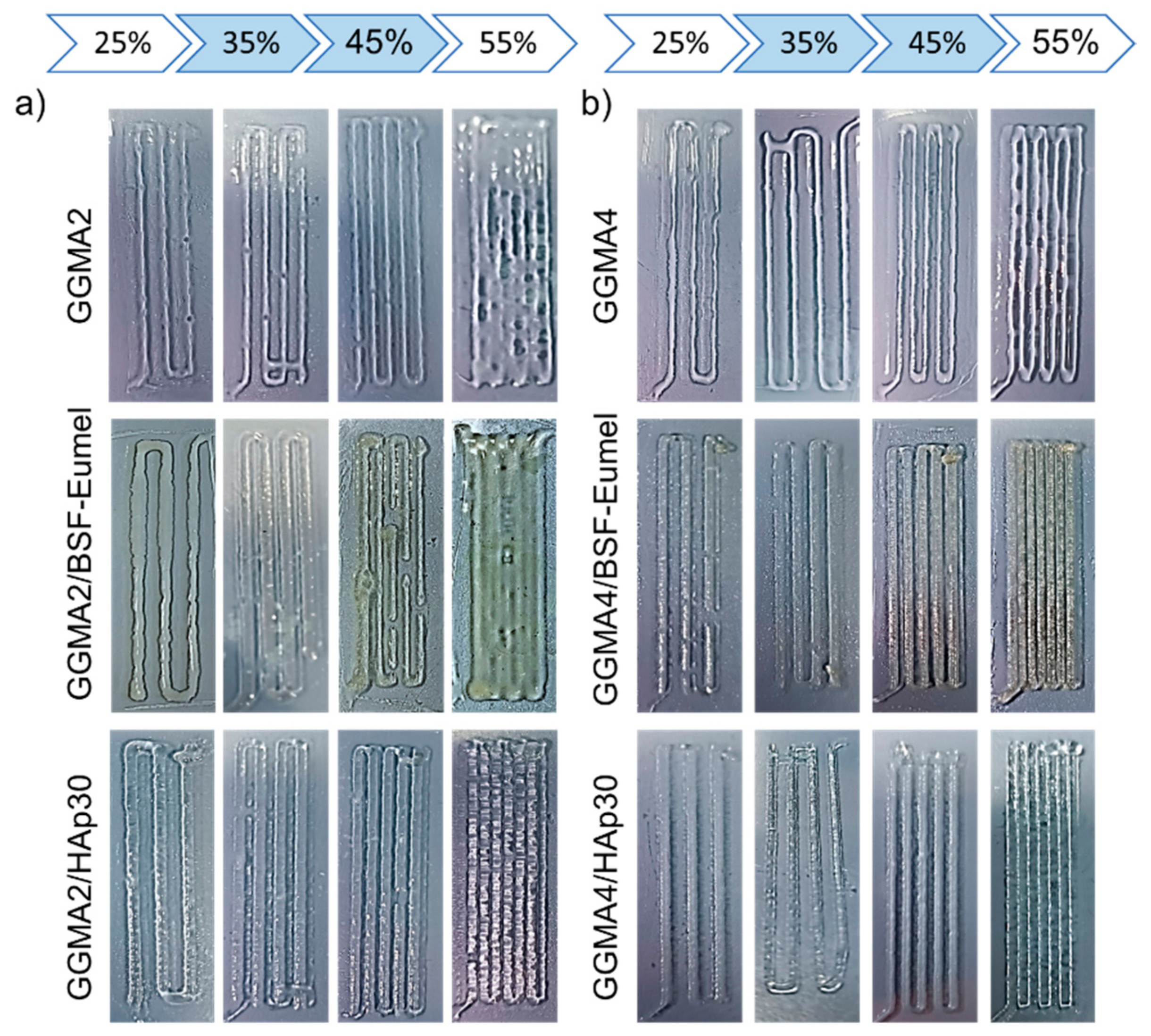
3.2. Qualitative Printability Assessment of GGMA-Based Ink
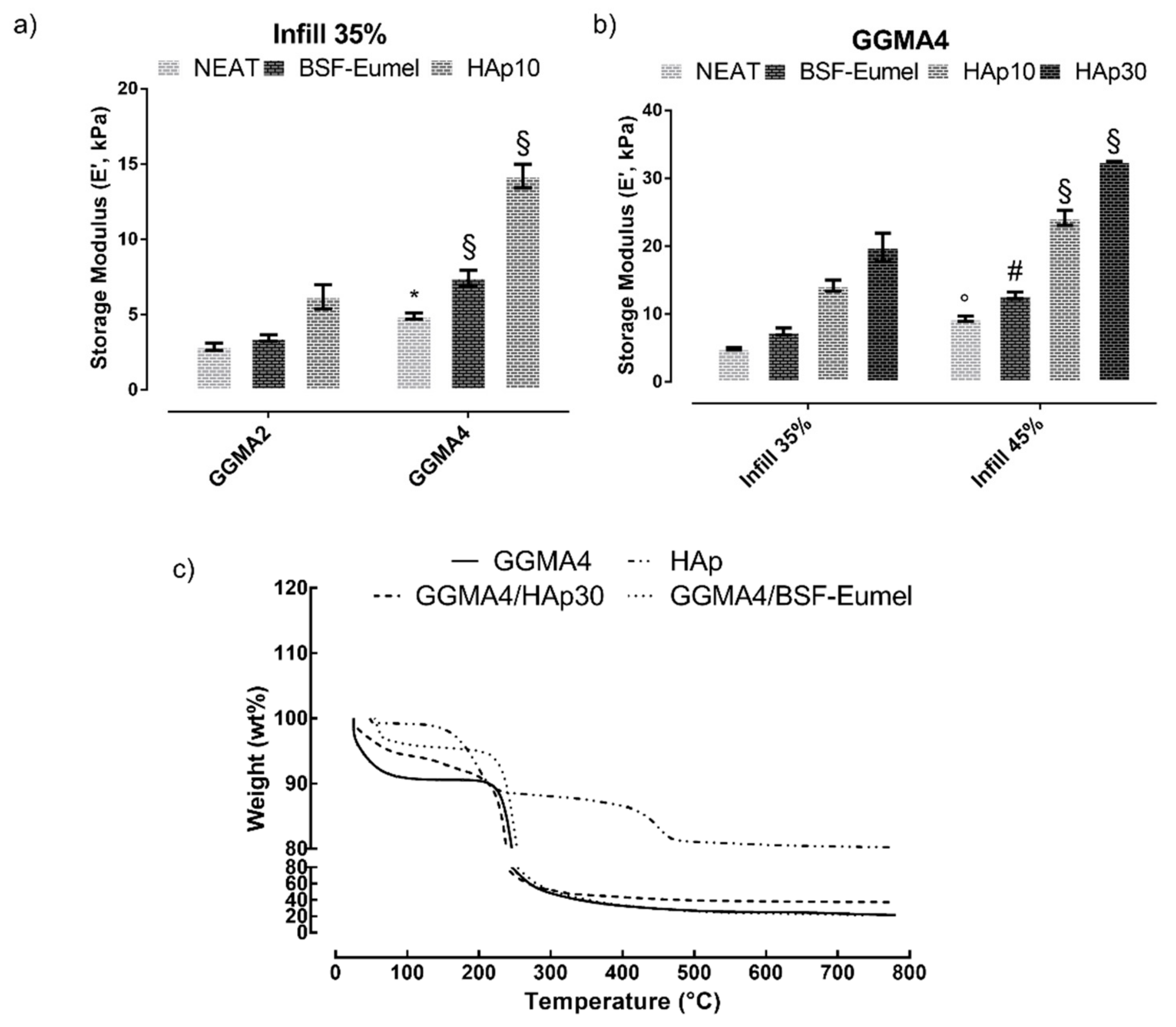
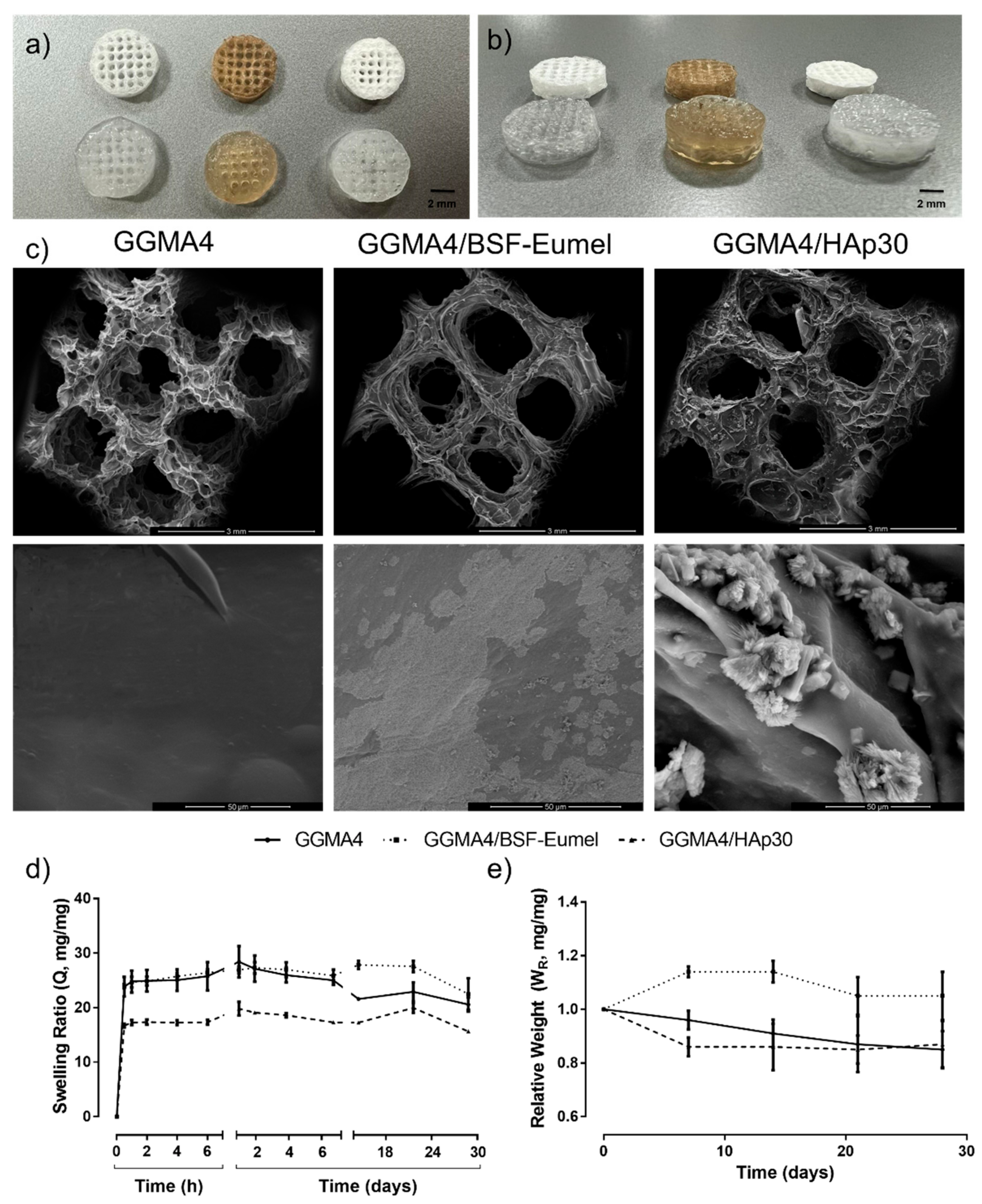
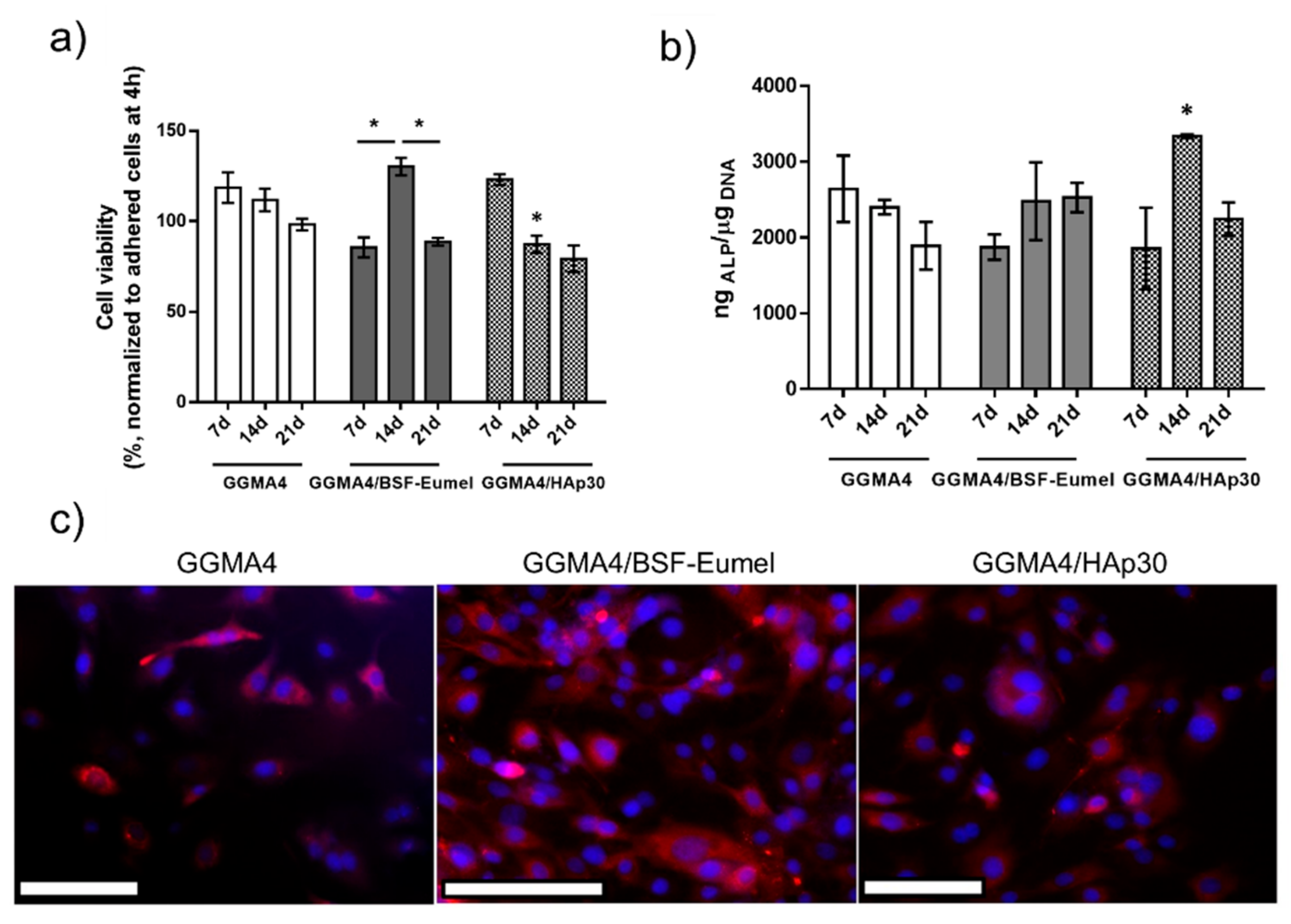
3.3. Three-Dimensional printing and Characterization of GGMA-Based Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef]
- He, H.-Y.; Zhang, J.-Y.; Mi, X.; Hu, Y.; Gu, X.-Y. Rapid Prototyping for Tissue-Engineered Bone Scaffold by 3D Printing and Biocompatibility Study. Int. J. Clin. Exp. Med. 2015, 8, 11777. [Google Scholar]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B. 3D Printing of Bone Tissue Engineering Scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Hu, D.; Wu, D.; Huang, L.; Jiao, Y.; Li, L.; Lu, L.; Zhou, C. 3D Bioprinting of Cell-Laden Scaffolds for Intervertebral Disc Regeneration. Mater. Lett. 2018, 223, 219–222. [Google Scholar] [CrossRef]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.; Eglin, D.; D’este, M. Hyaluronic Acid as a Bioink for Extrusion-Based 3D Printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef]
- Petta, D.; D’Amora, U.; D’Arrigo, D.; Tomasini, M.; Candrian, C.; Ambrosio, L.; Moretti, M. Musculoskeletal Tissues-on-a-Chip: Role of Natural Polymers in Reproducing Tissue-Specific Microenvironments. Biofabrication 2022, 14, 042001. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Bucchieri, F.; Fucarino, A.; Ronca, A.; D’Amora, U. Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources. J. Funct. Biomater. 2022, 13, 118. [Google Scholar] [CrossRef]
- Sworn, G.; Stouby, L. Gellan Gum. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 855–885. [Google Scholar]
- Ronca, A.; Ronca, S.; Forte, G.; Zeppetelli, S.; Gloria, A.; De Santis, R.; Ambrosio, L. Synthesis and Characterization of Divinyl-Fumarate Poly-ε-Caprolactone for Scaffolds with Controlled Architectures. J. Tissue Eng. Regen. Med. 2018, 12, 2322. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Saudagar, P.S.; Singhal, R.S.; Pandey, A. Statistical Approach to Optimization of Fermentative Production of Gellan Gum from Sphingomonas Paucimobilis ATCC 31461. J. Biosci. Bioeng. 2006, 102, 150–156. [Google Scholar] [CrossRef]
- Pitarresi, G.; Martorana, A.; Palumbo, F.S.; Fiorica, C.; Giammona, G. New Gellan Gum-Graft-Poly (D, L-Lactide-Co-Glycolide) Copolymers as Promising Bioinks: Synthesis and Characterization. Int. J. Biol. Macromol. 2020, 162, 1653–1667. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Jiang, S.; Bratlie, K.M. Chemically Modified Gellan Gum Hydrogels with Tunable Properties for Use as Tissue Engineering Scaffolds. ACS Omega 2018, 3, 6998–7007. [Google Scholar] [CrossRef]
- Martorana, A.; Pitarresi, G.; Palumbo, F.S.; Barberi, G.; Fiorica, C.; Giammona, G. Correlating Rheological Properties of a Gellan Gum-Based Bioink: A Study of the Impact of Cell Density. Polymers 2022, 14, 1844. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, T.; Wu, L.; Han, Q.; Chen, S.; Kong, Y.; Li, G.; Ma, L.; Wu, H.; Zhao, Y. Fabrication and Characterization of 3D-Printed Gellan Gum/Starch Composite Scaffold for Schwann Cells Growth. Nanotechnol. Rev. 2021, 10, 50–61. [Google Scholar] [CrossRef]
- Robinson, T.M.; Talebian, S.; Foroughi, J.; Yue, Z.; Fay, C.D.; Wallace, G.G. Fabrication of Aligned Biomimetic Gellan Gum-Chitosan Microstructures through 3D Printed Microfluidic Channels and Multiple in Situ Cross-Linking Mechanisms. ACS Biomater. Sci. Eng. 2020, 6, 3638–3648. [Google Scholar] [CrossRef]
- Zhuang, P.; Ng, W.L.; An, J.; Chua, C.K.; Tan, L.P. Layer-by-Layer Ultraviolet Assisted Extrusion-Based (UAE) Bioprinting of Hydrogel Constructs with High Aspect Ratio for Soft Tissue Engineering Applications. PLoS ONE 2019, 14, e0216776. [Google Scholar] [CrossRef]
- Akkineni, A.R.; Ahlfeld, T.; Funk, A.; Waske, A.; Lode, A.; Gelinsky, M. Highly Concentrated Alginate-Gellan Gum Composites for 3D Plotting of Complex Tissue Engineering Scaffolds. Polymers 2016, 8, 170. [Google Scholar] [CrossRef]
- Lee, H.; Fisher, S.; Kallos, M.S.; Hunter, C.J. Optimizing Gelling Parameters of Gellan Gum for Fibrocartilage Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 238–245. [Google Scholar] [CrossRef]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The Mechanical Properties and Cytotoxicity of Cell-Laden Double-Network Hydrogels Based on Photocrosslinkable Gelatin and Gellan Gum Biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.; Oliveira, J.; Sousa, R.; Mano, J.; Reis, R. Gellan Gum-Based Hydrogels for Intervertebral Disc Tissue-Engineering Applications. J. Tissue Eng. Regen. Med. 2011, 5, e97–e107. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Zavan, B.; Vindigni, V.; Silva, T.H.; Oliveira, J.M.; Abatangelo, G.; Reis, R.L. Biocompatibility Evaluation of Ionic-and Photo-Crosslinked Methacrylated Gellan Gum Hydrogels: In Vitro and in Vivo Study. Adv. Healthc. Mater. 2013, 2, 568–575. [Google Scholar] [CrossRef]
- Jongprasitkul, H.; Turunen, S.; Parihar, V.S.; Kellomäki, M. Two-Step Crosslinking to Enhance the Printability of Methacrylated Gellan Gum Biomaterial Ink for Extrusion-Based 3D Bioprinting. Bioprinting 2022, 25, e00185. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A.; Raucci, M.; Dozio, S.; Lin, H.; Fan, Y.; Zhang, X.; Ambrosio, L. In Situ Sol-Gel Synthesis of Hyaluronan Derivatives Bio-Nanocomposite Hydrogels. Regen. Biomater. 2019, 6, 249–258. [Google Scholar] [CrossRef]
- Guarino, V.; Veronesi, F.; Marrese, M.; Giavaresi, G.; Ronca, A.; Sandri, M.; Tampieri, A.; Fini, M.; Ambrosio, L. Needle-like Ion-Doped Hydroxyapatite Crystals Influence Osteogenic Properties of PCL Composite Scaffolds. Biomed. Mater. 2016, 11, 015018. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A.; Raucci, M.G.; Lin, H.; Soriente, A.; Fan, Y.; Zhang, X.; Ambrosio, L. Bioactive Composites Based on Double Network Approach with Tailored Mechanical, Physico-Chemical, and Biological Features. J. Biomed. Mater. Res. Part A 2018, 106, 3079–3089. [Google Scholar] [CrossRef]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum Hydrogels with Tunable Physical and Mechanical Properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Dreesen, I.; Kobayashi, S.; Vitalone, A.; Casadei, M.A. Injectable and Photocross-Linkable Gels Based on Gellan Gum Methacrylate: A New Tool for Biomedical Application. Int. J. Biol. Macromol. 2015, 72, 1335–1342. [Google Scholar] [CrossRef]
- Ronca, A.; Ambrosio, L.; Grijpma, D.W. Preparation of Designed Poly(d,l-Lactide)/Nanosized Hydroxyapatite Composite Structures by Stereolithography. Acta Biomater. 2013, 9, 5989–5996. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Łapa, A.; Reczyńska, K.; Krok-Borkowicz, M.; Pietryga, K.; Samal, S.K.; Declercq, H.A.; Schaubroeck, D.; Boone, M.; Van der Voort, P. Novel Injectable, Self-Gelling Hydrogel–Microparticle Composites for Bone Regeneration Consisting of Gellan Gum and Calcium and Magnesium Carbonate Microparticles. Biomed. Mater. 2016, 11, 65011. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and Structural Diversity in Eumelanins: Unexplored Bio-Optoelectronic Materials. Angew. Chem. Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef]
- d’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and Melanogenesis: Methods, Standards, Protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 1–28. [Google Scholar] [CrossRef]
- Pezzella, A.; Barra, M.; Musto, A.; Navarra, A.; Alfe, M.; Manini, P.; Parisi, S.; Cassinese, A.; Criscuolo, V.; d’Ischia, M. Stem Cell-Compatible Eumelanin Biointerface Fabricated by Chemically Controlled Solid State Polymerization. Mater. Horiz. 2015, 2, 212–220. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Oliveira, S.; Pirraco, R.P.; Santos, T.C.; Reis, R.L.; Marques, A.P.; Correlo, V.M. Eumelanin-Releasing Spongy-like Hydrogels for Skin Re-Epithelialization Purposes. Biomed. Mater. 2017, 12, 025010. [Google Scholar] [CrossRef]
- Migliaccio, L.; Manini, P.; Altamura, D.; Giannini, C.; Tassini, P.; Maglione, M.G.; Minarini, C.; Pezzella, A. Evidence of Unprecedented High Electronic Conductivity in Mammalian Pigment Based Eumelanin Thin Films after Thermal Annealing in Vacuum. Front. Chem. 2019, 7, 162. [Google Scholar] [CrossRef]
- Pira, A.; Amatucci, A.; Melis, C.; Pezzella, A.; Manini, P.; d’Ischia, M.; Mula, G. The Interplay of Chemical Structure, Physical Properties, and Structural Design as a Tool to Modulate the Properties of Melanins within Mesopores. Sci. Rep. 2022, 12, 11436 . [Google Scholar] [CrossRef]
- Kumar, P.; Di Mauro, E.; Zhang, S.; Pezzella, A.; Soavi, F.; Santato, C.; Cicoira, F. Melanin-Based Flexible Supercapacitors. J. Mater. Chem. C 2016, 4, 9516–9525. [Google Scholar] [CrossRef]
- Fasolino, I.; Bonadies, I.; Ambrosio, L.; Raucci, M.G.; Carfagna, C.; Caso, F.M.; Cimino, F.; Pezzella, A. Eumelanin Coated PLA Electrospun Micro Fibers as Bioinspired Cradle for SH-SY5Y Neuroblastoma Cells Growth and Maturation. ACS Appl. Mater. Interfaces 2017, 9, 40070–40076. [Google Scholar] [CrossRef]
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and Melanin-like Hybrid Materials in Regenerative Medicine. Nanomaterials 2020, 10, 1518. [Google Scholar] [CrossRef]
- Yoo, H.S.; Chung, K.H.; Lee, K.J.; Kim, D.H.; An, J.H. Melanin Extract from Gallus Gallus Domesticus Promotes Proliferation and Differentiation of Osteoblastic MG-63 Cells via Bone Morphogenetic Protein-2 Signaling. Nutr. Res. Pract. 2017, 11, 190–197. [Google Scholar] [CrossRef]
- D’Amora, U.; Soriente, A.; Ronca, A.; Scialla, S.; Perrella, M.; Manini, P.; Phua, J.W.; Ottenheim, C.; Di Girolamo, R.; Pezzella, A. Eumelanin from the Black Soldier Fly as Sustainable Biomaterial: Characterisation and Functional Benefits in Tissue-Engineered Composite Scaffolds. Biomedicines 2022, 10, 2945. [Google Scholar] [CrossRef]
- Raucci, M.G.; Fasolino, I.; Pastore, S.G.; Soriente, A.; Capeletti, L.B.; Dessuy, M.B.; Giannini, C.; Schrekker, H.S.; Ambrosio, L. Antimicrobial Imidazolium Ionic Liquids for the Development of Minimal Invasive Calcium Phosphate-Based Bionanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 42766–42776. [Google Scholar] [CrossRef]
- Cho, H.H.; Been, S.Y.; Kim, W.Y.; Choi, J.M.; Choi, J.H.; Song, C.U.; Song, J.E.; Bucciarelli, A.; Khang, G. Comparative Study on the Effect of the Different Harvesting Sources of Demineralized Bone Particles on the Bone Regeneration of a Composite Gellan Gum Scaffold for Bone Tissue Engineering Applications. ACS Appl. Bio Mater. 2021, 4, 1900–1911. [Google Scholar] [CrossRef]
- Manda, M.G.; da Silva, L.P.; Cerqueira, M.T.; Pereira, D.R.; Oliveira, M.B.; Mano, J.F.; Marques, A.P.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Gellan Gum-hydroxyapatite Composite Spongy-like Hydrogels for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2018, 106, 479–490. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Custódio, C.A.; Gasperini, L.; Reis, R.L.; Mano, J.F. Autonomous Osteogenic Differentiation of HASCs Encapsulated in Methacrylated Gellan-Gum Hydrogels. Acta Biomater. 2016, 41, 119–132. [Google Scholar] [CrossRef]
- Yu, I.; Kaonis, S.; Chen, R. A Study on Degradation Behavior of 3D Printed Gellan Gum Scaffolds. Procedia CIRP 2017, 65, 78–83. [Google Scholar] [CrossRef]
- Agibayeva, L.E.; Kaldybekov, D.B.; Porfiryeva, N.N.; Garipova, V.R.; Mangazbayeva, R.A.; Moustafine, R.I.; Semina, I.I.; Mun, G.A.; Kudaibergenov, S.E.; Khutoryanskiy, V.V. Gellan Gum and Its Methacrylated Derivatives as in Situ Gelling Mucoadhesive Formulations of Pilocarpine: In Vitro and in Vivo Studies. Int. J. Pharm. 2020, 577, 119093. [Google Scholar] [CrossRef]
- Modrogan, C.; Pandele, A.M.; Bobirică, C.; Dobrotǎ, D.; Dăncilă, A.M.; Gârleanu, G.; Orbuleţ, O.D.; Borda, C.; Gârleanu, D.; Orbeci, C. Synthesis, Characterization and Sorption Capacity Examination for a Novel Hydrogel Composite Based on Gellan Gum and Graphene Oxide (GG/GO). Polymers 2020, 12, 1182. [Google Scholar] [CrossRef]
- Raucci, M.; D’Antò, V.; Guarino, V.; Sardella, E.; Zeppetelli, S.; Favia, P.; Ambrosio, L. Biomineralized Porous Composite Scaffolds Prepared by Chemical Synthesis for Bone Tissue Regeneration. Acta Biomater. 2010, 6, 4090–4099. [Google Scholar] [CrossRef]
- Yan, G.; Chen, G.; Peng, Z.; Shen, Z.; Tang, X.; Sun, Y.; Zeng, X.; Lin, L. The Cross-Linking Mechanism and Applications of Catechol–Metal Polymer Materials. Adv. Mater. Interfaces 2021, 8, 2100239. [Google Scholar] [CrossRef]
- Muthukumar, T.; Song, J.; Molecules, G.K. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation in Vivo and in Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The Structural Unit of Melanin in the Cell Wall of the Fungal Pathogen Cryptococcus Neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Petralito, S.; Subham, S.; Gilmore, D.; Varani, G.; Yang, G.; Lin, D.; Casadei, M.A.; Paul, A. Investigating the Role of Polydopamine to Modulate Stem Cell Adhesion and Proliferation on Gellan Gum-Based Hydrogels. ACS Appl. Bio Mater. 2020, 3, 945–951. [Google Scholar] [CrossRef]
- da Silva, L.P.; Cerqueira, M.T.; Sousa, R.A.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Engineering Cell-Adhesive Gellan Gum Spongy-like Hydrogels for Regenerative Medicine Purposes. Acta Biomater. 2014, 10, 4787–4797. [Google Scholar] [CrossRef]
- Kannan, S.; Ghosh, J.; Dhara, S.K. Osteogenic Differentiation Potential and Marker Gene Expression of Different Porcine Bone Marrow Mesenchymal Stem Cell Subpopulations Selected in Different Basal Media. bioRxiv 2020, bioRxiv:2020-04. [Google Scholar]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amora, U.; Ronca, A.; Scialla, S.; Soriente, A.; Manini, P.; Phua, J.W.; Ottenheim, C.; Pezzella, A.; Calabrese, G.; Raucci, M.G.; et al. Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds. Nanomaterials 2023, 13, 772. https://doi.org/10.3390/nano13040772
D’Amora U, Ronca A, Scialla S, Soriente A, Manini P, Phua JW, Ottenheim C, Pezzella A, Calabrese G, Raucci MG, et al. Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds. Nanomaterials. 2023; 13(4):772. https://doi.org/10.3390/nano13040772
Chicago/Turabian StyleD’Amora, Ugo, Alfredo Ronca, Stefania Scialla, Alessandra Soriente, Paola Manini, Jun Wei Phua, Christoph Ottenheim, Alessandro Pezzella, Giovanna Calabrese, Maria Grazia Raucci, and et al. 2023. "Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds" Nanomaterials 13, no. 4: 772. https://doi.org/10.3390/nano13040772
APA StyleD’Amora, U., Ronca, A., Scialla, S., Soriente, A., Manini, P., Phua, J. W., Ottenheim, C., Pezzella, A., Calabrese, G., Raucci, M. G., & Ambrosio, L. (2023). Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds. Nanomaterials, 13(4), 772. https://doi.org/10.3390/nano13040772