Fabrication of Poly Dopamine@poly (Lactic Acid-Co-Glycolic Acid) Nanohybrids for Cancer Therapy via a Triple Collaboration Strategy
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
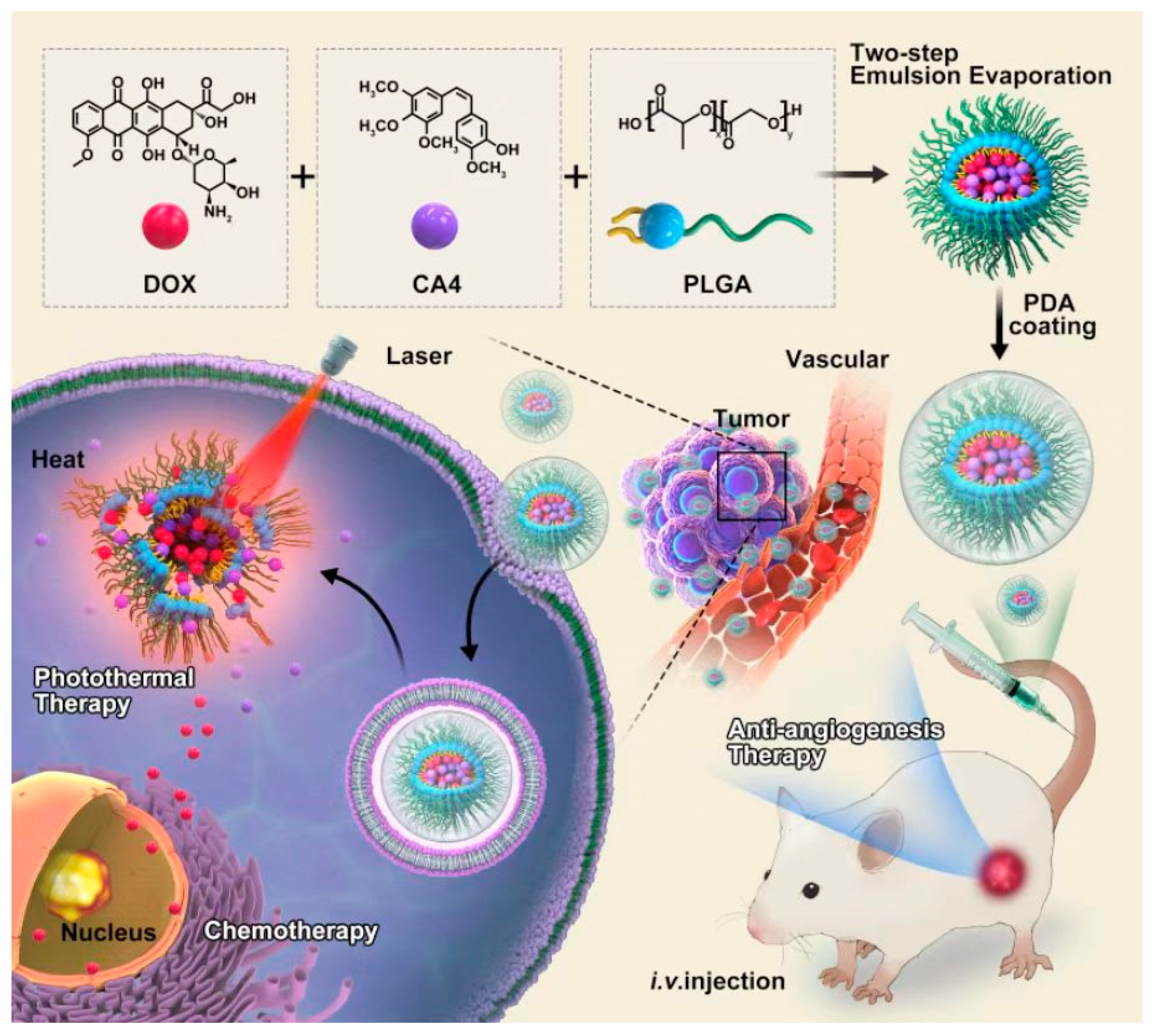
2.2. Preparation of PDA@PLGA/DC NPs
2.3. Physicochemical Characterizations of PDA@PLGA/DC NPs
2.4. Photothermal Effects of PDA@PLGA/DC NPs
2.5. In Vitro Release Behavior
2.6. Cellular Uptake In Vitro
2.7. Cytotoxicity Assay
2.8. Experimental Animals
2.9. In Vivo Imaging
2.10. In Vivo Antitumor Treatments
2.11. Biochemical Analysis and Histological
2.12. Statistic Analysis
3. Results and Discussion
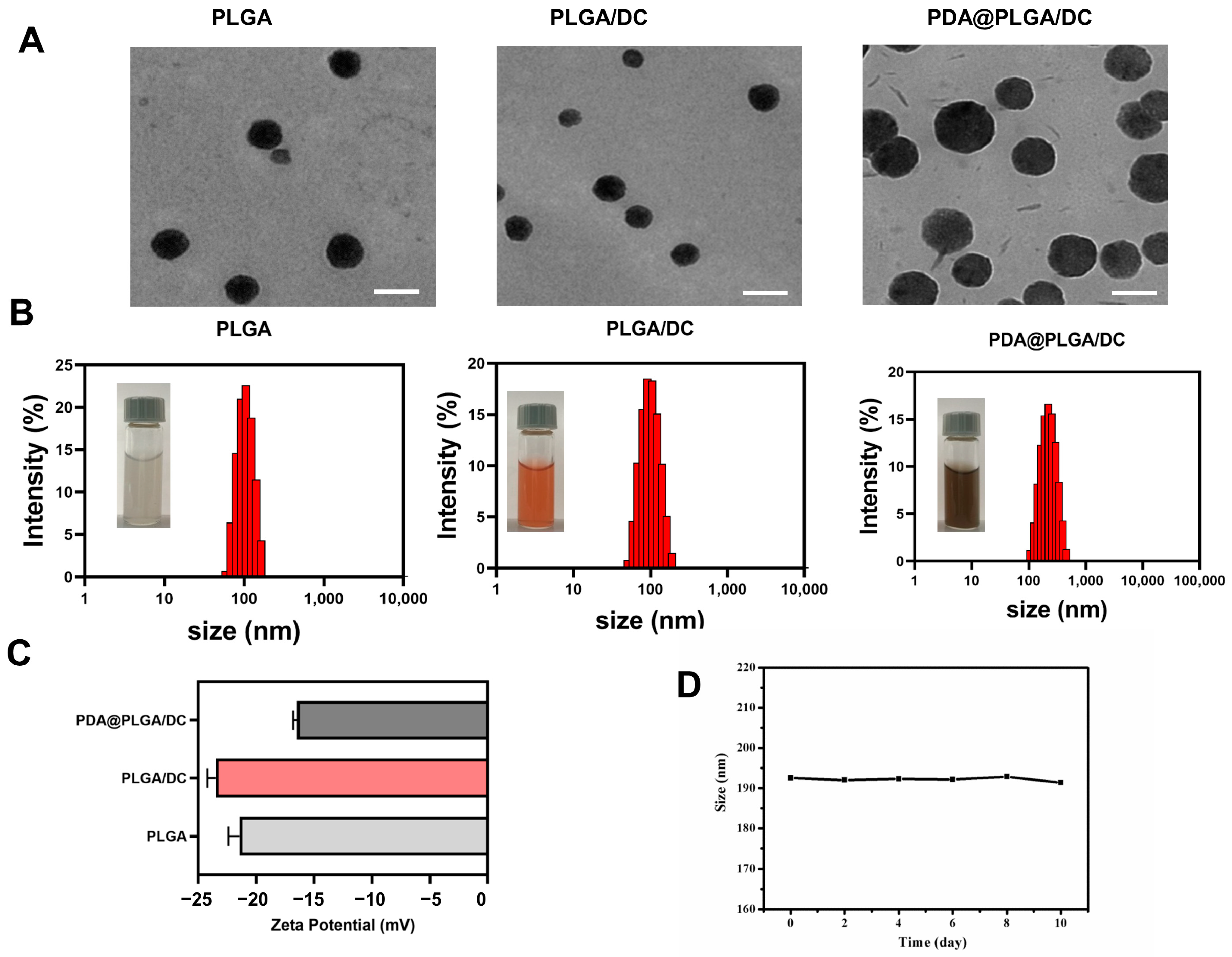
3.1. Construction and Characterization of PDA@PLGA/DC NPs
3.2. Photothermal Heating Effect
3.3. In Vitro Drug Release from PDA@PLGA/DC NPs
3.4. In Vitro Synergy Therapy
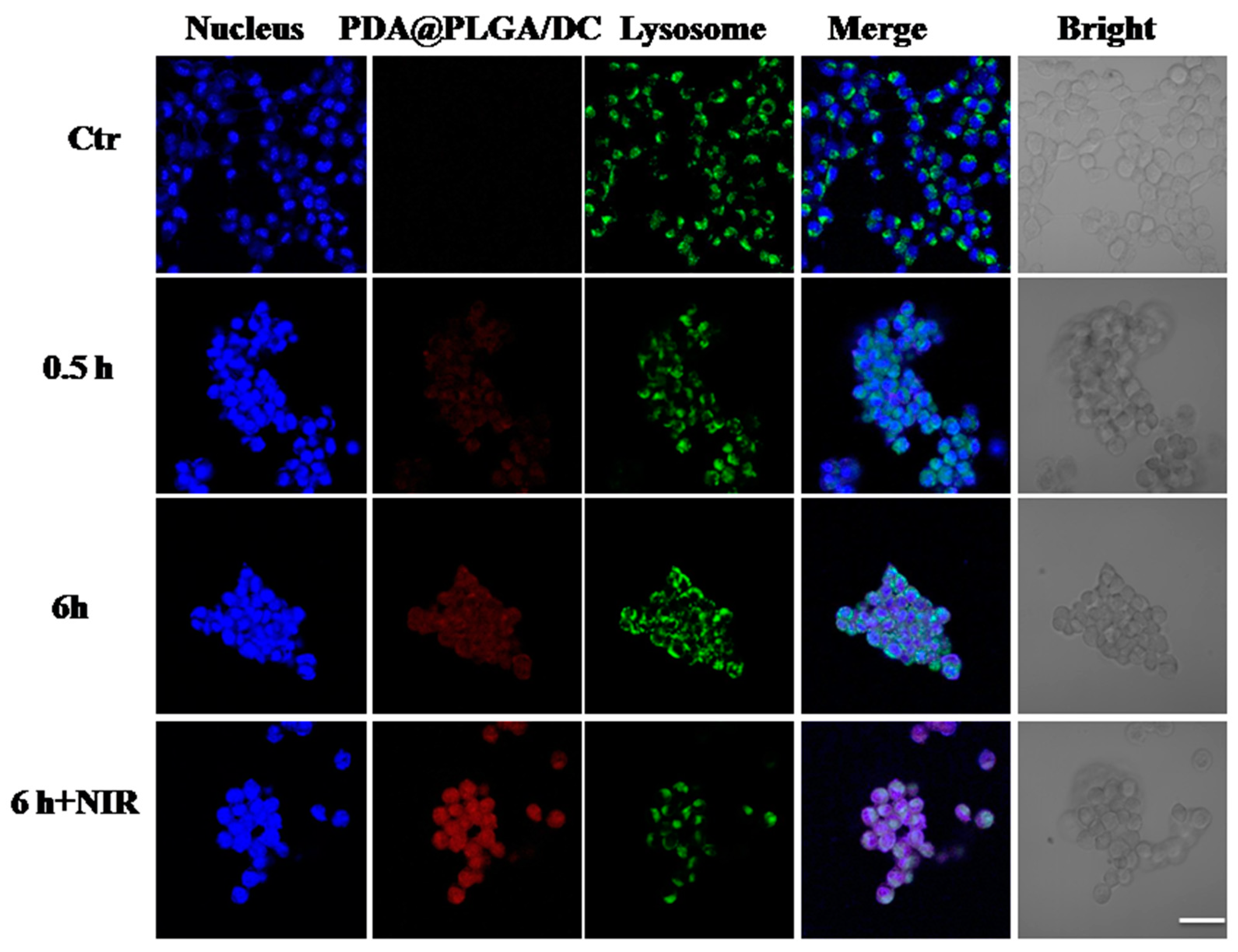
3.5. Intracellular Uptake Assays of PDA@PLGA/DC NPs
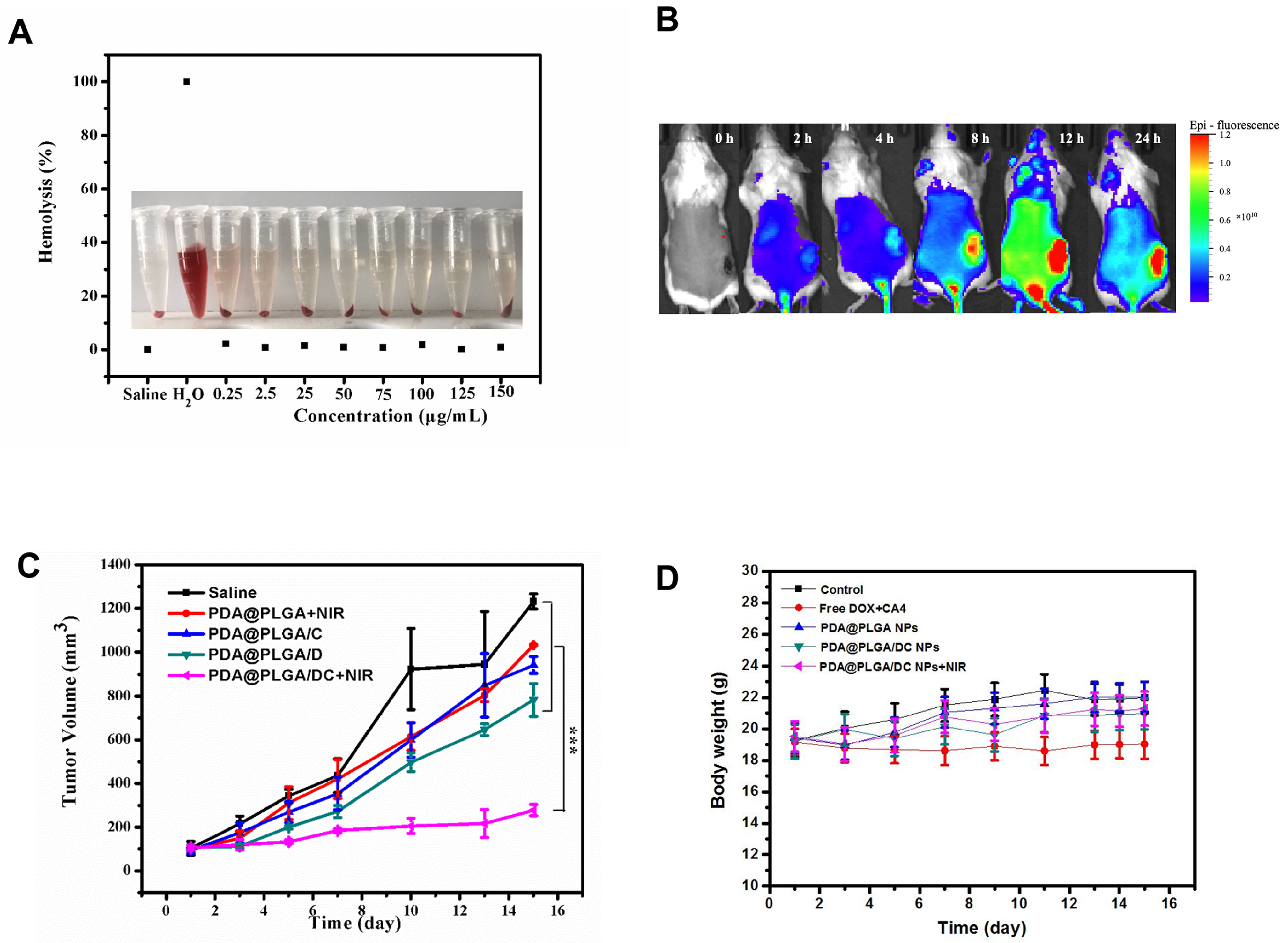
3.6. Synergistic Therapeutic Effect of PDA@PLGA/DC NPs In Vivo
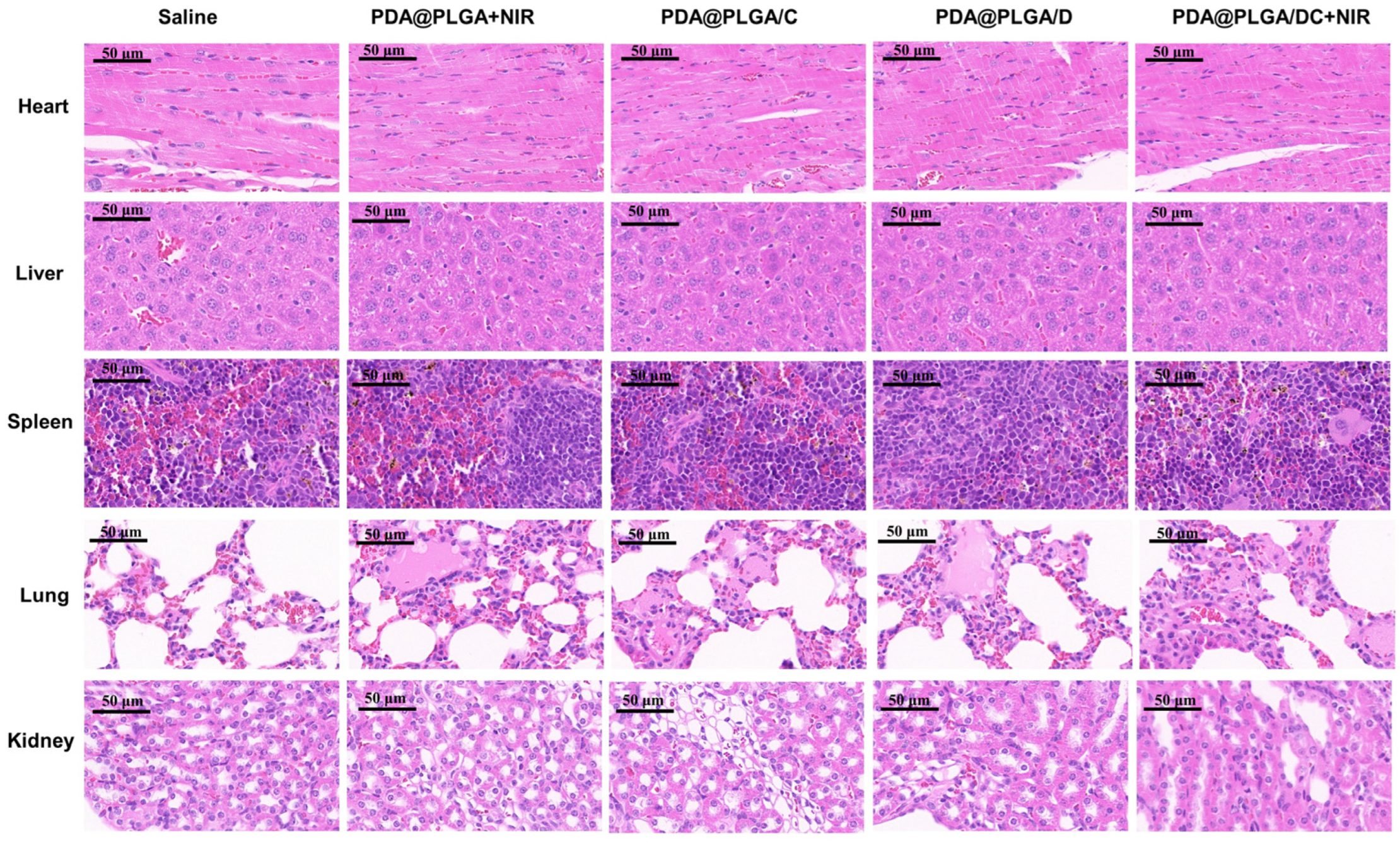
3.7. Potential Adverse Effects of PDA@PLGA/DC NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, H.K.K. Breast cancer: Presentation, investigation and management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safar, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, H.; Li, S.; Wu, P.; Mao, X. The Role of Progesterone Receptors in Breast Cancer. Drug Des. Dev. Ther. 2022, 2022, 305–314. [Google Scholar] [CrossRef]
- Derakhshani, A.; Rezaei, Z.; Safarpour, H.; Sabri, M.; Mir, A.; Sanati, M.A.; Vahidian, F.; Moghadam, A.G.; Aghadoukht, A.; Hajiasgharzadeh, K.; et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J. Cell Physiol. 2020, 235, 3142–3156. [Google Scholar] [CrossRef] [PubMed]
- Saxton, J.M.; Pickering, K.; Wane, S.; Humphreys, H.; Crank, H.; Anderson, A.S.; Cain, H.; Cohen, J.; Copeland, R.J.; Gray, J.; et al. Co-designed weight management intervention for women recovering from oestrogen-receptor positive breast cancer. BMC Cancer 2022, 22, 1202. [Google Scholar] [CrossRef]
- Morales, M.A.G.; Rodríguez, R.B.; Cruz, J.R.S.; Teran, L.M. Overview of New Treatments with Immunotherapy for Breast Cancer and a Proposal of a Combination Therapy. Molecules 2020, 25, 5686. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Jin, X.Y. A narrative review of combination therapy of PD-1/PD-L1 blockade with standard approaches for the treatment of breast cancer: Clinical application and immune mechanism. Ann. Palliat. Med. 2021, 10, 10075–10082. [Google Scholar] [CrossRef]
- Salam, N.M.A.; Abd-Rabou, A.A.; Sharada, H.M.; EL Samea, G.G.A.; Abdalla, M.S. Combination Therapy of TRAIL and Thymoquinone Induce Breast Cancer Cell Cytotoxicity-Mediated Apoptosis and Cell Cycle Arrest. Asian Pac. J. Cancer Prev. 2021, 22, 1513–1521. [Google Scholar] [CrossRef]
- Wang, Y.; Minden, A. Current Molecular Combination Therapies Used for the Treatment of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 11046. [Google Scholar] [CrossRef]
- Zou, L.; Liu, X.; Li, J.; Li, W.; Zhang, L.; Fu, C.; Zhang, J.; Gu, Z. Redox-sensitive carrier-free nanoparticles self-assembled by disulfide-linked paclitaxel-tetramethylpyrazine conjugate for combination cancer chemotherapy. Theranostics 2021, 11, 4171–4186. [Google Scholar] [CrossRef]
- He, P.; Yang, G.; Zhu, D.; Kong, H.; Corrales-Ureña, Y.R.; Ciacchi, L.C.; Wei, G. Biomolecule-mimetic nanomaterials for photothermal and photodynamic therapy of cancers: Bridging nanobiotechnology and biomedicine. J. Nanobiotechnol. 2022, 20, 483. [Google Scholar] [CrossRef]
- Heinhuis, K.; Ros, W.; Kok, M.; Steeghs, N.; Beijnen, J.; Schellens, J. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann. Oncol. 2019, 30, 219–235. [Google Scholar] [CrossRef]
- Jain, A.; Tiwari, A.; Verma, A.; Saraf, S.; Jain, S.K. Combination Cancer Therapy Using Multifunctional Liposomes. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 105–134. [Google Scholar] [CrossRef]
- Zhou, L.; Pi, W.; Hao, M.; Li, Y.; An, H.; Li, Q.; Zhang, P.; Wen, Y. An injectable and biodegradable nano-photothermal DNA hydrogel enhances penetration and efficacy of tumor therapy. Biomater. Sci. 2021, 9, 4904–4921. [Google Scholar] [CrossRef]
- Safari, A.; Sarikhani, A.; Shahbazi-Gahrouei, D.; Alamzadeh, Z.; Beik, J.; Dezfuli, A.S.; Mahabadi, V.P.; Tohfeh, M.; Shakeri-Zadeh, A. Optimal scheduling of the nanoparticle-mediated cancer photo-thermo-radiotherapy. Photodiagn. Photodyn. Ther. 2020, 32, 102061. [Google Scholar] [CrossRef]
- Beigi, F.H.; Jazi, S.S.; Shahbazi-Gahrouei, D.; Khaniabadi, P.M.; Hafezi, H.; Monajemi, R.; Amiri, G.R. Iron oxide nanoparticles coated with polydopamine as a potential nano-photothermal agent for treatment of melanoma cancer: An in vivo study. Lasers Med. Sci. 2022, 37, 3413–3421. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, D.-M.; Li, J.; Chen, X.; Xie, W.; Jiang, X.; Wu, L.; Wang, G.; Xiao, Y.; Liu, Z.; et al. Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics 2020, 10, 1281–1295. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef]
- Feng, J.; Xu, Z.; Dong, P.; Yu, W.; Liu, F.; Jiang, Q.; Wang, F.; Liu, X. Stimuli-responsive multifunctional metal–organic framework nanoparticles for enhanced chemo-photothermal therapy. J. Mater. Chem. B 2019, 7, 994–1004. [Google Scholar] [CrossRef]
- Park, S.; Lee, W.J.; Park, S.; Choi, D.; Kim, S.; Park, N. Reversibly pH-responsive gold nanoparticles and their applications for photothermal cancer therapy. Sci. Rep. 2019, 9, 20180. [Google Scholar] [CrossRef]
- Zhong, W.; Wong, K.H.; Xu, F.; Zhao, N.; Chen, M. NIR-responsive polydopamine-based calcium carbonate hybrid nanoparticles delivering artesunate for cancer chemo-photothermal therapy. Acta Biomater. 2022, 145, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-Based Simple and Versatile Surface Modification of Polymeric Nano Drug Carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef] [PubMed]
- Karan, A.; Khezerlou, E.; Rezaei, F.; Iasemidis, L.; DeCoster, M.A. Morphological Changes in Astrocytes by Self-Oxidation of Dopamine to Polydopamine and Quantification of Dopamine through Multivariate Regression Analysis of Polydopamine Images. Polymers 2020, 12, 2483. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Ding, B.; Li, G. Polydopamine-Incorporated Nanoformulations for Biomedical Applications. Macromol. Biosci. 2020, 20, e2000228. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.; Kandasubramanian, B. A polydopamine-based platform for anti-cancer drug delivery. Biomater. Sci. 2019, 7, 1776–1793. [Google Scholar] [CrossRef]
- Yakhlifi, S.E.; Ball, V. Polydopamine as a stable and functional nanomaterial. Colloids Surf. B Biointerfaces. 2020, 186, 110719. [Google Scholar] [CrossRef]
- Lu, J.; Cai, L.; Dai, Y.; Liu, Y.; Zuo, F.; Ni, C.; Shi, M.; Li, J. Polydopamine-Based Nanoparticles for Photothermal Therapy/Chemotherapy and their Synergistic Therapy with Autophagy Inhibitor to Promote Antitumor Treatment. Chem. Rec. 2021, 21, 781–796. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Z.; Xu, X.; Xu, M.; Yang, X.; Zhang, C.; Liu, J.; Zhang, F.; Shuai, X.; Wang, W.; et al. Polydopamine-Encapsulated Perfluorocarbon for Ultrasound Contrast Imaging and Photothermal Therapy. Mol. Pharm. 2020, 17, 817–826. [Google Scholar] [CrossRef]
- Chen, Y.; Su, M.; Jia, L.; Zhang, Z. Synergistic chemo-photothermal and ferroptosis therapy of polydopamine nanoparticles for esophageal cancer. Nanomedicine 2022, 17, 1115–1130. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, X.; Pei, Z.; Pei, Y. Cancer-Mitochondria Dual-Targeting Glycol/Ferrocenium-Based Polydopamine Nanoparticles for Synergistic Photothermal and Photodynamic Therapy. Chemmedchem 2022, 17, e202100548. [Google Scholar] [CrossRef]
- Li, H.; Yin, D.; Li, W.; Tang, Q.; Zou, L.; Peng, Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf. B Biointerfaces 2021, 199, 111502. [Google Scholar] [CrossRef]
- Tian, L.; Li, X.; Ji, H.; Yu, Q.; Yang, M.; Guo, L.; Huang, L.; Gao, W. Melanin-like nanoparticles: Advances in surface modification and tumour photothermal therapy. J. Nanobiotechnol. 2022, 20, 485. [Google Scholar] [CrossRef]
- Lee, C.K.; Atibalentja, D.F.; Yao, L.E.; Park, J.; Kuruvilla, S.; Felsher, D.W. Anti-PD-L1 F(ab) Conjugated PEG-PLGA Nanoparticle Enhances Immune Checkpoint Therapy. Nanotheranostics 2022, 6, 243–255. [Google Scholar] [CrossRef]
- Ahmad, A.; Gupta, A.; Ansari, M.; Vyawahare, A.; Jayamurugan, G.; Khan, R. Hyperbranched Polymer-Functionalized Magnetic Nanoparticle-Mediated Hyperthermia and Niclosamide Bimodal Therapy of Colorectal Cancer Cells. ACS Biomater. Sci. Eng. 2020, 6, 1102–1111. [Google Scholar] [CrossRef]
- Hashemi, M.; Shamshiri, A.; Saeedi, M.; Tayebi, L.; Yazdian-Robati, R. Aptamer-conjugated PLGA nanoparticles for delivery and imaging of cancer therapeutic drugs. Arch. Biochem. Biophys. 2020, 691, 108485. [Google Scholar] [CrossRef]
- Allavena, P.; Palmioli, A.; Avigni, R.; Sironi, M.; La Ferla, B.; Maeda, A. PLGA Based Nanoparticles for the Monocyte-Mediated Anti-Tumor Drug Delivery System. J. Biomed. Nanotechnol. 2020, 16, 212–223. [Google Scholar] [CrossRef]
- Huang, R.; Sheng, Y.; Xu, Z.; Wei, D.; Song, X.; Jiang, B.; Chen, H. Combretastatin A4-derived payloads for antibody-drug conjugates. Eur. J. Med. Chem. 2021, 216, 113355. [Google Scholar] [CrossRef]
- Cao, Y.; Ahmed, A.M.Q.; Du, H.H.; Sun, W.; Lu, X.J.; Xu, Z.; Tao, J.; Cao, Q.R. Combretastatin A4-loaded Poly (Lactic-co-glycolic Acid)/Soybean Lecithin Nanoparticles with Enhanced Drug Dissolution Rate and Antiproliferation Activity. Curr. Drug Deliv. 2022, 19, 918–927. [Google Scholar]
- Zhao, B.; Dong, Z.; Liu, W.; Lou, F.; Wang, Q.; Hong, H.; Wang, Y. Co-administration of combretastatin A4 nanoparticles and anti-PD-L1 for synergistic therapy of hepatocellular carcinoma. J. Nanobiotechnol. 2021, 19, 124. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, X.; He, P.; Xiao, C.; Yu, H.; Chen, X. Nanoparticles Composed of PEGylated Alternating Copolymer-Combretastatin A4 Conjugate for Cancer Therapy. Macromol. Biosci. 2021, 21, e2100077. [Google Scholar] [CrossRef]
- Song, M.-Y.; He, Q.-R.; Wang, Y.-L.; Wang, H.-R.; Jiang, T.-C.; Tang, J.-J.; Gao, J.-M. Exploring Diverse-Ring Analogues on Combretastatin A4 (CA-4) Olefin as Microtubule-Targeting Agents. Int. J. Mol. Sci. 2020, 21, 1817. [Google Scholar] [CrossRef] [PubMed]
- Mohyeddin, A.; Abdel, N.Z.; Naim, K.; Deema, H.; Johnny, A. Covalent functionalization of SWCNT with combretastatin A4 for cancer therapy. Nanotechnology 2018, 29, 245101. [Google Scholar]
- Bao, X.; Shen, N.; Lou, Y.; Yu, H.; Wang, Y.; Liu, L.; Tang, Z.; Chen, X. Enhanced anti-PD-1 therapy in hepatocellular carcinoma by tumor vascular disruption and normalization dependent on combretastatin A4 nanoparticles and DC101. Theranostics 2021, 11, 5955–5969. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, Q.; Zhao, C.; Lu, J.; Li, X.; Chen, L.; Deng, X.; Ge, G.; Wu, Y. Auto-fluorescent polymer nanotheranostics for self-monitoring of cancer therapy via triple-collaborative strategy. Biomaterials 2019, 194, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.S.; Lin, M. The Synthesis of Nano-Doxorubicin and its Anticancer Effect. Anticancer Agents Med. Chem. 2021, 21, 2466–2477. [Google Scholar] [CrossRef]
- Zoghebi, K.; Aliabadi, H.M.; Tiwari, R.K.; Parang, K. [(WR)8WKβA]-Doxorubicin Conjugate: A Delivery System to Overcome Multi-Drug Resistance against Doxorubicin. Cells 2022, 11, 301. [Google Scholar] [CrossRef]
- Su, R.; Yan, H.; Li, P.; Zhang, B.; Zhang, Y.; Su, W. Photo-enhanced antibacterial activity of polydopamine-curcumin nanocomposites with excellent photodynamic and photothermal abilities. Photodiagnosis Photodyn. Ther. 2021, 35, 102417. [Google Scholar] [CrossRef]
- Yao, M.Y.; Zou, Q.X.; Zou, W.W.; Xie, Z.Z.; Li, Z.H.; Zhao, X.J.; Du, C. Bifunctional scaffolds of hydroxyapatite/poly(dopamine)/carboxymethyl chitosan with osteogenesis and anti-osteosarcoma effect. Biomater Sci. 2021, 9, 3319–3333. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, R.; Guo, M.; Shao, L.; Liu, Y.; Zhao, Y.; Zhang, S.; Wu, Y.; Chen, C. Nucleosome-inspired nanocarrier obtains encapsulation efficiency enhancement and side effects reduction in chemotherapy by using fullerenol assembled with doxorubicin. Biomaterials 2018, 167, 205–215. [Google Scholar] [CrossRef]
- Ye, S.; Rao, J.; Qiu, S.; Zhao, J.; He, H.; Yan, Z.; Yang, T.; Deng, Y.; Ke, H.; Yang, H.; et al. Rational Design of Conjugated Photosensitizers with Controllable Photoconversion for Dually Cooperative Phototherapy. Adv. Mater. 2018, 30, e1801216. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Zhao, R.; Zhang, Q.; Kong, X. Multifunctional nanoparticles as photosensitizer delivery carriers for enhanced photodynamic cancer therapy. Mater. Sci. Eng. C 2020, 115, 111099. [Google Scholar] [CrossRef]
- Ding, J.X.; Chen, J.J.; Gao, L.Q.; Jiang, Z.Y.; Zhang, Y.; Li, M.Q.; Xiao, Q.C.; Lee, S.S.; Chen, X.S. Engineerednanomedicines with enhanced tumor penetration. Nano Today 2020, 29, 10080. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Gao, Y.; Pan, Z.; Jia, F.; Xu, C.; Cui, X.; Wang, X.; Wu, Y. Fabrication of Poly Dopamine@poly (Lactic Acid-Co-Glycolic Acid) Nanohybrids for Cancer Therapy via a Triple Collaboration Strategy. Nanomaterials 2023, 13, 1447. https://doi.org/10.3390/nano13091447
Li Y, Gao Y, Pan Z, Jia F, Xu C, Cui X, Wang X, Wu Y. Fabrication of Poly Dopamine@poly (Lactic Acid-Co-Glycolic Acid) Nanohybrids for Cancer Therapy via a Triple Collaboration Strategy. Nanomaterials. 2023; 13(9):1447. https://doi.org/10.3390/nano13091447
Chicago/Turabian StyleLi, Yunhao, Yujuan Gao, Zian Pan, Fan Jia, Chenlu Xu, Xinyue Cui, Xuan Wang, and Yan Wu. 2023. "Fabrication of Poly Dopamine@poly (Lactic Acid-Co-Glycolic Acid) Nanohybrids for Cancer Therapy via a Triple Collaboration Strategy" Nanomaterials 13, no. 9: 1447. https://doi.org/10.3390/nano13091447
APA StyleLi, Y., Gao, Y., Pan, Z., Jia, F., Xu, C., Cui, X., Wang, X., & Wu, Y. (2023). Fabrication of Poly Dopamine@poly (Lactic Acid-Co-Glycolic Acid) Nanohybrids for Cancer Therapy via a Triple Collaboration Strategy. Nanomaterials, 13(9), 1447. https://doi.org/10.3390/nano13091447



