Solution of the Drug Resistance Problem of Escherichia coli with Silver Nanoparticles: Efflux Effect and Susceptibility to 31 Antibiotics
Abstract
1. Introduction
2. Materials and Methods
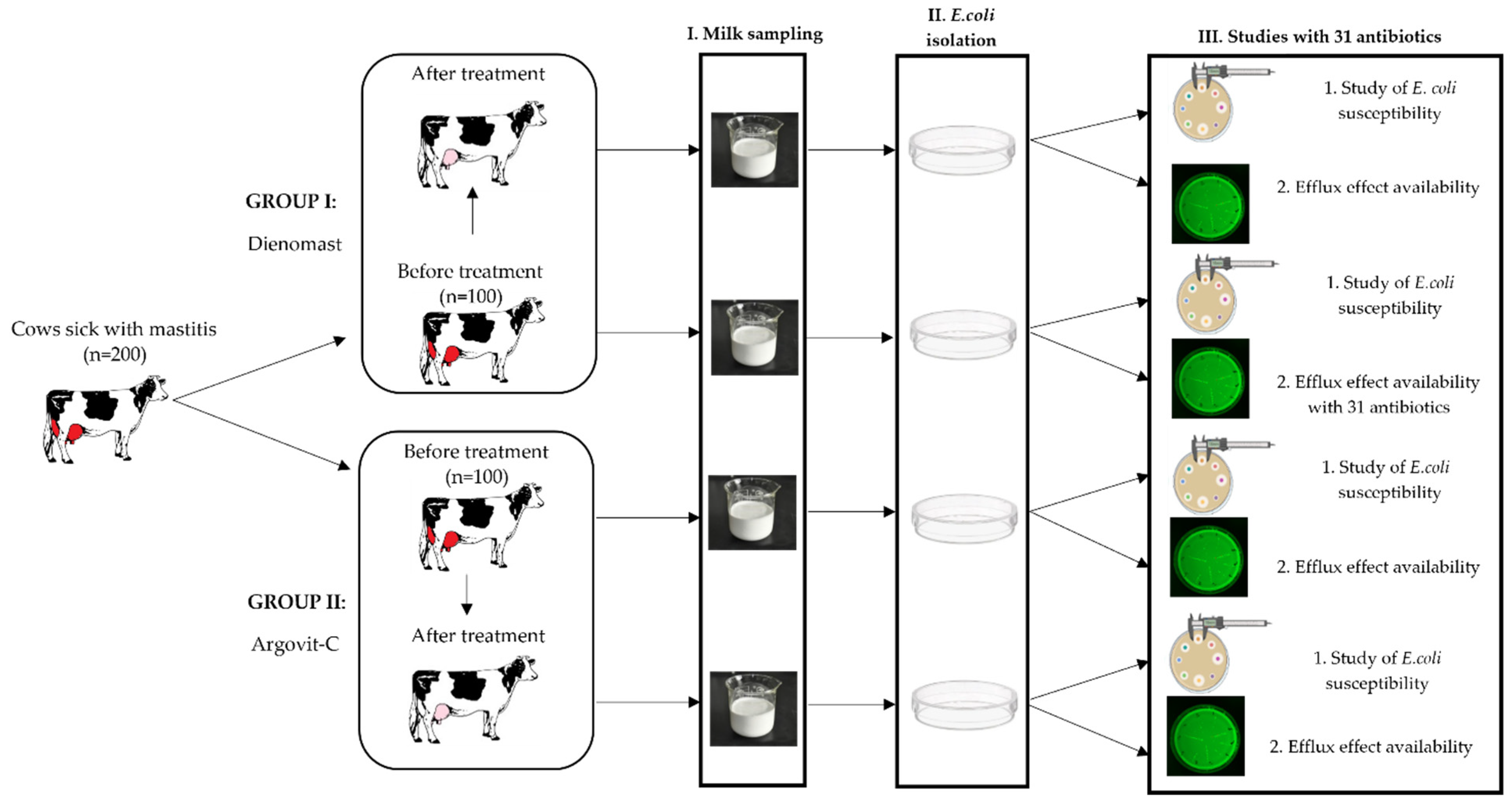
2.1. Experimental Procedure
2.2. Sampling
2.3. Treatment Formulations
2.4. Isolation and Identification of E. coli
2.5. Antimicrobial Sensitivity and Efflux Effect Testing
2.6. Ag Concentration in Milk and Blood
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Argovit-CTM AgNPs
3.2. Treatment Duration
3.3. Isolation and Identification of Bacteria
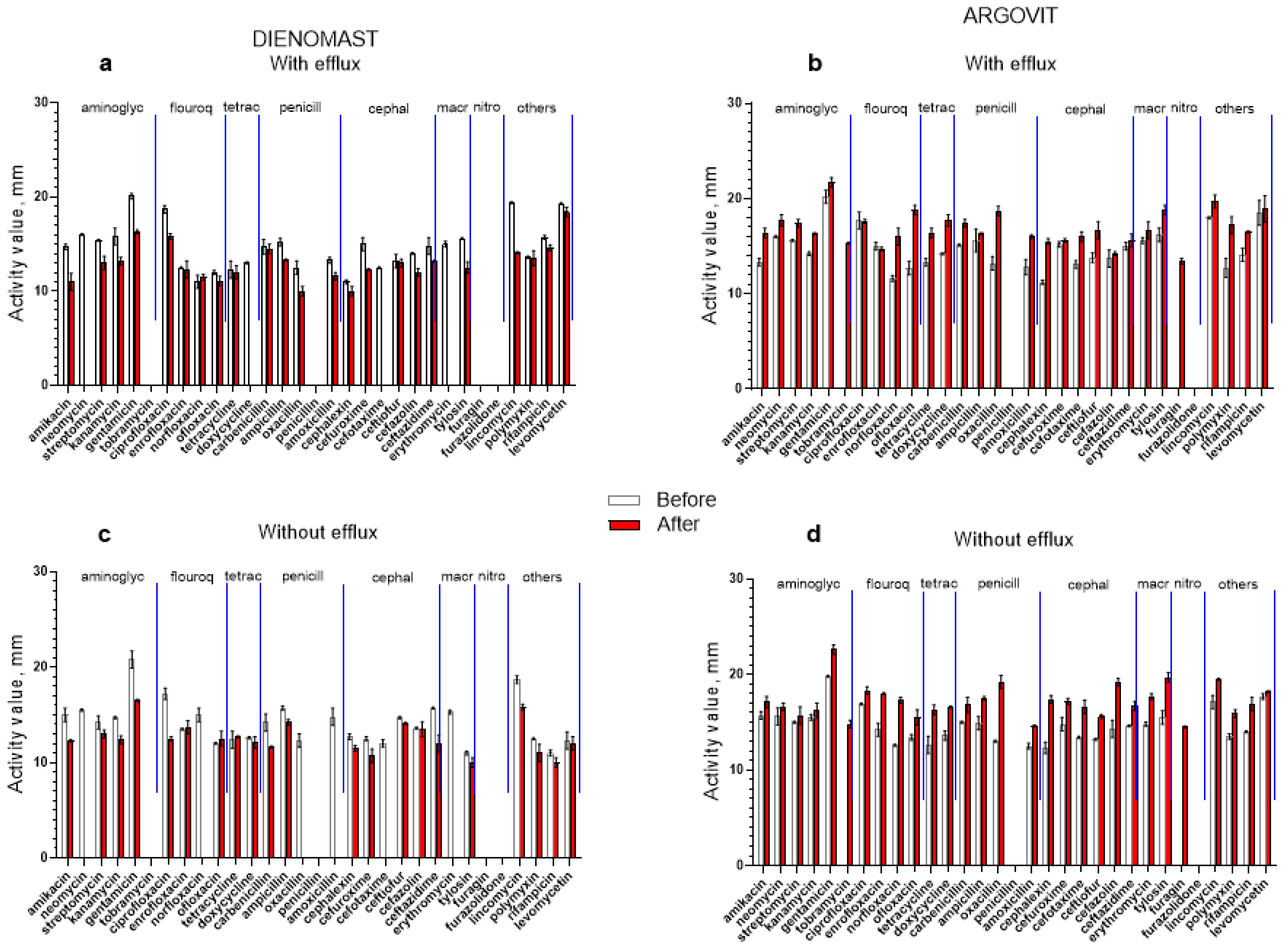
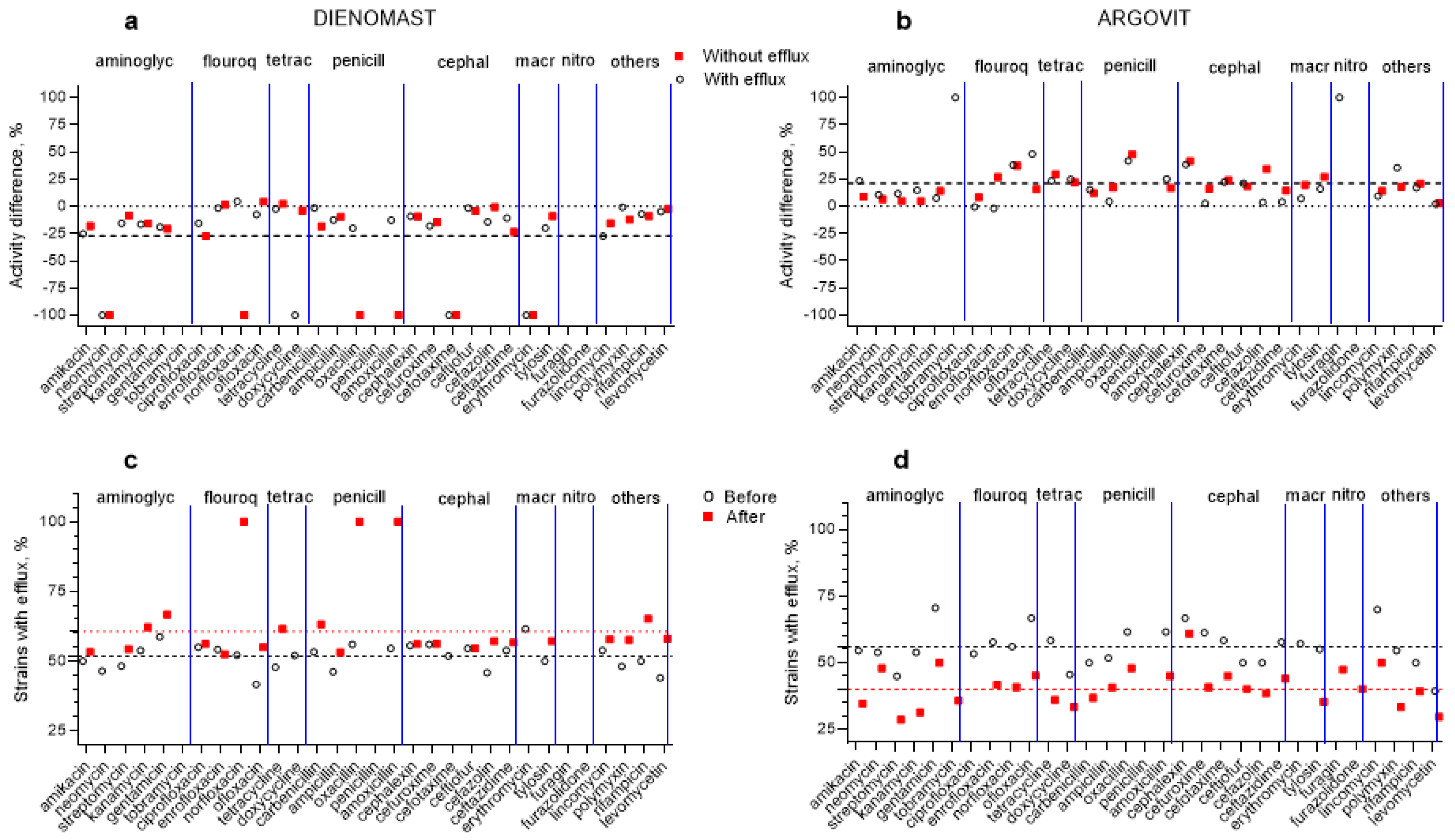
3.4. Antibiotic Susceptibility Changes after Treatments
3.5. DienomastTM
3.6. Argovit-CTM
3.7. Changes in the Contribution of Isolates with Efflux Effect after Treatments
3.8. Ag Concentration in Milk and Blood
4. Discussion
5. Conclusions
- The treatment of mastitis with antibiotic-containing medicines (LactobayTM, SpectromastTM LC, and DienomastTM) led to a 23–27% fall in S. aureus, Str. dysgalactiae, and E. coli sensitivity toward 31 antibiotics, while after Argovit-CTM treatment, their susceptibility toward the antibiotics increased by 11–19.4%.
- The total number of antibiotics for which the susceptibility of these three bacteria remained absent or disappeared after treatments with antibiotic-containing drugs was between 10 and 19, while for Argovit-CTM treatments, this number was 3 and 4.
- The portion of isolates showing the efflux effect for these three bacteria increased by 8–16% after treatment with antibiotic-containing drugs, while it decreased by 16–19% after Argovit-CTM treatment. The changes observed in susceptibility after treatments with Argovit-CTM and antibiotic-containing drugs can be at least partially explained by the alteration in the contribution of isolates with the efflux effect after treatments.
- Mastitis recovery with Argovit-CTM use occurred 33% to 50% faster than that with antibiotic-containing drugs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harikumar, G.; Krishanan, K. The growing menace of drug resistant pathogens and recent strategies to overcome drug resistance: A review. J. King Saud Univ. Sci. 2022, 34, 101979. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Ndayishimiye, J.; Kumeria, T.; Popat, A.; Falconer, J.R.; Blaskovich, M.A.T. Nanomaterials: The New Antimicrobial Magic Bullet. ACS Infect. Dis. 2022, 8, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cao, Z.; Liu, X.; Cui, Z.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. Noble metal-based nanomaterials as antibacterial agents. J. Alloys Compd. 2022, 904, 164091. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The Role of Streptococcus spp. in Bovine Mastitis. Microorganisms 2021, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Nefedova, E.; Shkil, N.; Luna Vazquez-Gomez, R.; Garibo, D.; Pestryakov, A.; Bogdanchikova, N. AgNPs Targeting the Drug Resistance Problem of Staphylococcus aureus: Susceptibility to Antibiotics and Efflux Effect. Pharmaceutics 2022, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Garibo Ruiz, D.; Nefedova, E.; Shkil, N.N.; Shkil, N.A.; Vazquez-Gomez, R.L.; Pestryakov, A.; Bogdanchikova, N. Silver Nanoparticles Targeting the Drug Resistance Problem of Streptococcus dysgalactiae: Susceptibility to Antibiotics and Efflux Effect. Int. J. Mol. Sci. 2022, 23, 6024. [Google Scholar] [CrossRef] [PubMed]
- Agrovetservis.ru. Available online: http://agrolab-nsk.ru/products/mastit-test-reaktiv (accessed on 12 October 2022).
- Holt, J. Determinant of bacteria Burgi M.: Mir; Williams & Wilkings: Baltimore, MD, USA, 1997; 432p. [Google Scholar]
- EUCAST 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf (accessed on 7 March 2023).
- García García, M.R.; Casares, N.; Martinez Perez, L.A.; Juarez Curiel, E.; Hernandez, A.A.J.; Bogdanchikova, N.; Garabo, D.; Rodriguez-Hernandez, A.G.; Pestryakov, A.; Castro Gamboa, S.; et al. Toxicological effects of silver nanoparticles and their effects in the induction of immunogenic cell death in cancer. J. Immunotoxicol. 2023, 20, 2175078. [Google Scholar] [CrossRef] [PubMed]
- Public Health Statement for Silver. Available online: https://wwwn.cdc.gov/TSP/PHS/PHS.aspx?phsid=537&toxid=97 (accessed on 7 March 2023).
- Zhanga, Q.; Wanga, R.; Wangb, M.; Liuc, C.; Koohi-Moghadama, M.; Wanga, H.; Hoc, P.L.; Lia, H.; Suna, H. Re-sensitization of mcr carrying multidrug resistant bacteria to colistin by silver. Proc. Natl. Acad. Sci. USA 2022, 119, e2119417119. [Google Scholar] [CrossRef] [PubMed]



| Microorganisms | Contribution of Isolates with Specific Microorganisms in 200 Milk Samples | |
|---|---|---|
| Number | Percentage, % | |
| E. coli | 140 | 70 |
| S. aureus | 20 | 10 |
| Str. dysgalactiae | 12 | 6 |
| S. epidermidis | 12 | 6 |
| Str. agalactiae | 10 | 5 |
| Str. pyogenes | 6 | 3 |
| Parameter | E. coli |
|---|---|
| Sodium citrate | - |
| Mannose | - |
| Sodium citrate with glucose | + |
| Arginine | - |
| Indole | + |
| Reaction Voges–Proskauer | - |
| Ornithine | + |
| Urea | - |
| H2S | - |
| Phenylalanine | - |
| Lysine | + |
| Glucose | + |
| Lactose | + |
| β-Galactosidase | + |
| Sodium malonate | + |
| Sucrose | + |
| Inositol | - |
| Sorbitol | + |
| Arabinose | + |
| Maltose | + |
| Temperature | 37 °C |
| Isolates without Efflux Effect | Isolates with Efflux Effect | ||||
|---|---|---|---|---|---|
| Activity Change Classification | Number of Antibiotics | Average Change in Activity | Number of Antibiotics | Average Change in Activity | |
| Cumulative change | |||||
| Activity variation | 27 | −30.2 % | 25 | −24.4% | |
| Total activity variation for 52 samples is −27.3% | |||||
| Details of changes | |||||
| Activity remained absent | 4 | 0 | 4 | 0 | |
| Activity disappeared (−100%) | 6 | −100% | 4 | −100% | |
| Activity appeared (+100%) | 0 | 0 | 0 | 0 | |
| Activity decreased (−∆%) | 18 | −12.3% | 22 | −12.0% | |
| Activity increased (+∆%) | 3 | +2.7% | 1 | +4.5% | |
| Activity remained constant (0%) | 0 | 0 | 0 | 0 | |
| Isolates without Efflux Effect | Isolates with Efflux Effect | ||||
|---|---|---|---|---|---|
| Activity Change Classification | Number of Antibiotics | Average Change in Activity | Number of Antibiotics | Average Change in Activity | |
| Cumulative change | |||||
| Activity change | 29 | +19.4% | 29 | +22.9% | |
| Total activity variation for 59 samples is +21.2% | |||||
| Changes detailed | |||||
| Activity remained absent | 2 | 0 | 2 | 0 | |
| Activity disappeared (−100%) | 0 | 0 | 0 | 0 | |
| Activity appeared (+100%) | 2 | +100% | 2 | +100% | |
| Activity decreased (−∆%) | 0 | 0 | 2 | −1.3 % | |
| Activity increased (+∆%) | 27 | +19.4% | 25 | +19.4% | |
| Activity remained constant (0%) | 0 | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nefedova, E.; Shkil, N.N.; Shkil, N.A.; Garibo, D.; Luna Vazquez-Gomez, R.; Pestryakov, A.; Bogdanchikova, N. Solution of the Drug Resistance Problem of Escherichia coli with Silver Nanoparticles: Efflux Effect and Susceptibility to 31 Antibiotics. Nanomaterials 2023, 13, 1088. https://doi.org/10.3390/nano13061088
Nefedova E, Shkil NN, Shkil NA, Garibo D, Luna Vazquez-Gomez R, Pestryakov A, Bogdanchikova N. Solution of the Drug Resistance Problem of Escherichia coli with Silver Nanoparticles: Efflux Effect and Susceptibility to 31 Antibiotics. Nanomaterials. 2023; 13(6):1088. https://doi.org/10.3390/nano13061088
Chicago/Turabian StyleNefedova, Ekaterina, Nikolay N. Shkil, Nikolay A. Shkil, Diana Garibo, Roberto Luna Vazquez-Gomez, Alexey Pestryakov, and Nina Bogdanchikova. 2023. "Solution of the Drug Resistance Problem of Escherichia coli with Silver Nanoparticles: Efflux Effect and Susceptibility to 31 Antibiotics" Nanomaterials 13, no. 6: 1088. https://doi.org/10.3390/nano13061088
APA StyleNefedova, E., Shkil, N. N., Shkil, N. A., Garibo, D., Luna Vazquez-Gomez, R., Pestryakov, A., & Bogdanchikova, N. (2023). Solution of the Drug Resistance Problem of Escherichia coli with Silver Nanoparticles: Efflux Effect and Susceptibility to 31 Antibiotics. Nanomaterials, 13(6), 1088. https://doi.org/10.3390/nano13061088









