Silver and Samaria-Doped Ceria (Ag-SDC) Cermet Cathode for Low-Temperature Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
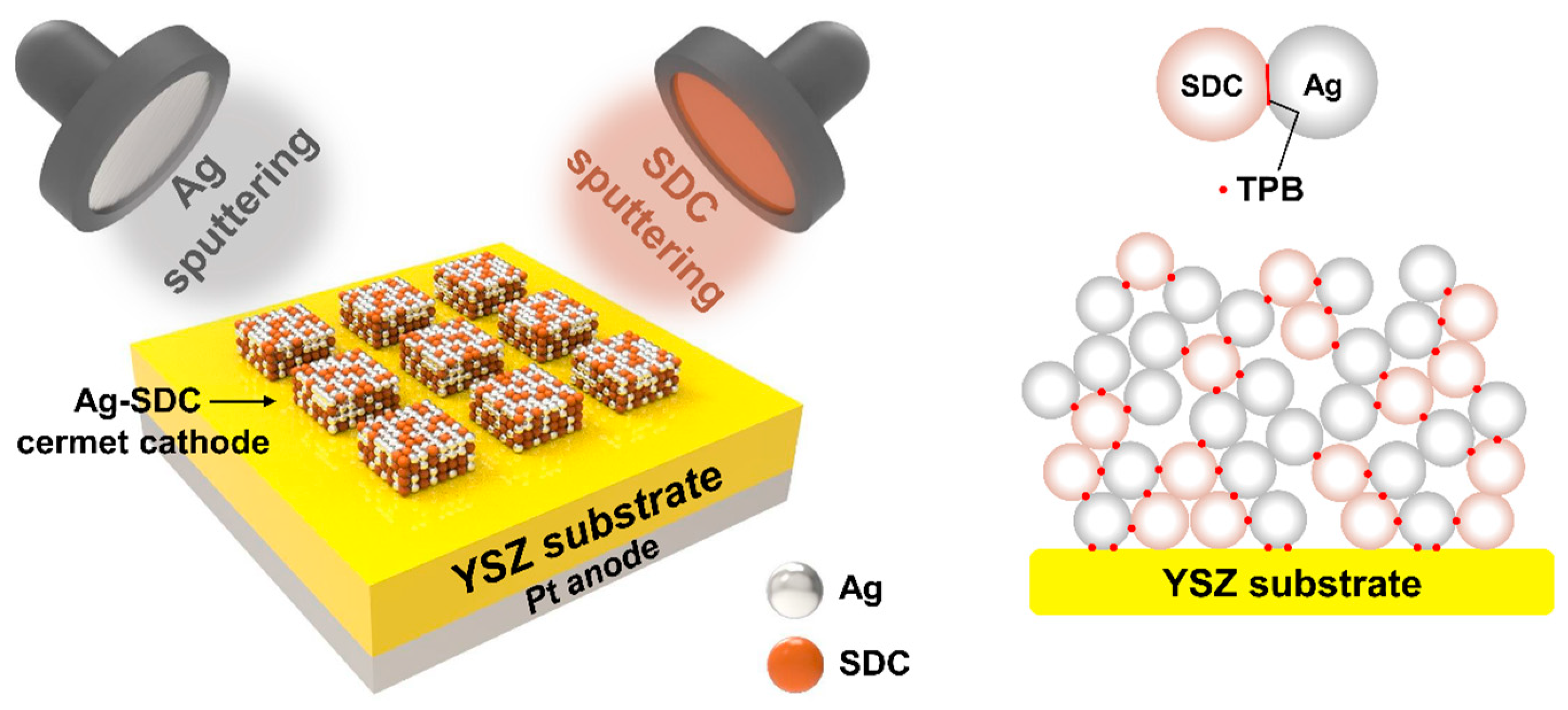
2.1. Sample Preparation
2.2. Fuel Cell Characterization
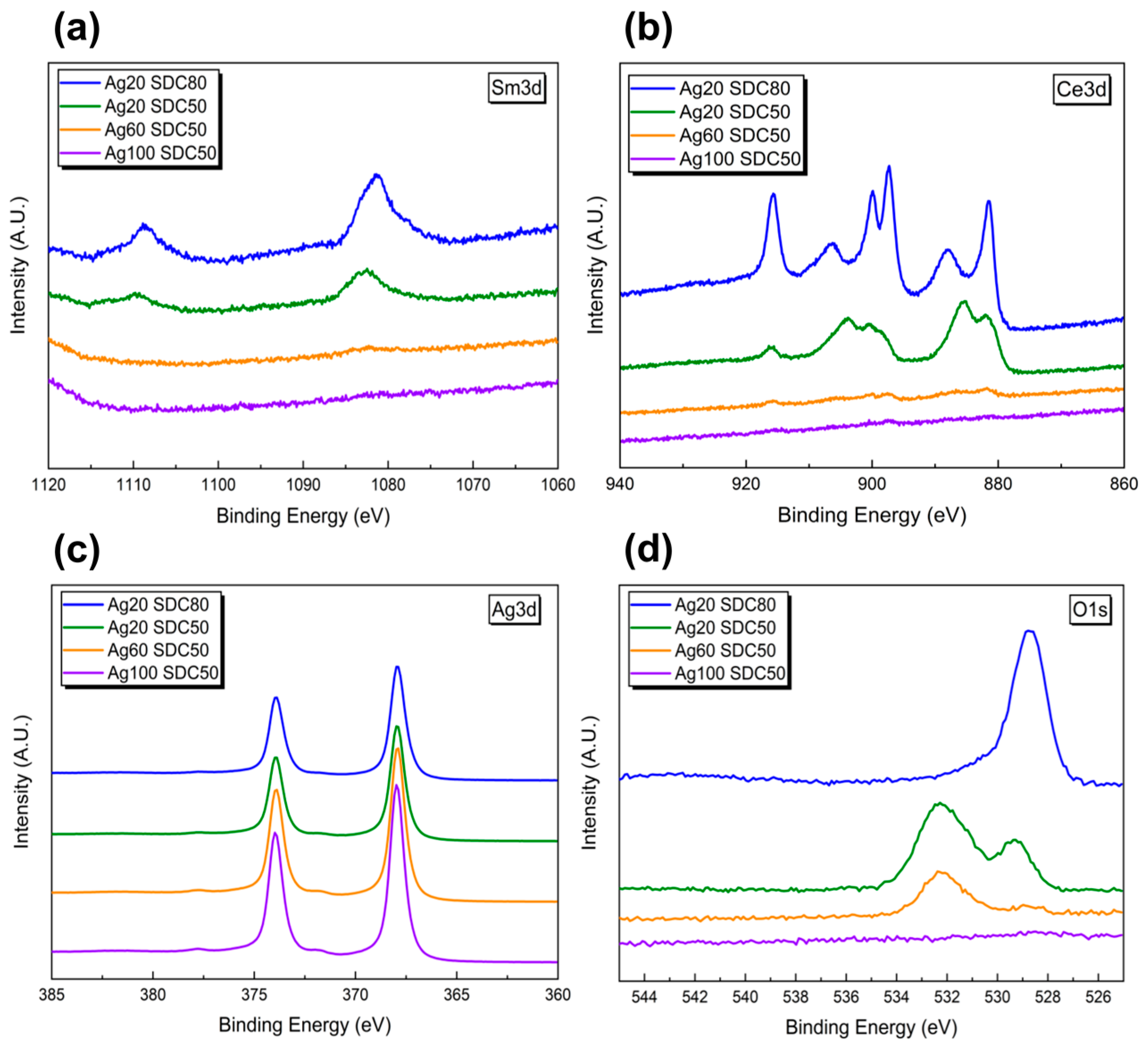
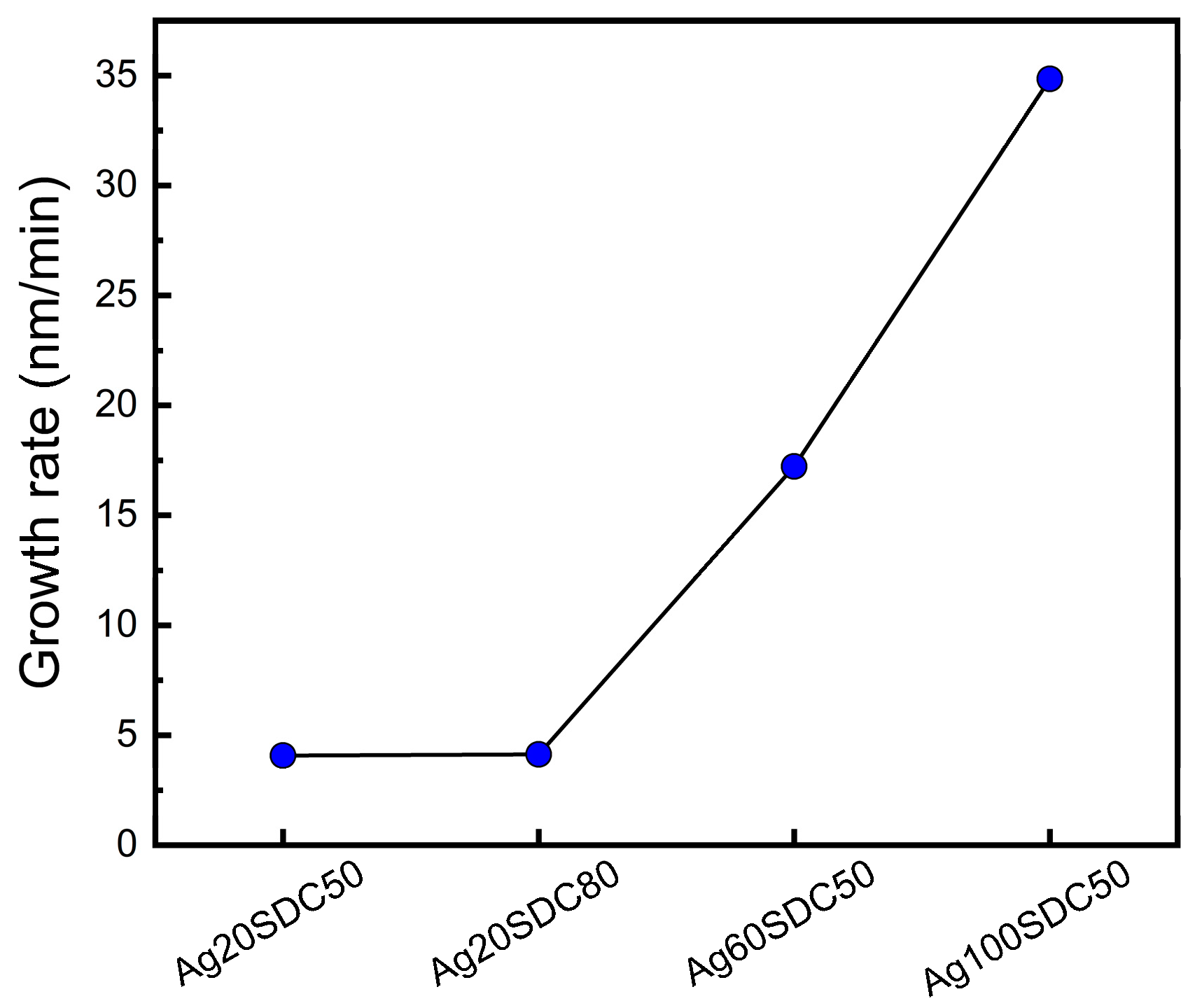
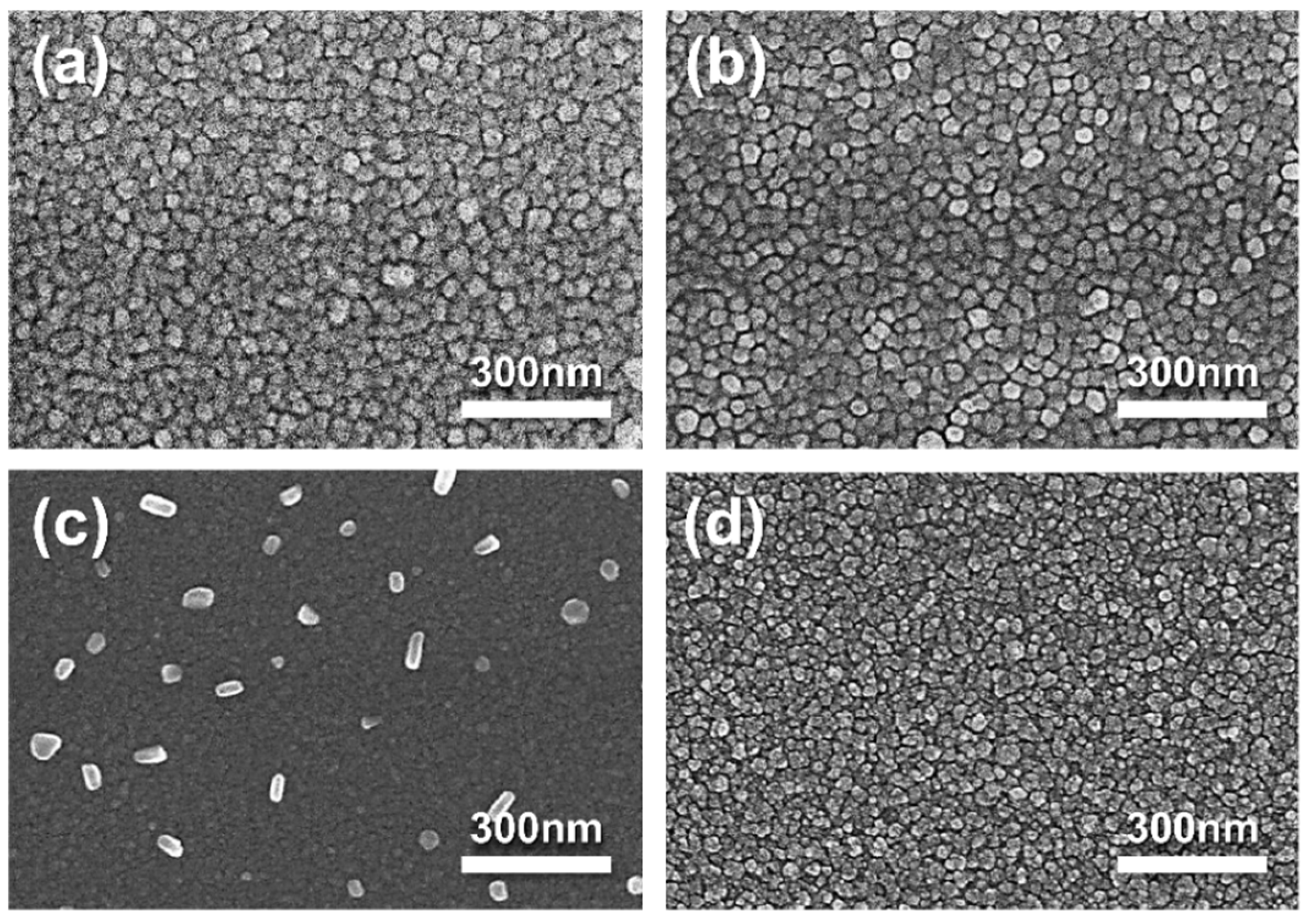
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venezia, E.; Viviani, M.; Presto, S.; Kumar, V.; Tomov, R.I. Inkjet printing functionalization of SOFC LSCF cathodes. Nanomaterials 2019, 9, 654. [Google Scholar] [CrossRef]
- Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Horri, B.A. Progress in material development for low-temperature solid oxide fuel cells: A review. Energies 2021, 14, 1280. [Google Scholar] [CrossRef]
- Filonova, E.; Medvedev, D. Recent progress in the design, characterization and application of LaAlO3- and LaGaO3-based solid oxide fuel cell electrolytes. Nanomaterials 2022, 12, 1991. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mi, Y.; Li, J.; Qi, F.; Yan, S.; Dong, W. Recent progress in semiconductor-ionic conductor nanomaterial as a membrane for low-temperature solid oxide fuel cells. Nanomaterials 2021, 11, 2290. [Google Scholar] [CrossRef] [PubMed]
- Raza, T.; Yang, J.; Wang, R.; Xia, C.; Raza, R.; Zhu, B.; Yun, S. Recent advance in physical description and material development for single component SOFC: A mini-review. Chem. Eng. J. 2022, 444, 136533. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Albert, T. Strategies for lowering solid oxide fuel cells operating temperature. Energies 2009, 2, 1130–1150. [Google Scholar]
- Raza, R.; Zhu, B.; Rafique, A.; Muhammad, R.N.; Lund, P. Functional ceria-based nanocomposites for advanced low-temperature (300–600 °C) solid oxide fuel cell: A comprehensive review. Mater. Today Energy 2020, 15, 100373. [Google Scholar] [CrossRef]
- An, J.; Kim, Y.-B.; Park, J.; Gür, T.M.; Prinz, F.B. Three-dimensional nanostructured bilayer solid oxide fuel cell with 1.3 W/cm2 at 450 °C. Nano Lett. 2013, 13, 4551–4555. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Kang, S.; Cha, S.-W.; Lee, W.; Kim, Y.-B.; Park, J.S.; Gür, T.M.; Prinz, F.B.; Chao, C.-C.; An, J. Atomic later deposition of thin-film ceramic electrolytes for high-performance fuel cells. J. Mater. Chem. A 2013, 1, 12695–12705. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Baker, J.; Majumdar, P.; Yang, Z.; Han, M.; Chen, F. Hierarchically oriented macroporous anode-supported solid oxide fuel cell with thin ceria electrolyte film. ACS Appl. Mater. Interfaces 2014, 6, 5130–5136. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Hong, S.; Son, J.; Lim, Y.; Yang, H.; Prinz, F.B.; Kim, Y.B. A homogeneous grain-controlled ScSZ functional layer for high performance low-temperature solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 16506–16514. [Google Scholar] [CrossRef]
- Hong, S.; Yang, H.; Lim, Y.; Prinz, F.B.; Kim, Y.-B. Grain-controlled gadolinia-doped ceria (GDC) functional layer for interface reaction enhanced low-temperature solid oxide fuel cells. ACS Appl. Mater. Interfaces 2019, 11, 41338–41346. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Sayagués, M.J.; Gotor, F.J. A Novel, Simple and Highly Efficient Route to Obtain PrBaMn2O5+δ Double Perovskite: Mechanochemical Synthesis. Nanomaterials 2021, 11, 380. [Google Scholar] [CrossRef]
- Lee, C.-H.; Yeh, B.-S.; Yang, T.-N. Study of the La1-xSrxMnO3 cathode film prepared by a low power plasma spray method with liquid solution precursor for a solid oxide fuel cell. Crystals 2022, 12, 1633. [Google Scholar] [CrossRef]
- Wang, S.; Yoon, J.; Kim, G.; Huang, D.; Wang, H.; Jacobson, A.J. Electrochemical properties of nanocrystalline La0.5Sr0.5CoO3-x thin films. Chem. Mater. 2010, 3, 776–782. [Google Scholar] [CrossRef]
- Hong, J.; Heo, S.J.; Singh, P. Combined Cr and S poisoning behaviors of La1-xSrxMnO3±δ and La1-xSrxCo1-yFeyO3-δ cathodes in solid oxide fuel cells. Appl. Surf. Sci. 2020, 530, 147253. [Google Scholar] [CrossRef]
- Wang, S.; Kato, T.; Nagata, S.; Honda, T.; Kaneko, T.; Iwashita, N.; Dokiya, M. Performance of a La0.6Sr0.4Co0.8Fe0.2O3-Ce0.8Gd0.2O1.9 Ag cathode for ceria electrolyte SOFCs. Solid State Ion. 2002, 146, 203–210. [Google Scholar] [CrossRef]
- Herle, J.V.; McEvoy, A.J. Oxygen diffusion through silver cathodes for solid oxide fuel cells. J. Phys. Chem. Solids 1994, 55, 339–347. [Google Scholar] [CrossRef]
- Wang, J.-H.; Liu, M.; Lin, M.C. Oxygen reduction reactions in the SOFC cathode of Ag/CeO2. Solid State Ion. 2006, 177, 939–947. [Google Scholar] [CrossRef]
- Yu, C.-C.; Beak, J.D.; Fan, L.; Liao, Y.-C.; Su, P.-C. Inkjet-printed porous silver thin film as a cathode for a low-temperature solid oxide fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 10343–10349. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, M.; Neoh, K.C.; Jang, D.Y.; Kim, H.J.; Shin, J.M.; Kim, G.-T.; Shim, J.H. High-performance silver cathode surface treated with Scandia-stabilized zirconia nanoparticles for intermediate temperature solid oxide fuel cells. Adv. Energy Mater. 2017, 7, 1601956. [Google Scholar] [CrossRef]
- Kamlungsua, K.; Lee, T.H.; Lee, S.; Su, P.C.; Yoon, Y.J. Inkjet-printed Ag@ SDC core-shell nanoparticles as a high-performance cathode for low-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 60–30853. [Google Scholar] [CrossRef]
- Kim, D.H.; Bae, K.; Choi, H.J.; Shim, J.H. Ag surface-coated with nano-YSZ as an alternative to Pt catalyst for low-temperature solid oxide fuel cells. J. Alloys Compd. 2018, 769, 545–551. [Google Scholar] [CrossRef]
- Neoh, K.C.; Han, G.D.; Kim, M.; Kim, J.W.; Choi, H.J.; Park, S.W.; Shim, J.H. Nanoporous silver cathode surface treated by atomic layer deposition of CeOx for low-temperature solid oxide fuel cells. Nanotechnology 2016, 27, 185403. [Google Scholar] [CrossRef]
- Chen, C.J.; Huang, J.C.; Chou, H.S.; Lai, Y.H.; Chang, L.W.; Du, X.H.; Chu, J.P.; Nieh, T.G. On the amorphous and nanocrystalline Zr-Cu and Zr-Ti co-sputtered thin films. J. Alloys Compd. 2009, 483, 337–340. [Google Scholar] [CrossRef]
- Mazur, M.; Wojcieszak, D.; Wiatrowski, A.; Kaczmarek, D.; Lubańska, A.; Domaradzki, J.; Mazur, P.; Kalisz, M. Analysis of amorphous tungsten oxide thin films deposited by magnetron sputtering for application in transparent electronics. Appl. Surf. Sci. 2021, 570, 151151. [Google Scholar] [CrossRef]
- Velasco, S.C.; Cavaleiro, A.; Carvalho, S. Functional properties of ceramic-Ag nanocomposite coatings produced by magnetron sputtering. Prog. Mater. Sci. 2016, 84, 159–191. [Google Scholar]
- Liang, Y.-C.; Deng, X.-S. Structure dependent luminescence evolution of c-axis-oriented ZnO nanofilms embedded with silver nanoparticles and clusters prepared by sputtering. J. Alloys Compd. 2013, 569, 144–149. [Google Scholar] [CrossRef]
- Xu, K.; Hao, L.; Du, M.; Mi, J.; Yu, Q.; Li, S.; Wang, J.; Li, S. Thermal emittance of Ag films deposited by magnetron sputtering. Vacuum 2020, 174, 109200. [Google Scholar] [CrossRef]
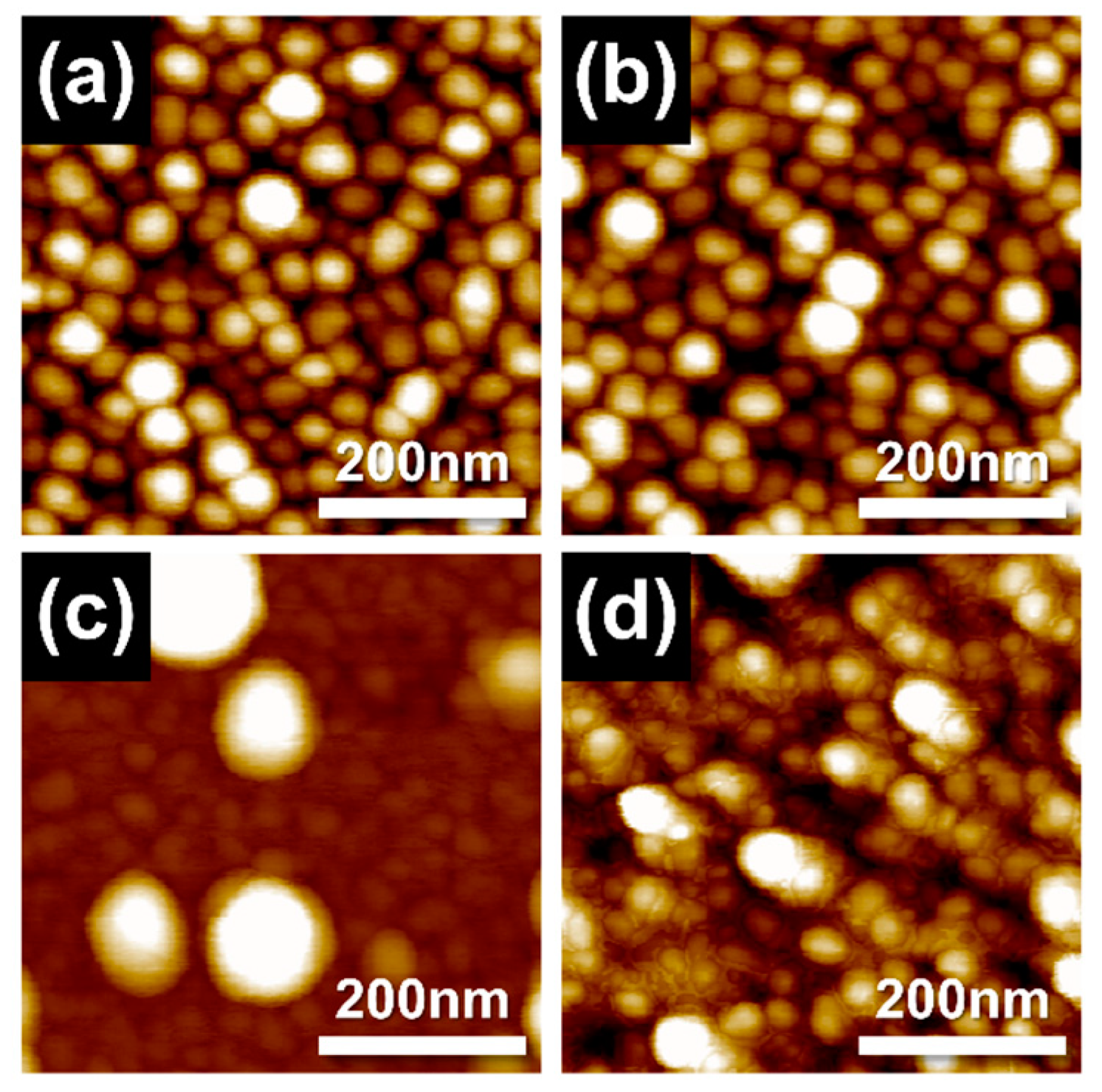

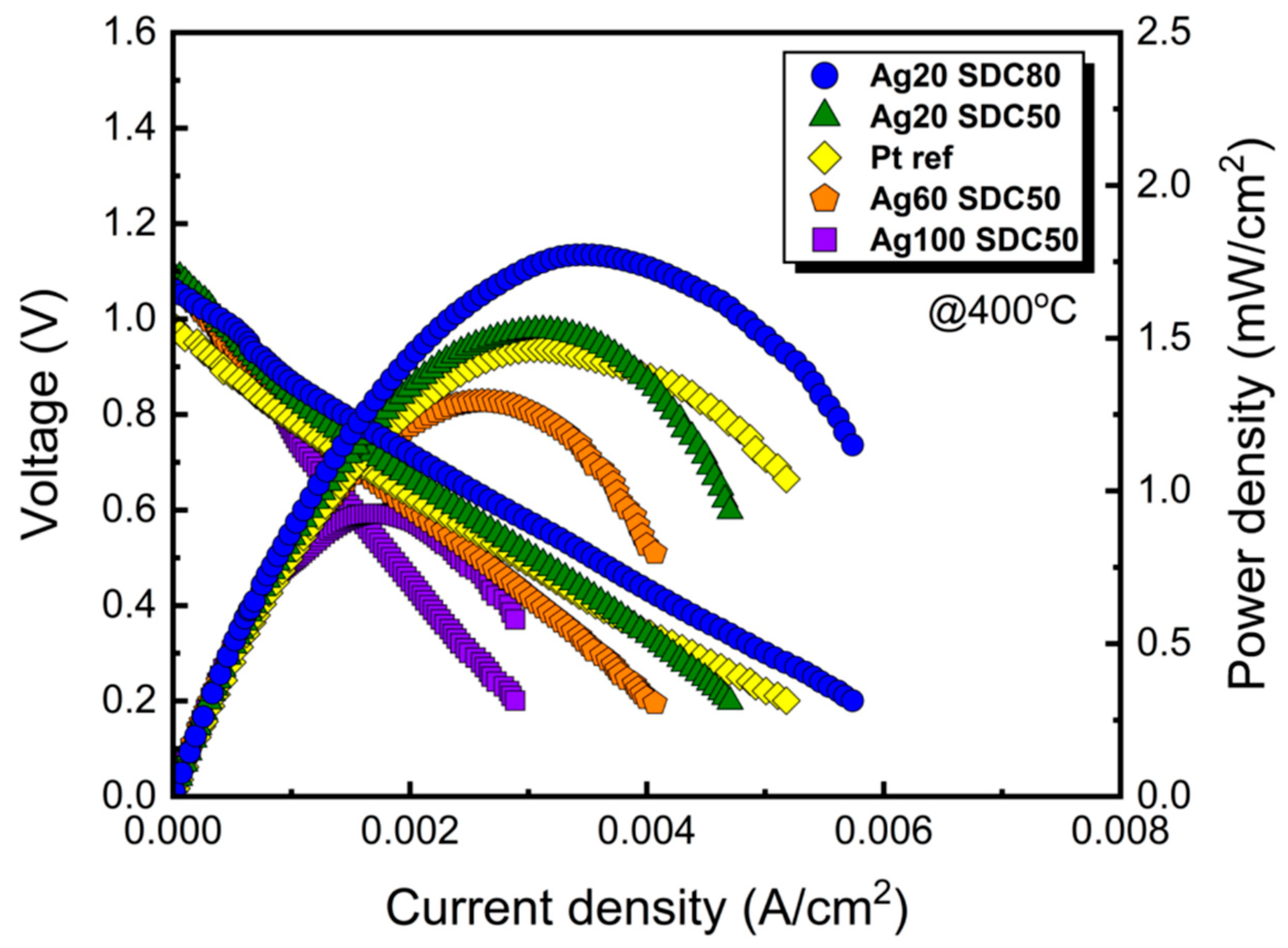
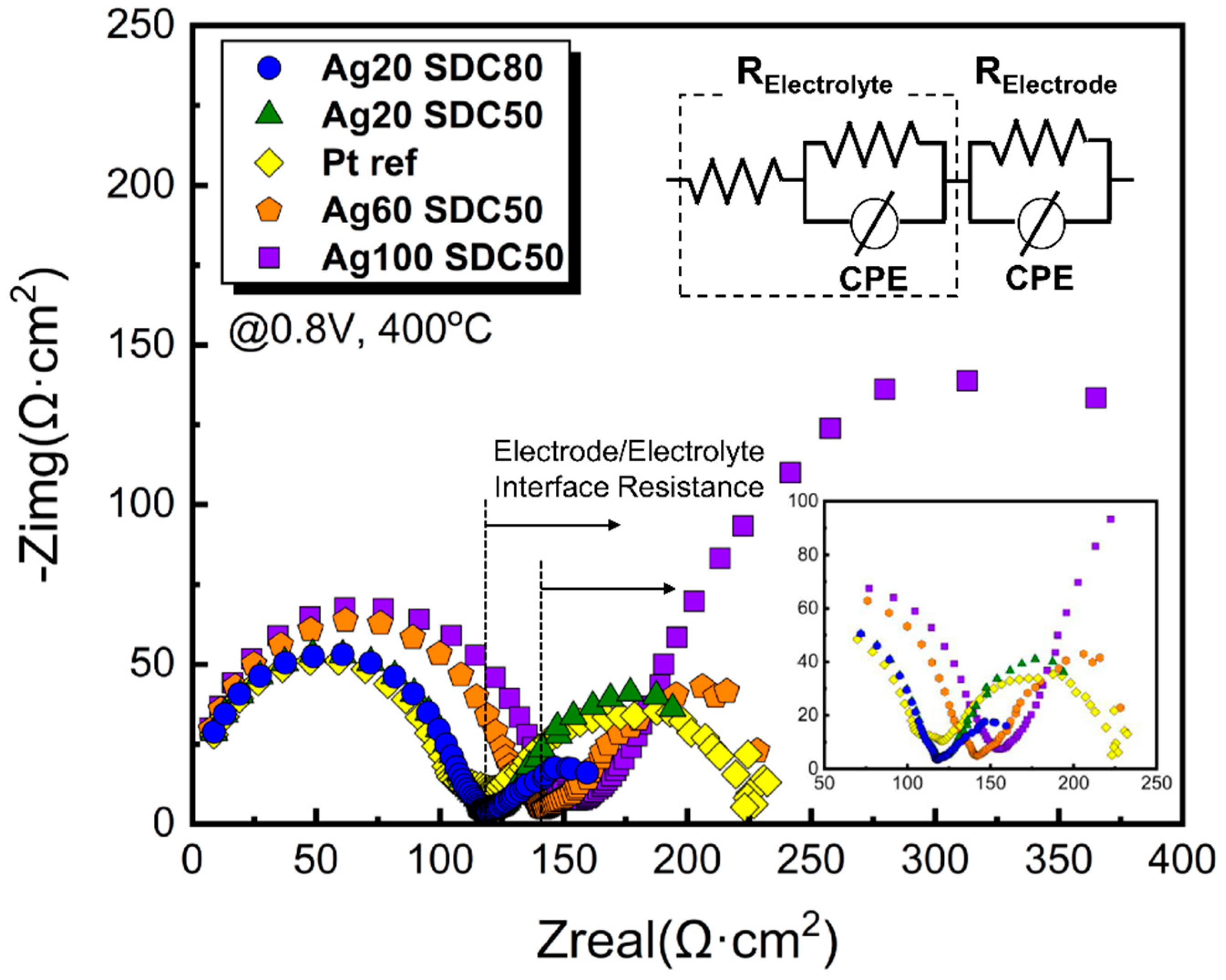
| Power of Ag (DC) | 20 W | 20 W | 60 W | 100 W |
| Power of SDC (RF) | 50 W | 80 W | 50 W | 50 W |
| Ag3d (at%) | 65.55 | 38.24 | 88.86 | 99.79 |
| Ce3d (at%) | 5.34 | 11.78 | 0.87 | 0.21 |
| Sm3d (at%) | 0.81 | 1.45 | 0 | 0 |
| O1s (at%) | 28.3 | 48.53 | 10.27 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, D.; Lim, Y.; Kim, H.; Park, Y.; Hong, S. Silver and Samaria-Doped Ceria (Ag-SDC) Cermet Cathode for Low-Temperature Solid Oxide Fuel Cells. Nanomaterials 2023, 13, 886. https://doi.org/10.3390/nano13050886
Jeong D, Lim Y, Kim H, Park Y, Hong S. Silver and Samaria-Doped Ceria (Ag-SDC) Cermet Cathode for Low-Temperature Solid Oxide Fuel Cells. Nanomaterials. 2023; 13(5):886. https://doi.org/10.3390/nano13050886
Chicago/Turabian StyleJeong, Davin, Yonghyun Lim, Hyeontaek Kim, Yongchan Park, and Soonwook Hong. 2023. "Silver and Samaria-Doped Ceria (Ag-SDC) Cermet Cathode for Low-Temperature Solid Oxide Fuel Cells" Nanomaterials 13, no. 5: 886. https://doi.org/10.3390/nano13050886
APA StyleJeong, D., Lim, Y., Kim, H., Park, Y., & Hong, S. (2023). Silver and Samaria-Doped Ceria (Ag-SDC) Cermet Cathode for Low-Temperature Solid Oxide Fuel Cells. Nanomaterials, 13(5), 886. https://doi.org/10.3390/nano13050886







