Abstract
We explored two methods for synthesizing Pd nanoparticles using three different carbide-derived carbon (CDC) support materials, one of which was nitrogen-doped. These materials were studied for oxygen reduction reaction (ORR) in 0.1 M KOH solution, and the resulting CDC/Pd catalysts were characterized using TEM, XRD, and XPS. The citrate method and the polyol method using polyvinylpyrrolidone (PVP) as a capping agent were employed to elucidate the impact of the support material on the final catalyst. The N-doping of the CDC material resulted in smaller Pd nanoparticles, but only in the case of the citrate method. This suggests that the influence of support is weaker when using the polyol method. The citrate method with CDC1, which is predominantly microporous, led to a higher degree of agglomeration and formation of larger particles in comparison to supports, which possessed a higher degree of mesoporosity. We achieved smaller Pd particle sizes using citrate and NaBH4 compared to the ethylene glycol PVP method. Pd deposited on CDC2 and CDC3 supports showed similar specific activity (SA), suggesting that the N-doping did not significantly influence the ORR process. The highest SA value was observed for CDC1/Pd_Cit, which could be attributed to the formation of larger Pd particles and agglomerates.
1. Introduction
Pd has been researched as a substitute for Pt as the oxygen reduction reaction (ORR) catalyst for the fuel cell cathode side due to its structural similarity and greater availability in the Earth’s crust [1,2,3,4]. However, the comparison of Pd with Pt is only competitive in alkaline conditions [5,6,7]. Kim et al. and Arriaga et al. noted higher electrocatalytic ORR activity in alkaline media on Pd/graphene in comparison to Pt/graphene [5,7].
There are three routes for improving the utilization of noble metals in the ORR catalyst material: reduction of loading of noble metal, nano-alloying, and improving dispersion and stability through substrate interactions [1,2,3,4]. This work focuses on the third category, which employs carbide-derived carbon (CDC) materials to support Pd particles.
Pd nanoparticle (PdNP) sizes and shapes are generally limited to a small pool of suitable sizes for the ORR. Jiang et. al. studied the ORR in alkaline media while varying Pd particle sizes between 3 and 16.7 nm, with mass activity showing a volcano-type plot with a maximum of around 5 nm and the specific activity (SA) increasing with Pd particle size [8]. The SA effects have also been noted on thin Pd films [9]. Furthermore, different facets of Pd possess varied ORR activity, and as such, shape-controlled Pd particles dominated by a specific facet exhibit different ORR activity, with the benefiting shape being cuboid [10,11,12,13,14,15]. The particle shape effect observed in acidic conditions is conclusive; however, in alkaline media, Shao and co-workers observed no difference in electrocatalytic activity for their cubic PdNPs compared to spherical ones [16]. This is also supported by a single-crystal study carried out by Hoshi and co-workers on Pd monocrystals in alkaline media, as it was shown that the ORR activity increases in the order Pd (110) < Pd (100) < Pd (111) [17]. However, the differences in the electrocatalytic ORR activity are not as drastic as in HClO4 [18].
Various synthesis methods for PdNP preparation have been studied, which include methods such as microbial and other green methods using plant extracts; however, these methods are somewhat impractical and rarely produce suitable particle sizes for ORR application [19,20,21,22,23,24,25]. The noble metal particle size must be below 5 nm to obtain any reasonable mass activity for ORR. Still, the number of synthesis methods for achieving these small particles is limited. Methods such as water-in-oil microemulsion require a lot of solvents and are generally not green approaches for particle synthesis; however, these are suitable to achieve the desired particle size of 5 nm and less [26,27]. Reduction of Pd precursors with alcohol usually leads to larger particles except for anhydrous methanol [28,29,30], but reduction with ethanol produces particles of 3–12 nm [24]. However, variation of ethanol content with polyvinylalcohol capping agent could lead to better particle size control [31]. There are two suitable methods of PdNP synthesis based on NaBH4 reduction in the presence of citrate ions and reduction with H2 at elevated temperature [32]. Ethylene glycol (EG) has been used as a reducing agent for PdNP synthesis; however, typically, larger particles are formed. With the addition of polyvinylpyrrolidone (PVP), Pd particles of 4–14 nm have been obtained [33]. However, the addition of hydroxide has been shown to produce a suitable Pd particle size [34], and this method was used in this work for comparison.
Since the substrate can influence the particle size during the synthesis, one-pot synthesizes can be challenging to reproduce [35,36,37]. It has been shown that the presence of nitrogen groups on the support can lead to smaller PdNPs compared to the non-doped counterpart, which usually leads to a better dispersion of particles and improved catalyst mass activity. According to a study by Perini et al., the use of mesoporous carbon and nitrogen doping of the carbon support resulted in a decrease in particle size from 5–6 to 2–3 nm without the need for surfactants [38]. Similarly, a reduction in particle size was observed by depositing the Pd catalyst on graphene and N-doped graphene using plasma synthesis, as it decreased particle size from 2.8–3.2 to 2.6–2.9 nm [37]. This effect has also been noted using a PtM (Pd, Fe, Ni) catalyst, as smaller nanoparticles were observed for all alloys on N-doped carbon black compared to regular undoped carbon black [39]. Another benefit of N-doping has been noted by Perini et al. and Zhang et al., showing an increase in stability with N-doped support material [38,40], with contradicting results shown for HOPG, in which case N-doping lowered stability [41]. Various materials have been used to support metal nanoparticles for ORR, including covalent organic frameworks and oxides, which can benefit catalyst stability [42,43].
Two one-pot synthesis methods were used: one with EG as the reducing agent and PVP as the capping agent, and the other with NaBH4 as the reducing agent and citrate as the capping agent. Three different CDC materials were chosen as support materials, of which CDC3 was nitrogen-doped. EG synthesis was only used for CDC2 and CDC3 since these performed best using the citrate method. The prepared catalyst materials were characterized by TEM, XPS, MP-AES, and electrochemically using a rotating disc electrode.
2. Materials and Methods
Three carbide-derived carbon (CDC) support materials were employed in this work. The CDC materials were synthesized by chlorine treatment at a high temperature. The starting carbides used for the CDC preparation are TiC for CDC1 [44], B4C for CDC2 [45], and TiCN for CDC3 [46]. The porosities of these materials are provided in Table S1 [45], and corresponding pore-size distribution (PSD) graphs are shown in Figure S1. These CDC materials were ball-milled according to a previous procedure using 4 mL of ethanol, 200 mg of CDC, and 400 rpm for four cycles of 30 min and 5 min cool-down periods with 0.5 mm balls [47].
CDC/Pd_Cit materials were prepared using a simple one-pot synthesis. A certain amount of PdCl2 (Sigma Aldrich, St. Louis, MO, USA) was dissolved in water (Millipore, Inc., Burlington, MA, USA) and HCl (Sigma Aldrich), to which sodium citrate (Sigma Aldrich) and CDC support were added. PdCl42− and citrate concentrations were 0.25 mM, in which 80 mg of a CDC material was dispersed. For all catalysts, the nominal 20 wt% Pd loading was used. During stirring, 22.3 mL of freshly prepared ice-cold 0.1 M NaBH4 (Aldrich) was added, followed by stirring for 30 min. After stirring, KOH pellets were added to remove citrate, and the material was filtered and dried in an oven overnight at 60 °C. Catalysts are named CDCx/Pd_Cit with x representing the used CDC.
CDC/Pd_EG materials were synthesized by dissolving 20.9 mg of PVP (MW = 8000) and 20.9 mg of PdCl2 in 25 mL of EG, where 50 mg of CDC had already been dispersed for acidification; 21 μL of conc. HCl solution was used in the case of CDC2 and conc. H2SO4 in the case of CDC3. After that, 7.6 mL of EG in which KOH was dissolved was added to reach 0.3 M final hydroxide concentration. The reaction bath was heated to 190 °C for 1 h. After that, EG was evaporated at 200 °C in a N2 atmosphere. Carbon material was re-dispersed in 3 M KOH to remove remnants of PVP and centrifuged three times, followed by filtering and drying overnight at 60 °C. Pd loading for the catalysts was aimed at 20 wt%. Catalysts are named CDCx/Pd_EG, with x designating the used CDC.
CDC/Pd catalyst materials were dispersed in isopropanol (99.8% Honeywell)/Nafion (5%, Aldrich)/water mixture at a volume ratio of 0.975:0.025:9, of which 6 μL was pipetted onto a glassy carbon (GC) disk electrode (d = 5 mm, Origalys, Rillieux-la-Pape, France) rotating at 700 rpm and dried in N2 flow. For comparison, a commercial Pd/C catalyst (20 wt% Premetek, Cherry Hill, NJ, USA) was employed in this work.
For X-ray photoelectron spectroscopy (XPS) measurements, the samples were drop-casted onto GC plates and measured using Scienta SES-100 electron energy analyzer (at 200 eV pass energy, Gammadata Scienta AB, Uppsala, Sweden) using Mg Kα radiation (incident energy 1256.6 eV) from twin anode X-ray source XR3E2 (Thermo Electron Corporation, East Grinstead, UK) with the total energy resolution of about 0.7 eV. The incident angle was 45°, and the take-off angle was 0° relative to the sample normal. The pressure in the analysis chamber was about 5 × 10−10 Torr. Spectra were calibrated using Au 4f photoelectron peaks. CasaXPS software (2.3.22PR1.0) was used for data analysis. Before fitting, the X-ray satellite was subtracted. For curve fitting, we used the combination of Gaussian and Lorentzian line shapes (GL30) for line shape and for the background correction the combination of linear and Shirley backgrounds. For sp2-C the asymmetry was added by convolution with a Doniach–Sunjic line shape. Scanning transmission electron microscopy (STEM) images were obtained by imaging samples drop-casted onto a lacey carbon-coated copper grid using a transmission electron microscope Titan Themis 200 (FEI) (Thermo Fisher Scientific, Waltham, MA, USA) operating at 200 kV using HAADF and BF detectors. Imaging was carried out in scanning mode and Gatan Digital Micrograph software (2.12.1785.0) was used for image analysis. The same operating voltage and spot size were implemented for elemental mapping with the SuperX (Bruker, Billerica, MA, USA) energy-dispersive X-ray spectroscopy (EDX) system. The signal was acquired for 10 min.
For microwave plasma atomic emission spectroscopy (MP-AES) analysis, the Anton Par Multiwave PRO microwave digestion system with NXF100 digestion vessel was used to dissolve 10 mg of catalyst material in 8 mL aqua regia. The metal content of the catalysts was determined using Agilent MP-AES 4210 (Agilent, Santa Clara, CA, USA). The crystallographic structure of catalysts was studied by X-ray diffraction (XRD) using a Bruker D8 Advance diffractometer (Bruker, Billerica, MA, USA) with Cu Kα radiation and 1D-detector (LynxEye XE-T). Profile fitting was carried out using Bruker TOPAS 6.
A standard three-electrode system was used for electrochemical measurements. The following working, counter, and reference electrodes were used: GC electrode covered with a catalyst layer, Pt-wire, and Pt wire in an electrolyte solution bubbled with continuous H2 flow and separated from the working electrode compartment with Luggin capillary. Experiments were carried out in 0.1 M KOH solution (p.a., Aldrich) and saturated with Ar (99.999%, Linde Gas, Dublin, Ireland), O2 (99.999%, Linde Gas) or CO (99.97%, Linde Gas). For the rotating disk electrode (RDE) measurements, a rotator and speed control unit (OrigaLys ElectroChem, SAS, Rillieux-la-Pape, France) were used, and electrode potential was controlled with PGSTAT128N potentiostat/galvanostat (Metrohm Autolab, Utrecht, The Netherlands). The electrochemistry data were collected using GPES software. Cyclic voltammograms (CVs) were measured between 0.1 and 1.4 V vs. RHE at a scan rate of 50 mV s−1. The electrodes were conditioned between 0.1 and 0.8 V for 10 cycles at 50 mV s−1. CO stripping was carried out at 20 mV s−1 between 0.1 and 1 V. The ORR polarization curves were measured between 0.1 and 1.1 V vs. RHE at a potential scan rate (v) of 10 mV s−1. The electrode rotation rate (ω) was varied between 360 and 4600 rpm. Electrochemical measurements were made at room temperature (23 ± 1 °C).
3. Results and Discussion
3.1. TEM and MP-AES Studies
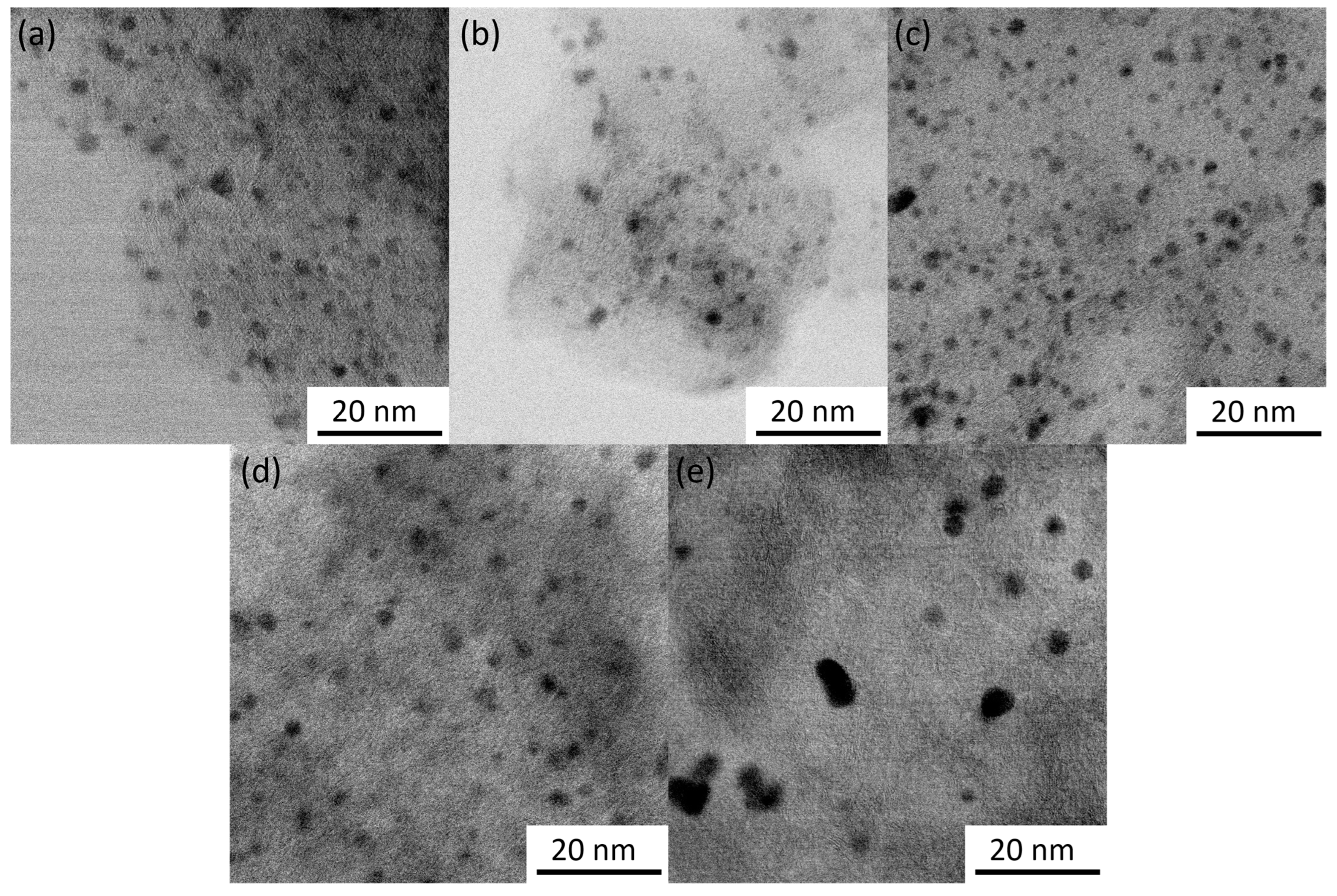
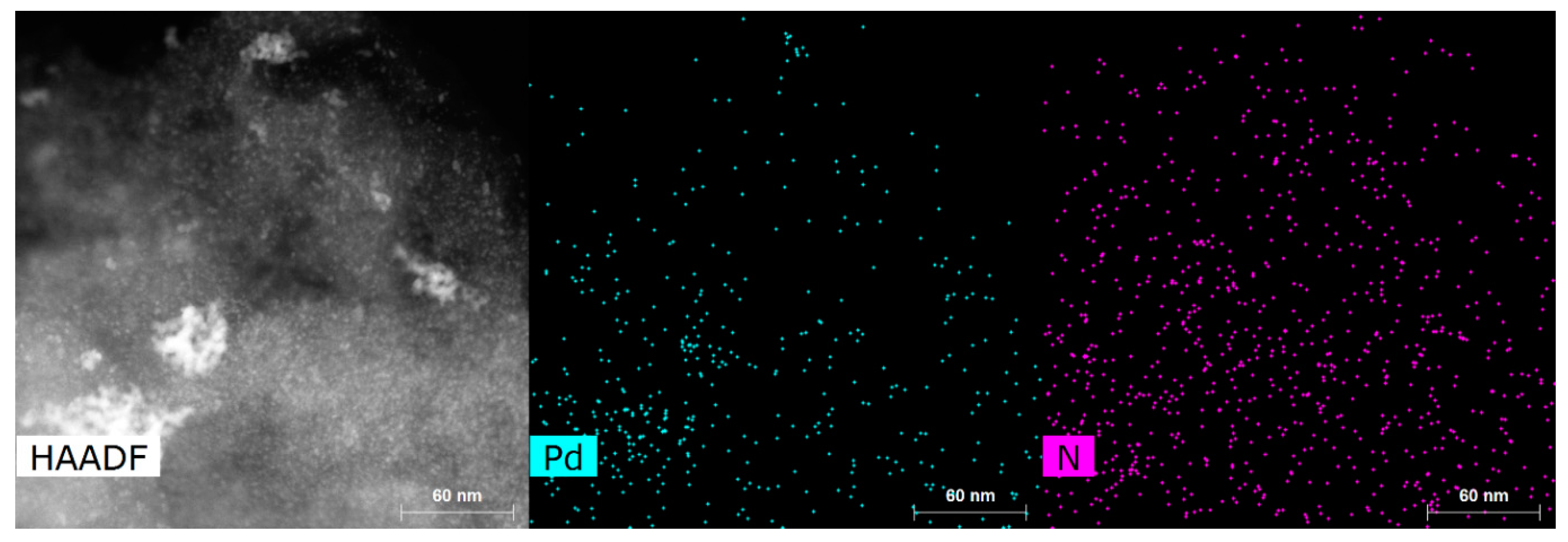
The average lattice spacing of 2.27 Å with standard variation of 0.06 Å was determined from STEM images of the particles. Examples of lattice fringes are shown in Figure S2. This distance can be attributed to the (111) planes of face-centered cubic Pd (2.227 Å) [48], which is also supported by XRD data (see next section). Particle sizes were counted from the TEM images and are provided in Table 1 with particle size distribution histograms in Figure S3. The citrate method resulted in smaller Pd particles in comparison to the EG/PVP method for both CDC2 and CDC3 substrates. Pd nanoparticles deposited on CDC1 showed similar particle sizes. However, a higher degree of agglomeration was observed. Generally, it has been shown that the presence of nitrogen functional groups in the carbon material leads to smaller PdNPs in the one-pot synthesis methods [38,39,49]; however, in the case of the EG/PVP method used in this work, smaller Pd particles were obtained on the CDC2 support, while the citrate method gives a conventional result. Furthermore, another driving factor for the deposition of metal nanoparticles is the defectiveness of the support material, as shown by Asanova et al., where significantly better nanoparticle dispersion and smaller size were achieved on a support with more defects [50]; this has also been observed in our electrodeposition work [36]. Figure 1e shows that the PdNPs for CDC3/Pd_EG are not spherical compared to the CDC2/Pd_EG material (Figure 1d), suggesting that the support has played an essential role in shaping these Pd particles. Figure 2 demonstrates the N-doping of the CDC3 material, showing well-distributed nitrogen species across the entire material.

Table 1.
Physical parameters of CDCx/Pd catalysts.

Figure 1.
STEM images of CDCx/Pd catalysts: (a) CDC1/Pd_Cit, (b) CDC2/Pd_Cit, (c) CDC3/Pd_Cit, (d) CDC2/Pd_EG and (e) CDC3/Pd_EG. Scale bar 20 nm.

Figure 2.
STEM image and elemental mapping of CDC3/Pd_Cit.
Pd content was determined by MP-AES (Table 1), and we can observe that for the one-pot synthesis of Pd particles, the support material has played a role in Pd metal uptake with microporous CDC1 having lower Pd loading compared to mesoporous CDC2 and CDC3.
3.2. XRD and XPS Analysis
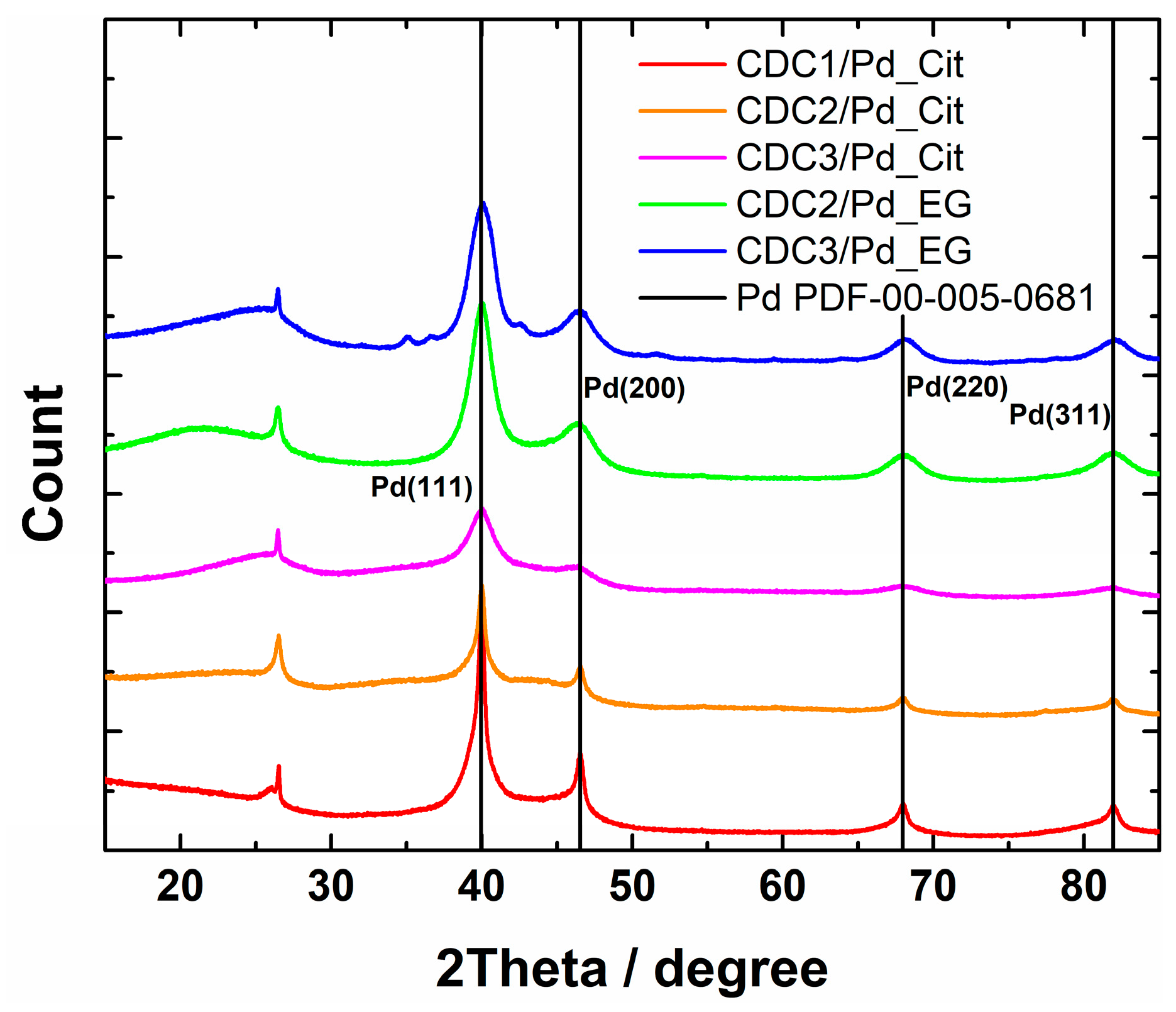
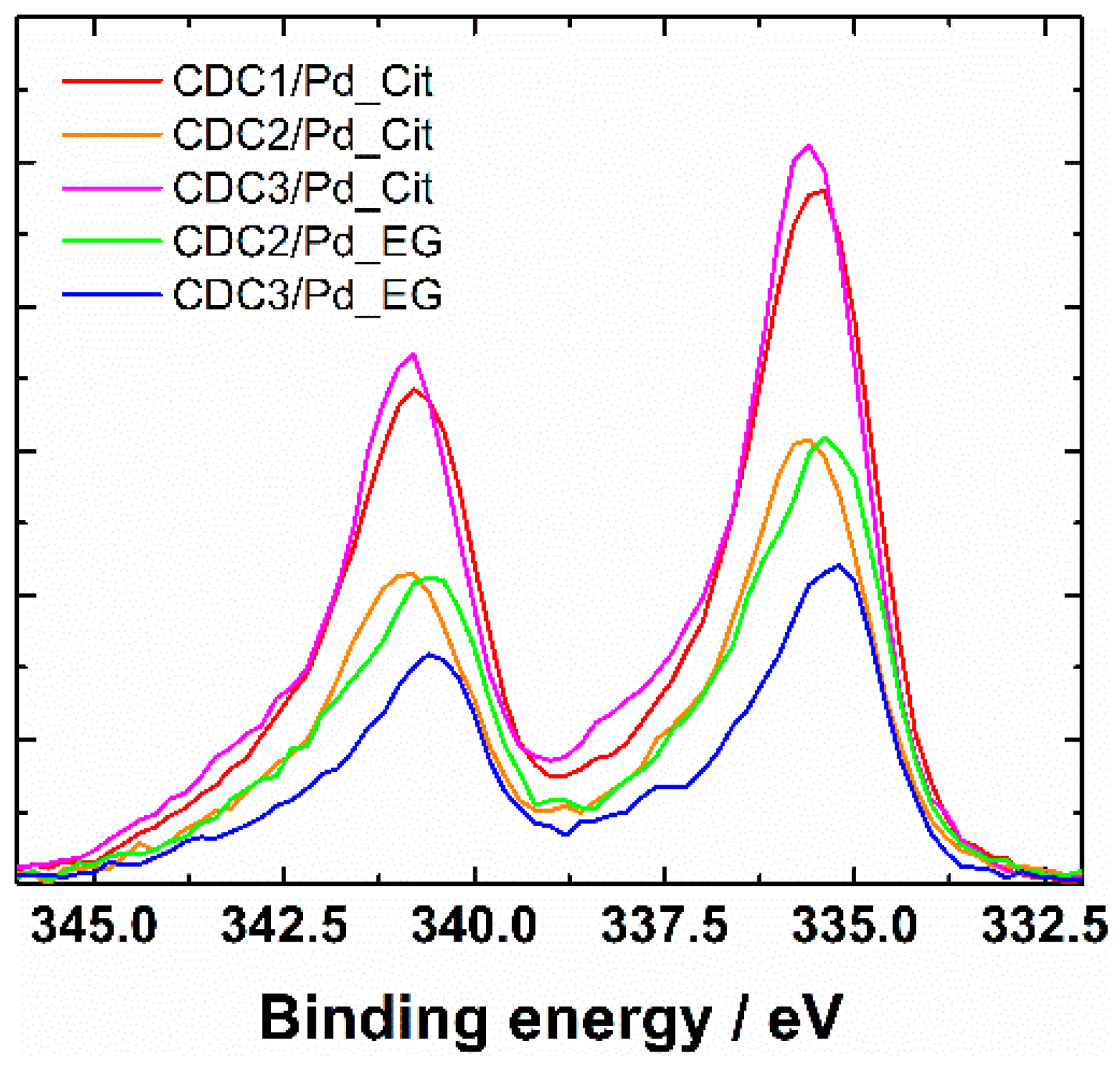
XRD patterns showed the presence of Pd4S in the case of CDC3/Pd_EG (reflections at 35°, 37.5°, and 42.7° can be attributed to Pd4S, Figure 3). All the samples show loosely connected layered carbon material with some graphitized carbon represented by (002) reflection at 26.4°. In the case of the citrate synthesis method, the palladium catalyst could be separated into two fractions, highly dispersed tiny crystallites or amorphous Pd and larger crystallites, listed in Table 1. Lattice constants (Table 1) observed for catalysts prepared by the citrate method are typical to that of Pd at 3.859 Å [48] for one of the phases; however, in the case of CDC1, palladium crystallite size is more variable. Two palladium structures were used to approximate its characterization. CDC2 and CDC3 had very finely dispersed Pd, which made determining lattice constant inaccurate, and thus only lattice constant for larger particles are provided. We can observe the differences in crystallite size distributions by comparing the XRD patterns and particle sizes counted from TEM images. Since the XRD method encompasses a more significant part of the sample, the average crystallite size for CDC3/Pd_EG is comparable to that of CDC2/Pd_EG, with N-doped CDC3 support providing a narrower PdNP size distribution. The difference in the TEM data could be due to larger Pd4S particles. CDCx/Pd-material XPS-survey spectra are shown in Figure S4, and Pd3d XPS spectra are provided in Figure 4 (with deconvolutions, Figure S5), and the presence of N-doping was also confirmed by XPS [51] (Figure S6 and Table S2). A slight difference is observed in the Pd3d XPS peaks between the EG and citrate synthesis methods on CDC2 and CDC3 substrates. The observation of the 3d3/2 peak at ca. 333.4 eV shows that different samples have different ratios of metallic palladium (at 335.4 eV) and Pd2+ (336.4 eV [52]). Palladium species and their distribution are provided in Table S3.

Figure 3.
XRD patterns of CDCx/Pd materials.

Figure 4.
XPS Pd3d spectra of CDCx/Pd materials.
3.3. CV and CO-Stripping Measurements
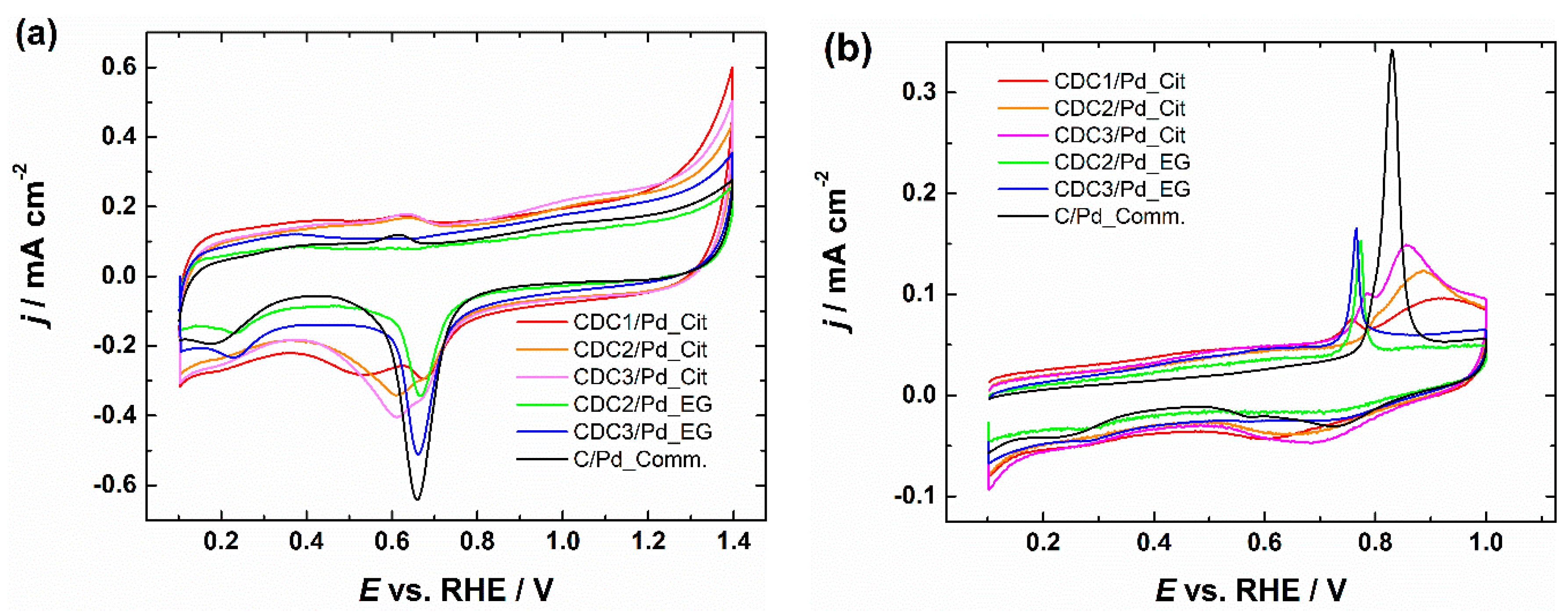
From CVs, we can see that the typical PdO reduction peak is broadened by a second peak in the case of the citrate synthesis method, which can be due to smaller Pd crystallites, as it has been shown that Pd oxide species are generally more stable on smaller particles, leading to a shift in the peak location [8]. This effect does not appear in the case of the EG synthesis method, as these smaller crystallites were observed during XRD only in the case of the citrate method. The CV profile of the CDCx/Pd_EG catalyst looks typical (Figure 5a) and is comparable to the CV of commercial Pd/C [53]. After the ORR measurement, we can observe the change in the CV shape in the case of the citrate synthesis method (Figures S7–S11), while the EG method yields a similar CV to the commercial Pd/C. Since the measurements are carried out in an alkaline solution and CO stripping should be able to clean the PdNP surface from adsorbed citrate if the particles were contaminated [54], it would be observable during the CV studies; however, no cleaning effect is observed in the hydrogen desorption area even when CO stripping was carried out in acidic conditions (Figures S12–S16). However, changes in the peak shape closer to that of the commercial Pd/C can be observed, which can be attributed to the low stability of these smaller crystallites (Figures S7–S16). The problem with these CVs is that the typical method of using charge integration under the PdO reduction peak for the electrochemical surface area (ESA) determination is disrupted in the case of materials synthesized using the citrate method. The shape of the CO-stripping curves further complicates this. At the same time, Pd NPs prepared by the citrate method gives the typical single peak for the CO oxidation profile similar to commercial Pd/C, albeit at a lower potential (Figure 5b). For the EG synthesis method, two peaks can be observed, one at around the same potential as for the CDCx/Pd_Cit material and the second broader peak stretching to higher potentials. We have previously observed similar CO-stripping behavior for Pd-based catalysts deposited on CDC and other highly porous carbon materials. The effect seems to be independent of N-doping [55,56]. One possible explanation for the tailing of the CO-stripping peak can be the particle size, as the tailing of CO-stripping peaks has been previously observed by comparing two commercial Pd/C materials with two different particle size distributions [57]. With the double peak, the first peak in the case of Pt has been previously attributed to agglomeration [54]; another explanation for the first one is that catalytically active OH formed on defect sites [58]. However, this further complicates the determination of ESA, as the second method typically used for this purpose is unsuitable for comparing these catalyst materials. The specific activity (SA) values summarized in Table 2 were obtained using the PdO reduction method for ESA determination in the case of the EG synthesis method and the CO oxidation method for the citrate synthesis method, as determining ESA based on Hudp in alkaline media on Pd electrodes is unreliable.

Figure 5.
(a) CV curves of CDC/Pd catalysts in Ar-saturated 0.1 M KOH, v = 50 mV s−1. (b) Oxidation of pre-adsorbed CO on CDC/Pd catalysts, v = 20 mV s−1. Current densities are normalized to the geometric area of GC.

Table 2.
Kinetic parameters of the prepared Pd-based catalysts for ORR in 0.1 M KOH solution.
3.4. RDE Measurements
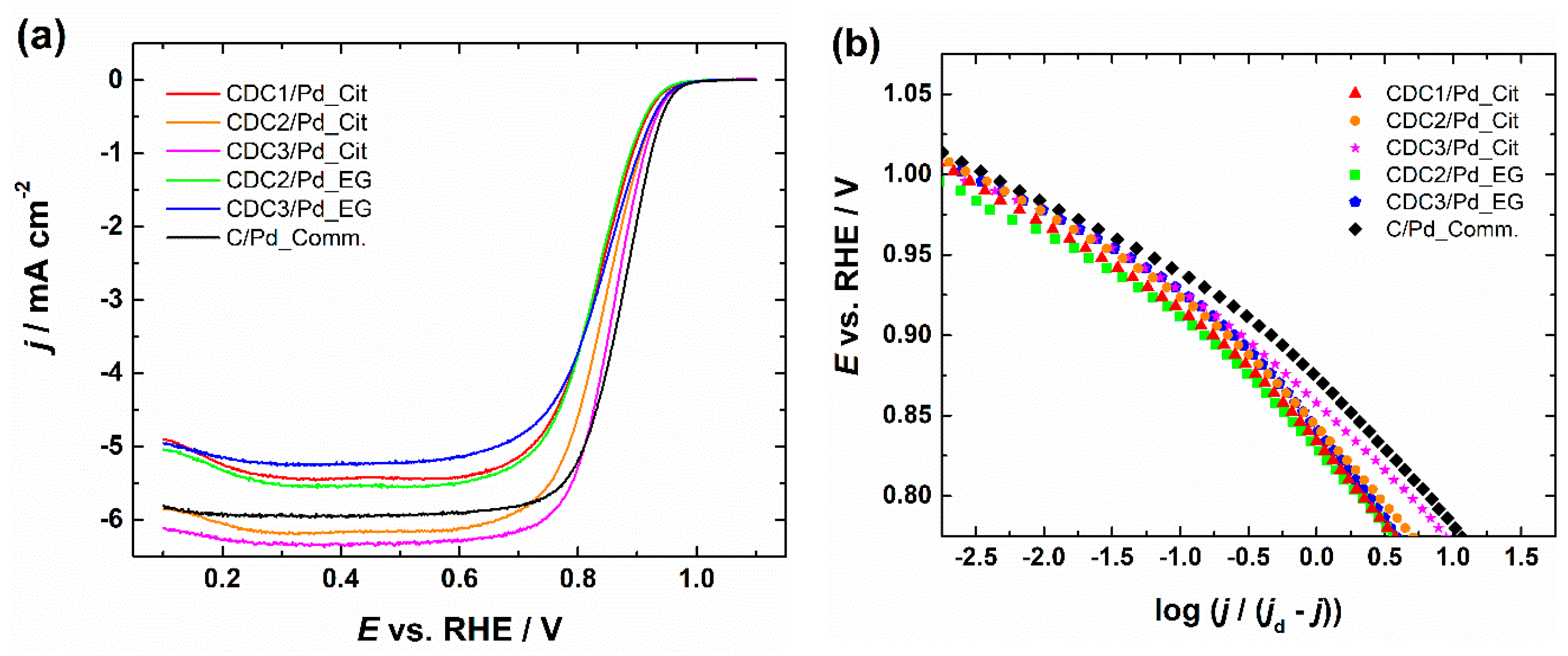
RDE polarization curves for ORR and the corresponding Koutecky–Levich plots are shown in Figures S17–S21, with insets showing the number of electrons transferred (n) calculated by Equation (S1) using constants [59,60]; all of the catalysts gave n value ~4, which is typical for a Pd catalyst. A comparison of catalyst materials in 0.1 M KOH solution at 1900 rpm can be seen in Figure 6. The ORR activity in terms of half-wave potentials (E1/2) of the catalysts prepared by the citrate method follows the order of CDC3/Pd > CDC2/Pd > CDC1/Pd, and the Pd catalyst supported on predominantly microporous CDC is performing significantly worse than those deposited on the other two CDC supports with mostly mesopores. Furthermore, CDC3/Pd material with N-doped support outperforms non-doped counterparts; a similar effect can also be observed for the EG synthesis method, as CDC3/Pd outperforms CDC2/Pd.

Figure 6.
(a) Comparison of RDE polarization curves for oxygen reduction in O2-saturated 0.1 M KOH solution and (b) corresponding Tafel plots, ω = 1900 rpm, v = 10 mV s−1. Current densities are normalized to the geometric area of GC.
Equations (1) and (2) were used to calculate specific (SA) and mass activity (MA):
where Ik is the kinetic current at a specific potential, and ESA is the surface area of Pd determined by CO stripping or PdO reduction methods. m represents Pd mass obtained by MP-AES measurement.
SA = Ik/ESA
MA = Ik/m,
Furthermore, we can see that the citrate synthesis method, which produced smaller PdNPs, is showing higher specific activity compared to EG synthesized counterparts as comparing the Pd particle sizes (average particle sizes for CDC2/Pd were 2.8 ± 0.5, 2.2 ± 0.3 nm and for CDC3/Pd 3.4 ± 1.6, 1.8 ± 0.4 nm for EG and citrate synthesis, respectively). However, since CDC2/Pd_EG particles were smaller than those of CDC3/Pd_EG, this would suggest that the presence of N-doping has a more substantial impact on the ORR activity than just decreasing Pd particle size, but this could also be due to the presence of Pd4S. Several researchers have discussed various effects of N-doping on the electrocatalytic activity of ORR. Gracia-Espino et al. suggested that N-doped graphene reduces the energy barrier of O2 dissociation [61]; however, a typical explanation is based on the increase in the number of anchoring sites [62,63]. Comparing the specific activities between the two synthesis methods is inaccurate as the electrochemical surface area is determined using different methods depending on the synthesis; for the CDC2- and CDC3-supported Pd catalysts, we can observe somewhat similar SA values, but compared to the PdNPs deposited on CDC1, which was predominantly microporous, a higher specific activity can be seen. This increase in SA can be attributed to the increased degree of agglomeration and growth of larger Pd crystallites that were determined by the XRD measurements, as it has been shown that larger Pd particles generally possess higher specific activity [8]. Mass activities provided in Table 2 show that different deposition methods resulted in similar MA values; furthermore, the effect of sulfur for the CDC3/Pd_EG is negligible. A comparison with the literature can be found in Table S4 [8,63,64,65,66,67,68,69,70].
Tafel slope values listed in Table 2 were determined in the potential range of 0.89–0.94 V. Tafel slope values are near −60 mV dec−1, which is typical for Pd/C catalysts [71,72]. A slightly higher Tafel slope of −69.2 mV dec−1 was calculated in the case of CDC3/Pd_EG material, which could be due to the presence of Pd4S as determined by XRD. A similar increase in Tafel slope was observed by Huang et al. in 0.5 M H2SO4 [73]. The −60 mV dec−1 slope is attributed to the oxide-covered Pd surface [74] with the rate-determining step for ORR being the transfer of the first electron to the O2 molecule [71,72]. The increase in Tafel slope has been previously attributed to the lowering of the oxide coverage on the Pd nanoparticles [75], which can be beneficial as PdO has been shown to promote the 2e− ORR pathway [76].
4. Conclusions
Two synthesis approaches were used in this study to prepare Pd nanoparticles on the carbide-derived carbon supports, one of which was doped with nitrogen. No significant effect from N-doping was observed if SA was considered. However, smaller Pd particles were observed on the N-doped CDC, improving mass activity. An increase in the specific activity for ORR was noted for CDC1/Pd_Cit; however, this could be attributed to agglomeration and the formation of larger Pd particles, which was also observed by XRD. Both the CDC2 and CDC3 support materials had significant mesoporosity, which seems to facilitate better dispersion of the Pd nanoparticles. This is further improved in the case of N-doped support materials, resulting in higher ESA values as compared to the non-doped counterpart. While in the case of CDC3/Pd_Cit this could be attributed to smaller Pd particle size, in the case of CDC3/Pd_EG the particles were larger compared to the CDC2/Pd_EG material, which could suggest a lower degree of agglomeration of Pd nanoparticles in the N-doped CDC material.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano14120994/s1, CDC_Pd_SI.docx. Table S1. Texture characteristics of CDC materials measured using standard methodology [1]. Table S2. Various surface species of the CDC3 support material determined by XPS. Table S3. Pd species in the catalyst materials determined by XPS. Table S4. Comparison with various Pd/C materials from literature. Figure S1. PSD graph of CDC materials. Figure S2. Lattice fringes for a) CDC1/Pd_Cit, b) CDC2/Pd_Cit, c) CDC3/Pd_Cit, d) CDC2/Pd_EG, and e) CDC3/Pd_EG samples. Figure S3. Pd particle size distribution for catalyst materials. Figure S4. Cascade of survey spectra for CDCx/Pd catalysts. Figure S5. Deconvolution of the Pd3d peak for a) CDC1/Pd_Cit, b) CDC2/Pd_Cit, c) CDC3/Pd_Cit, d) CDC2/Pd_EG, and e) CDC3/Pd_EG samples. Figure S6. (a) C1s, (b) O1s, and (c) N1s XPS spectra for CDC3 sample. Figure S7. CV curves of CDC1/Pd_Cit catalyst in Ar-saturated 0.1 M KOH solution, v = 50 mV s−1. Figure S8. CV curves of CDC2/Pd_Cit catalyst in Ar-saturated 0.1 M KOH solution, v = 50 mV s−1. Figure S9. CV curves of CDC3/Pd_Cit catalyst in Ar-saturated 0.1 M KOH solution, v = 50 mV s−1. Figure S10. CV curves of CDC2/Pd_EG catalyst in Ar-saturated 0.1 M KOH solution, v = 50 mV s−1. Figure S11. CV curves of CDC3/Pd_EG catalyst in Ar-saturated 0.1 M KOH solution, v = 50 mV s−1. Figure S12. CV curves of CDC1/Pd_Cit catalyst in Ar-saturated 0.5 M H2SO4 solution, v = 50 mV s−1. Figure S13. CV curves of CDC2/Pd_Cit catalyst in Ar-saturated 0.5 M H2SO4 solution, v = 50 mV s−1. Figure S14. CV curves of CDC3/Pd_Cit catalyst in Ar-saturated 0.5 M H2SO4 solution, v = 50 mV s−1. Figure S15. CV curves of CDC2/Pd_EG catalyst in Ar-saturated 0.5 M H2SO4 solution, v = 50 mV s−1. Figure S16. CV curves of CDC3/Pd_EG catalyst in Ar-saturated 0.5 M H2SO4 solution, v = 50 mV s−1. Figure S17. a) RDE results of CDC1/Pd_Cit in O2-saturated 0.1 M KOH and b) corresponding K–L plots, inset shows the potential dependence of n. Figure S18. a) RDE results of CDC2/Pd_Cit in O2-saturated 0.1 M KOH and b) corresponding K–L plots, inset shows the potential dependence of n. Figure S19. a) RDE results of CDC3/Pd_Cit in O2-saturated 0.1 M KOH and b) corresponding K–L plots, inset shows the potential dependence of n. Figure S20. a) RDE results of CDC2/Pd_EG in O2-saturated 0.1 M KOH and b) corresponding K–L plots, inset shows the potential dependence of n. Figure S21. a) RDE results of CDC3/Pd_EG in O2-saturated 0.1 M KOH and b) corresponding K–L plots, inset shows the potential dependence of n. References [8,45,51,59,60,63,64,65,66,67,68,69,70] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, K.T.; methodology, M.L., H.E., M.K., H.-M.P., J.A., A.K., V.K., J.L., K.K. and K.T.; validation, M.L., M.K., H.-M.P., J.A. and A.K.; formal analysis, M.L., M.K., H.-M.P., J.A. and A.K.; investigation, M.L., M.K., H.-M.P., J.A. and A.K.; resources, V.K., J.L., K.K. and K.T.; data curation, M.L.; writing—original draft preparation, M.L.; writing—review and editing, M.L., H.E., M.K., H.-M.P., J.A., A.K., V.K., J.L., K.K. and K.T.; funding acquisition, V.K., J.L., K.K. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Estonian Research Council, grant number PRG723, PRG753 and PRG1509. This work was also supported by the Estonian Ministry of Education and Research, grant number TK210 (Centre of Excellence in Sustainable Green Hydrogen and Energy Technologies).
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
We thank Peeter Paaver (Institute of Ecology and Earth Sciences of the University of Tartu) for the MP-AES measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Shao, M. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Meng, H.; Zeng, D.; Xie, F. Recent Development of Pd-Based Electrocatalysts for Proton Exchange Membrane Fuel Cells. Catalysts 2015, 5, 1221–1274. [Google Scholar] [CrossRef]
- Luo, M.; Sun, Y.; Qin, Y.; Li, Y.; Li, C.; Yang, Y.; Xu, N.; Wang, L.; Guo, S. Palladium-based nanoelectrocatalysts for renewable energy generation and conversion. Mater. Today Nano 2018, 1, 29–40. [Google Scholar] [CrossRef]
- Carrera-Cerritos, R.; Baglio, V.; Aricò, A.S.; Ledesma-García, J.; Sgroi, M.F.; Pullini, D.; Pruna, A.J.; Mataix, D.B.; Fuentes-Ramírez, R.; Arriaga, L.G. Improved Pd electro-catalysis for oxygen reduction reaction in direct methanol fuel cell by reduced graphene oxide. Appl. Catal. B Environ. 2014, 144, 554–560. [Google Scholar] [CrossRef]
- Jiang, L.; Hsu, A.; Chu, D.; Chen, R. Oxygen Reduction Reaction on Carbon Supported Pt and Pd in Alkaline Solutions. J. Electrochem. Soc. 2009, 156, B370–B376. [Google Scholar] [CrossRef]
- Seo, M.H.; Choi, S.M.; Kim, H.J.; Kim, W.B. The graphene-supported Pd and Pt catalysts for highly active oxygen reduction reaction in an alkaline condition. Electrochem. Commun. 2011, 13, 182–185. [Google Scholar] [CrossRef]
- Jiang, L.; Hsu, A.; Chu, D.; Chen, R. Size-Dependent Activity of Palladium Nanoparticles for Oxygen Electroreduction in Alkaline Solutions. J. Electrochem. Soc. 2009, 156, B643–B649. [Google Scholar] [CrossRef]
- Erikson, H.; Kasikov, A.; Johans, C.; Kontturi, K.; Tammeveski, K.; Sarapuu, A. Oxygen reduction on Nafion-coated thin-film palladium electrodes. J. Electroanal. Chem. 2011, 652, 1–7. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Tammeveski, K.; Solla-Gullon, J.; Feliu, J.M. Enhanced electrocatalytic activity of cubic Pd nanoparticles towards the oxygen reduction reaction in acid media. Electrochem. Commun. 2011, 13, 734–737. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Alexeyeva, N.; Tammeveski, K.; Solla-Gullón, J.; Feliu, J.M. Electrochemical reduction of oxygen on palladium nanocubes in acid and alkaline solutions. Electrochim. Acta 2012, 59, 329–335. [Google Scholar] [CrossRef]
- Lee, C.-L.; Chiou, H.-P. Methanol-tolerant Pd nanocubes for catalyzing oxygen reduction reaction in H2SO4 electrolyte. Appl. Catal. B Environ. 2012, 117–118, 204–211. [Google Scholar] [CrossRef]
- Lee, C.-L.; Chiou, H.-P.; Liu, C.-R. Palladium nanocubes enclosed by (100) planes as electrocatalyst for alkaline oxygen electroreduction. Int. J. Hydrogen Energy 2012, 37, 3993–3997. [Google Scholar] [CrossRef]
- Arjona, N.; Guerra-Balcázar, M.; Ortiz-Frade, L.; Osorio-Monreal, G.; Álvarez-Contreras, L.; Ledesma-García, J.; Arriaga, L.G. Electrocatalytic activity of well-defined and homogeneous cubic-shaped Pd nanoparticles. J. Mater. Chem. A 2013, 1, 15524–15529. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Z.; Lee, C. Truncated palladium nanocubes: Synthesis and the effect of OH- concentration on their catalysis of the alkaline oxygen reduction reaction. Electrochim. Acta 2015, 157, 78–87. [Google Scholar] [CrossRef]
- Shao, M.H.; Odell, J.; Humbert, M.; Yu, T.Y.; Xia, Y.N. Electrocatalysis on Shape-Controlled Palladium Nanocrystals: Oxygen Reduction Reaction and Formic Acid Oxidation. J. Phys. Chem. C 2013, 117, 4172–4180. [Google Scholar] [CrossRef]
- Kiguchi, F.; Nakamura, M.; Hoshi, N. Cation Effects on ORR Activity on Low-index Planes of Pd in Alkaline Solution. Electrochemistry 2021, 89, 145–147. [Google Scholar] [CrossRef]
- Kondo, S.; Nakamura, M.; Maki, N.; Hoshi, N. Active sites for the oxygen reduction reaction on the low and high index planes of palladium. J. Phys. Chem. C 2009, 113, 12625–12628. [Google Scholar] [CrossRef]
- Li, C.-J.; Shan, G.-C.; Guo, C.-X.; Ma, R.-G. Design strategies of Pd-based electrocatalysts for efficient oxygen reduction. Rare Met. 2023, 42, 1778–1799. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chang, Q.W.; Chen, H.M.; Shao, M.H. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Kumar, G.; Das, S.K.; Nayak, C.; Dey, R.S. Pd “Kills Two Birds with One Stone” for the Synthesis of Catalyst: Dual Active Sites of Pd Triggers the Kinetics of O2 Electrocatalysis. Small 2023, 20, e2307110. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Lao, M.; Zhou, H.; Huang, J.; Li, S.; Liang, Y.; Yin, S.; Xie, Z. Pd Nanoparticles on Carbon Supports as Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2023, 6, 20320–20328. [Google Scholar] [CrossRef]
- Vishnukumar, P.; Vivekanandhan, S.; Muthuramkumar, S. Plant-Mediated Biogenic Synthesis of Palladium Nanoparticles: Recent Trends and Emerging Opportunities. ChemBioEng Rev. 2017, 4, 18–36. [Google Scholar] [CrossRef]
- Jukk, K.; Alexeyeva, N.; Johans, C.; Kontturi, K.; Tammeveski, K. Oxygen reduction on Pd nanoparticle/multi-walled carbon nanotube composites. J. Electroanal. Chem. 2012, 666, 67–75. [Google Scholar] [CrossRef]
- Jukk, K.; Alexeyeva, N.; Sarapuu, A.; Ritslaid, P.; Kozlova, J.; Sammelselg, V.; Tammeveski, K. Electroreduction of oxygen on sputter-deposited Pd nanolayers on multi-walled carbon nanotubes. Int. J. Hydrogen Energy 2013, 38, 3614–3620. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chen, D.-H.; Huang, T.-C. Synthesis of palladium nanoparticles in water-in-oil microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2001, 189, 145–154. [Google Scholar] [CrossRef]
- Chen, M.; Feng, Y.-g.; Wang, L.-y.; Zhang, L.; Zhang, J.-Y. Study of palladium nanoparticles prepared from water-in-oil microemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2006, 281, 119–124. [Google Scholar] [CrossRef]
- Hirai, H.; Yakura, N. Protecting polymers in suspension of metal nanoparticles. Polym. Adv. Technol. 2001, 12, 724–733. [Google Scholar] [CrossRef]
- Burton, P.D.; Boyle, T.J.; Datye, A.K. Facile, surfactant-free synthesis of Pd nanoparticles for heterogeneous catalysts. J. Catal. 2011, 280, 145–149. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Tsumori, N.; Xu, Q. Immobilizing Extremely Catalytically Active Palladium Nanoparticles to Carbon Nanospheres: A Weakly-Capping Growth Approach. J. Am. Chem. Soc. 2015, 137, 11743–11748. [Google Scholar] [CrossRef]
- Roy Chowdhury, S.; Sarathi Roy, P.; Bhattacharya, S.K. Green synthesis and characterization of polyvinyl alcohol stabilized palladium nanoparticles: Effect of solvent on diameter and catalytic activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025002. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, H.; Li, Y.; Zhou, Y.; Fang, B. Ligand-free synthesis of noble metal nanocatalysts for electrocatalysis. Chem. Eng. J. 2023, 451, 138668. [Google Scholar] [CrossRef]
- Bonet, F.; Delmas, V.; Grugeon, S.; Herrera Urbina, R.; Silvert, P.Y.; Tekaia-Elhsissen, K. Synthesis of monodisperse Au, Pt, Pd, Ru and Ir nanoparticles in ethylene glycol. Nanostruct. Mater. 1999, 11, 1277–1284. [Google Scholar] [CrossRef]
- Chen, L.-J.; Wan, C.-C.; Wang, Y.-Y. Chemical preparation of Pd nanoparticles in room temperature ethylene glycol system and its application to electroless copper deposition. J. Colloid Interface Sci. 2006, 297, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Sanij, F.D.; Balakrishnan, P.; Leung, P.; Shah, A.; Su, H.; Xu, Q. Advanced Pd-based nanomaterials for electro-catalytic oxygen reduction in fuel cells: A review. Int. J. Hydrogen Energy 2021, 46, 14596–14627. [Google Scholar] [CrossRef]
- Lüsi, M.; Erikson, H.; Merisalu, M.; Rähn, M.; Sammelselg, V.; Tammeveski, K. Electrochemical reduction of oxygen in alkaline solution on Pd/C catalysts prepared by electrodeposition on various carbon nanomaterials. J. Electroanal. Chem. 2019, 834, 223–232. [Google Scholar] [CrossRef]
- Lüsi, M.; Erikson, H.; Treshchalov, A.; Rähn, M.; Merisalu, M.; Kikas, A.; Kisand, V.; Sammelselg, V.; Tammeveski, K. Oxygen reduction reaction on Pd nanocatalysts prepared by plasma-assisted synthesis on different carbon nanomaterials. Nanotechnology 2020, 32, 035401. [Google Scholar] [CrossRef]
- Perini, L.; Durante, C.; Favaro, M.; Perazzolo, V.; Agnoli, S.; Schneider, O.; Granozzi, G.; Gennaro, A. Metal–Support Interaction in Platinum and Palladium Nanoparticles Loaded on Nitrogen-Doped Mesoporous Carbon for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ko, Y.; Lee, W.; Züttel, A.; Kim, W. Nitrogen-doped carbon black supported Pt–M (M = Pd, Fe, Ni) alloy catalysts for oxygen reduction reaction in proton exchange membrane fuel cell. Mater. Today Energy 2019, 13, 374–381. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Wang, L.K.; Fang, L.P.; Tian, Y.L.; Tang, Y.; Niu, X.L.; Hao, Y.P.; Li, Z.F. A Facile Method to Prepare Ultrafine Pd Nanoparticles Embedded into N-Doped Porous Carbon Nanosheets as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction. J. Electrochem. Soc. 2020, 167, 054508. [Google Scholar] [CrossRef]
- Ju, W.; Favaro, M.; Durante, C.; Perini, L.; Agnoli, S.; Schneider, O.; Stimming, U.; Granozzi, G. Pd Nanoparticles deposited on nitrogen-doped HOPG: New Insights into the Pd-catalyzed Oxygen Reduction Reaction. Electrochim. Acta 2014, 141, 89–101. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Wang, T.; Zhang, Z.; Wang, X.; Cheng, C.; Liu, X. Inner-pore reduction nucleation of palladium nanoparticles in highly conductive wurster-type covalent organic frameworks for efficient oxygen reduction electrocatalysis. J. Energy Chem. 2023, 77, 543–552. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Zhuo, H.; Wei, Z.Z.; Zhuang, G.L.; Zhong, X.; Deng, S.W.; Li, X.N.; Wang, J.G. Oxygen vacancies on TiO2 promoted the activity and stability of supported Pd nanoparticles for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2264–2272. [Google Scholar] [CrossRef]
- Käärik, M.; Arulepp, M.; Kook, M.; Mäeorg, U.; Kozlova, J.; Sammelselg, V.; Perkson, A.; Leis, J. Characterisation of steam-treated nanoporous carbide-derived carbon of TiC origin: Structure and enhanced electrochemical performance. J. Porous Mater. 2018, 25, 1057–1070. [Google Scholar] [CrossRef]
- Käärik, M.; Arulepp, M.; Käärik, M.; Maran, U.; Leis, J. Characterization and prediction of double-layer capacitance of nanoporous carbon materials using the Quantitative nano-Structure-Property Relationship approach based on experimentally determined porosity descriptors. Carbon 2020, 158, 494–504. [Google Scholar] [CrossRef]
- Leis, J.; Arulepp, M.; Käärik, M.; Perkson, A. Method of Synthesis of Electrocatalytically Active Porous Carbon Material for Oxygen Reduction in Low-Temperature Fuel Cells. WO2013011146A2, 21 July 2012. [Google Scholar]
- Ratso, S.; Zitolo, A.; Käärik, M.; Merisalu, M.; Kikas, A.; Kisand, V.; Rähn, M.; Paiste, P.; Leis, J.; Sammelselg, V.; et al. Non-precious metal cathodes for anion exchange membrane fuel cells from ball-milled iron and nitrogen doped carbide-derived carbons. Renew. Energy 2021, 167, 800–810. [Google Scholar] [CrossRef]
- Davey, W.P. Precision Measurements of the Lattice Constants of Twelve Common Metals. Phys. Rev. B 1925, 25, 753–761. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Treshchalov, A.; Rahn, M.; Kook, M.; Merisalu, M.; Matisen, L.; Sammelselg, V.; Tammeveski, K. Oxygen Electroreduction on Pt Nanoparticles Deposited on Reduced Graphene Oxide and N-doped Reduced Graphene Oxide Prepared by Plasma-assisted Synthesis in Aqueous Solution. Chemelectrochem 2018, 5, 2902–2911. [Google Scholar] [CrossRef]
- Asanova, T.I.; Asanov, I.P.; Tur, V.A.; Gerasimov, E.Y.; Brzhezinskaya, M. PtPd-nanoparticles supported by new carbon materials. J. Struct. Chem. 2016, 57, 1398–1406. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Belenkov, E.A.; Greshnyakov, V.A.; Yalovega, G.E.; Bashkin, I.O. New aspects in the study of carbon-hydrogen interaction in hydrogenated carbon nanotubes for energy storage applications. J. Alloys Compd. 2019, 792, 713–720. [Google Scholar] [CrossRef]
- X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: https://www.xpsfitting.com/2017/10/palladium.html (accessed on 12 January 2024).
- Grden, M.; Lukaszewski, M.; Jerkiewicz, G.; Czerwinski, A. Electrochemical behaviour of palladium electrode: Oxidation, electrodissolution and ionic adsorption. Electrochim. Acta 2008, 53, 7583–7598. [Google Scholar] [CrossRef]
- López-Cudero, A.; Solla-Gullón, J.; Herrero, E.; Aldaz, A.; Feliu, J.M. CO electrooxidation on carbon supported platinum nanoparticles: Effect of aggregation. J. Electroanal. Chem. 2010, 644, 117–126. [Google Scholar] [CrossRef]
- Lüsi, M.; Erikson, H.; Sarapuu, A.; Merisalu, M.; Rähn, M.; Treshchalov, A.; Paiste, P.; Käärik, M.; Leis, J.; Sammelselg, V.; et al. Electroreduction of Oxygen on Carbide-Derived Carbon Supported Pd Catalysts. Chemelectrochem 2020, 7, 546–554. [Google Scholar] [CrossRef]
- Lüsi, M.; Erikson, H.; Tammeveski, K.; Treshchalov, A.; Kikas, A.; Piirsoo, H.-M.; Kisand, V.; Tamm, A.; Aruväli, J.; Solla-Gullón, J.; et al. Oxygen reduction reaction on Pd nanoparticles supported on novel mesoporous carbon materials. Electrochim. Acta 2021, 394, 139132. [Google Scholar] [CrossRef]
- Zadick, A.; Dubau, L.; Demirci, U.B.; Chatenet, M. Effects of Pd Nanoparticle Size and Solution Reducer Strength on Pd/C Electrocatalyst Stability in Alkaline Electrolyte. J. Electrochem. Soc. 2016, 163, F781–F787. [Google Scholar] [CrossRef]
- Martins, C.A.; Fernández, P.S.; Troiani, H.E.; Martins, M.E.; Arenillas, A.; Camara, G.A. Agglomeration and Cleaning of Carbon Supported Palladium Nanoparticles in Electrochemical Environment. Electrocatalysis 2014, 5, 204–212. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry, Physics; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- Gracia-Espino, E.; Jia, X.; Wågberg, T. Improved Oxygen Reduction Performance of Pt–Ni Nanoparticles by Adhesion on Nitrogen-Doped Graphene. J. Phys. Chem. C 2014, 118, 2804–2811. [Google Scholar] [CrossRef]
- Ejaz, A.; Jeon, S. The individual role of pyrrolic, pyridinic and graphitic nitrogen in the growth kinetics of Pd NPs on N-rGO followed by a comprehensive study on ORR. Int. J. Hydrogen Energy 2018, 43, 5690–5702. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Chen, S.; Wang, H.; Huang, M. Nitrogen and Oxygen Co-Doping Assisted Synthesis of Highly Dispersed Pd Nanoparticles on Hollow Carbon Spheres as Efficient Electrocatalysts for Oxygen Reduction Reaction. Chem. Eur. J. 2020, 26, 12589–12595. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, J.; Li, P.; Zhao, G.; Wang, G.; Jiang, Y.; Chen, J.; Dou, S.X.; Pan, H.; Sun, W. Hexagonal Boron Nitride as a Multifunctional Support for Engineering Efficient Electrocatalysts toward the Oxygen Reduction Reaction. Nano Lett. 2020, 20, 6807–6814. [Google Scholar] [CrossRef]
- Wang, M.; Qin, X.; Jiang, K.; Dong, Y.; Shao, M.; Cai, W.-B. Electrocatalytic Activities of Oxygen Reduction Reaction on Pd/C and Pd–B/C Catalysts. J. Phys. Chem. C 2017, 121, 3416–3423. [Google Scholar] [CrossRef]
- Yan, W.; Tang, Z.; Li, L.; Wang, L.; Yang, H.; Wang, Q.; Wu, W.; Chen, S. Ultrasmall Palladium Nanoclusters Encapsulated in Porous Carbon Nanosheets for Oxygen Electroreduction in Alkaline Media. ChemElectroChem 2017, 4, 1349–1355. [Google Scholar] [CrossRef]
- Khalily, M.A.; Patil, B.; Yilmaz, E.; Uyar, T. Atomic Layer Deposition of Pd Nanoparticles on N-Doped Electrospun Carbon Nanofibers: Optimization of ORR Activity of Pd-Based Nanocatalysts by Tuning Their Nanoparticle Size and Loading. ChemNanoMat 2019, 5, 1540–1546. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, G.; Zeng, R.; Villarino, A.M.; DiSalvo, F.J.; van Dover, R.B.; Abruña, H.D. Combinatorial Studies of Palladium-Based Oxygen Reduction Electrocatalysts for Alkaline Fuel Cells. J. Am. Chem. Soc. 2020, 142, 3980–3988. [Google Scholar] [CrossRef]
- Si, W.; Yang, Z.; Hu, X.; Lv, Q.; Li, X.; Zhao, F.; He, J.; Huang, C. Preparation of zero valence Pd nanoparticles with ultra-efficient electrocatalytic activity for ORR. J. Mater. Chem. A 2021, 9, 14507–14514. [Google Scholar] [CrossRef]
- Lv, H.; Xu, D.; Sun, L.; Henzie, J.; Suib, S.L.; Yamauchi, Y.; Liu, B. Ternary Palladium–Boron–Phosphorus Alloy Mesoporous Nanospheres for Highly Efficient Electrocatalysis. ACS Nano 2019, 13, 12052–12061. [Google Scholar] [CrossRef] [PubMed]
- Vracar, L.M.; Sepa, D.B.; Damjanovic, A. Palladium Electrode in Oxygen-Saturated Aqueous Solutions: Reduction of Oxygen in the Activation-Controlled Region. J. Electrochem. Soc. 1986, 133, 1835–1839. [Google Scholar] [CrossRef]
- Vracar, L.M.; Sepa, D.B.; Damjanovic, A. Palladium Electrode in Oxygen-Saturated Aqueous Solutions: Potential Dependent Adsorption of Oxygen Containing Species and Their Effect on Oxygen Reduction. J. Electrochem. Soc. 1989, 136, 1973–1977. [Google Scholar] [CrossRef]
- Huang, Y.; Seo, K.-D.; Park, D.-S.; Park, H.; Shim, Y.-B. Hydrogen Evolution and Oxygen Reduction Reactions in Acidic Media Catalyzed by Pd4S Decorated N/S Doped Carbon Derived from Pd Coordination Polymer. Small 2021, 17, 2007511. [Google Scholar] [CrossRef]
- Alvarez, G.F.; Mamlouk, M.; Kumar, S.M.S.; Scott, K. Preparation and characterisation of carbon-supported palladium nanoparticles for oxygen reduction in low temperature PEM fuel cells. J. Appl. Electrochem. 2011, 41, 925–937. [Google Scholar] [CrossRef]
- Shao, M.; Yu, T.; Odell, J.H.; Jin, M.; Xia, Y. Structural dependence of oxygen reduction reaction on palladium nanocrystals. Chem. Commun. 2011, 47, 6566–6568. [Google Scholar] [CrossRef]
- Srejic, I.; Rakocevic, Z.; Nenadovic, M.; Strbac, S. Oxygen reduction on polycrystalline palladium in acid and alkaline solutions: Topographical and chemical Pd surface changes. Electrochim. Acta 2015, 169, 22–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).