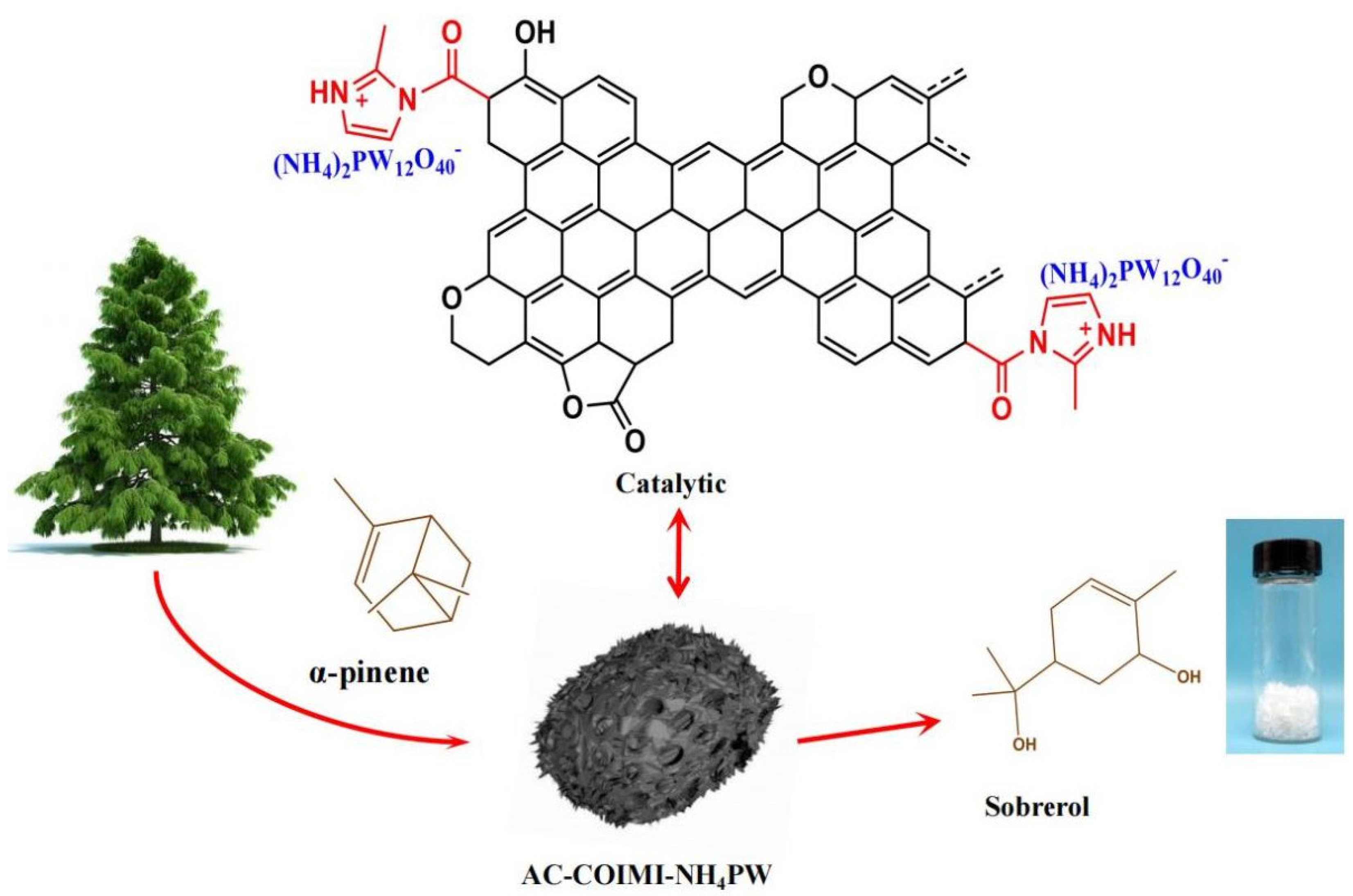
Selective Catalytic Epoxidation–Hydration of α-Pinene with Hydrogen Peroxide to Sobrerol by Durable Ammonium Phosphotungstate Immobilized on Imidazolized Activated Carbon
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Reagents
2.2. Catalyst Preparation
2.3. Catalytic Materials Characterization
2.4. Catalytic Performance Test
3. Results and Discussion
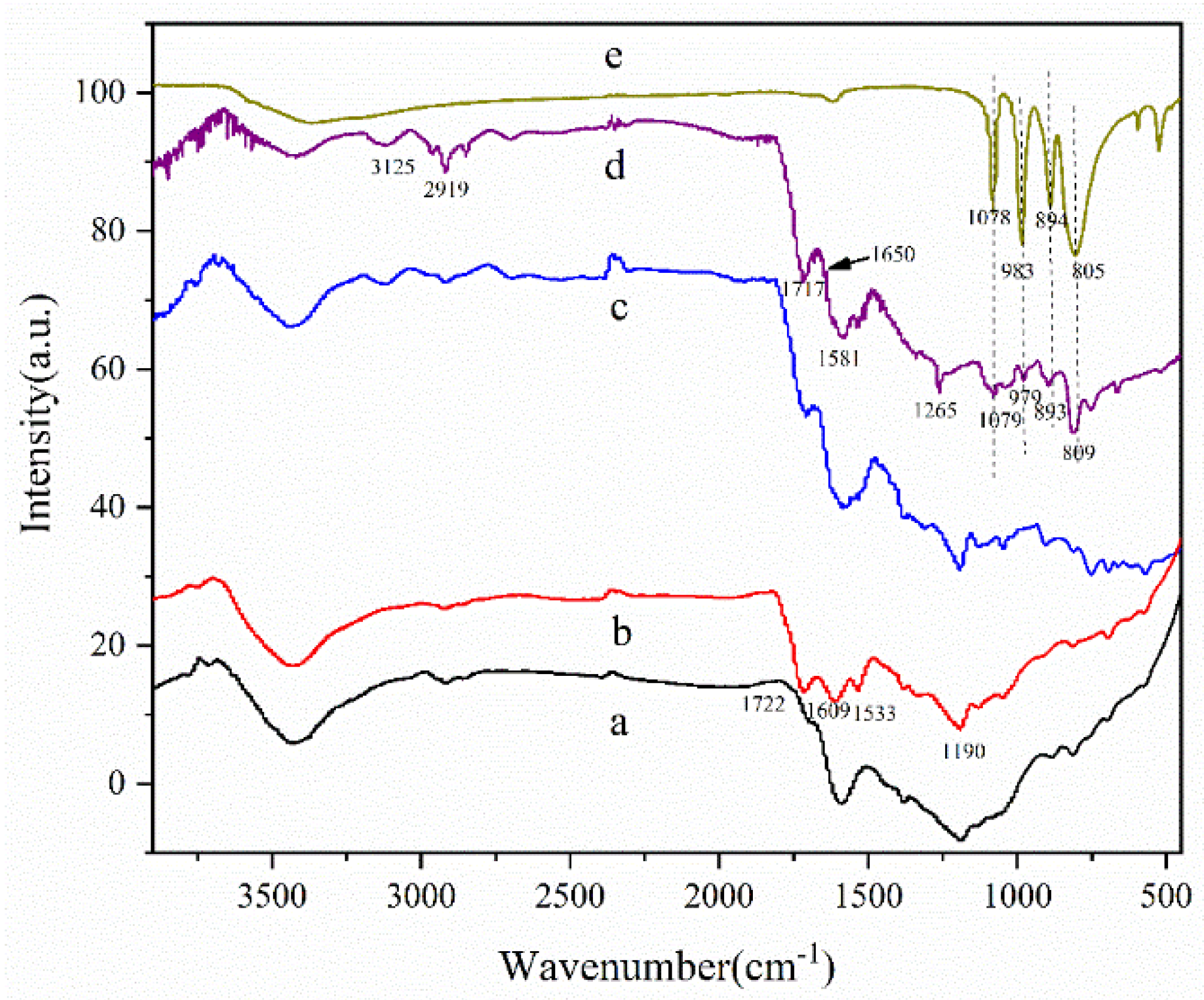
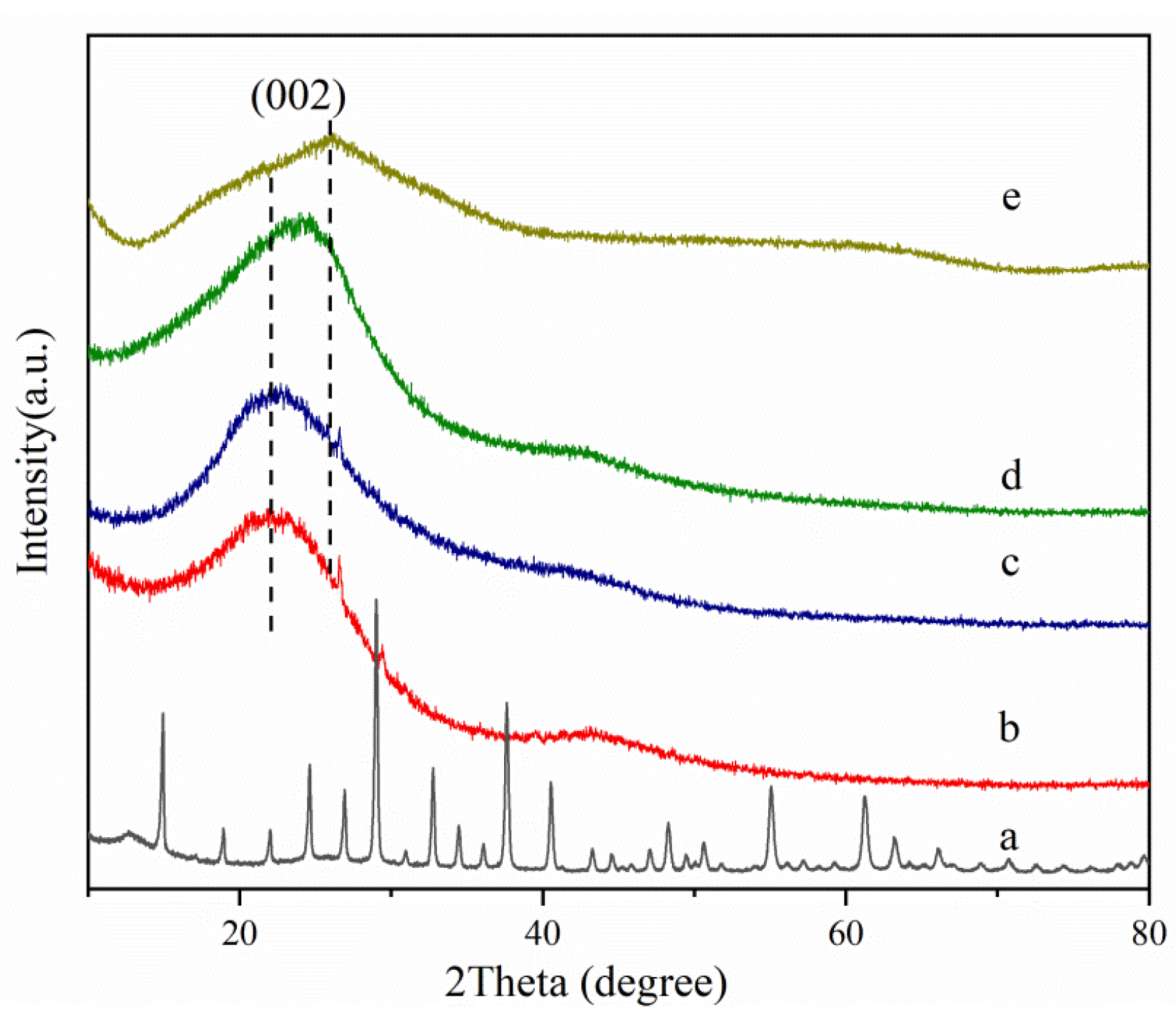
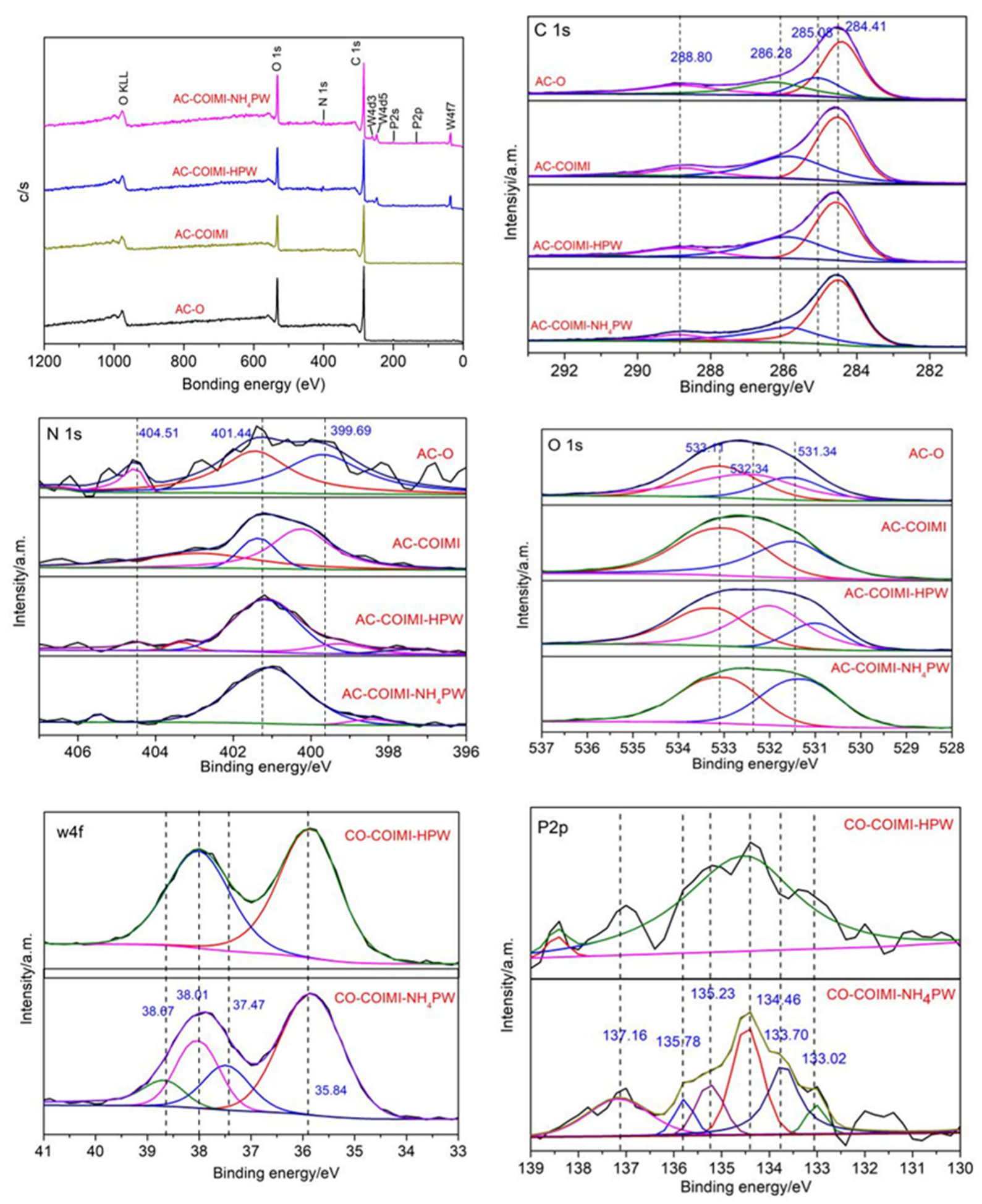
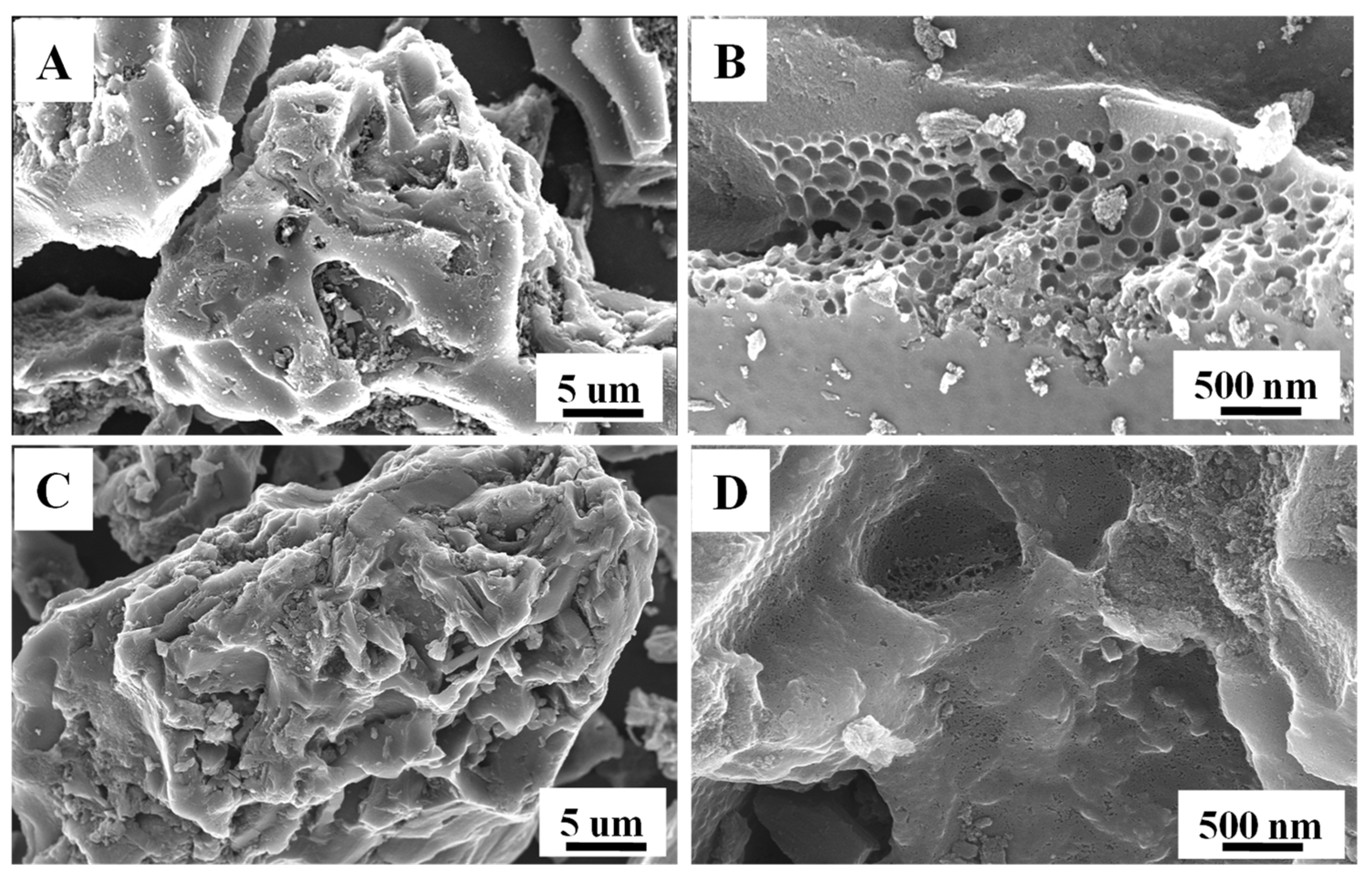
3.1. Characterization
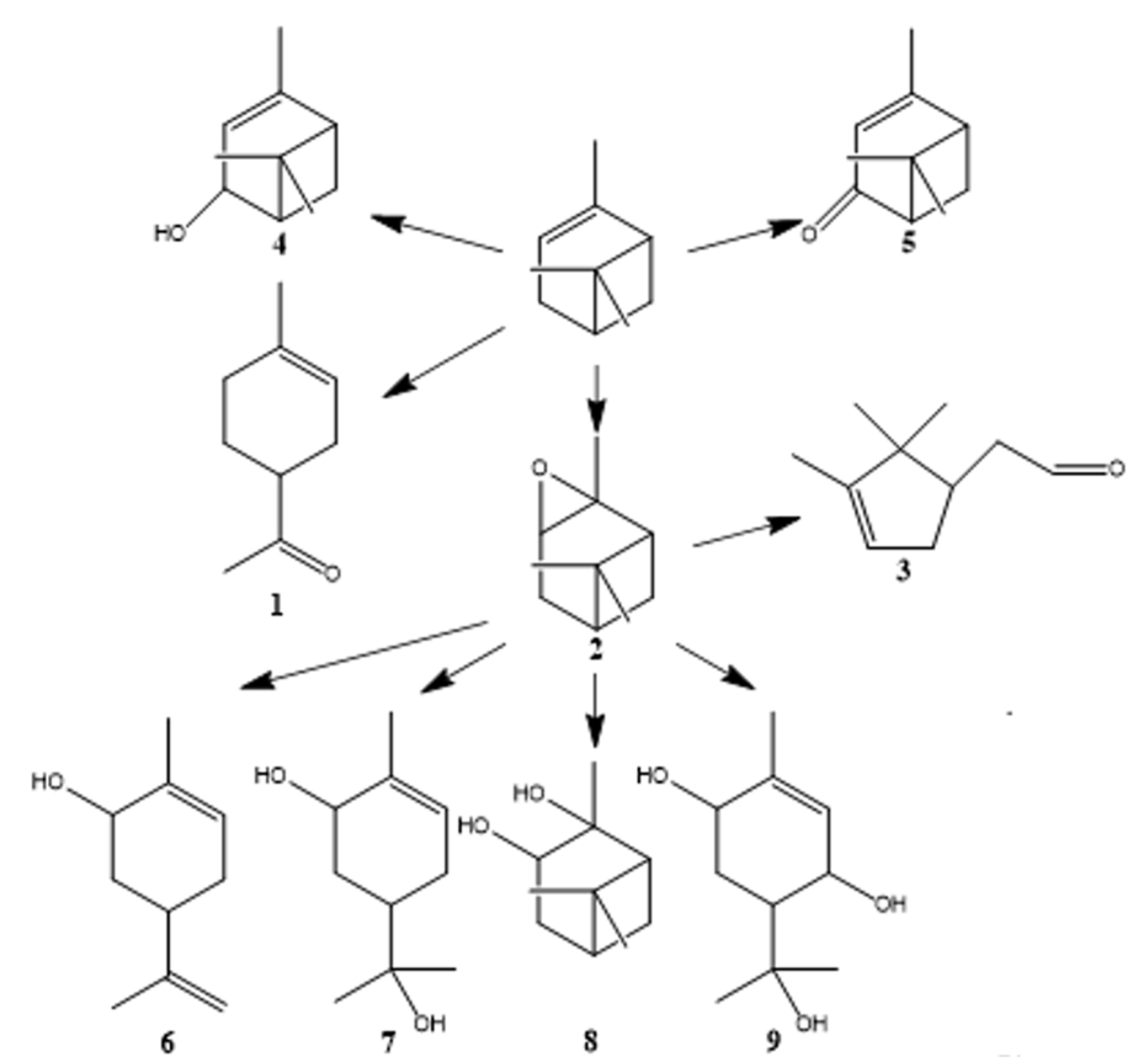
3.2. Construction of Catalytic Sites for Tandem Epoxidation–Hydration
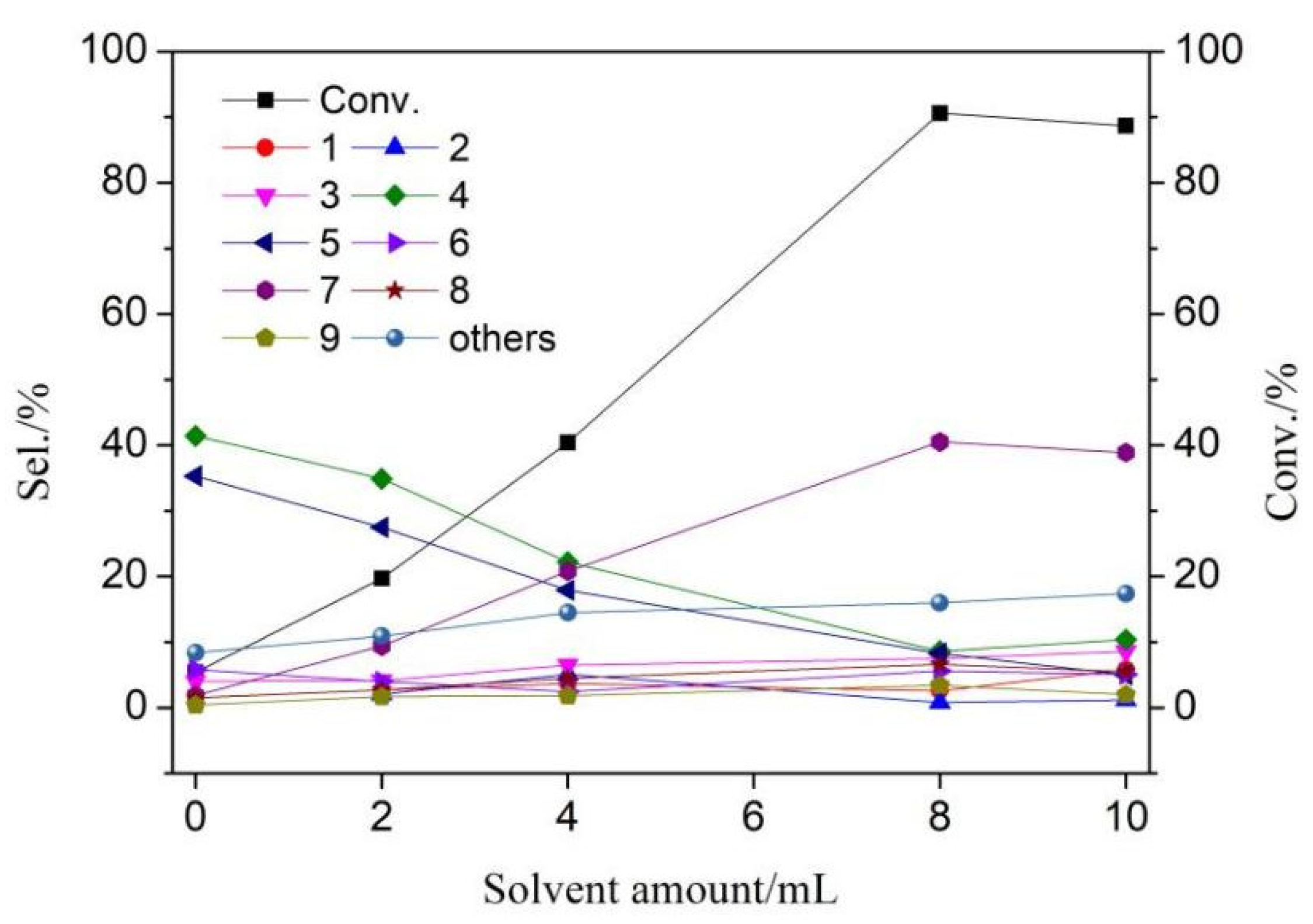
3.3. Solvent Modification of Catalytic Performance
3.4. Effect of Reaction Conditions on the Process
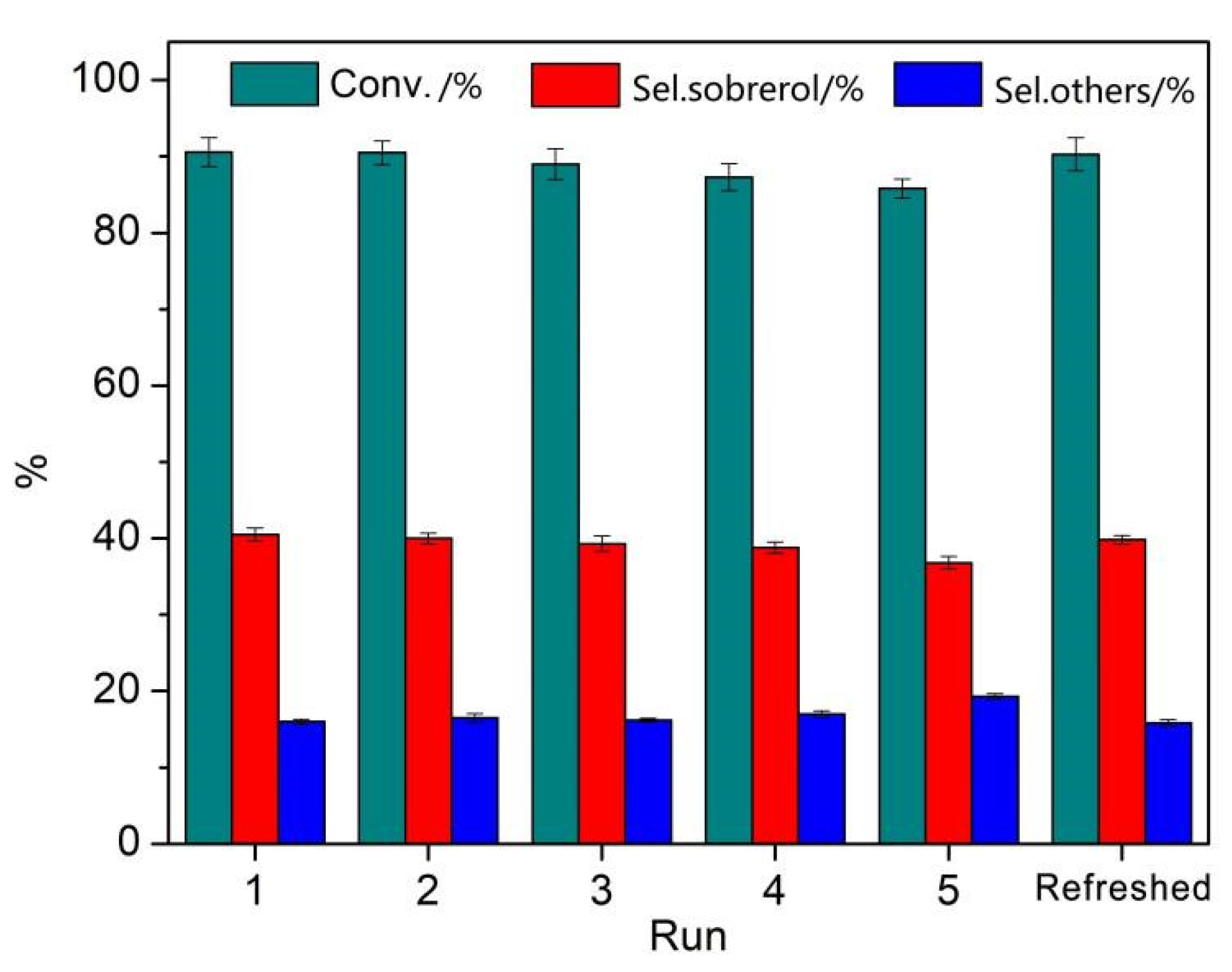
3.5. Catalytic Stability
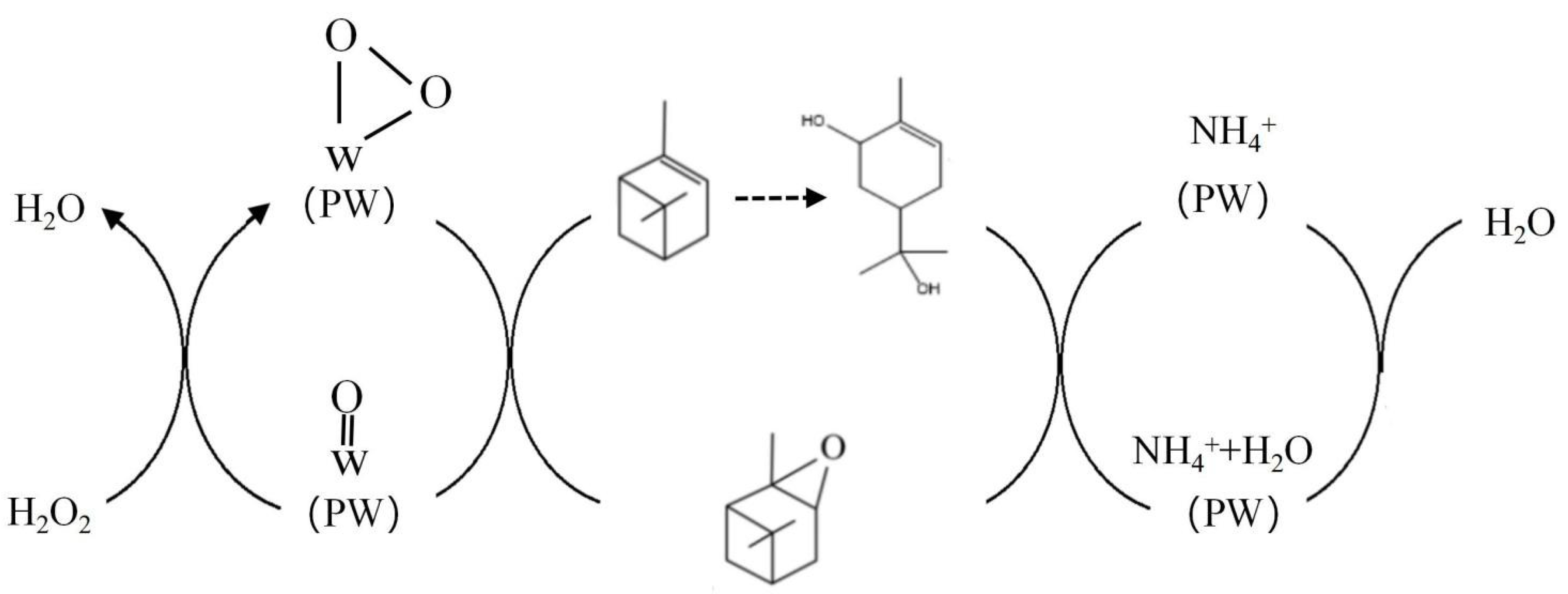
3.6. Catalytic Mechanism
3.7. Comparison of Catalytic Performance with the Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellussi, L.; Bernocchi, D.; Ciferri, G.; Manini, G.; Passali, D. Sobrerol in the Treatment of Secretory Otitis Media in Childhood. J. Int. Med. Res. 1989, 17, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Kazanov, D.; Arber, N. Monoterpenes: A Novel and Promising Therapeutic Agent, Found in Essential Oils, for Human Colorectal Cancer. Gastroenterology 2016, 150, S140. [Google Scholar] [CrossRef]
- Demeke, C.A.; Woldeyohanins, A.E.; Kifle, Z.D. Herbal medicine use for the management of COVID-19: A review article. Metab. Open 2020, 12, 100141. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.A.P.; Santos, D.M.; de Carvalho, F.O.; Menezes, I.A.C.; Barreto, A.S.; Souza, D.S.; Quintans-Júnior, L.J.; Santos, M.R.V. Monoterpenes and their derivatives as agents for cardiovascular disease management: A systematic review and meta-analysis. Phytomedicine 2021, 88, 153451. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.S.; Costa, C.S.M.F.; Coelho, J.F.J.; Fonseca, A.C.; Serra, A.C. A simple strategy toward the substitution of styrene by sobrerol-based monomers in unsaturated polyester resins. Green Chem. 2018, 20, 4880–4890. [Google Scholar] [CrossRef]
- Stamm, A.; Öhlin, J.; Mosbech, C.; Olsén, P.; Guo, B.; Söderberg, E.; Biundo, A.; Fogelström, L.; Bhattacharyya, S.; Bornscheuer, U.T.; et al. Pinene-based oxidative synthetic toolbox for scalable polyester synthesis. JACS Au 2021, 1, 1949–1960. [Google Scholar] [CrossRef]
- Esteban, J.L.; Martínez-Castro, I.; Morales, R.; Fabrellas, B.; Sanz, J. Rapid identification of volatile compounds in aromatic plants by automatic thermal desorption—GC-MS. Chromatographia 1996, 43, 63–72. [Google Scholar] [CrossRef]
- Mohafrash, S.M.M.M.; Fallatah, S.A.; Farag, S.M.F.; Mossa, A.-T.H.M. Mentha spicata essential oil nanoformulation and its larvicidal application against Culex pipiens and Musca domestica. Ind. Crops Prod. 2020, 157, 112944. [Google Scholar] [CrossRef]
- Liu, J.-F.; Li, H.-J.; Wang, L.-X.; Liu, M.-Q.; Bi, Y.-F.; Zhang, Y.-B. Two new monoterpenes from the fruits of Illicium lanceolatum. Molecules 2013, 18, 11866–11872. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Chen, Q. A Convenient, Large Scale Synthesis of trans-(+)-Sobrerol. Synth. Commun. 2023, 33, 2125–2134. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, S.; Yi, X.; Wang, J.; Zhao, Z.; Jiang, J. High value-added application of turpentine as a potential renewable source for the synthesis of heterocyclic Schiff base derivatives of cis-1,8-p-menthane-diamine serving as botanical herbicides. Ind. Crops Prod. 2018, 115, 111–116. [Google Scholar] [CrossRef]
- Mochida, T.; Ohnishi, R.; Horita, N.; Kamiya, Y.; Okuhara, T. Hydration of α-pinene over hydrophobic zeolites in 1,4-dioxane-water and in water. Microporous Mesoporous Mater. 2017, 101, 176–183. [Google Scholar] [CrossRef]
- Salvador, V.T.; Silva, E.S.; Gonçalves, P.G.C.; Cella, R. Biomass transformation: Hydration and isomerization reactions of turpentine oil using ion exchange resins as catalyst. Sustain. Chem. Pharm. 2020, 15, 100214. [Google Scholar] [CrossRef]
- Prakoso, T.; Putra, I.A.; Handojo, L.; Soerawidjaja, T.H.; Winoto, H.P.; Indarto, A. A method to control terpineol production from turpentine by acid catalysts mixing. Heliyon 2020, 6, e04984. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhang, D.-Q.; Xie, C.-X.; Yu, F.-L.; Yu, S.-T. Hydration of α-pinene catalyzed by oxalic acid/polyethylene glycol deep eutectic solvents. J. Fuel Chem. Technol. 2021, 49, 330–338. [Google Scholar] [CrossRef]
- Deng, X.; Zhi, Y.; Shan, S.; Miao, Y.; Jia, Q.; Ni, Y. Effective and reusable microcrystalline cellulosic Salen complexes for epoxidation of alpha-pinene. Cellulose 2018, 25, 1281–1289. [Google Scholar] [CrossRef]
- Su, W.; Li, Q.; Liu, Y.; Qin, Y.; Liu, H.; Tang, A. Improved efficiency of lipase-mediated epoxidation of α-pinene using H2O2 in single-phase systems. Mol. Catal. 2021, 508, 111585. [Google Scholar] [CrossRef]
- Castellanos, N.J.; Martínez, Q.H.; Martínez, O.F.; Leus, K.; Voort, P.V.D. Photo-epoxidation of (α, β)-pinene with molecular O2 catalyzed by a dioxo-molybdenum (VI)-based Metal–Organic Framework. Res. Chem. Intermed. 2021, 47, 4227–4244. [Google Scholar] [CrossRef]
- Huang, P.; Chen, T.; Zheng, Y.; Yang, C.; Wang, Y.; Ran, S.; Zhi, Y.; Shan, S.; Jiang, L. Aerobic epoxidation of α-pinene using Mn/SAPO-34 catalyst: Optimization via response surface methodology (RSM). Mol. Catal. 2023, 535, 112872. [Google Scholar] [CrossRef]
- Costa, V.V.; da Silva Rocha, K.A.; de Sousa, L.F.; Robles-Dutenhefner, P.A.; Gusevskaya, E.V. Isomerization of α-pinene oxide over cerium and tin catalysts: Selective synthesis of trans-carveol and trans-sobrerol. J. Mol. Catal. A Chem. 2011, 345, 69–74. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Hasan, Z.; Panchenko, V.N.; Prosvirin, I.P.; Jhung, S.H. Vanadium-containing nickel phosphate molecular sieves as catalysts for α-pinene oxidation with molecular oxygen: A study of the effect of vanadium content on activity and selectivity. J. Mol. Catal. A: Chem. 2012, 363–364, 328–334. [Google Scholar] [CrossRef]
- da Silva Rocha, K.A.; Hoehne, J.L.; Gusevskaya, E.V. Phosphotungstic Acid as a Versatile Catalyst for the Synthesis of Fragrance Compounds by α-Pinene Oxide Isomerization: Solvent-Induced Chemoselectivity. Chem. A Eur. J. 2008, 14, 6166–6172. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.J.A.; Pereira, M.M.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V.; da Silva Rocha, K.A. Heteropoly acid catalysts in upgrading of biorenewables: Synthesis of para-menthenic fragrance compounds from α-pinene oxide. Catal. Today 2020, 344, 166–170. [Google Scholar] [CrossRef]
- Draczyńska, B.; Cagara, C.; Siewiński, A.; Rymkiewicz, A.; Zabźa, A.; Leufvèn, A. Biotransformation of pinenes XVII. Transformation of α-and β-pinenes by means of Armillariella mellea (honey fungus), a parasite of woodlands. J. Basic Microbiol. 1985, 25, 487–492. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, G.; He, H.; Xu, Q.; Yin, D. Biomimeti cconversion of α-pinene with H2O2 to sobrerol over V2O5: Dihydroxylation by a peroxovanadiumper acid vectoring gentle synergistic oxidation. Catal. Commun. 2020, 142, 106041. [Google Scholar] [CrossRef]
- He, H.; Zheng, M.; Liu, Q.; Liu, J.; Zhao, J.; Zhuang, Y.; Liu, X.; Xu, Q.; Kirk, S.R.; Yin, D. Hydroxyl-assisted selective epoxidation of perillyl alcohol with hydrogen peroxide by vanadium-substituted phosphotungstic acid hinged on imidazolyl activated carbon. New J. Chem. 2022, 46, 6636–6645. [Google Scholar] [CrossRef]
- Zheng, M.; He, H.; Li, X.; Yin, D.L. Imidazolized activated carbon anchoring phosphotungstic acid as recyclable catalyst for oxidation of alcohols with aqueous hydrogen peroxide. Front. Chem. 2022, 10, 925622. [Google Scholar] [CrossRef]
- Liu, W.; Guo, R.; Peng, G.; Yin, D. Sulfuric acid immobilized on activated carbon aminated with ethylenediamine: An efficient reusable catalyst for the synthesis of acetals (Ketals). Nanomaterials 2022, 12, 1462. [Google Scholar] [CrossRef]
- Hosseini-Eshbala, F.; Sedrpoushan, A.; Breit, B.; Mohanazadeh, F.; Veisi, H. Ionic-liquid-modified CMK-3 as a support for the immobilization of molybdate ions (MoO42−): Heterogeneous nanocatalyst for selective oxidation of sulfides and benzylic alcohols. Mater. Sci. Eng. C 2020, 110, 110577. [Google Scholar] [CrossRef]
- Analía, L.C.; Corina, M.C.; Virginia, M.V.; Eimer, G.A.; Casuscelli, S.G. Biomass toward fine chemical products: Oxidation α-pinene over sieves nanostructured modified with vanadium. J. Mol. Catal. A Chem. 2015, 404–405, 65–73. [Google Scholar]
- Maria, N.T.; Valentin, N.P. Rearrangement of α-pinene oxide to campholenic aldehyde over the trimesate metal-organic frameworks MIL-100, MIL-110 and MIL-96. J. Catal. 2014, 311, 114–120. [Google Scholar]
- Sidorenko, A.Y.; Kravtsova, A.V.; Aho, A.; Heinmaa, I.; Kuznetsova, T.F.; Murzin, D.Y.; Agabekov, V.E. Catalytic isomerization of α-pinene oxide in the presence of acid-modified. Mol. Catal. 2018, 448, 18–29. [Google Scholar] [CrossRef]
- Coelho, J.V.; de Meireles, A.L.P.; da Silva Rocha, K.A.; Pereira, M.C.; Oliveira, L.C.A.; Gusevskaya, E.V. Isomerization of α-pinene oxide catalyzed by iron-modified mesoporous silicates. Appl. Catal. A Gen. 2012, 443–444, 125–132. [Google Scholar] [CrossRef]
- Panchenko, V.N.; Kirillov, V.L.; Gerasimov, E.Y. Isomerization of α-pinene oxide to campholenic aldehyde in the presence of Al-SiO2 and magnetic Al-SiO2/Fe3O4 catalysts. React. Kinet. Mech. Catal. 2020, 130, 919–934. [Google Scholar] [CrossRef]

| Cat. | Conv. /% | Sel. a/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Others b | ||
| None | 5.4 | - | <0.1 | 4.2 | 37.1 | 37.3 | 5.8 | 2.2 | 1.6 | 0.4 | 11.4 |
| AC | 8.4 | - | <0.1 | 4.2 | 32.6 | 38.3 | 6.8 | 2.8 | 1.8 | 0.4 | 13.0 |
| AC-O | 17.9 | - | 0.2 | 4.2 | 8.1 | 7.3 | 2.8 | 3.2 | 2.6 | 0.8 | 71.8 |
| AC-COIMI | 2.4 | - | 2.1 | 0.2 | 47.1 | 46.9 | 1.8 | 0.5 | - | - | 1.4 |
| AC-COIMI-HPW | 89.3 | 4.3 | 0.1 | 3.2 | 10.1 | 7.7 | 2.2 | 10.5. | 4.3 | 2.1 | 55.8 |
| AC-COIMI-NH4PW | 90.6 | 2.6 | 0.8 | 7.5 | 8.6 | 8.3 | 5.6 | 40.5 | 6.6 | 3.4 | 16.0 |
| Solvents a | Conv. /% | Sel./% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Others | ||
| None | 5.4 | - | <0.1 | 4.2 | 37.1 | 37.3 | 5.8 | 2.2 | 1.6 | 0.4 | 11.4 |
| DCM (1.60) | 8.7 | - | <0.1 | 4.7 | 40.6 | 21.2 | <0.1 | 2.6 | 7.7 | 0.4 | 22.8 |
| HAC (1.74) | 31.5 | - | <0.1 | 11.8 | 3.2 | 17.1 | 1.7 | 19.1 | 8.9 | 2.3 | 35.9 |
| EA (1.78) | 22.1 | - | <0.1 | 10.7 | 24.6 | 22.1 | 4.3 | 18.8 | 4.6 | 1.3 | 13.6 |
| THF (1.63) | 52.0 | 6.9 | 11.6 | 7.5 | 14.1 | 31.9 | 1.2 | 9.7 | 3.1 | 0.9 | 13.1 |
| Dioxane (0) | 45.3 | 4.6 | 12.3 | 8.2 | 15.8 | 27.4 | 2.3 | 8.3 | 2.5 | 2.2 | 16.4 |
| Acetone (2.88) | 66.2 | 3.3 | <0.1 | 16.0 | 4.8 | 8.8 | 3.1 | 28.8 | 6.7 | 3.8 | 24.8 |
| Butanone (2.78) | 46.1 | 9.6 | 10.0 | 6.7 | 11.8 | 2.1 | 2.0 | 24.5 | 11. | 5.1 | 12.9 |
| CAN (3.92) | 90.6 | 2.6 | 0.8 | 7.5 | 8.6 | 8.3 | 5.6 | 40.5 | 6.6 | 3.4 | 16.0 |
| Substrate/Catalyst | T/°C | t/h | Conv.% | Sel./% | Times a | Ref. |
|---|---|---|---|---|---|---|
| α-Pinene/AC-COIMI-NH4PW | 40 | 3 | 90.6 | 40.5 | 6 | This work |
| α-Epoxypinane/Ce/SiO2 | 25 | 3 | 98 | 72 | 1 | [20] |
| α-Epoxypinane/HPW | 60 | 3 | 100 | 38 | 1 | [22] |
| α-Epoxypinane/CsPW | 25 | 5 | 100 | 48 | 1 | [23] |
| α-Pinene/V2O5 | 25 | 2 | 85.7 | 38.0 | 1 | [25] |
| α-Epoxypinane/MIL-100 | 30 | 0.5 | 98 | 7 | 1 | [30] |
| α-Epoxypinane/acidic clays | 50 | 2 | 99.9 | 8.3 | 1 | [31] |
| α-Epoxypinane/Fe2+-MCM | 40 | 3 | 96 | 56 | 1 | [32] |
| α-Epoxypinane/12%Al-SiO2 | 30 | 0.5 | 80 | 8 | 1 | [33] |
| α-Pinene/V-M(x) | 70 | 7 | 84.5 | 36.3 | 4 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Li, X.; Xun, Y.; Wang, J.; Yin, D. Selective Catalytic Epoxidation–Hydration of α-Pinene with Hydrogen Peroxide to Sobrerol by Durable Ammonium Phosphotungstate Immobilized on Imidazolized Activated Carbon. Nanomaterials 2023, 13, 1554. https://doi.org/10.3390/nano13091554
Zheng M, Li X, Xun Y, Wang J, Yin D. Selective Catalytic Epoxidation–Hydration of α-Pinene with Hydrogen Peroxide to Sobrerol by Durable Ammonium Phosphotungstate Immobilized on Imidazolized Activated Carbon. Nanomaterials. 2023; 13(9):1554. https://doi.org/10.3390/nano13091554
Chicago/Turabian StyleZheng, Min, Xiangzhou Li, Youyi Xun, Jianhua Wang, and Dulin Yin. 2023. "Selective Catalytic Epoxidation–Hydration of α-Pinene with Hydrogen Peroxide to Sobrerol by Durable Ammonium Phosphotungstate Immobilized on Imidazolized Activated Carbon" Nanomaterials 13, no. 9: 1554. https://doi.org/10.3390/nano13091554
APA StyleZheng, M., Li, X., Xun, Y., Wang, J., & Yin, D. (2023). Selective Catalytic Epoxidation–Hydration of α-Pinene with Hydrogen Peroxide to Sobrerol by Durable Ammonium Phosphotungstate Immobilized on Imidazolized Activated Carbon. Nanomaterials, 13(9), 1554. https://doi.org/10.3390/nano13091554







