Construction of CNT-MgO-Ag-BaO Nanocomposite with Enhanced Field Emission and Hydrogen Sensing Performances
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CNT-Based Nanocomposite Cathodes on Alloy Substrates
2.2. Research Methodology
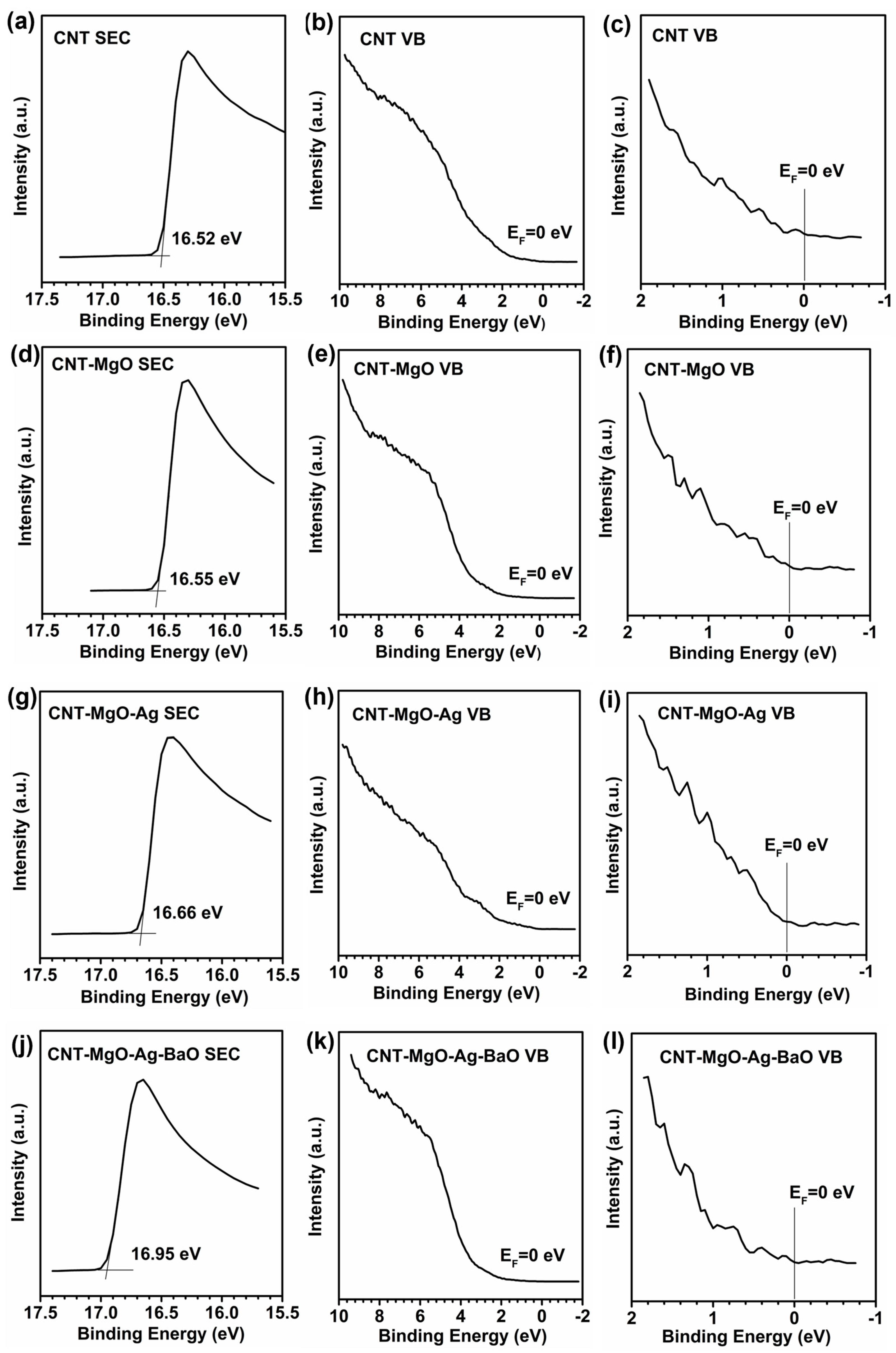
2.3. UPS Measurements
2.4. Tensile Test
2.5. Field Emission and Hydrogen Sensing Test
3. Results
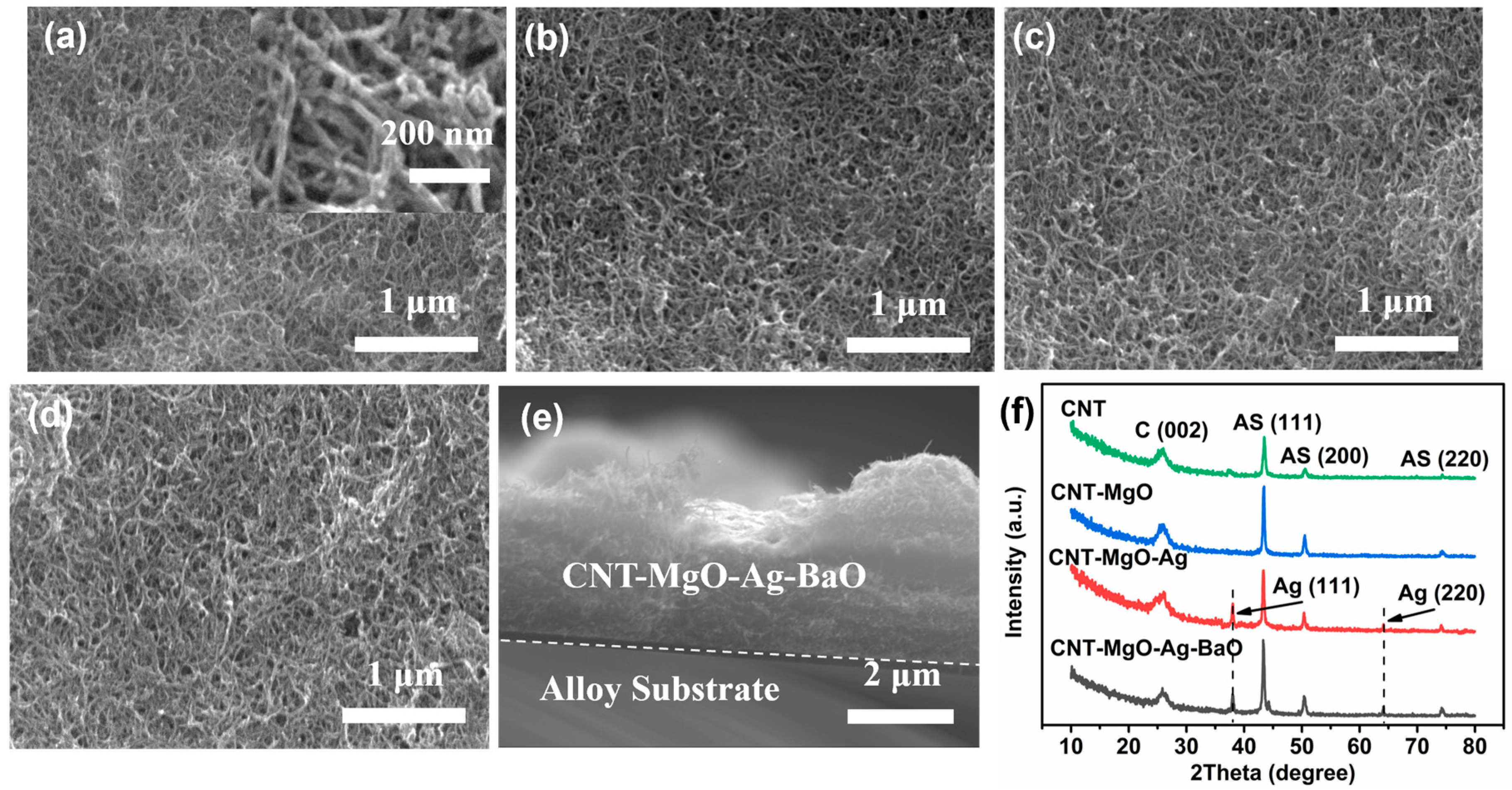
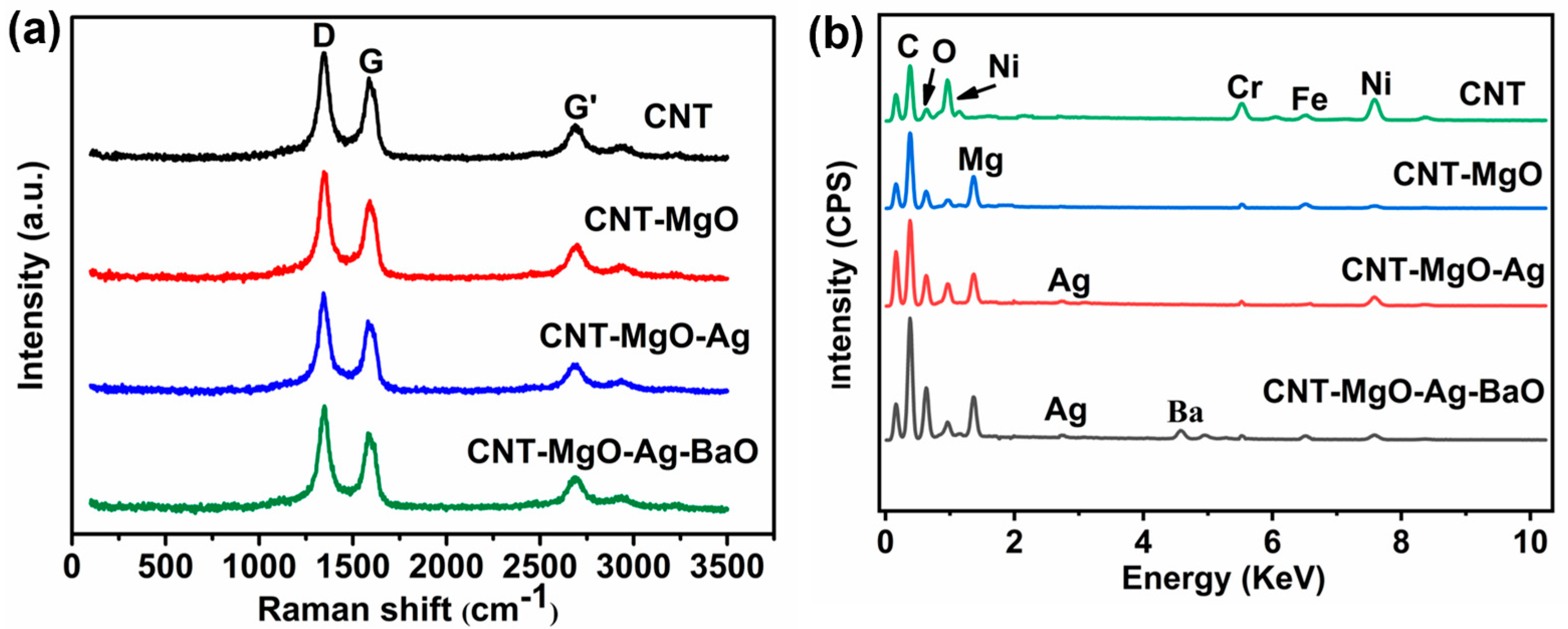
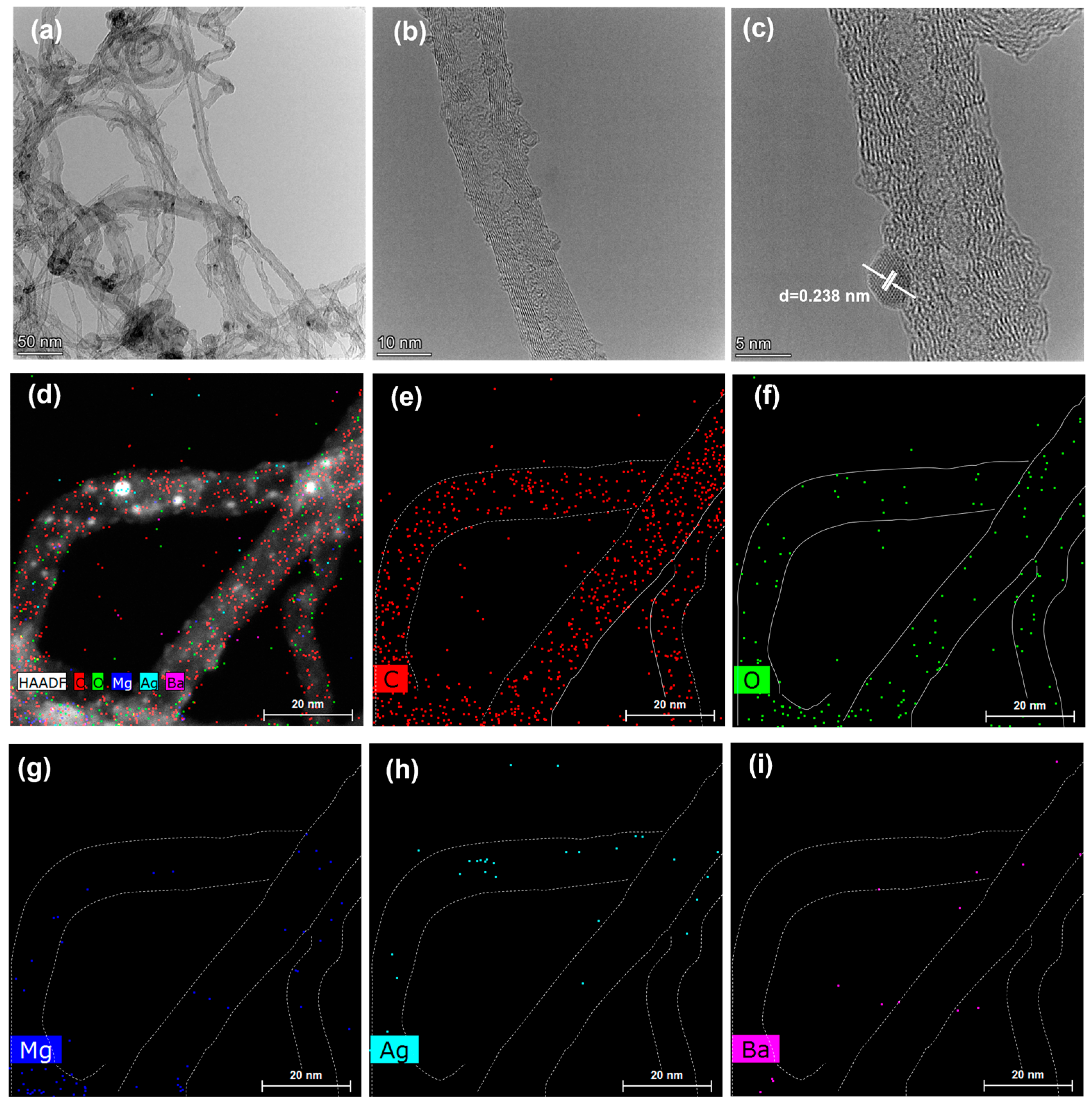
3.1. Morphologic and Structural Characterizations of CNT and CNT-Based Nanocomposites
3.2. Field Emission Performance Test
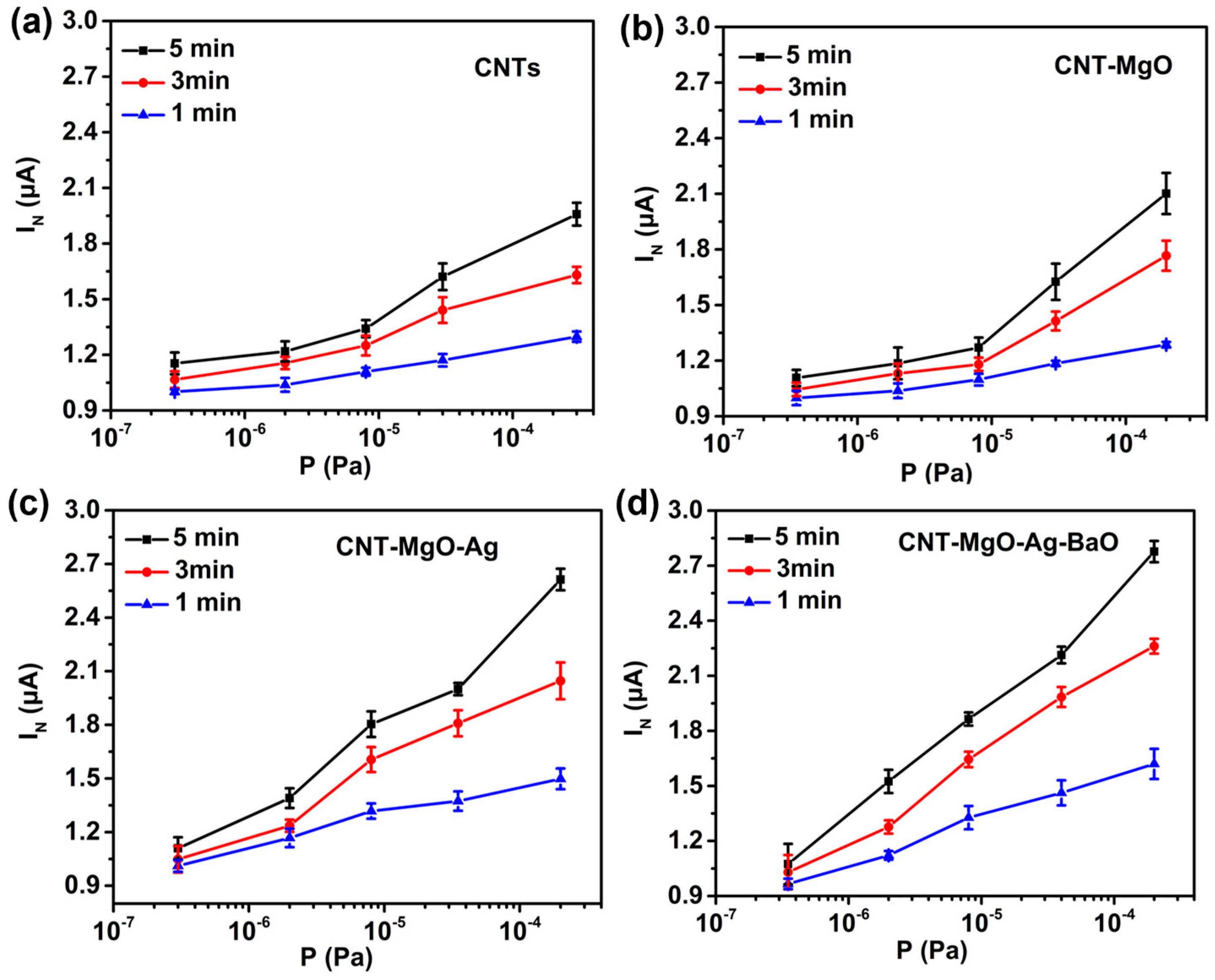
3.3. Hydrogen Sensing Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, C.; Gupta, M. Influences of the surface reactions on the field emission from multiwall carbon nanotubes. Appl. Phys. Lett. 2003, 83, 159–161. [Google Scholar]
- Zhang, Q.; Wang, X.J.; Meng, P.; Yue, H.X.; Zheng, R.T.; Wu, X.L.; Cheng, G.A. High current density and low emission field of carbon nanotube array microbundle. Appl. Phys. Lett. 2018, 112, 013101. [Google Scholar] [CrossRef]
- Thapa, A.; Poudel, Y.R.; Guo, R.; Jungjohann, K.L.; Li, W. Direct synthesis of micropillars of vertically aligned carbon nanotubes on stainless-steel and their excellent field emission properties. Carbon 2021, 171, 188–200. [Google Scholar] [CrossRef]
- Shao, X.; Srinivasan, A.; Ang, W.K.; Khursheed, A. A high-brightness large-diameter graphene coated point cathode field emission electron source. Nat. Commun. 2018, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, J.T.; Jeong, J.W.; Kim, J.W.; Yun, K.N.; Jeon, H.; Go, E.; Lee, J.W.; Ahn, Y.; Yeon, J.H. A Fully Closed Nano-Focus X-Ray Source With Carbon Nanotube Field Emitters. IEEE Electr. Device L. 2018, 39, 1936–1939. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, J.; Zhao, P.; Yang, K.; Zhao, J.; Chen, T.; Gong, Y.; Zeng, B. Design of a Ka-Band Traveling Wave Tube Using Low Turn-On Field Emission Electron Source Made by Carbon Nanotubes. IEEE T. Plasma Sci. 2021, 50, 29–35. [Google Scholar] [CrossRef]
- Huang, W.; Huang, Y.; Liu, R.; Zhu, W.; Kang, S.; Qian, W.; Dong, C. A dual-functional micro-focus X-ray source based on carbon nanotube field emission. Diam. Relat. Mater. 2022, 125, 108970. [Google Scholar] [CrossRef]
- Samareh, J.A.; Siochi, E.J. Systems Analysis of Carbon Nanotubes: Opportunities and Challenges for Space Applications. Nanotechnology 2017, 28, 372001. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, H.; Iijima, S. Single-wall carbon nanotubes synthesized by laser ablation in a nitrogen atmosphere. Appl. Phys. Lett. 1998, 73, 3827–3829. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Tang, H.; Liu, R.; Huang, W.; Zhu, W.; Qian, W.; Dong, C. Field Emission of Multi-Walled Carbon Nanotubes from Pt-Assisted Chemical Vapor Deposition. Nanomaterials 2022, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Wisitsoraat, A.; Phokharatkul, D.; Komin, K.; Jaruwongrangsee, K.; Tuantranont, A. Effect of Anodization Voltage on Electron Field Emission from Carbon Nanotubes in Anodized Alumina Template. J. Nanosci. Nanotechnol. 2011, 11, 10774–10777. [Google Scholar] [CrossRef]
- Li, M.; Luo, H.; Qian, W.; Huang, W.; Wan, L.; Dong, C. Catalyst free N-doped carbon nanotube arrays based on a ZnO nanorod template with high performance field emission. J. Mater. Chem. C 2019, 7, 8730–8736. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.; Minay, E.J.; Shaffer, M. Electrophoretic deposition of carbon nanotubes. Carbon 2006, 44, 3149–3160. [Google Scholar] [CrossRef]
- Dong, M.; Qian, W.; Liu, X.; Chen, Y.; Huang, W.; Dong, C. Enhanced thermo cell properties from N-doped carbon nanotube-Pd composite electrode. Surf. Coat. Technol. 2022, 434, 128–213. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, W.; Huang, W.; Liu, B.; Lin, H.; Dong, C. Carbon Nanotube-Graphene Hybrid Electrodes with Enhanced Thermo-Electrochemical Cell Properties. Nanomaterials 2019, 9, 1450. [Google Scholar] [CrossRef]
- Qian, W.; Cao, M.; Fei, X.; Dong, C. Thermo-Electrochemical Cells Based on Carbon Nanotube Electrodes by Electrophoretic Deposition. Nano-micro lett. 2016, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Kim, J.-P.; Park, J.-S. Effects of Al interlayer coating and thermal treatment on electron emission characteristics of carbon nanotubes deposited by electrophoretic method. Nanoscale Res. Lett. 2014, 9, 236. [Google Scholar]
- Zhang, Y.A.; Wu, C.X.; Lin, J.Y.; Lin, Z.X.; Guo, T.L. An improved planar-gate triode with CNTs field emitters by electrophoretic deposition. Appl. Surf. Sci. 2011, 257, 3259–3264. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Lin, J.Y.; Wu, C.X.; Li, F.S.; Guo, T.L. Stable field emission from planar-gate electron source with MWNTs by electrophoretic deposition. Solid State Electron. 2012, 67, 6–10. [Google Scholar] [CrossRef]
- Qian, W.; Li, M.; Chen, L.; Zhang, J.; Dong, C. Improving thermo-electrochemical cell performance by constructing Ag–MgO–CNTs nanocomposite electrodes. RSC Adv. 2015, 5, 97982–97987. [Google Scholar] [CrossRef]
- Dong, R.X.; Liu, C.T.; Huang, K.C.; Chiu, W.Y.; Ho, K.C.; Lin, J.J. Controlling formation of silver/carbon nanotube networks for highly conductive film surface. ACS Appl. Mater. Interfaces 2012, 4, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Li, L. Decoration of carbon nanotubes with silver nanoparticles for advanced CNT/polymer nanocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 961–967. [Google Scholar] [CrossRef]
- Halas, S.; Durakiewicz, T. Physical foundations of the oxide cathodes. Appl. Surf. Sci. 2006, 252, 6119–6121. [Google Scholar] [CrossRef]
- Dong, C.; Luo, H.; Cai, J.; Wang, F.; Zhao, Y.; Li, D. Hydrogen sensing characteristics from carbon nanotube field emissions. Nanoscale 2016, 8, 5599–5604. [Google Scholar] [CrossRef]
- Kang, S.; Qian, W.; Liu, R.; Yu, H.; Zhu, W.; Liao, X.; Wang, F.; Huang, W.; Dong, C. Miniature vacuum sensor based on gas adsorptions from carbon nanotube field emitters. Vacuum 2023, 207, 111663. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, J.; Luo, H.; Kang, S.; Qian, W.; Dong, C. Low pressure hydrogen sensing based on carbon nanotube field emission: Mechanism of atomic adsorption induced work function effects. Carbon 2017, 124, 669–674. [Google Scholar] [CrossRef]
- Zhao, F.; Qian, W.; Li, M.; Li, W.; Chen, L.; Zhong, F.; Huang, W.; Dong, C. Directly grown carbon nanotube based hybrid electrodes with enhanced thermo-cell performances. RSC Adv. 2017, 7, 23890–23895. [Google Scholar] [CrossRef]
- Mehta, K.K.; Mukhopadhyay, P.; Mandal, R.K.; Singh, A.K. Mechanical Properties Anisotropy of Cold-Rolled and Solution-Annealed Ni-Based Hastelloy C-276 Alloy. Metall. Mater. Trans. A 2014, 45, 3493–3504. [Google Scholar] [CrossRef]
- Nachimuthu, P.; Kim, Y.J.; Kuchibhatla, S.V.N.T.; Yu, Z.Q.; Jiang, W.; Engelhard, M.H.; Shutthanandan, V.; Szanyi, J.; Thevuthasan, S. Growth and Characterization of Barium Oxide Nanoclusters on YSZ(111). J. Phys. Chem. C 2009, 113, 14324–14328. [Google Scholar] [CrossRef]
- Renukadevi, R.; Sundaram, R.; Kaviyarasu, K. Barium Oxide nanoparticles with Robust Catalytic, Photocatalytic and Humidity Sensing Properties. J. Nanostruct. 2020, 10, 167–176. [Google Scholar]
- Li, M.; Huang, W.; Qian, W.; Liu, B.; Lin, H.; Li, W.; Wan, L.; Dong, C. Controllable Ag nanoparticle coated ZnO nanorod arrays on an alloy substrate with enhanced field emission performance. RSC Adv. 2017, 7, 46760–46766. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.; Day, C.M.; Little, S.A. Enhanced electron emission from functionalized carbon nanotubes with a barium strontium oxide coating produced by magnetron sputtering. Carbon 2007, 45, 587–593. [Google Scholar]
- Pelella, A.; Grillo, A.; Urban, F.; Giubileo, F.; Passacantando, M.; Pollmann, E.; Sleziona, S.; Schleberger, M.; Di Bartolomeo, A. Gate-Controlled Field Emission Current from MoS2 Nanosheets. Adv. Electron. Mater. 2021, 7, 200083. [Google Scholar] [CrossRef]
- Huang, W.; Qian, W.; Luo, H.; Dong, M.; Shao, H.; Chen, Y.; Liu, X.; Dong, C. Field emission enhancement from directly grown N-doped carbon nanotubes on stainless steel substrates. Vacuum 2022, 198, 110900. [Google Scholar] [CrossRef]
- Yang, G.W.; Gao, G.Y.; Wang, C.; Xu, C.L.; Li, H.L. Controllable Deposition of Ag Nanoparticles on Carbon Nanotubes as a Catalyst for Hydrazine Oxidation. Carbon 2008, 46, 747–752. [Google Scholar] [CrossRef]


| Sample | Turn-on Field (V/µm) | Threshold Field (V/µm) | β |
|---|---|---|---|
| CNT | 4.99 | / | 1117 |
| CNT−MgO | 3.99 | 6.59 | 1380 |
| CNT-MgO-Ag | 3.65 | 6.33 | 1812 |
| CNT-MgO-Ag-BaO | 3.32 | 5.92 | 1417 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Qian, W.; Chen, Y.; Dong, M.; Yu, T.; Huang, W.; Dong, C. Construction of CNT-MgO-Ag-BaO Nanocomposite with Enhanced Field Emission and Hydrogen Sensing Performances. Nanomaterials 2023, 13, 885. https://doi.org/10.3390/nano13050885
Liu X, Qian W, Chen Y, Dong M, Yu T, Huang W, Dong C. Construction of CNT-MgO-Ag-BaO Nanocomposite with Enhanced Field Emission and Hydrogen Sensing Performances. Nanomaterials. 2023; 13(5):885. https://doi.org/10.3390/nano13050885
Chicago/Turabian StyleLiu, Xingzhen, Weijin Qian, Yawei Chen, Mingliang Dong, Taxue Yu, Weijun Huang, and Changkun Dong. 2023. "Construction of CNT-MgO-Ag-BaO Nanocomposite with Enhanced Field Emission and Hydrogen Sensing Performances" Nanomaterials 13, no. 5: 885. https://doi.org/10.3390/nano13050885
APA StyleLiu, X., Qian, W., Chen, Y., Dong, M., Yu, T., Huang, W., & Dong, C. (2023). Construction of CNT-MgO-Ag-BaO Nanocomposite with Enhanced Field Emission and Hydrogen Sensing Performances. Nanomaterials, 13(5), 885. https://doi.org/10.3390/nano13050885






