Plasma-Etched Vertically Aligned CNTs with Enhanced Antibacterial Power
Abstract
:1. Introduction
2. Materials and Methods
2.1. CVD Synthesis of VA-CNTs
2.2. Plasma Etching Treatment
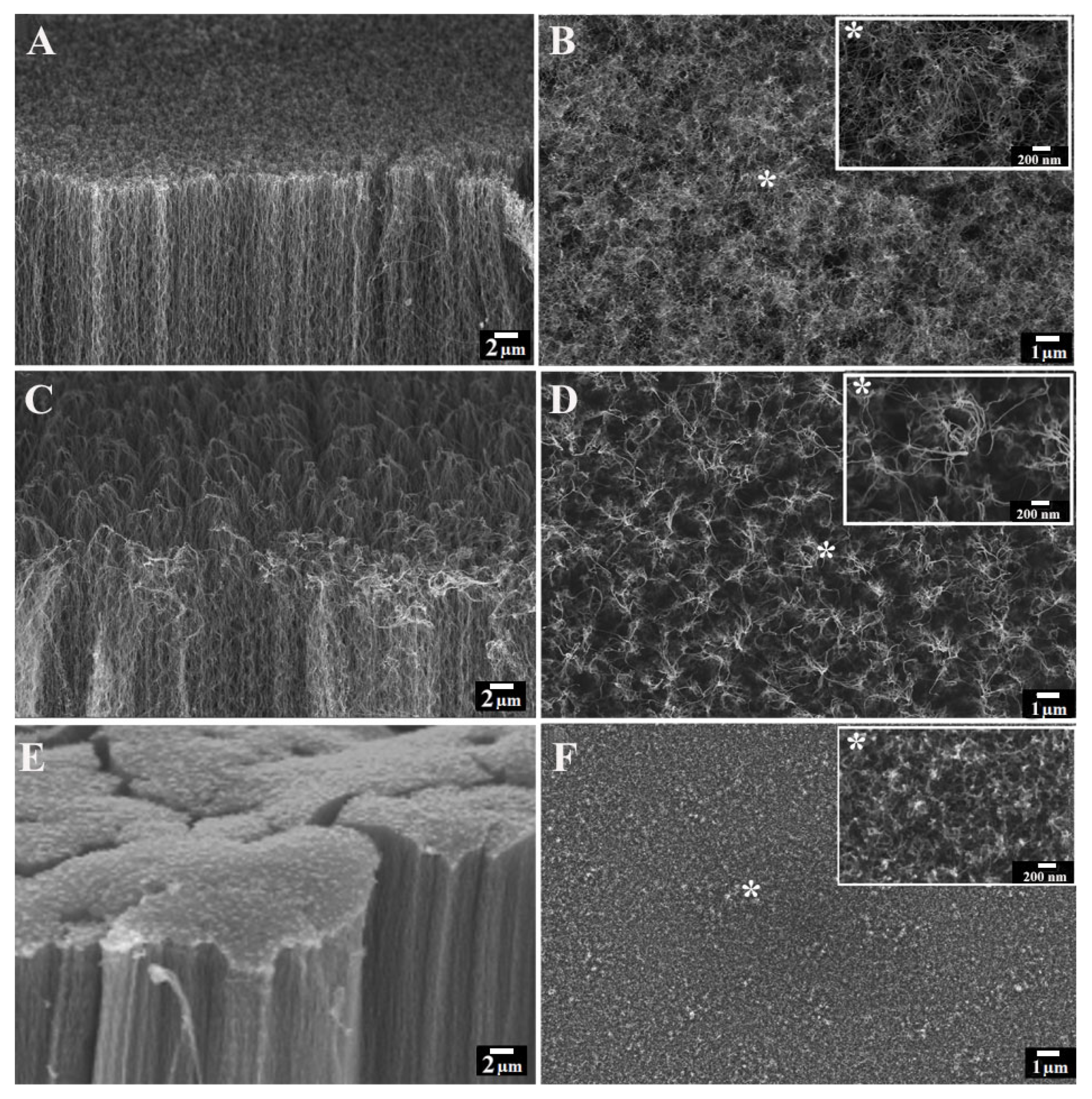
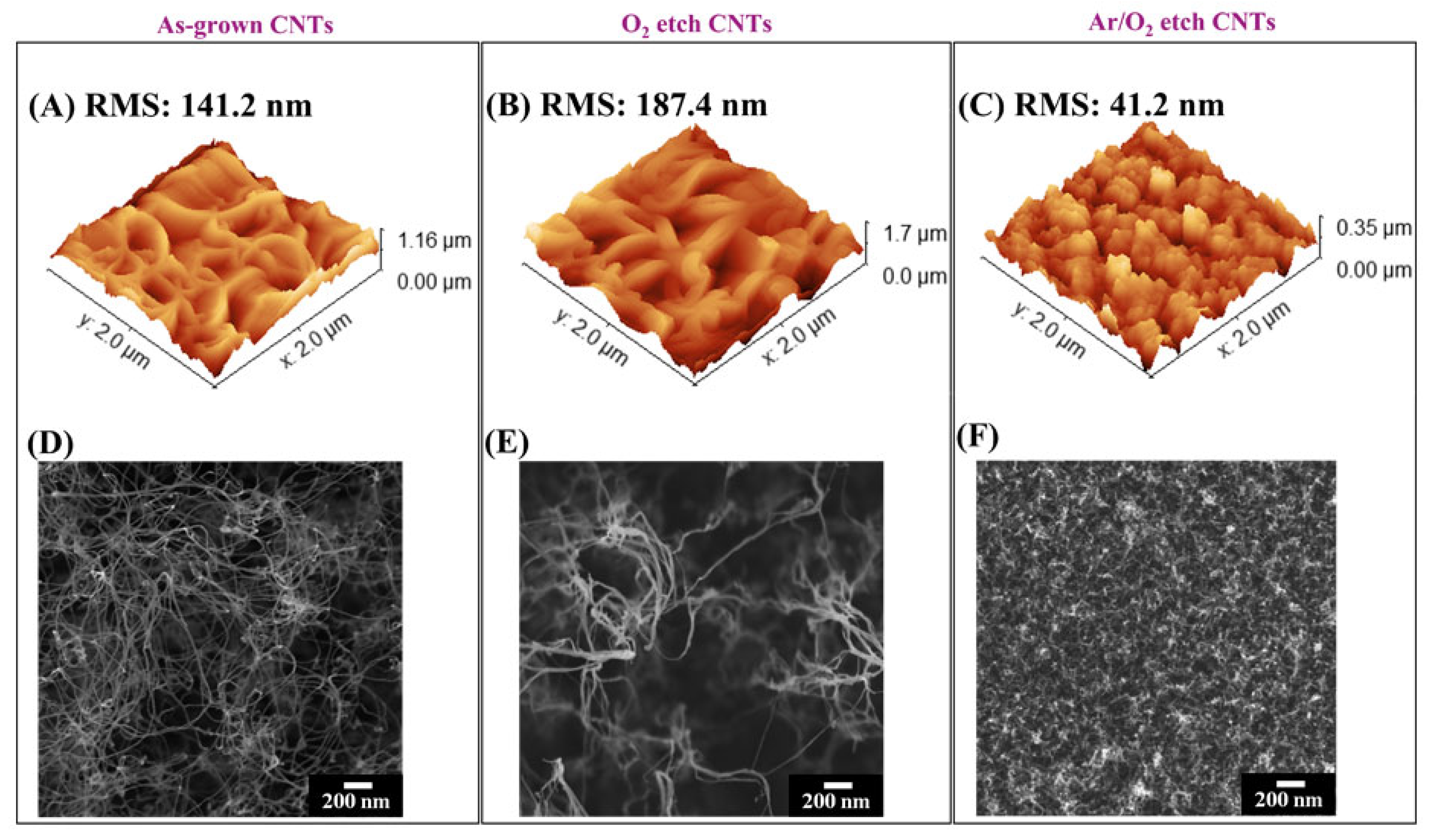
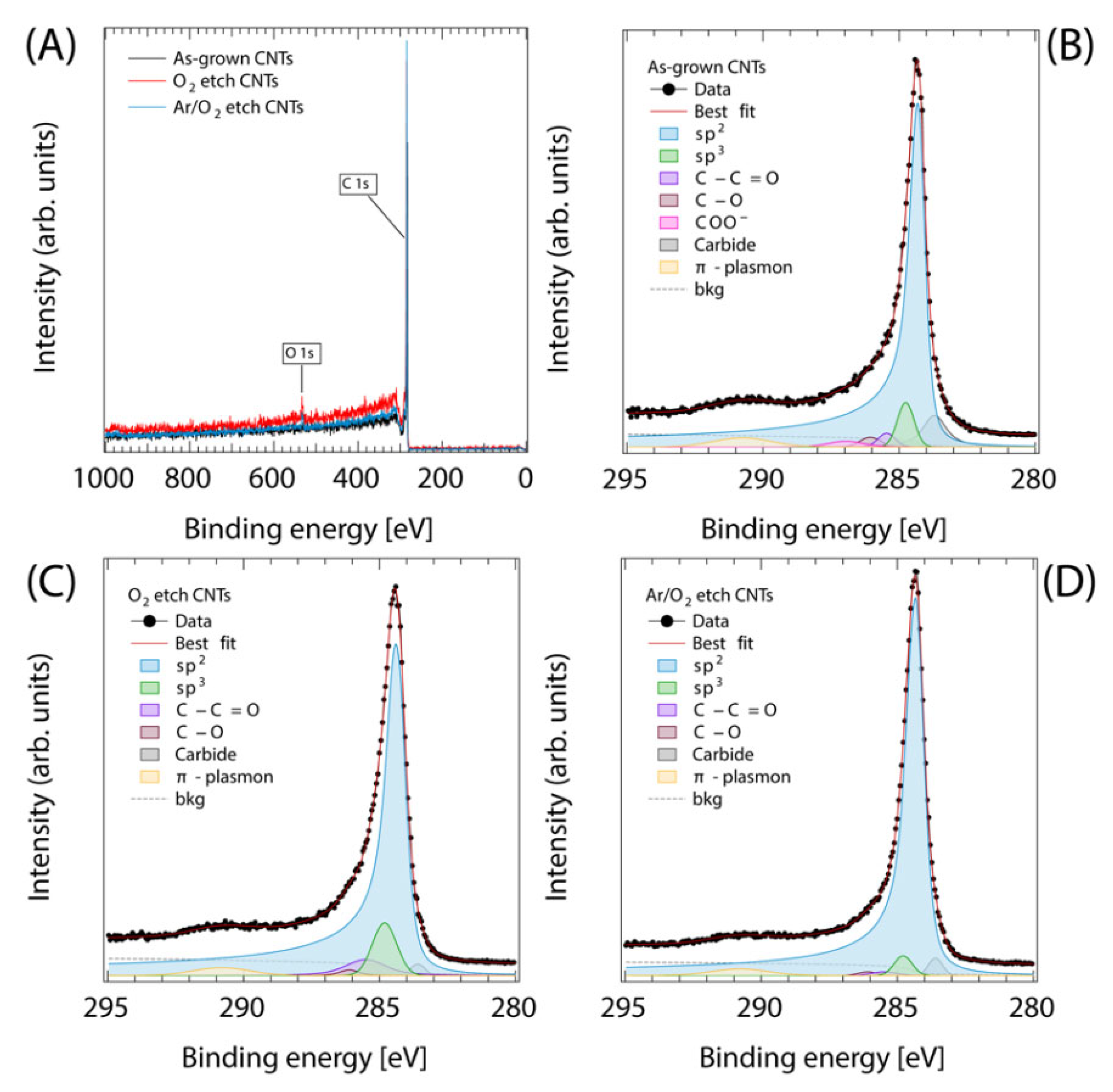
2.3. Characterization of VA-CNTs
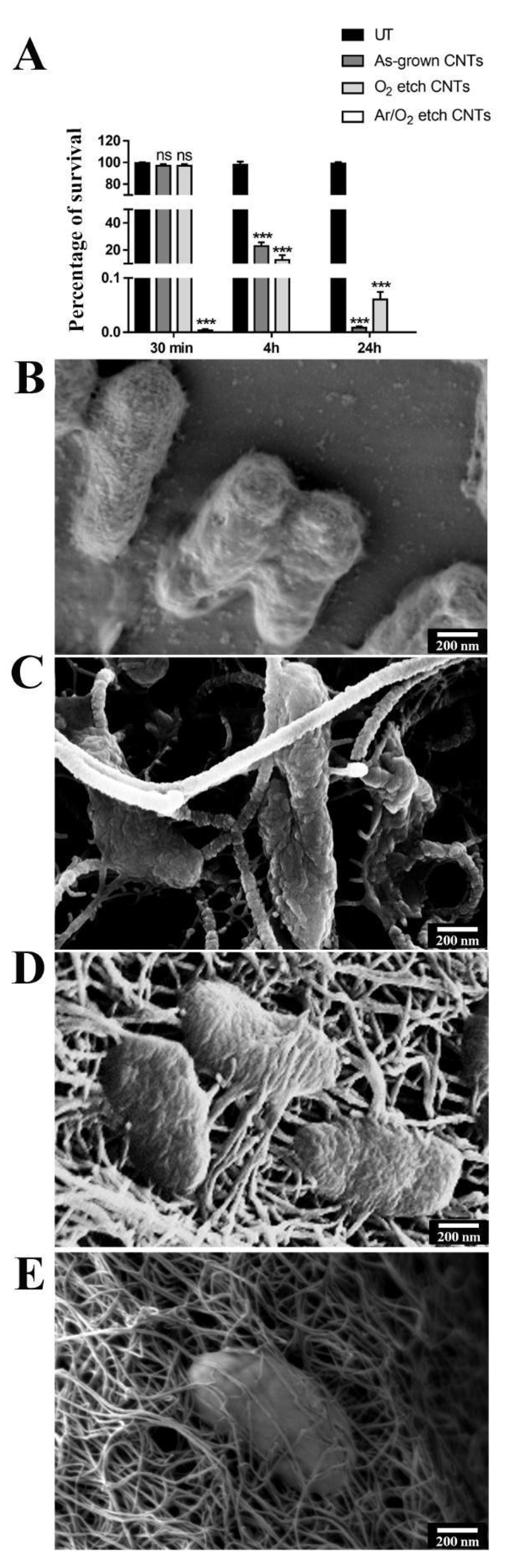
2.4. Bacteria Growth Conditions and Cell Viability Test
2.5. Biofilm Formation
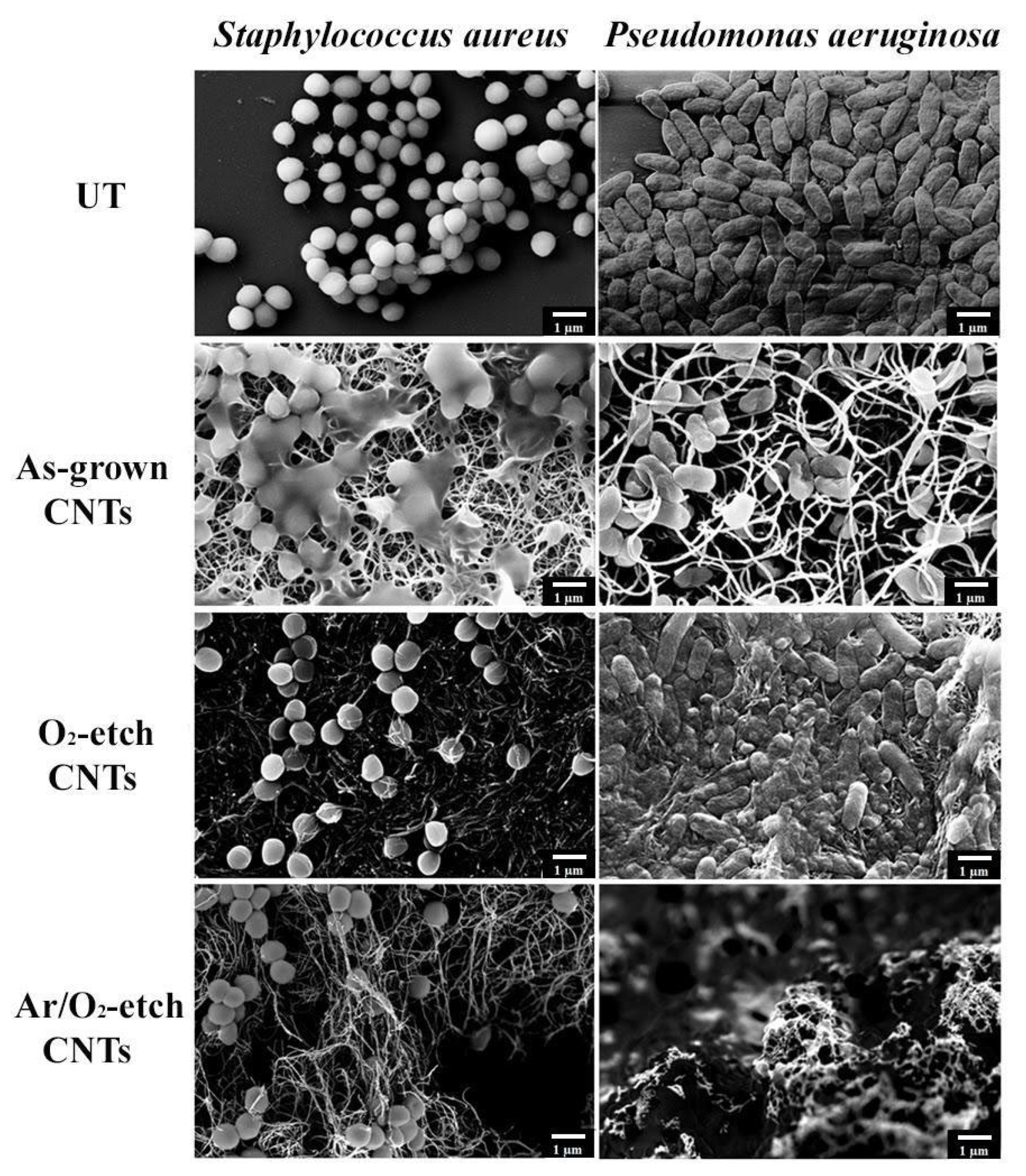
2.6. Bacteria SEM Analysis
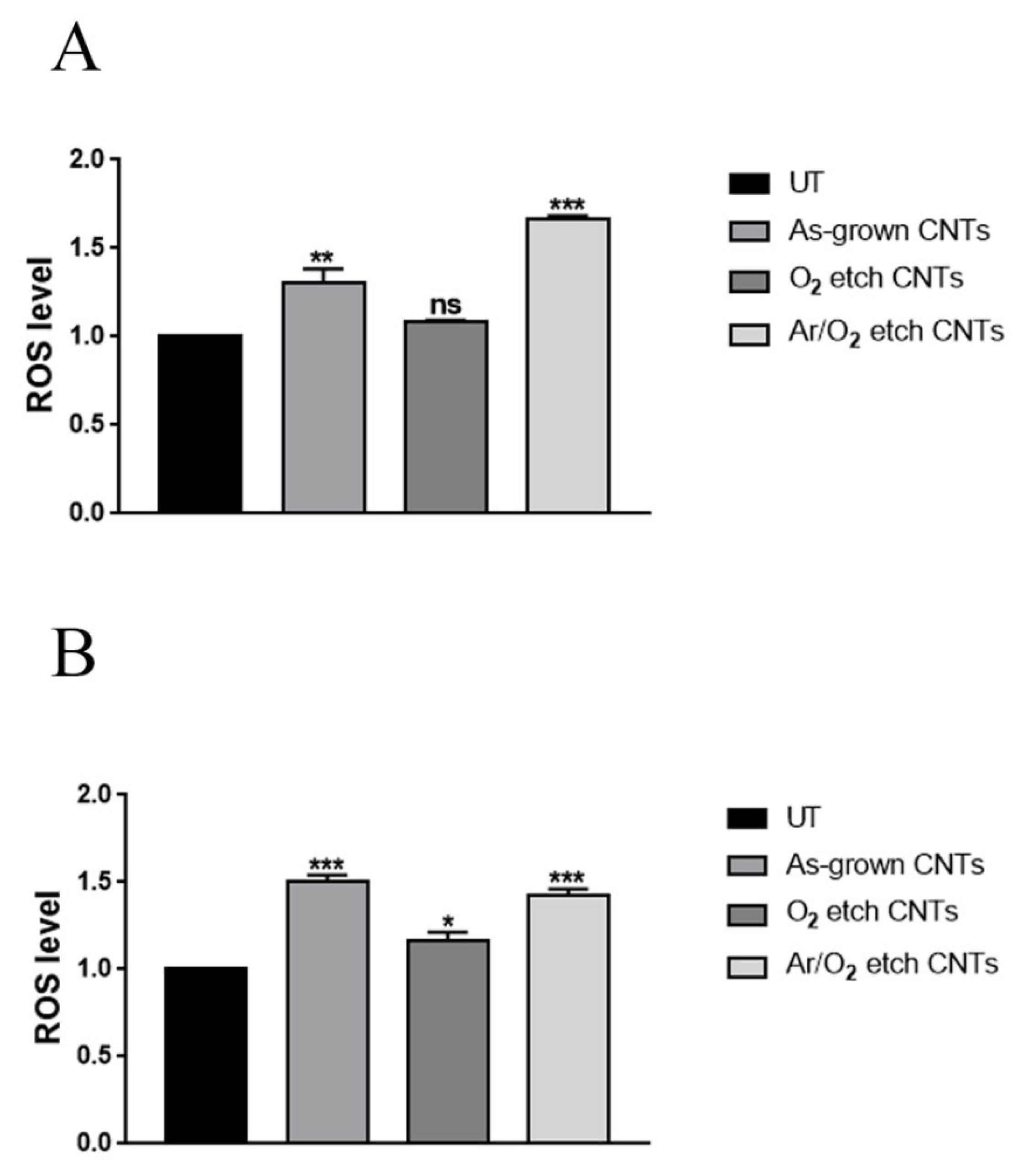
2.7. Reactive Oxygen Species Evaluation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heuer, H.; Smalla, K. Horizontal gene transfer between bacteria. Env. Biosaf. Res. 2007, 6, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, R.; Lim, C.; Li, S.; Wang, Y.; Song, J.; Lin, T.; Muir, B.W.; Hsu, H.; Shen, H. Recent Advances in the Development of Lipid-, Metal-, Carbon-, and Polymer-Based Nanomaterials for Antibacterial Applications. Nanomaterials 2022, 12, 3855. [Google Scholar] [CrossRef]
- Lucien, M.A.B.; Canarie, M.F.; Kilgore, P.E.; Jean-Denis, G.; Fénélon, N.; Pierre, M.; Cerpa, M.; Joseph, G.A.; Maki, G.; Zervos, M.J.; et al. Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings. Int. J. Infect. Dis. 2021, 104, 250–254. [Google Scholar] [CrossRef]
- Deep, K.K.; Olivier, H. Death at the interface: Nanotechnology’s challenging frontier against microbial surface colonization. Front. Chem. 2022, 10, 1003234. [Google Scholar]
- Vadrucci, M.; De Bellis, G.; Mazzuca, C.; Mercuri, F.; Borgognoni, F.; Schifano, E.; Uccelletti, D.; Cicero, C. Effects of the Ionizing Radiation Disinfection Treatment on Historical Leather. Front. Mater. 2020, 7, 00021. [Google Scholar] [CrossRef] [Green Version]
- Naskar, A.; Kim, K. Nanomaterials as Delivery Vehicles and Components of New Strategies to Combat Bacterial Infections: Advantages and Limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of Antibiotic Penetration Limitation in Klebsiella Pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [Green Version]
- Linklater, D.P.; Juodkazis, S.; Rubanov, S.; Ivanova, E.P. Comment on “Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli”. ACS Appl. Mater. Interfaces 2017, 9, 29387. [Google Scholar] [CrossRef] [Green Version]
- Ishak, M.I.; Jenkins, J.; Kulkarni, S.; Keller, T.F.; Briscoe, W.H.; Nobbs, A.H.; Su, B. Insights into complex nanopillar-bacteria interactions: Roles of nanotopography and bacterial surface proteins. J. Colloid Interface Sci. 2021, 604, 91–103. [Google Scholar] [CrossRef]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [Green Version]
- Watson, G.S.; Green, D.W.; Watson, J.A.; Zhou, Z.; Li, X.; Cheung, G.S.P.; Gellender, M. A simple model for binding and rupture of bacterial cells on nanopillar surfaces. Adv. Mater. Interfaces 2019, 6, 1801646. [Google Scholar] [CrossRef]
- Ayazi, M.; Ebrahimi, N.G.; Nodoushan, E.J. Bacterial adhesion reduction on the surface with a simulated pattern: An insight into extrand model. Int. J. Adhes. Adhes. 2019, 88, 66–73. [Google Scholar] [CrossRef]
- Li, X. Bactericidal mechanism of nanopatterned surfaces. Phys. Chem. Chem. Phys. 2016, 18, 1311–1316. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.P.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Olivi, M.; Zanni, E.; De Bellis, G.; Talora, C.; Sarto, M.S.; Palleschi, C.; Flahaut, E.; Monthioux, M.; Rapino, S.; Uccelletti, D.; et al. Inhibition of microbial growth by carbon nanotube networks. Nanoscale 2013, 5, 9023. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Guo, Y.; Baulin, V.A.; Al Kobaisi, M. Graphene induces formation of pores that kill spherical and rod- shaped bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Werber, J.R.; Chu, C.; Zucker, I.; Kim, J.; Osuji, C.O.; Elimelech, M. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc. Natl Acad. Sci. USA 2017, 114, E9793–E9801. [Google Scholar] [CrossRef] [Green Version]
- Pandit, S.; Gaska, K.; Mokkapati, V.R.S.S.; Celauro, E.; Derouiche, A.; Forsberg, S.; Svensson, M.; Kádár, R.; Mijakovic, I. Precontrolled alignment of graphite nanoplatelets in polymeric composites prevents bacterial attachment. Small 2020, 16, 1904756. [Google Scholar] [CrossRef]
- Linklater, D.P.; Ivanova, E.P. Nanostructured antibacterial surfaces—What can be achieved? Nano Today 2022, 43, 101404. [Google Scholar] [CrossRef]
- Linklater, D.P.; Juodkazis, S.; Ivanova, E.P. Nanofabrication of mechano-bactericidal surfaces. Nanoscale 2017, 9, 16564–16585. [Google Scholar] [CrossRef]
- Linklater, D.P.; Nguyen, H.K.D.; Bhadra, C.M.; Juodkazis, S.; Ivanova, E.P. Influence of nanoscale topology on bactericidal efficiency of black silicon surfaces. Nanotechnology 2017, 28, 245301. [Google Scholar] [CrossRef]
- Bhadra, C.M.; Werner, M.; Baulin, V.A.; Truong, V.K.; Kobaisi, M.A.; Nguyen, S.H.; Balcytis, A.; Juodkazis, S.; Wang, J.Y.; Mainwaring, D.E.; et al. Subtle Variations in Surface Properties of Black Silicon Surfaces Influence the Degree of Bactericidal Efficiency. Nano-Micro Lett. 2018, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; et al. Bactericidal Activity of Black Silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [Green Version]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef]
- Malek, I.; Schaber, C.F.; Heinlein, T.; Schneider, J.J.; Gorb, S.N.; Schmitz, R.A. Vertically aligned multi walled carbon nanotubes prevent biofilm formation of medically relevant bacteria. J. Mater. Chem. B 2016, 4, 5228–5235. [Google Scholar] [CrossRef] [Green Version]
- Corletto, A.; Shapter, J.G. Nanoscale Patterning of Carbon Nanotubes: Techniques, Applications, and Future. Adv. Sci. 2021, 8, 2001778. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial Activity of Single-Walled Carbon Nanotubes: Length Effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-Spectrum Antibacterial Activity of Carbon Nanotubes to Human Gut Bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef]
- Le, T.T.A.; McEvoy, J.; Khan, E. Mitigation of Bactericidal Effect of Carbon Nanotubes by Cell Entrapment. Sci. Total Environ. 2016, 565, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Kohls, A.; Ditty, M.M.; Dehghandehnavi, F.; Zheng, S.Y. Vertically Aligned Carbon Nanotubes as a Unique Material for Biomedical Applications. ACS Appl. Mater. Interfaces 2022, 14, 6287–6306. [Google Scholar] [CrossRef]
- Allegri, M.; Perivoliotis, D.K.; Bianchi, M.G.; Chiu, M.; Pagliaro, A.; Koklioti, M.A.; Trompeta, A.A.; Bergamaschi, E.; Bussolati, O.; Charitidis, C.A. Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicol. Rep. 2016, 19, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the toxicity of carbon nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Rago, I.; Rauti, R.; Bevilacqua, M.; Calaresu, I.; Pozzato, A.; Cibinel, M.; Dalmiglio, M.; Tavagnacco, C.; Goldoni, A.; Scaini, D. Carbon nanotubes, directly grown on supporting surfaces, improve neuronal activity in hippocampal neuronal networks. Adv. Biosyst. 2019, 3, 1800286. [Google Scholar] [CrossRef]
- Pampaloni, N.P.; Rago, I.; Calaresu, I.; Cozzarini, L.; Casalis, L.; Goldoni, A.; Ballerini, L.; Scaini, D. Transparent carbon nanotubes promote the outgrowth of enthorino-dentate projections in lesioned organ slice cultures. Dev. Neurobiol. 2020, 80, 316–331. [Google Scholar] [CrossRef]
- Ulloa, L.S.; Perissinotto, F.; Rago, I.; Goldoni, A.; Santoro, R.; Pesce, M.; Casalis, L.; Scaini, D. Carbon nanotubes substrates alleviate pro-calcific evolution in porcine valve interstitial cells. Nanomaterials 2021, 11, 2724. [Google Scholar] [CrossRef]
- Goh, G.L.; Agarwala, S.; Yeong, W.Y. Directed and On-Demand Alignment of Carbon Nanotube: A Review toward 3D Printing of Electronics. Adv. Mater. Interfaces 2019, 6, 1801318. [Google Scholar] [CrossRef]
- Seo, S.; Kim, S.; Yamamoto, S.; Cui, K.; Kodama, T.; Shiomi, J.; Inoue, T.; Chiashi, S.; Maruyama, S.; Hart, A.J. Tailoring the surface morphology of carbon nanotube forests by plasma etching: A parametric study. Carbon 2021, 180, 204–214. [Google Scholar] [CrossRef]
- Schneider, J.J. Vertically Aligned Carbon Nanotubes as Platform for Biomimetically Inspired Mechanical Sensing, Bioactive Surfaces, and Electrical Cell Interfacing. Adv. Biosys. 2017, 1, 1700101. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Pavelyev, V.; Kumar, S.; Mishra, P.; Islam, S.S.; Tripathi, N. Analysis on the synthesis of vertically aligned carbon nanotubes: Growth mechanism and techniques. J. Mater. Sci. Mater. Electron. 2020, 31, 4399–4443. [Google Scholar] [CrossRef]
- Sarasini, F.; Tirillò, J.; Lilli, M.; Bracciale, M.P.; Vullum, P.E.; Berto, F.; De Bellis, G.; Tamburrano, A.; Cavoto, G.; Pandolfi, F.; et al. Highly aligned growth of carbon nanotube forests with in-situ catalyst generation: A route to multifunctional basalt fibres. Compos. Part B Eng. 2022, 243, 110136. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, W.; Qian, W.; Xiang, R.; Huang, J.; Wang, D.; Wei, F. Synchronous Growth of Vertically Aligned Carbon Nanotubes with Pristine Stress in the Heterogeneous Catalysis Process. J. Phys. Chem. C 2007, 111, 14638–14643. [Google Scholar] [CrossRef]
- Xu, M.; Futaba, D.N.; Yumura, M.; Hata, K. Alignment control of carbon nanotube forest from random to nearly perfectly aligned by utilizing the crowding effect. ACS Nano 2012, 6, 5837–5844. [Google Scholar] [CrossRef]
- Landois, P.; Pinault, M.; Rouzière, S.; Porterat, D.; Mocuta, C.; Elkaim, E.; Mayne-L’Hermite, M.; Launois, P. In situ time resolved wide angle X-ray diffraction study of nanotube carpet growth: Nature of catalyst particles and progressive nanotube alignment. Carbon 2015, 87, 246–256. [Google Scholar] [CrossRef]
- Gbordzoe, S.; Yarmolenko, S.; Kanakaraj, S.; Haase, M.R.; Alvarez, N.T.; Borgemenke, R.; Adusei, P.K.; Shanov, V. Effects of laser cutting on the structural and mechanical properties of carbon nanotube assemblages. Mater. Sci. Eng. B 2017, 223, 143–152. [Google Scholar] [CrossRef]
- Wu, X.; Yin, H.; Li, Q. Ablation and Patterning of Carbon Nanotube Film by Femtosecond Laser Irradiation. Appl. Sci. 2019, 9, 3045. [Google Scholar] [CrossRef] [Green Version]
- De Volder, M.; Hart, A.J. Engineering hierarchical nanostructures by elastocapillary self-assembly. Angew. Chem. Int. Ed. 2013, 52, 2412–2425. [Google Scholar] [CrossRef] [Green Version]
- Sears, K.; Skourtis, C.; Atkinson, K.; Finn, N.; Humphries, W. Focused ion beam milling of carbon nanotube yarns to study the relationship between structure and strength. Carbon 2010, 48, 4450–4456. [Google Scholar] [CrossRef]
- Xu, M.; Du, F.; Ganguli, S.; Roy, A.; Dai, L. Carbon nanotube dry adhesives with temperature-enhanced adhesion over a large temperature range. Nat. Commun. 2016, 7, 13450. [Google Scholar] [CrossRef] [Green Version]
- Hou, Z.; Cai, B.; Liu, H.; Xu, D. Ar, O₂, CHF3, and SF6 plasma treatments of screen-printed carbon nanotube films for electrode applications. Carbon 2008, 46, 405–413. [Google Scholar] [CrossRef]
- Cavoto, G.; Luchetta, F.; Polosa, A.D. Sub-GeV dark matter detection with electron recoils in carbon nanotubes. Phys. Lett. B 2018, 776, 338–344. [Google Scholar] [CrossRef]
- Capparelli, L.M.; Cavoto, G.; Mazzilli, D.; Polosa, A.D. Directional dark matter searches with carbon nanotubes. Phys. Dark Universe 2015, 9, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Cavoto, G.; Cirilllo, E.N.M.; Cocina, F.; Ferretti, J.; Polosa, A.D. WIMP detection and slow ion dynamics in carbon nanotube arrays. Eur. Phys. J. C 2016, 76, 76. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Tan, Y.; Lolli, G.; Sakulchaicharoen, N.; Requejo, F.G.; Mun, B.S.; Resasco, D.E. Influence of a Top Crust of Entangled Nanotubes on the Structure of Vertically Aligned Forests of Single-Walled Carbon Nanotubes. Chem. Mater. 2006, 18, 5624–5629. [Google Scholar] [CrossRef]
- He, B.; Yang, Y.; Yuen, M.F.; Chen, X.F.; Lee, C.S.; Zhang, W.J. Vertical nanostructure arrays by plasma etching for applications in biology, energy, and electronics. Nano Today 2013, 8, 265–289. [Google Scholar] [CrossRef]
- Puliyalil, H.; Cvelbar, U. Selective plasma etching of polymeric substrates for advanced applications. Nanomaterials 2016, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Kim, J.P.; Park, J.S. Effects of Al interlayer coating and thermal treatment on electron emission characteristics of carbon nanotubes deposited by electrophoretic method. Nanoscale Res. Lett. 2014, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N.M.D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Schifano, E.; Cavallini, D.; De Bellis, G.; Bracciale, M.P.; Felici, A.C.; Santarelli, M.L.; Sarto, M.S.; Uccelletti, D. Antibacterial Effect of Zinc Oxide-Based Nanomaterials on Environmental Biodeteriogens Affecting Historical Buildings. Nanomaterials 2020, 10, 335. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Kodama, T.; Won, Y.; Dogbe, S.; Pan, L.; Goodson, K.E. Impact of nanotube density and alignment on the elastic modulus near the top and base surfaces of aligned multi-walled carbon nanotube films. Carbon 2012, 50, 3789–3798. [Google Scholar] [CrossRef]
- Bedewy, M.; Meshot, E.R.; Guo, H.; Verploegen, E.A.; Lu, W.; Hart, A.J. Collective mechanism for the evolution and self-termination of vertically aligned carbon nanotube growth. J. Phys. Chem. C 2009, 113, 20576–20582. [Google Scholar] [CrossRef]
- Wang, B.N.; Bennett, R.D.; Verploegen, E.; Hart, A.J.; Cohen, R.E. Quantitative characterization of the morphology of multiwall carbon nanotube films by small-angle x-ray scattering. J. Phys. Chem. C 2007, 111, 5859–5865. [Google Scholar] [CrossRef]
- Malik, H.; Stephenson, K.; Bahr, D.; Field, D. Quantitative characterization of carbon nanotube turf topology by SEM analysis. J. Mater. Sci. 2011, 46, 3119–3126. [Google Scholar] [CrossRef]
- Seifi, T.; Kamali, A.R. 3D-graphene nanosheets as efficient antibacterial agent. Mater. Lett. 2022, 321, 132406. [Google Scholar] [CrossRef]
- Stegarescu, A.; Lung, I.; Ciorî, A.; Kacso, I.; Opri, O.; Soran, M.L.; Soran, A. The Antibacterial Properties of Nanocomposites Based on Carbon Nanotubes and Metal Oxides Functionalized with Azithromycin and Ciprofloxacin. Nanomaterials 2022, 12, 4115. [Google Scholar] [CrossRef]
- Noor, M.M.; Santana-Pereira, A.L.R.; Liles, M.R.; Davis, V.A. Dispersant Effects on Single-Walled Carbon Nanotube Antibacterial Activity. Molecules 2022, 27, 1606. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef] [Green Version]
- Linklater, D.P.; De Volder, M.; Baulin, V.A.; Werner, M.; Jessl, S.; Golozar, M.; Maggini, L.; Rubanov, S.; Hanssen, E.; Juodkazis, S.; et al. High Aspect Ratio Nanostructures Kill Bacteria via Storage and Release of Mechanical Energy. ACS Nano 2018, 12, 6657–6667. [Google Scholar] [CrossRef]
- Nie, C.; Cheng, C.; Ma, L.; Deng, J.; Zhao, C. Mussel-Inspired Antibacterial and Biocompatible Silver–Carbon Nanotube Composites: Green and Universal Nanointerfacial Functionalization. Langmuir 2016, 32, 5955–5965. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef]
- Abo-Neima, S.E.; Motaweh, H.A.; Elsehly, E.M. Antimicrobial activity of functionalised carbon nanotubes against pathogenic microorganisms. IET Nanobiotechnol. 2020, 14, 457–464. [Google Scholar] [CrossRef]
- Liu, D.; Mao, Y.; Ding, L. Carbon nanotubes as antimicrobial agents for water disinfection and pathogen control. J. Water Health 2018, 16, 171–180. [Google Scholar] [CrossRef]
- Nepal, D.; Balasubramanian, S.; Simonian, A.L.; Davis, V.A. Strong Antimicrobial Coatings: Single-Walled Carbon Nanotubes Armored with Biopolymers. Nano Lett. 2008, 8, 1896–1901. [Google Scholar] [CrossRef]
- Hartono, M.R.; Kushmaro, A.; Chen, X.; Marks, R.S. Probing the toxicity mechanism of multiwalled carbon nanotubes on bacteria. Env. Sci. Pollut. Res. 2018, 25, 5003–5012. [Google Scholar] [CrossRef]
- Rajavel, K.; Gomathi, R.; Manian, S.; Kumar, R.T.R. In Vitro Bacterial Cytotoxicity of CNTs: Reactive Oxygen Species Mediate Cell Damage Edges over Direct Physical Puncturing. Langmuir 2014, 30, 592–601. [Google Scholar] [CrossRef]
- Sahoo, B.M.; Banik, B.K.; Borah, P.; Jain, A. Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anti-Cancer Agents Med. Chem. 2022, 2, 215–222. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, S.; Pan, F.; Chen, S.; Zhong, L.; Wang, J.; Pei, X. Nanomaterial-based ROS-mediated strategies for combating bacteria and biofilms. J. Mater. Res. 2021, 36, 822–845. [Google Scholar] [CrossRef]
- Kurilich, M.R.; Thapa, A.; Moilanen, A.; Miller, J.L.; Li, W.; Neupane, S. Comparative study of electron field emission from randomly-oriented and vertically-aligned carbon nanotubes synthesized on stainless steel substrates. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2019, 37, 041202. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schifano, E.; Cavoto, G.; Pandolfi, F.; Pettinari, G.; Apponi, A.; Ruocco, A.; Uccelletti, D.; Rago, I. Plasma-Etched Vertically Aligned CNTs with Enhanced Antibacterial Power. Nanomaterials 2023, 13, 1081. https://doi.org/10.3390/nano13061081
Schifano E, Cavoto G, Pandolfi F, Pettinari G, Apponi A, Ruocco A, Uccelletti D, Rago I. Plasma-Etched Vertically Aligned CNTs with Enhanced Antibacterial Power. Nanomaterials. 2023; 13(6):1081. https://doi.org/10.3390/nano13061081
Chicago/Turabian StyleSchifano, Emily, Gianluca Cavoto, Francesco Pandolfi, Giorgio Pettinari, Alice Apponi, Alessandro Ruocco, Daniela Uccelletti, and Ilaria Rago. 2023. "Plasma-Etched Vertically Aligned CNTs with Enhanced Antibacterial Power" Nanomaterials 13, no. 6: 1081. https://doi.org/10.3390/nano13061081




