Facile Synthesis of Hydrogen-Substituted Graphdiyne Powder via Dehalogenative Homocoupling Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Components
2.2. Synthesis of tBEP Polymer
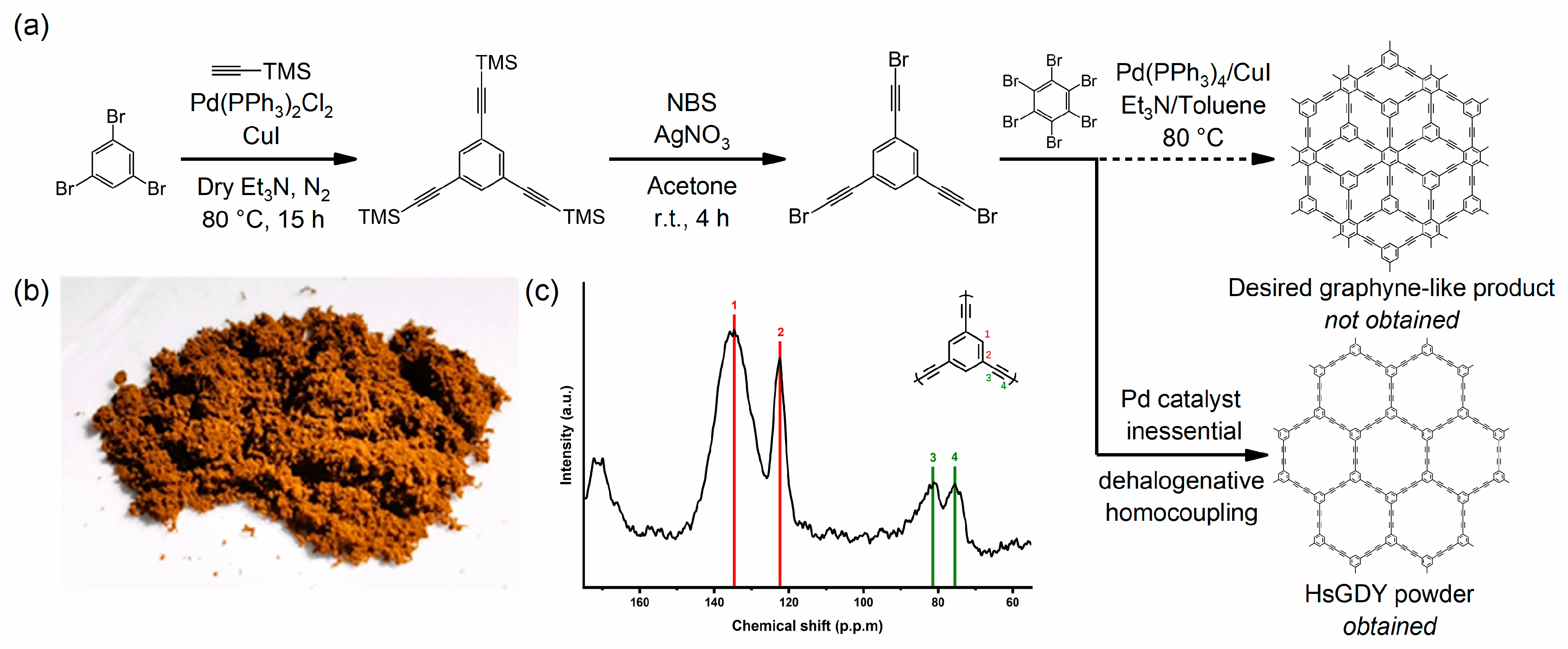
2.2.1. 1,3,5-Tris((trimethylsilyl)ethynyl)benzene
2.2.2. 1,3,5-Tris(bromoethynyl)benzene (tBEP)
2.3. Synthesis of HsGDY Powder
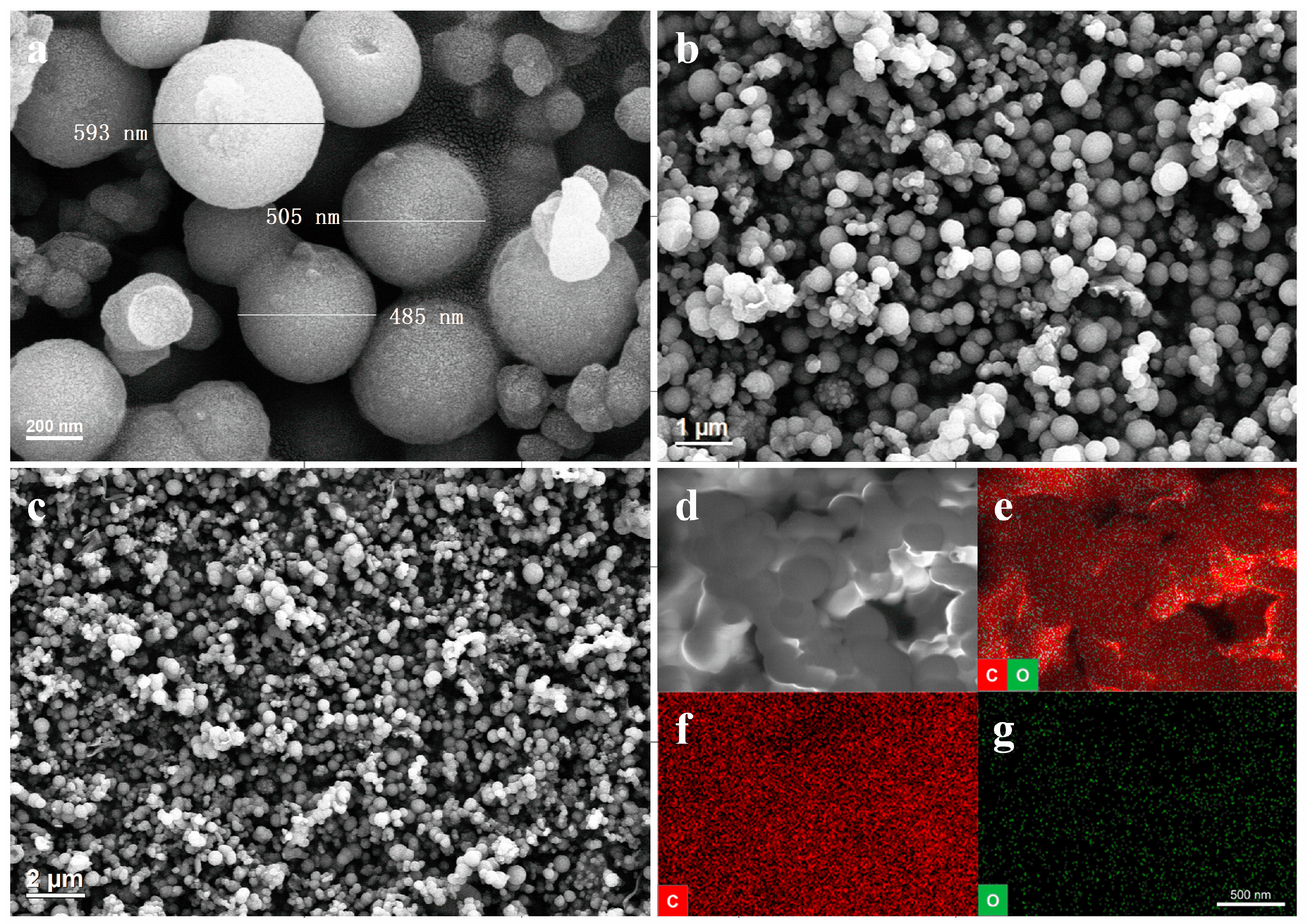
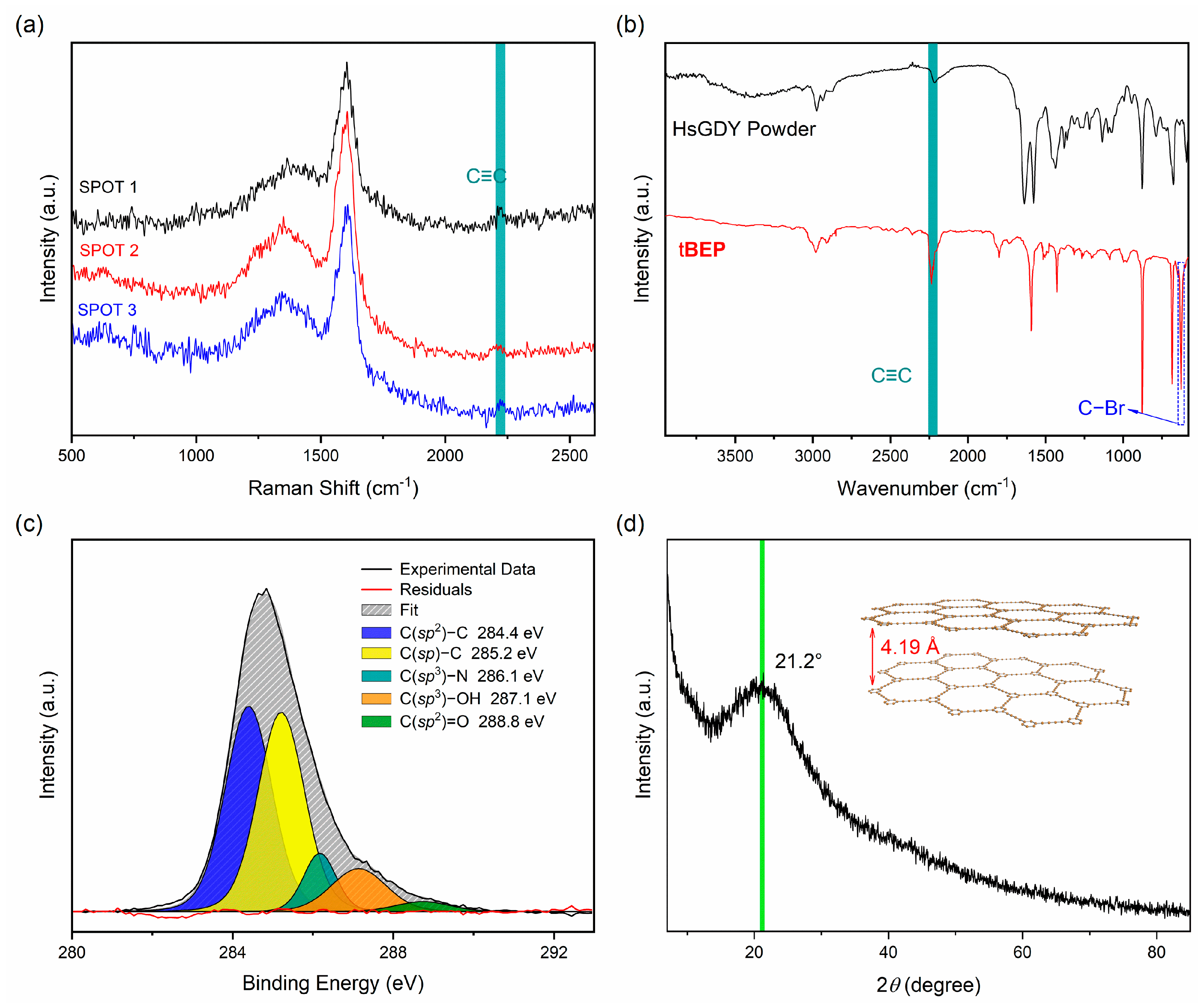
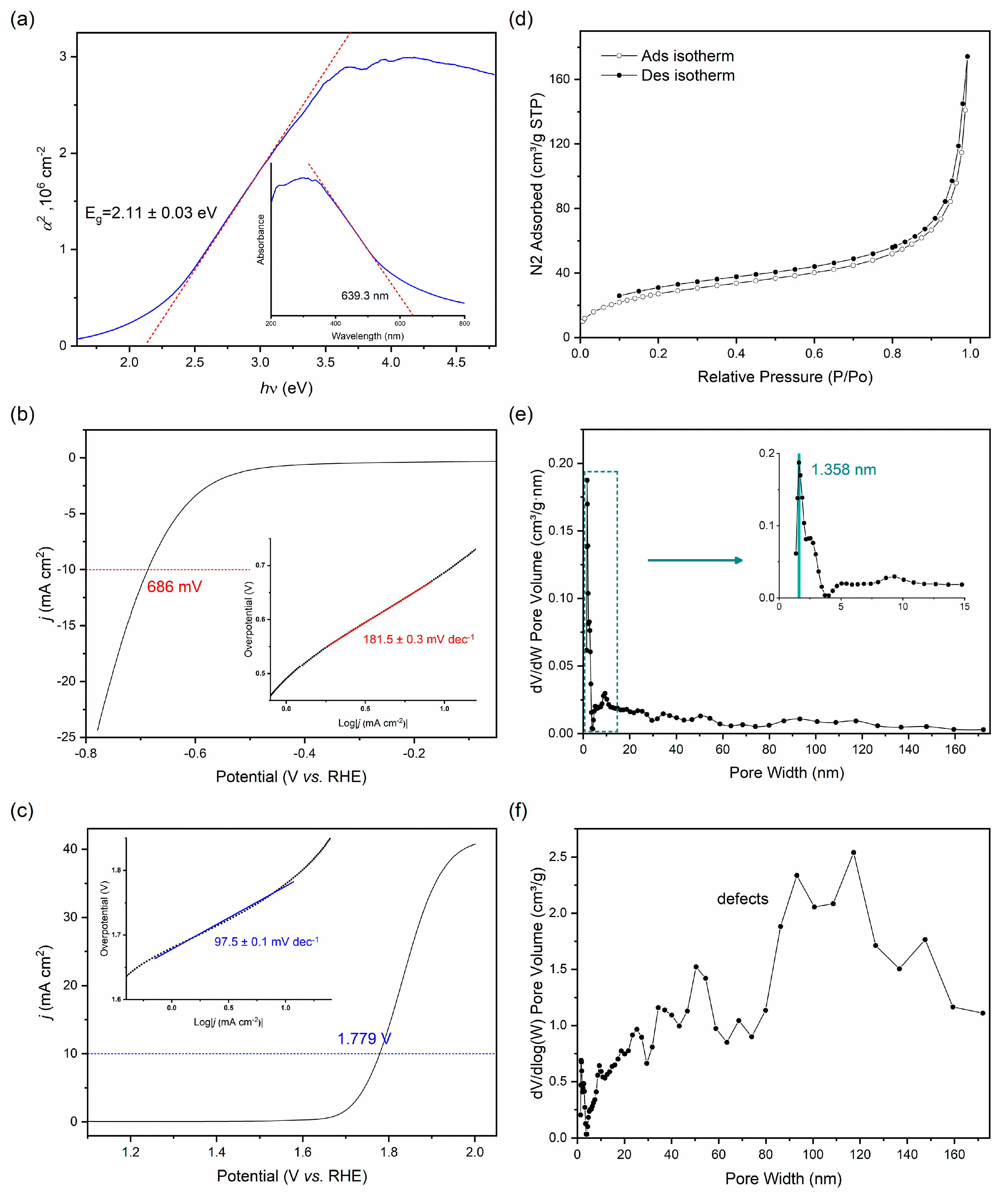
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes–the route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Fukui, N.; Maeda, H.; Matsuoka, R.; Toyoda, R.; Nishihara, H. The accelerating world of Graphdiynes. Adv. Mater. 2019, 31, e1804211. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871. [Google Scholar] [CrossRef]
- Baughman, R.H.; Eckhardt, H.; Kertesz, M. Structure-property predictions for new planar forms of carbon: Layered phases containing sp2 and sp atoms. J. Chem. Phys. 1987, 87, 6687–6699. [Google Scholar] [CrossRef]
- Kim, B.G.; Choi, H.J. Graphyne: Hexagonal network of carbon with versatile Dirac cones. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 2012, 86, 115435. [Google Scholar] [CrossRef]
- Luo, G.; Qian, X.; Liu, H.; Qin, R.; Zhou, J.; Li, L.; Gao, Z.; Wang, E.; Mei, W.-N.; Lu, J.; et al. Quasiparticle energies and excitonic effects of the two-dimensional carbon allotrope graphdiyne: Theory and experiment. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 2011, 84, 075439. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Yin, C.; Tong, L.; Chen, C.; Zhang, J. Synthesis of Hydrogen-Substituted Graphyne Film for Lithium–Sulfur Battery Applications. Small 2019, 15, 1805344. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; He, J.; Tu, Z.; Yang, Z.; Zhao, F.; Li, X.; Huang, C.; Wang, K.; Jiu, T.; Yi, Y.; et al. Synthesis of Chlorine-Substituted Graphdiyne and Applications for Lithium-Ion Storage. Angew. Chem. Int. Ed. 2017, 56, 10740–10745. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, X.; Wang, N.; He, J.; Li, X.; Wang, X.; Hou, Z.; Wang, K.; Gao, J.; Jiu, T.; et al. Graphdiyne Containing Atomically Precise N Atoms for Efficient Anchoring of Lithium Ion. ACS Appl. Mater. Interfaces 2019, 11, 2608–2617. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Liu, H.; Li, Y. Graphdiyne and graphyne: From theoretical predictions to practical construction. Chem. Soc. Rev. 2014, 43, 2572–2586. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, J.; Yang, Q.; Ma, L.; Zhao, Y.; Huang, Z.; Li, X.; Dong, B.; Fu, X.-Z.; Zhi, C. Metal-Tuned Acetylene Linkages in Hydrogen Substituted Graphdiyne Boosting the Electrochemical Oxygen Reduction. Small 2020, 16, 1907341. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Xie, Z.; Gao, X.; Zhao, J.; Xiong, Y.; Chen, C.; Zhang, J.; Liu, Z. Diatomite-Templated Synthesis of Freestanding 3D Graphdiyne for Energy Storage and Catalysis Application. Adv. Mater. 2018, 30, e1800548. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xue, Y.; Hui, L.; He, F.; Zhang, C.; Liu, Y.; Fang, Y.; Xing, C.; Li, Y.; Liu, H.; et al. Graphdiyne-engineered heterostructures for efficient overall water-splitting. Nano Energy 2019, 64, 103928. [Google Scholar] [CrossRef]
- Hui, L.; Huang, B.; Yu, H.; Zhang, C.; Zhang, D.; Jia, D.; Zhao, Y.; Li, Y.; Liu, H.; Li, Y. Overall water splitting by graphdiyne-exfoliated and -sandwiched layered double-hydroxide nanosheet arrays. Nat. Commun. 2018, 9, 5309. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Shi, Y.; Liu, L.; Li, R.; Shi, L.; Anjum, D.H.; Han, Y.; Wang, P. Dual-template engineering of triple-layered nanoarray electrode of metal chalcogenides sandwiched with hydrogen-substituted graphdiyne. Nat. Commun. 2018, 9, 3132. [Google Scholar] [CrossRef]
- Kan, X.; Ban, Y.; Wu, C.; Pan, Q.; Liu, H.; Song, J.; Zuo, Z.; Li, Z.; Zhao, Y. Interfacial Synthesis of Conjugated Two-Dimensional N-Graphdiyne. ACS Appl. Mater. Interfaces 2018, 10, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ren, X.; Ma, X.; Song, Y.; Hu, X.; Zhang, M.; Li, Y.; Xie, C.; Li, X.; Li, J.; et al. Fast Preparation of Controllable Nitrogen Atoms Substituted Graphyne Film Applied for FET Devices. Mater. Chem. Front. 2021, 5, 7993–8001. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Yang, Z.; Wang, J.; Liu, C.; Xie, C.; Wang, H.; Huang, C. A facile liquid/liquid interface method to synthesize graphyne analogs. Chem. Commun. 2019, 55, 6571–6574. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, X.; Liu, R.; Xie, Z.; Yang, J.; Zhang, S.; Zhang, G.; Liu, H.; Li, Y.; Zhang, J.; et al. Synthesis of Graphdiyne Nanowalls Using Acetylenic Coupling Reaction. J. Am. Chem. Soc. 2015, 137, 7596–7599. [Google Scholar] [CrossRef]
- Matsuoka, R.; Sakamoto, R.; Hoshiko, K.; Sasaki, S.; Masunaga, H.; Nagashio, K.; Nishihara, H. Crystalline Graphdiyne Nanosheets Produced at a Gas/Liquid or Liquid/Liquid Interface. J. Am. Chem. Soc. 2017, 139, 3145–3152. [Google Scholar] [CrossRef]
- Wu, B.; Li, M.; Xiao, S.; Qu, Y.; Qiu, X.; Liu, T.; Tian, F.; Li, H.; Xiao, S. A graphyne-like porous carbon-rich network synthesized via alkyne metathesis. Nanoscale 2017, 9, 11939–11943. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, J.; Du, R.; Zhou, J.; Huang, M.-Y.; Liu, R.; Li, J.; Xie, Z.; Wu, L.-Z.; Liu, Z.; et al. Direct Synthesis of Graphdiyne Nanowalls on Arbitrary Substrates and Its Application for Photoelectrochemical Water Splitting Cell. Adv. Mater. 2017, 29, 1605308. [Google Scholar] [CrossRef]
- Li, J.; Xie, Z.; Xiong, Y.; Li, Z.; Huang, Q.; Zhang, S.; Zhou, J.; Liu, R.; Gao, X.; Chen, C.; et al. Architecture of β-Graphdiyne-Containing Thin Film Using Modified Glaser–Hay Coupling Reaction for Enhanced Photocatalytic Property of TiO2. Adv. Mater. 2017, 29, 1700421. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, N.; Cui, Z.; Du, H.; Fu, L.; Huang, C.; Yang, Z.; Shen, X.; Yi, Y.; Tu, Z.; et al. Hydrogen substituted graphdiyne as carbon-rich flexible electrode for lithium and sodium ion batteries. Nat. Commun. 2017, 8, 1172. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhu, Y.; Yi, D.; Zhou, J.; Zhang, S.; Yin, C.; Ding, F.; Zhang, S.; Yi, X.; Wang, J.; et al. Ultrathin graphdiyne film on graphene through solution-phase van der Waals epitaxy. Sci. Adv. 2018, 4, eaat6378. [Google Scholar] [CrossRef]
- Desykatkin, V.G.; Martin, W.B.; Aliev, A.E.; Chapman, N.E.; Fonseca, A.F.; Galvão, D.S.; Miller, E.R.; Stone, K.H.; Wang, Z.; Zakhidov, D.; et al. Scalable Synthesis and Characterization of Multilayer γ-Graphyne, New Carbon Crystals with a Small Direct Band Gap. J. Am. Chem. Soc. 2022, 144, 17999–18008. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, Z.; Liu, R.; Gao, X.; Li, J.; Xiong, Y.; Tong, L.; Zhang, J.; Liu, Z. Synthesis of Ultrathin Graphdiyne Film Using a Surface Template. ACS Appl. Mater. Interfaces 2019, 11, 2632–2637. [Google Scholar] [CrossRef]
- Ikegashira, K.; Nishihara, Y.; Hirabayashi, K.; Mori, A.; Hiyama, T. Copper(i) salt promoted homo-coupling reaction of organosilanes. Chem. Commun. 1997, 11, 1039–1040. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, L.; Ma, H.; Yuan, C.; Xu, W. Dehalogenative Homocoupling of Terminal Alkynyl Bromides on Au(111): Incorporation of Acetylenic Scaffolding into Surface Nanostructures. ACS Nano 2016, 10, 7023–7030. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liang, J.; Zhang, Y.; Zhao, X.; Yuan, C. Synthesis of hydrogen-substituted graphydines via dehalogenative homocoupling reactions. Chem. Commun. 2021, 57, 5036–5039. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Zhou, J.; Liu, R.; Du, R.; Xu, H.; Liu, Z.; Zhang, J.; Liu, Z. Identifying sp–sp2 carbon materials by Raman and infrared spectroscopies. Phys. Chem. Chem. Phys. 2014, 16, 11303–11309. [Google Scholar] [CrossRef]
- Varga, M.; Izak, T.; Vretenar, V.; Kozak, H.; Holovsky, J.; Artemenko, A.; Hulman, M.; Skakalova, V.; Lee, D.S.; Kromka, A. Diamond/carbon nanotube composites: Raman, FTIR and XPS spectroscopic studies. Carbon 2017, 111, 54–61. [Google Scholar] [CrossRef]
- Popov, V.N.; Lambin, P. Theoretical Raman fingerprints of α-, β-, and γ-graphyne. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 2013, 88, 075427. [Google Scholar] [CrossRef]
- Bao, H.; Wang, L.; Li, C.; Luo, J. Structural Characterization and Identification of Graphdiyne and Graphdiyne-Based Materials. ACS Appl. Mater. Interfaces 2019, 11, 2717–2729. [Google Scholar] [CrossRef]
- Xing, T.; Li, L.H.; Hou, L.; Hu, X.; Zhou, S.; Peter, R.; Petravic, M.; Chen, Y. Disorder in ball-milled graphite revealed by Raman spectroscopy. Carbon 2013, 57, 515–519. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, S.; Liu, H.; Li, Y.; Cui, G.; Li, Y. Graphdiyne for high capacity and long-life lithium storage. Nano Energy 2015, 11, 481–489. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, Y.; Gu, J.; Li, N.; Wang, C.; Liu, Z.; Li, X.; Huang, Z.; Wei, S.; Xu, S.; et al. Scalable synthesis of 2D hydrogen-substituted graphdiyne on Zn substrate for high-yield N2 fixation. Nano Energy 2020, 78, 105283. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, J.; Song, X.; Wang, C.; Hua, W.; Ma, Y. Identification of oxidation states in γ-graphyne by computational XPS and NEXAFS spectra. Appl. Surf. Sci. 2023, 609, 155134. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Zanatta, A.R. Revisiting the optical band gap of semiconductors and the proposal of a unified methodology to its determination. Sci. Rep. 2019, 9, 11225. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, J.; Xue, Z.; Li, Y.; Liu, H.; Li, D.; Yang, W.; Li, Y. Extraordinarily Durable Graphdiyne-Supported Electrocatalyst with High Activity for Hydrogen Production at All Values of pH. ACS Appl. Mater. Interfaces 2016, 8, 31083–31091. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, H.; Liu, Y.; Chen, J.-F.; Li, Y.; Dai, L. Graphdiyne with tunable activity towards hydrogen evolution reaction. Nano Energy 2019, 63, 103874. [Google Scholar] [CrossRef]
- Yan, H.; Guo, S.; Wu, F.; Yu, P.; Liu, H.; Li, Y.; Mao, L. Carbon Atom Hybridization Matters: Ultrafast Humidity Response of Graphdiyne Oxides. Angew. Chem. Int. Ed. 2018, 57, 3922–3926. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Bojdys, M.J.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Porous, Fluorescent, Covalent Triazine-Based Frameworks Via Room-Temperature and Microwave-Assisted Synthesis. Adv. Mater. 2012, 24, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Datar, A.; Jeong, C.; Deng, X.; Chung, Y.G.; Lin, L.-C. Surface Area Determination of Porous Materials Using the Brunauer–Emmett–Teller (BET) Method: Limitations and Improvements. J. Phys. Chem. C 2019, 123, 20195–20209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Liang, J.; Yuan, C.; Zheng, W. Facile Synthesis of Hydrogen-Substituted Graphdiyne Powder via Dehalogenative Homocoupling Reaction. Nanomaterials 2023, 13, 1018. https://doi.org/10.3390/nano13061018
Yin J, Liang J, Yuan C, Zheng W. Facile Synthesis of Hydrogen-Substituted Graphdiyne Powder via Dehalogenative Homocoupling Reaction. Nanomaterials. 2023; 13(6):1018. https://doi.org/10.3390/nano13061018
Chicago/Turabian StyleYin, Jiayi, Jizhe Liang, Chunxue Yuan, and Wei Zheng. 2023. "Facile Synthesis of Hydrogen-Substituted Graphdiyne Powder via Dehalogenative Homocoupling Reaction" Nanomaterials 13, no. 6: 1018. https://doi.org/10.3390/nano13061018
APA StyleYin, J., Liang, J., Yuan, C., & Zheng, W. (2023). Facile Synthesis of Hydrogen-Substituted Graphdiyne Powder via Dehalogenative Homocoupling Reaction. Nanomaterials, 13(6), 1018. https://doi.org/10.3390/nano13061018






