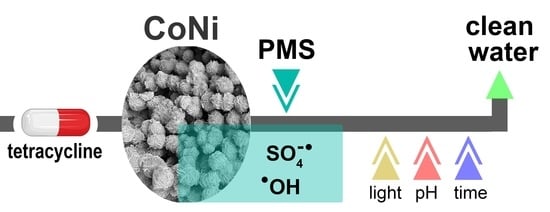
Enhanced Activation of Peroxymonosulfate for Tetracycline Degradation Using CoNi-Based Electrodeposited Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PMS-Catalysts
2.3. Characterization of PMS Catalysts
2.4. Catalytic Experiments
3. Results and Discussion
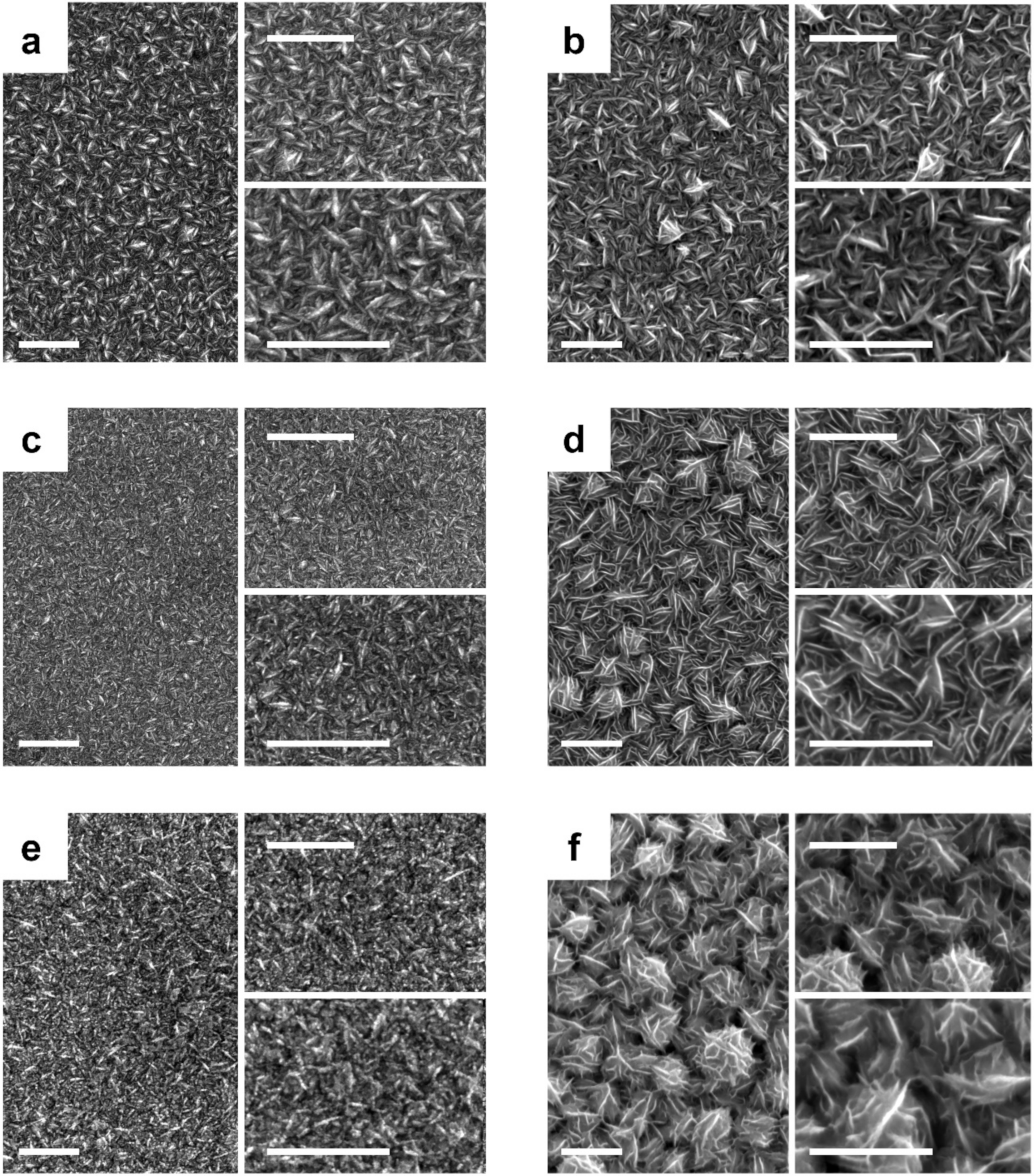
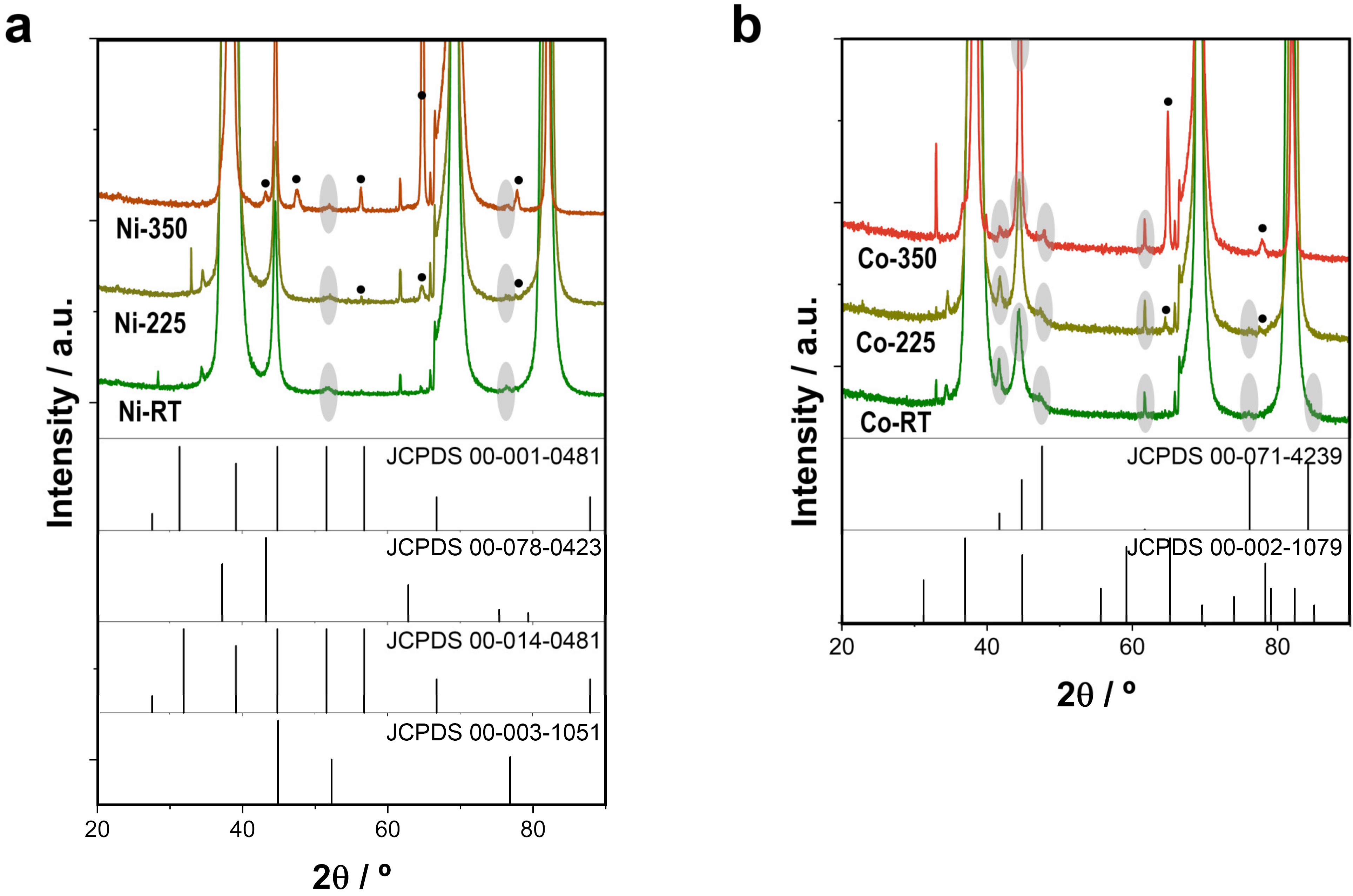
3.1. Synthesis and Characterization of PMS-Catalysts
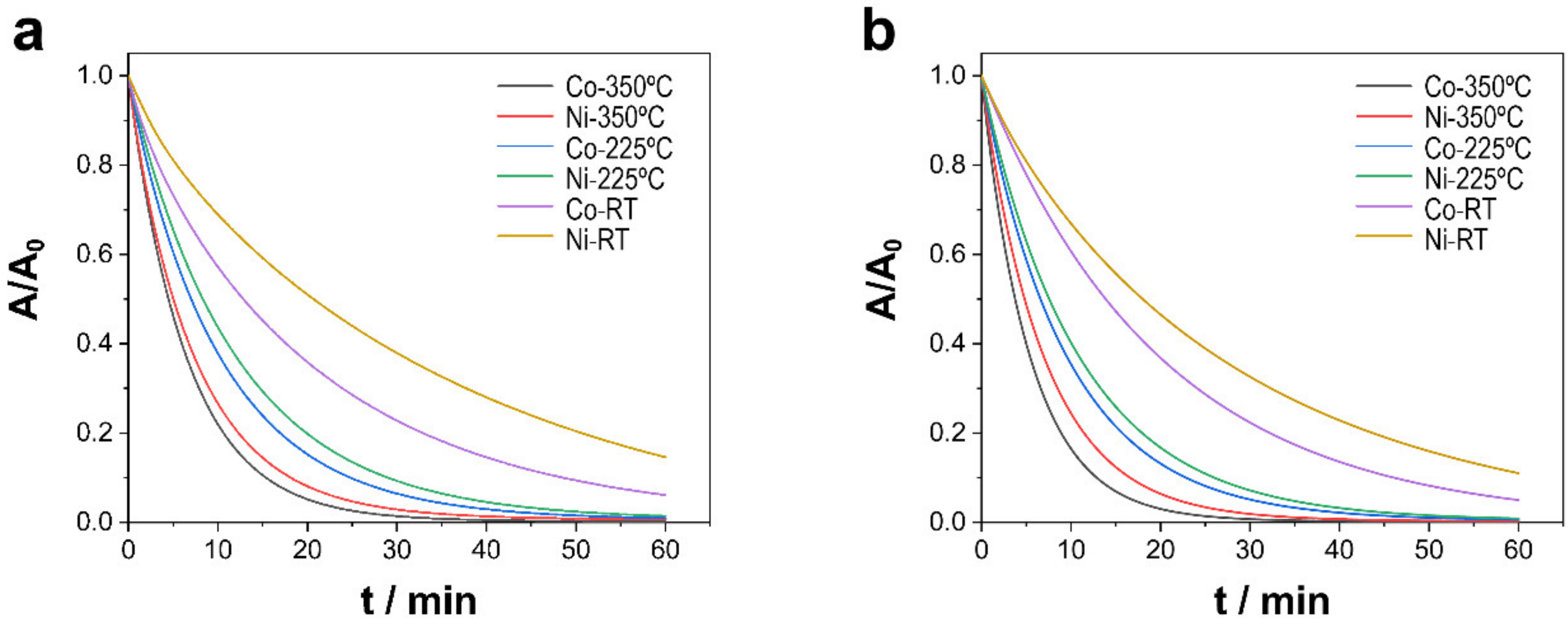
3.2. Mineralization of TCs via PMS Catalysis
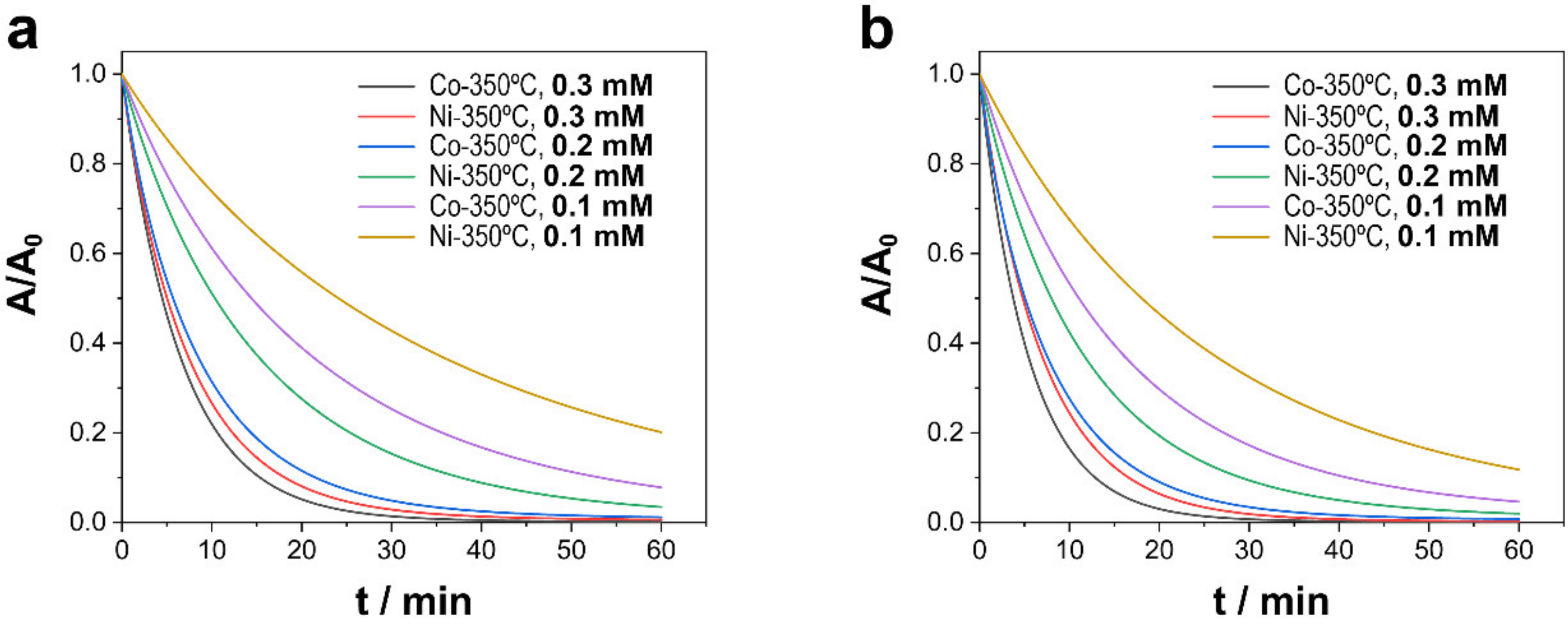
3.2.1. Effect of PMS Concentration
3.2.2. Effect of Light Irradiation
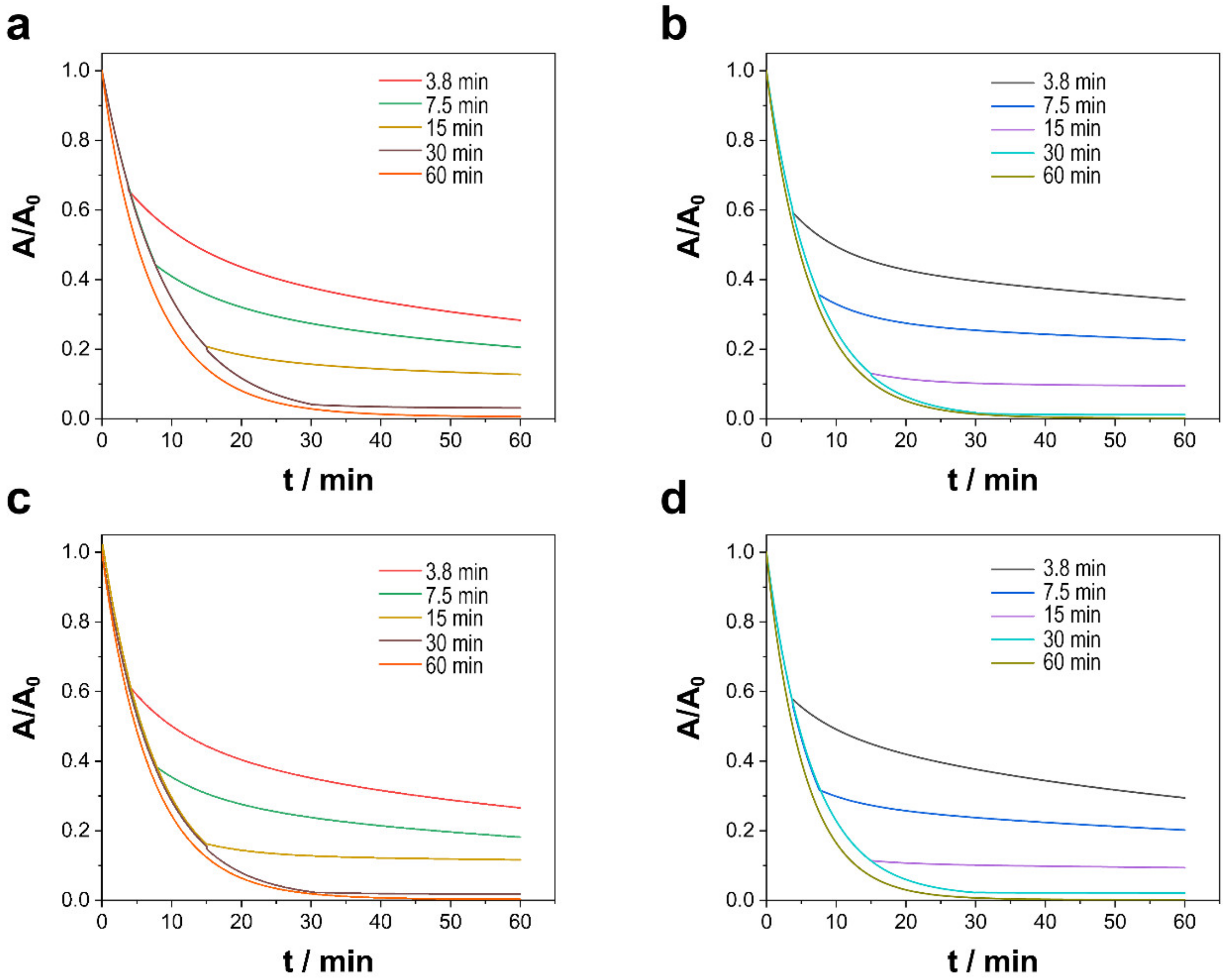
3.2.3. Effect of Contact Duration
3.2.4. Identification of Free Radicals
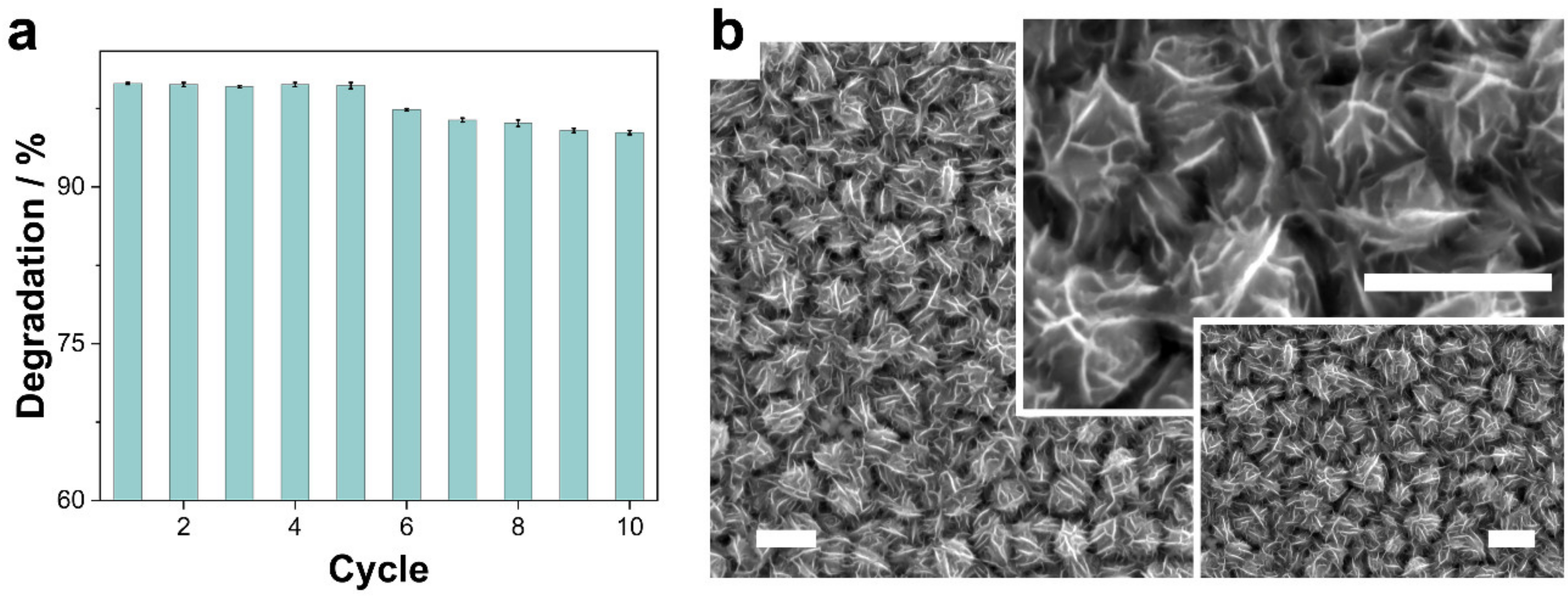
3.2.5. Stability and Reusability of Co-CoNi (350 °C)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jury, W.A.; Vaux, H.J. The Emerging Global Water Crisis: Managing Scarcity and Conflict Between Water Users. Adv. Agron. 2007, 95, 1–76. [Google Scholar] [CrossRef]
- Serrà, A.; Philippe, L.; Perreault, F.; Garcia-Segura, S. Photocatalytic treatment of natural waters. Reality or hype? The case of cyanotoxins remediation. Water Res. 2021, 188, 116543. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Tugaoen, H.O.N.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Challenges in photocatalytic reduction of nitrate as a water treatment technology. Sci. Total Environ. 2017, 599–600, 1524–1551. [Google Scholar] [CrossRef]
- Cao, J.; Lai, L.; Lai, B.; Yao, G.; Chen, X.; Song, L. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism. Chem. Eng. J. 2019, 364, 45–56. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, T.B.; Chen, C.W.; Hung, C.M.; Huang, C.P.; Dong, C. Di Cobalt-impregnated biochar (Co-SCG) for heterogeneous activation of peroxymonosulfate for removal of tetracycline in water. Bioresour. Technol. 2019, 292, 121954. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Chu, W. Degradation of atrazine by cobalt-mediated activation of peroxymonosulfate: Different cobalt counteranions in homogenous process and cobalt oxide catalysts in photolytic heterogeneous process. Water Res. 2009, 43, 2513–2521. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Lin, C.; Zhang, H.; Li, X.; Ma, J. Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem. Eng. J. 2017, 315, 201–209. [Google Scholar] [CrossRef]
- Shahzad, A.; Ali, J.; Ifthikar, J.; Aregay, G.G.; Zhu, J.; Chen, Z.; Chen, Z. Non-radical PMS activation by the nanohybrid material with periodic confinement of reduced graphene oxide (rGO) and Cu hydroxides. J. Hazard. Mater. 2020, 392, 122316. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Zhang, Z.; Wei, T.; Wang, J.; Ma, J.; Ren, Y.; Zhang, H. Co3O4 crystal plane regulation to efficiently activate peroxymonosulfate in water: The role of oxygen vacancies. J. Colloid Interface Sci. 2022, 623, 520–531. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Shi, J.; et al. Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: Reaction kinetics, mechanisms and pathways. Chem. Eng. J. 2021, 409, 128176. [Google Scholar] [CrossRef]
- Qi, C.; Wen, Y.; Zhao, Y.; Dai, Y.; Li, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of organic contaminants by Fe(III)/peroxymonosulfate process with L-cysteine. Chin. Chem. Lett. 2022, 33, 2125–2128. [Google Scholar] [CrossRef]
- Gao, J.; Yang, H.B.; Huang, X.; Hung, S.F.; Cai, W.; Jia, C.; Miao, S.; Chen, H.M.; Yang, X.; Huang, Y.; et al. Enabling Direct H2O2 Production in Acidic Media through Rational Design of Transition Metal Single Atom Catalyst. Chem 2020, 6, 658–674. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Luo, C.; Luo, M.; Wang, Q.; Wang, J.; Liao, Z.; Chen, Z.; Chen, Z. Understanding the synergetic effect from foreign metals in bimetallic oxides for PMS activation: A common strategy to increase the stoichiometric efficiency of oxidants. Chem. Eng. J. 2020, 381, 122587. [Google Scholar] [CrossRef]
- Wang, J.; Peng, R.; Chen, K.; Wang, Y.; Xie, T.; Zhu, Q.; Peng, Y.; Liu, S.; Yao, Z. A novel CoNi7O8/MnO2 nanocomposite supported on Ni foam as a peroxymonosulfate activator for the highly efficient singlet oxygen mediated removal of methylene blue. New J. Chem. 2022, 7569–7579. [Google Scholar] [CrossRef]
- Chen, L.; Ji, H.; Qi, J.; Huang, T.; Wang, C.C.; Liu, W. Degradation of acetaminophen by activated peroxymonosulfate using Co(OH)2 hollow microsphere supported titanate nanotubes: Insights into sulfate radical production pathway through CoOH+ activation. Chem. Eng. J. 2021, 406, 126877. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, H.; Pan, Z.; Liu, Y.; Yao, G.; Guo, Y.; Lai, B. Degradation of sulfamethoxazole by cobalt-nickel powder composite catalyst coupled with peroxymonosulfate: Performance, degradation pathways and mechanistic consideration. J. Hazard. Mater. 2020, 400, 123322. [Google Scholar] [CrossRef]
- Dong, X.; Ren, B.; Zhang, X.; Liu, X.; Sun, Z.; Li, C.; Tan, Y.; Yang, S.; Zheng, S.; Dionysiou, D.D. Diatomite supported hierarchical 2D CoNi3O4 nanoribbons as highly efficient peroxymonosulfate catalyst for atrazine degradation. Appl. Catal. B Environ. 2020, 272, 118971. [Google Scholar] [CrossRef]
- Gómez, E.; Fons, A.; Cestaro, R.; Serrà, A. Electrodeposition of CoNi alloys in a biocompatible DES and its suitability for activating the formation of sulfate radicals. Electrochim. Acta 2022, 435, 141428. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Michler, J.; Philippe, L. Facile cost-effective fabrication of Cu@Cu2O@CuO–microalgae photocatalyst with enhanced visible light degradation of tetracycline. Chem. Eng. J. 2021, 413, 127477. [Google Scholar] [CrossRef]
- Bembibre, A.; Benamara, M.; Hjiri, M.; Gómez, E.; Alamri, H.R.; Dhahri, R.; Serrà, A. Visible-light driven sonophotocatalytic removal of tetracycline using Ca-doped ZnO nanoparticles. Chem. Eng. J. 2022, 427, 132006. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Winckler, C.; Grafe, A. Use of veterinary drugs in intensive animal production: Evidence for persistence of tetracycline in pig slurry. J. Soils Sediments 2001, 1, 66–70. [Google Scholar] [CrossRef]
- Cestaro, R.; Philippe, L.; Serr, A. Applied Catalysis B: Environmental Electrodeposition of nanostructured Bi2MoO6@Bi2MoO6 − x homojunction films for the enhanced visible-light-driven photocatalytic degradation of antibiotics. Appl. Catal. B Environ. 2022, 317, 121703. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Serrà, A.; Sebastián-Pascual, P.; Landa-Castro, M.; Gómez, E. Electrochemical assessment of high active area of cobalt deposited in deep eutectic solvent. J. Electroanal. Chem. 2021, 896, 115177. [Google Scholar] [CrossRef]
- Landa-Castro, M.; Sebastián, P.; Giannotti, M.I.; Serrà, A.; Gómez, E. Electrodeposition of nanostructured cobalt films from a deep eutectic solvent: Influence of the substrate and deposition potential range. Electrochim. Acta 2020, 359, 136928. [Google Scholar] [CrossRef]
- Li, J.; Ni, Z.; Gao, Q.; Yang, X.; Fang, Y.; Qiu, R.; Zhu, M.; Zhang, S. Core-shell structured cobalt–nickel bimetallic sulfide with dual redox cycles to activate peroxymonosulfate for glyphosate removal. Chem. Eng. J. 2023, 453, 139972. [Google Scholar] [CrossRef]
- Zhai, S.; Chen, Y.; Ge, M. Efficient polyvinyl alcohol degradation via peroxymonosulfate activated by natural illite supported CoNi3O4 nanosheets. Chem. Eng. J. 2023, 452, 139155. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Zhou, R.; Lin, S.; Zhou, L. High-efficiency carbamazepine degradation using a Ni/Co-LDH as the peroxymonosulfate activator: Performance, mechanism and degradation pathway. Appl. Surf. Sci. 2022, 574, 151580. [Google Scholar] [CrossRef]
- Cai, P.; Zhao, J.; Zhang, X.; Zhang, T.; Yin, G.; Chen, S.; Dong, C.L.; Huang, Y.C.; Sun, Y.; Yang, D.; et al. Synergy between cobalt and nickel on NiCo2O4 nanosheets promotes peroxymonosulfate activation for efficient norfloxacin degradation. Appl. Catal. B Environ. 2022, 306, 121091. [Google Scholar] [CrossRef]
- Wu, Z.; Niu, H.; Chen, J.; Chen, J. Metal-organic frameworks-derived hierarchical Co3O4/CoNi-layered double oxides nanocages with the enhanced catalytic activity for toluene oxidation. Chemosphere 2021, 280, 130801. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin Spinel-Structured Nanosheets Rich in Oxygen Deficiencies for Enhanced Electrocatalytic Water Oxidation. Angew. Chemie-Int. Ed. 2015, 54, 7399–7404. [Google Scholar] [CrossRef]
- Wan, L.L.; Zang, G.L.; Wang, X.; Zhou, L.A.; Li, T.; Zhou, Q.X. Tiny crystalline grain nanocrystal NiCo2O4/N-doped graphene composite for efficient oxygen reduction reaction. J. Power Sources 2017, 345, 41–49. [Google Scholar] [CrossRef]
- Yang, J.; Yu, C.; Liang, S.; Li, S.; Huang, H.; Han, X.; Zhao, C.; Song, X.; Hao, C.; Ajayan, P.M.; et al. Bridging of Ultrathin NiCo2O4 Nanosheets and Graphene with Polyaniline: A Theoretical and Experimental Study. Chem. Mater. 2016, 28, 5855–5863. [Google Scholar] [CrossRef]
- Lim, T.H.; Cho, S.J.; Yang, H.S.; Engelhard, M.H.; Kim, D.H. Effect of Co/Ni ratios in cobalt nickel mixed oxide catalysts on methane combustion. Appl. Catal. A Gen. 2015, 505, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Jiang, Z.; Xu, H.; Liu, L.; Gu, X.; Wang, Y. Crystal-plane engineering of NiCo2O4 electrocatalysts towards efficient overall water splitting. J. Catal. 2018, 357, 238–246. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Jian, P.; Wang, L.; Yan, X. Hollow urchin-like NiO/NiCo2O4 heterostructures as highly efficient catalysts for selective oxidation of styrene. J. Colloid Interface Sci. 2018, 526, 295–301. [Google Scholar] [CrossRef]
- Stephens, C.R.; Murai, K.; Brunings, K.J.; Woodward, R.B. Acidity Constants of the Tetracycline Antibiotics. J. Am. Chem. Soc. 1956, 78, 4155–4158. [Google Scholar] [CrossRef]
- Li, Q.; Ren, Z.; Liu, Y.; Zhang, C.; Liu, J.; Zhou, R.; Bu, Y.; Mao, F.; Wu, H. Petal-like hierarchical Co3O4/N-doped porous carbon derived from Co-MOF for enhanced peroxymonosulfate activation to remove tetracycline hydrochloride. Chem. Eng. J. 2023, 452, 139545. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, K.; Shang, M.Y.; Zhang, Y.Z.; Zhang, Y.; Li, B.L.; Kan, Y.J.; Cao, X.Q.; Zhang, J. A novel partially carbonized Fe3O4@PANI-p catalyst for tetracycline degradation via peroxymonosulfate activation. Chem. Eng. J. 2023, 451, 138655. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Kang, J.; Tang, Y.; Wang, J.; Xu, Z.; Liu, J. Enhanced tetracycline degradation by N[sbnd]C codoped Fe2O3 with rich oxygen vacancies in peroxymonosulfate assisting photoelectrochemical oxidation system: Performance, mechanism and degradation pathway. Chem. Eng. J. 2023, 451, 138611. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, A.; Wang, S.; Long, Y.; Fan, G.; Yu, X. Efficient tetracycline degradation via peroxymonosulfate activation by magnetic Co/N co-doped biochar: Emphasizing the important role of biochar graphitization. Chem. Eng. J. 2022, 450, 138428. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Guo, Y.; Song, W.; Li, Y.; Yan, L. Goethite-MoS2 hybrid with dual active sites boosted peroxymonosulfate activation for removal of tetracycline: The vital roles of hydroxyl radicals and singlet oxygen. Chem. Eng. J. 2022, 450, 138104. [Google Scholar] [CrossRef]
- Xin, J.; Liu, Y.; Niu, L.; Zhang, F.; Li, X.; Shao, C.; Li, X.; Liu, Y. Three-dimensional porous CuFe2O4 for visible-light-driven peroxymonosulfate activation with superior performance for the degradation of tetracycline hydrochloride. Chem. Eng. J. 2022, 445, 136616. [Google Scholar] [CrossRef]
- Xiong, M.; Yan, J.; Fan, G.; Liu, Y.; Chai, B.; Wang, C.; Song, G. Built-in electric field mediated peroxymonosulfate activation over biochar supported-Co3O4 catalyst for tetracycline hydrochloride degradation. Chem. Eng. J. 2022, 444, 136589. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; González, R.; Expósito, A.J. In situ chemical oxidation of carbamazepine solutions using persulfate simultaneously activated by heat energy, UV light, Fe2+ ions, and H2O2. Appl. Catal. B Environ. 2015, 176–177, 120–129. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, T.; Mao, J.; Wu, X. Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe0/persulfate Fenton-like system. Chem. Eng. J. 2014, 257, 36–44. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, H.; Wang, Z.; Fu, Y.; Liu, Y. Kinetics and reaction mechanism of photochemical degradation of diclofenac by UV-activated peroxymonosulfate. RSC Adv. 2021, 11, 6804–6817. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Yan, J.; Li, J.; Lai, B. Co/Al2O3-EPM as peroxymonosulfate activator for sulfamethoxazole removal: Performance, biotoxicity, degradation pathways and mechanism. Chem. Eng. J. 2018, 343, 676–688. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ji, Q.; Lai, L.; Yao, G.; Lai, B. Degradation of p-nitrophenol (PNP)in aqueous solution by mFe/Cu-air-PS system. Chin. Chem. Lett. 2019, 30, 1129–1132. [Google Scholar] [CrossRef]


| Electrochemical Media | E/V (vs. Ag|AgCl) | Co/at. % | Ni/at. % | ||
|---|---|---|---|---|---|
| EDS | ICP | EDS | ICP | ||
| 0.14 M NiCl2 and 0.03 M CoCl2 in a 1 ChCl:2 urea (molar ratio) deep eutectic solvent | −0.95 | 16.2 | 15.4 | 83.8 | 84.6 |
| 0.03 M NiCl2 and 0.14 M CoCl2 in a 1 ChCl:2 urea (molar ratio) deep eutectic solvent | −1.05 | 84.0 | 84.2 | 16.0 | 15.8 |
| pH = 6.0 | pH = 8.0 | |||
|---|---|---|---|---|
| k/min−1 | Mineralization/% | k/min−1 | Mineralization/% | |
| Ni-CoNi (RT) | 0.031 | 65.9 ± 0.2 | 0.045 | 70.4 ± 0.3 |
| Ni-CoNi (225 °C) | 0.077 | 85.9 ± 0.1 | 0.088 | 89.8 ± 0.2 |
| Ni-CoNi (350 °C) | 0.105 | 96.0 ± 0.2 | 0.147 | 97.8 ± 0.3 |
| CoNi (RT) | 0.037 | 78.4 ± 0.4 | 0.050 | 81.0 ± 0.4 |
| Co-CoNi (225 °C) | 0.086 | 90.1 ± 0.3 | 0.096 | 94.4 ± 0.2 |
| Co-CoNi (350 °C) | 0.132 | 99.4 ± 0.3 | 0.173 | 99.9 ± 0.1 |
| PMS Concentration/mM | k/min−1 Ni-CoNi (350 °C) | k/min−1 Co-CoNi (350 °C) | ||
|---|---|---|---|---|
| pH = 6.0 | pH = 8.0 | pH = 6.0 | pH = 8.0 | |
| 0.1 | 0.027 | 0.044 | 0.036 | 0.055 |
| 0.2 | 0.060 | 0.103 | 0.075 | 0.115 |
| 0.3 | 0.105 | 0.147 | 0.132 | 0.173 |
| 0.4 | 0.077 | 0.109 | 0.081 | 0.122 |
| 1.0 | 0.016 | 0.026 | 0.023 | 0.034 |
| Conditions | k/min−1 Ni-CoNi (350 °C) | k/min−1 Co-CoNi (350 °C) | ||
|---|---|---|---|---|
| pH = 6.0 | pH = 8.0 | pH = 6.0 | pH = 8.0 | |
| Dark conditions | 0.105 | 0.147 | 0.132 | 0.173 |
| Visible light irradiation | 0.199 | 0.292 | 0.288 | 0.388 |
| Contact Time/min | Mineralization/% Ni-CoNi (350 °C) | Mineralization/% Co-CoNi (350 °C) | ||
|---|---|---|---|---|
| pH = 6.0 | pH = 8.0 | pH = 6.0 | pH = 8.0 | |
| 3.8 | 43 ± 2 | 46 ± 3 | 42 ± 1 | 51 ± 2 |
| 7.5 | 56 ± 2 | 59 ± 1 | 61 ± 1 | 65 ± 2 |
| 15 | 73 ± 3 | 78 ± 2 | 69 ± 2 | 75 ± 3 |
| 30 | 82 ± 2 | 86 ± 1 | 83 ± 2 | 89 ± 1 |
| 60 | 96.0 ± 0.2 | 97.8 ± 0.3 | 99.4 ± 0.3 | 99.9 ± 0.1 |
| Scavenger | Degradation/% | |||
|---|---|---|---|---|
| Dark Conditions | Visible Light Irradiation | |||
| pH = 6.0 | pH = 8.0 | pH = 6.0 | pH = 8.0 | |
| No scavenger | 99 | 99 | 99 | ~100 |
| MeOH | 43.2 ± 0.2 | 48.4 ± 0.1 | 99 | 99 |
| TBA | 78.1 ± 0.3 | 82.6 ± 0.2 | 99 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, E.; Fons, A.; Cestaro, R.; Serrà, A. Enhanced Activation of Peroxymonosulfate for Tetracycline Degradation Using CoNi-Based Electrodeposited Films. Nanomaterials 2023, 13, 790. https://doi.org/10.3390/nano13050790
Gómez E, Fons A, Cestaro R, Serrà A. Enhanced Activation of Peroxymonosulfate for Tetracycline Degradation Using CoNi-Based Electrodeposited Films. Nanomaterials. 2023; 13(5):790. https://doi.org/10.3390/nano13050790
Chicago/Turabian StyleGómez, Elvira, Arnau Fons, Roberto Cestaro, and Albert Serrà. 2023. "Enhanced Activation of Peroxymonosulfate for Tetracycline Degradation Using CoNi-Based Electrodeposited Films" Nanomaterials 13, no. 5: 790. https://doi.org/10.3390/nano13050790
APA StyleGómez, E., Fons, A., Cestaro, R., & Serrà, A. (2023). Enhanced Activation of Peroxymonosulfate for Tetracycline Degradation Using CoNi-Based Electrodeposited Films. Nanomaterials, 13(5), 790. https://doi.org/10.3390/nano13050790