Eco-Friendly Preparation of Carbon-Bonded Carbon Fiber Based on Glucose-Polyacrylamide Hydrogel Derived Carbon as Binder
Abstract
1. Introduction
2. Experiments
2.1. Preparation of Glucose-Acrylamide Hydrogel Binder
2.2. Preparation of Dopamine-Modified Carbon Fiber
2.3. Preparation of CBCF Composite Slurry and Molding of CBCF Composites
2.4. Characterization
3. Results
3.1. Formation and Pyrolysis Mechanism of Glu-PAM Hydrogels
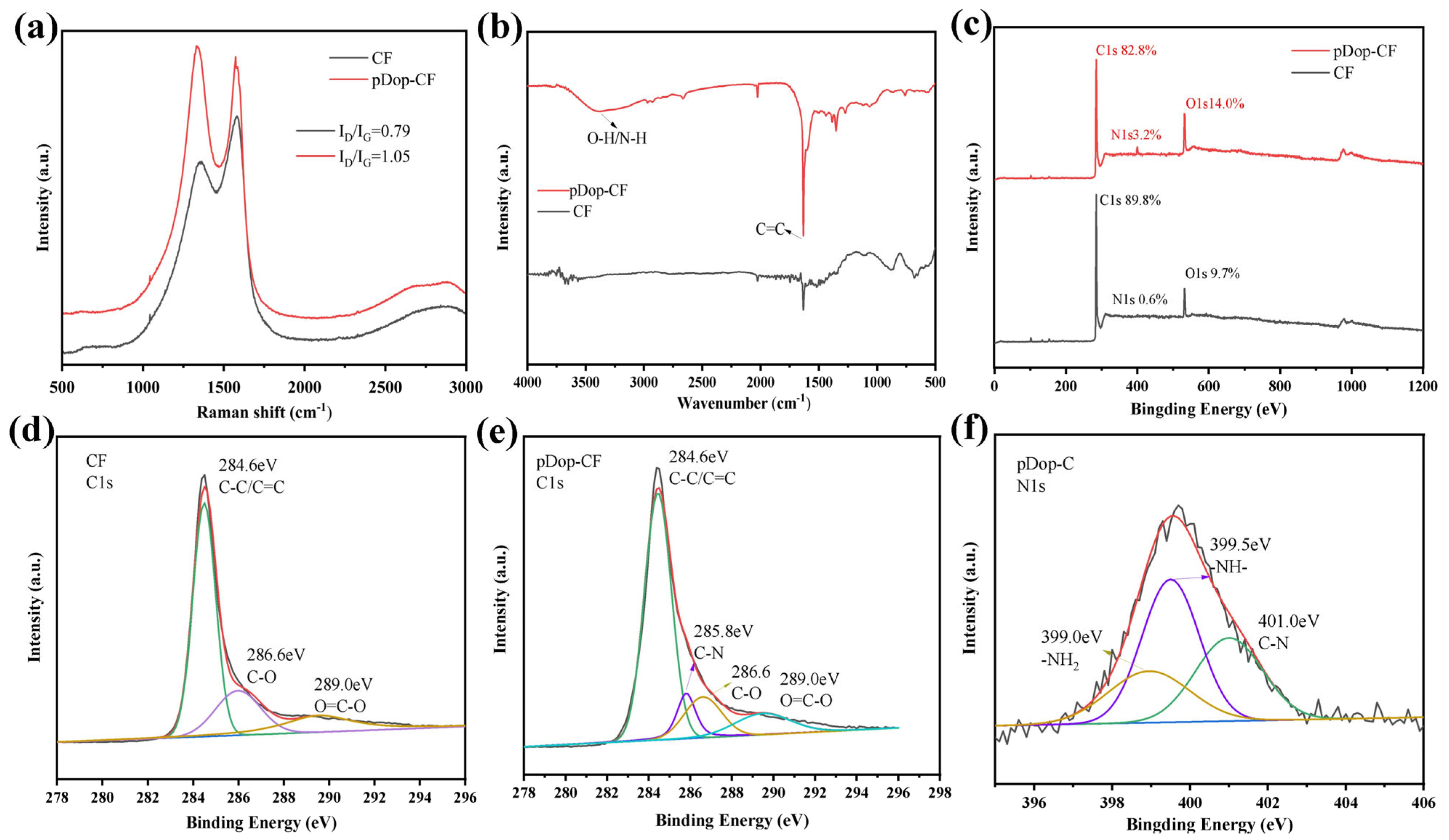
3.2. Characterization of Dopamine-Modified Carbon Fiber
3.3. Microstructure of CBCF Composites
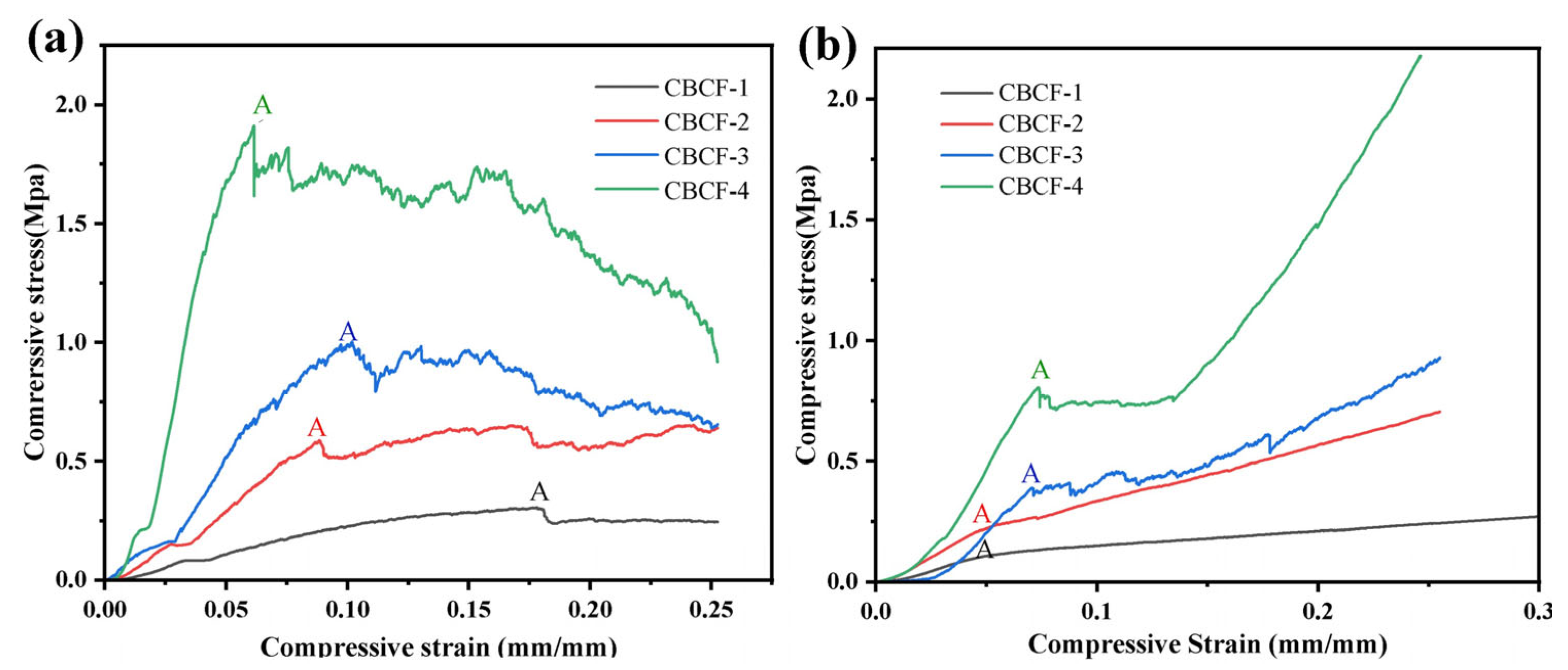
3.4. Mechanical Properties of CBCF Composites
3.5. Thermal Properties of CBCF Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, B.; Zhou, S.; Hong, C.; Han, J.; Zhang, X. Mechanical Enhancement of Lightweight ZrB2-Modified Carbon-Bonded Carbon Fiber Composites with Self-Grown Carbon Nanotubes. Carbon 2016, 102, 487–493. [Google Scholar] [CrossRef]
- Yang, D.; Dong, S.; Hong, C.; Zhang, X. Preparation, Modification, and Coating for Carbon-Bonded Carbon Fiber Composites: A Review. Ceram. Int. 2022, 48, 14935–14958. [Google Scholar] [CrossRef]
- Li, J.; Sha, J.; Dai, J.; Lv, Z.; Shao, J.; Wang, S.; Zhang, Z. Fabrication and Characterization of Carbon-Bonded Carbon Fiber Composites with in-Situ Grown SiC Nanowires. Carbon 2017, 118, 148–155. [Google Scholar] [CrossRef]
- Liu, H.; Li, L.; Yang, J.; Zhou, Y.; Ai, Y.; Qi, Z.; Gao, Y.; Jiao, J. Characterization and Modeling Damage and Fracture of Prepreg-MI SiC/SiC Composites under Tensile Loading at Room Temperature. Appl. Compos. Mater. 2022, 29, 1167–1193. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Liu, D.; Tao, R.; Cheng, T.; Ai, S.; Xu, B.; Yang, Y.; Fang, D. Fabrication and Ultra-High-Temperature Properties of Lightweight Carbon-Bonded Carbon Fiber Composites. Ceram. Int. 2019, 45, 22249–22252. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.; Yang, Z.; Zhang, D.; Shi, J.; Yuan, Z.; Liu, Q. Compression Behaviors of Carbon-Bonded Carbon Fiber Composites: Experimental and Numerical Investigations. Carbon 2017, 116, 398–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.; Yang, Z.; Zhang, D.; Li, J.; Shi, J.; Yuan, Z.; Chen, H. Resilient Carbon Fiber Network Materials under Cyclic Compression. Carbon 2019, 155, 344–352. [Google Scholar] [CrossRef]
- Cheng, H.; Hong, C.; Zhang, X.; Xue, H. Lightweight Carbon-Bonded Carbon Fiber Composites with Quasi-Layered and Network Structure. Mater. Des. 2015, 86, 156–159. [Google Scholar] [CrossRef]
- Du, B.; Hong, C.; Zhang, X.; Wang, J.; Qu, Q. Preparation and Mechanical Behaviors of SiOC-Modified Carbon-Bonded Carbon Fiber Composite with in-Situ Growth of Three-Dimensional SiC Nanowires. J. Eur. Ceram. Soc. 2018, 38, 2272–2278. [Google Scholar] [CrossRef]
- Wang, C.; Jin, X.; Cheng, H.; Hong, C.; Zhang, X. Organic Aerogel-Impregnated Low-Density Carbon/Carbon Composites: Preparation, Properties and Response under Simulated Atmospheric Re-Entry Conditions. Mater. Des. 2017, 131, 177–185. [Google Scholar] [CrossRef]
- Liu, C.; Han, J.; Zhang, X.; Hong, C.; Du, S. Lightweight Carbon-Bonded Carbon Fiber Composites Prepared by Pressure Filtration Technique. Carbon 2013, 59, 551–554. [Google Scholar] [CrossRef]
- Cheng, H.; Xue, H.; Hong, C.; Zhang, X. Preparation, Mechanical, Thermal and Ablative Properties of Lightweight Needled Carbon Fibre Felt/Phenolic Resin Aerogel Composite with a Bird’s Nest Structure. Compos. Sci. Technol. 2017, 140, 63–72. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B.; Ding, S.; Zhuang, Y.; Liu, J.; Li, L. Functionally Graded Polyetheretherketone-Based Composites Additively Manufactured by Material Extrusion Using a Transition Interface Design Method. Compos. Part A Appl. Sci. Manuf. 2022, 158, 106977. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Hu, Z.; Ye, X.; Wang, S.; Zhao, Y.; Wang, B.; Ou, Y.; Zhang, J. High Strength Carbon-Fiber Reinforced Polyamide 6 Composites Additively Manufactured by Screw-Based Extrusion. Compos. Sci. Technol. 2022, 229, 109707. [Google Scholar] [CrossRef]
- Hmeidat, N.S.; Elkins, D.S.; Peter, H.R.; Kumar, V.; Compton, B.G. Processing and Mechanical Characterization of Short Carbon Fiber-Reinforced Epoxy Composites for Material Extrusion Additive Manufacturing. Compos. Part B Eng. 2021, 223, 109122. [Google Scholar] [CrossRef]
- Wright, W.J.; Koerner, H.; Rapking, D.; Abbott, A.; Celik, E. Rapid Fiber Alignment Quantification in Direct Write Printing of Short Fiber Reinforced Composites. Compos. Part B Eng. 2022, 236, 109814. [Google Scholar] [CrossRef]
- Goh, G.D.; Toh, W.; Yap, Y.L.; Ng, T.Y.; Yeong, W.Y. Additively Manufactured Continuous Carbon Fiber-Reinforced Thermoplastic for Topology Optimized Unmanned Aerial Vehicle Structures. Compos. Part B Eng. 2021, 216, 108840. [Google Scholar] [CrossRef]
- Spoerk, M.; Savandaiah, C.; Arbeiter, F.; Traxler, G.; Cardon, L.; Holzer, C.; Sapkota, J. Anisotropic Properties of Oriented Short Carbon Fibre Filled Polypropylene Parts Fabricated by Extrusion-Based Additive Manufacturing. Compos. Part A Appl. Sci. Manuf. 2018, 113, 95–104. [Google Scholar] [CrossRef]
- Nawafleh, N.; Celik, E. Additive Manufacturing of Short Fiber Reinforced Thermoset Composites with Unprecedented Mechanical Performance. Addit. Manuf. 2020, 33, 101109. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Acharya, J.; Pant, H.R.; Park, M. Lokta Paper-Derived Free-Standing Carbon as a Binder-Free Electrode Material for High-Performance Supercapacitors. Sustain. Mater. Technol. 2022, 33, e00450. [Google Scholar] [CrossRef]
- Ischia, G.; Cutillo, M.; Guella, G.; Bazzanella, N.; Cazzanelli, M.; Orlandi, M.; Miotello, A.; Fiori, L. Hydrothermal Carbonization of Glucose: Secondary Char Properties, Reaction Pathways, and Kinetics. Chem. Eng. J. 2022, 449, 137827. [Google Scholar] [CrossRef]
- Chen, X.; Eugene Chong, J.J.; Celine Fah, Z.W.; Hong, L. Glucose-Derived Carbon Molecular Sieve Membrane: An Inspiration from Cooking. Carbon 2017, 111, 334–337. [Google Scholar] [CrossRef]
- Liu, H.; Luo, S.H.; Yan, S.X.; Wang, Q.; Hu, D.B.; Wang, Y.L.; Feng, J.; Yi, T.F. High-Performance α-Fe2O3/C Composite Anodes for Lithium-Ion Batteries Synthesized by Hydrothermal Carbonization Glucose Method Used Pickled Iron Oxide Red as Raw Material. Compos. Part B Eng. 2019, 164, 576–582. [Google Scholar] [CrossRef]
- Gong, Y.; Wei, Z.; Wang, J.; Zhang, P.; Li, H.; Wang, Y. Design and Fabrication of Hierarchically Porous Carbon with a Template-Free Method. Sci. Rep. 2014, 4, 2206314. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Chen, D.; Cheng, Y.; Fei, W.; Jiang, D.; Tang, S.; Zhao, G.; Song, J.; Hou, C.; Zhang, W.; et al. Sugar-Derived Isotropic Nanoscale Polycrystalline Graphite Capable of Considerable Plastic Deformation. Adv. Mater. 2022, 34, 200363. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, D.; Cheng, Y.; Sun, B.; Zhao, G.; Fei, W.; Han, W.; Han, J.; Zhang, X. Eco-Friendly and Sustainable Approach of Assembling Sugars into Biobased Carbon Fibers. Green Chem. 2022, 24, 5097–5106. [Google Scholar] [CrossRef]
- Wu, S.; Chen, D.; Zhao, G.; Cheng, Y.; Sun, B.; Yan, X.; Han, W.; Chen, G.; Zhang, X. Controllable Synthesis of a Robust Sucrose-Derived Bio-Carbon Foam with 3D Hierarchical Porous Structure for Thermal Insulation, Flame Retardancy and Oil Absorption. Chem. Eng. J. 2022, 434, 134514. [Google Scholar] [CrossRef]
- Wu, S.; Chen, D.; Han, W.; Xie, Y.; Zhao, G.; Dong, S.; Tan, M.; Huang, H.; Xu, S.; Chen, G.; et al. Ultralight and Hydrophobic MXene/Chitosan-Derived Hybrid Carbon Aerogel with Hierarchical Pore Structure for Durable Electromagnetic Interference Shielding and Thermal Insulation. Chem. Eng. J. 2022, 446, 137093. [Google Scholar] [CrossRef]
- Tan, M.; Chen, D.; Cheng, Y.; Sun, H.; Chen, G.; Dong, S.; Zhao, G.; Sun, B.; Wu, S.; Zhang, W.; et al. Anisotropically Oriented Carbon Films with Dual-Function of Efficient Heat Dissipation and Excellent Electromagnetic Interference Shielding Performances. Adv. Funct. Mater. 2022, 32, 2202057. [Google Scholar] [CrossRef]
- Huan, X.; Shi, K.; Yan, J.; Lin, S.; Li, Y.; Jia, X.; Yang, X. High Performance Epoxy Composites Prepared Using Recycled Short Carbon Fiber with Enhanced Dispersibility and Interfacial Bonding through Polydopamine Surface-Modification. Compos. Part B Eng. 2020, 193, 107987. [Google Scholar] [CrossRef]
- Zhang, R.; Hou, X.; Ye, C.; Wang, B. Enhanced Mechanical and Thermal Properties of Anisotropic Fibrous Porous Mullite–Zirconia Composites Produced Using Sol-Gel Impregnation. J. Alloys Compd. 2017, 699, 511–516. [Google Scholar] [CrossRef]
- Petrov, V.A. Combined radiation and conduction heat transfer in high temperature fiber thermal insulation. Int. J. Heat Mass Tran. 1997, 40, 2241–2247. [Google Scholar] [CrossRef]
- Tsirlin, A.M.; Shcherbakova, G.I.; Florina, E.K.; Popova, N.A.; Gubin, S.P.; Moroz, E.M.; Riedel, R.; Kroke, E.; Steen, M. Nano-structured metal-containing polymer precursors for high temperature non-oxide cer, mics and ceramic fibers-syntheses, pyrolyses and properties. J. Eur. Ceram. Soc. 2002, 22, 2577–2585. [Google Scholar] [CrossRef]
- Wiener, M.; Reichenauer, G.; Braxmeier, S.; Hemberger, F.; Ebert, H.P. Carbon aerogel-based high-temperature thermal insulation. Int. J. Thermophys. 2009, 30, 1372. [Google Scholar] [CrossRef]
- Tseng, C.-J.; Kuo, K.-T. Thermal radiative properties of phenolic foam insulation. J. Quant. Pectrosc. Ra. 2002, 72, 349–359. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, C.; Gu, Y.; Xie, Y.; Hu, W.; Huang, M.; Liao, G.; Yang, J.; Fan, Z.; Tan, R. Eco-Friendly Preparation of Carbon-Bonded Carbon Fiber Based on Glucose-Polyacrylamide Hydrogel Derived Carbon as Binder. Nanomaterials 2023, 13, 1045. https://doi.org/10.3390/nano13061045
Zeng C, Gu Y, Xie Y, Hu W, Huang M, Liao G, Yang J, Fan Z, Tan R. Eco-Friendly Preparation of Carbon-Bonded Carbon Fiber Based on Glucose-Polyacrylamide Hydrogel Derived Carbon as Binder. Nanomaterials. 2023; 13(6):1045. https://doi.org/10.3390/nano13061045
Chicago/Turabian StyleZeng, Chen, Yanju Gu, You Xie, Weiqin Hu, Min Huang, Gen Liao, Jianxiao Yang, Zheqiong Fan, and Ruixuan Tan. 2023. "Eco-Friendly Preparation of Carbon-Bonded Carbon Fiber Based on Glucose-Polyacrylamide Hydrogel Derived Carbon as Binder" Nanomaterials 13, no. 6: 1045. https://doi.org/10.3390/nano13061045
APA StyleZeng, C., Gu, Y., Xie, Y., Hu, W., Huang, M., Liao, G., Yang, J., Fan, Z., & Tan, R. (2023). Eco-Friendly Preparation of Carbon-Bonded Carbon Fiber Based on Glucose-Polyacrylamide Hydrogel Derived Carbon as Binder. Nanomaterials, 13(6), 1045. https://doi.org/10.3390/nano13061045





