Charge Injection and Energy Transfer of Surface-Engineered InP/ZnSe/ZnS Quantum Dots
Abstract
:1. Introduction
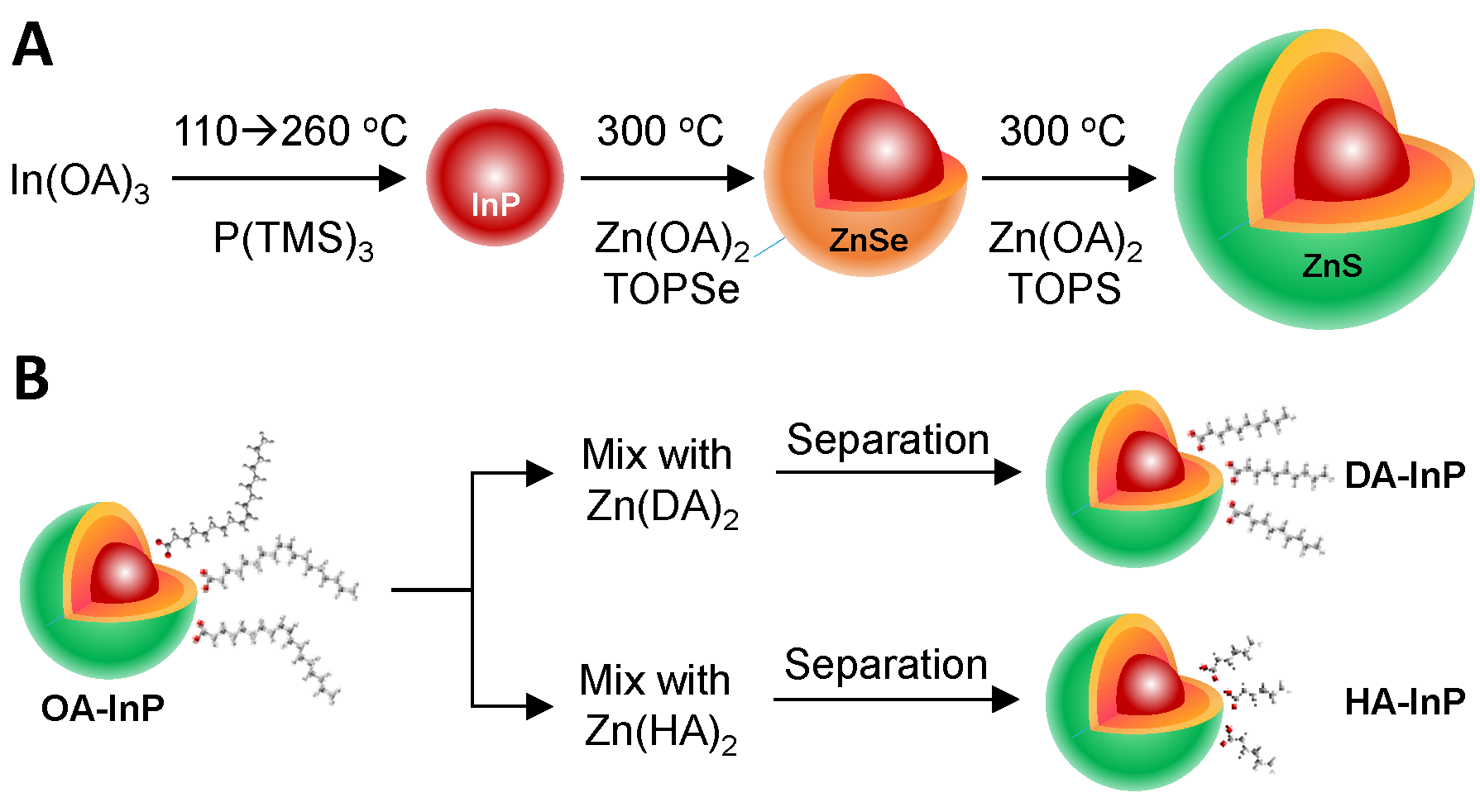
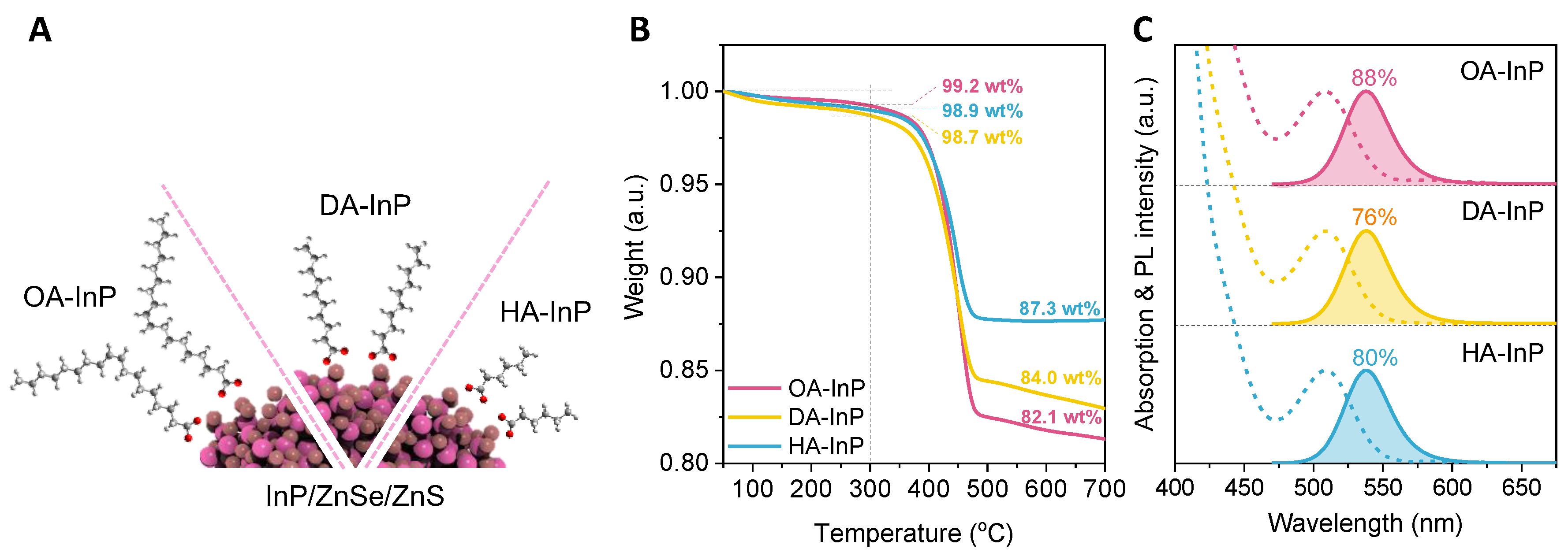
2. Materials and Methods
3. Results and Discussion
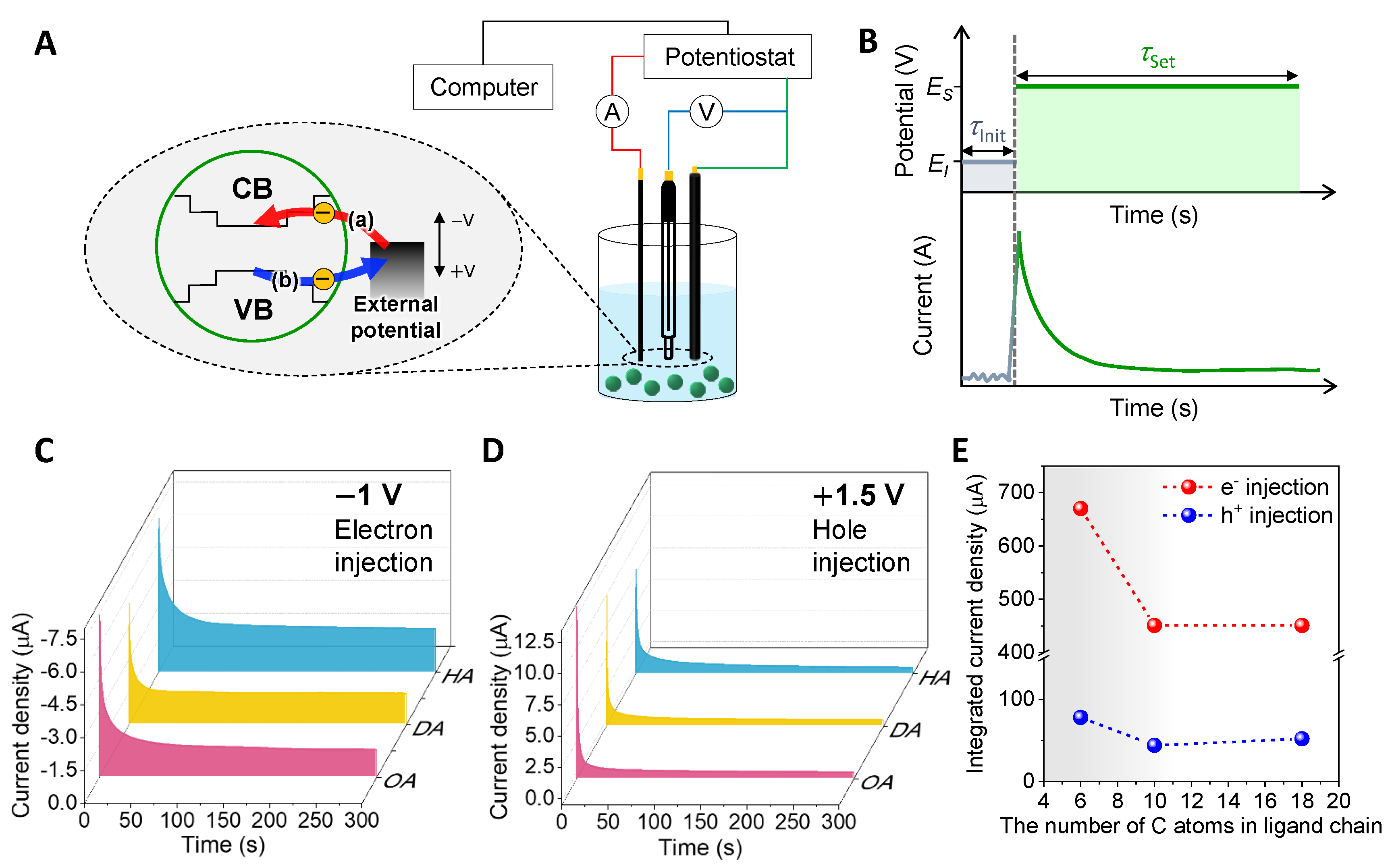
3.1. Chronoamperometry
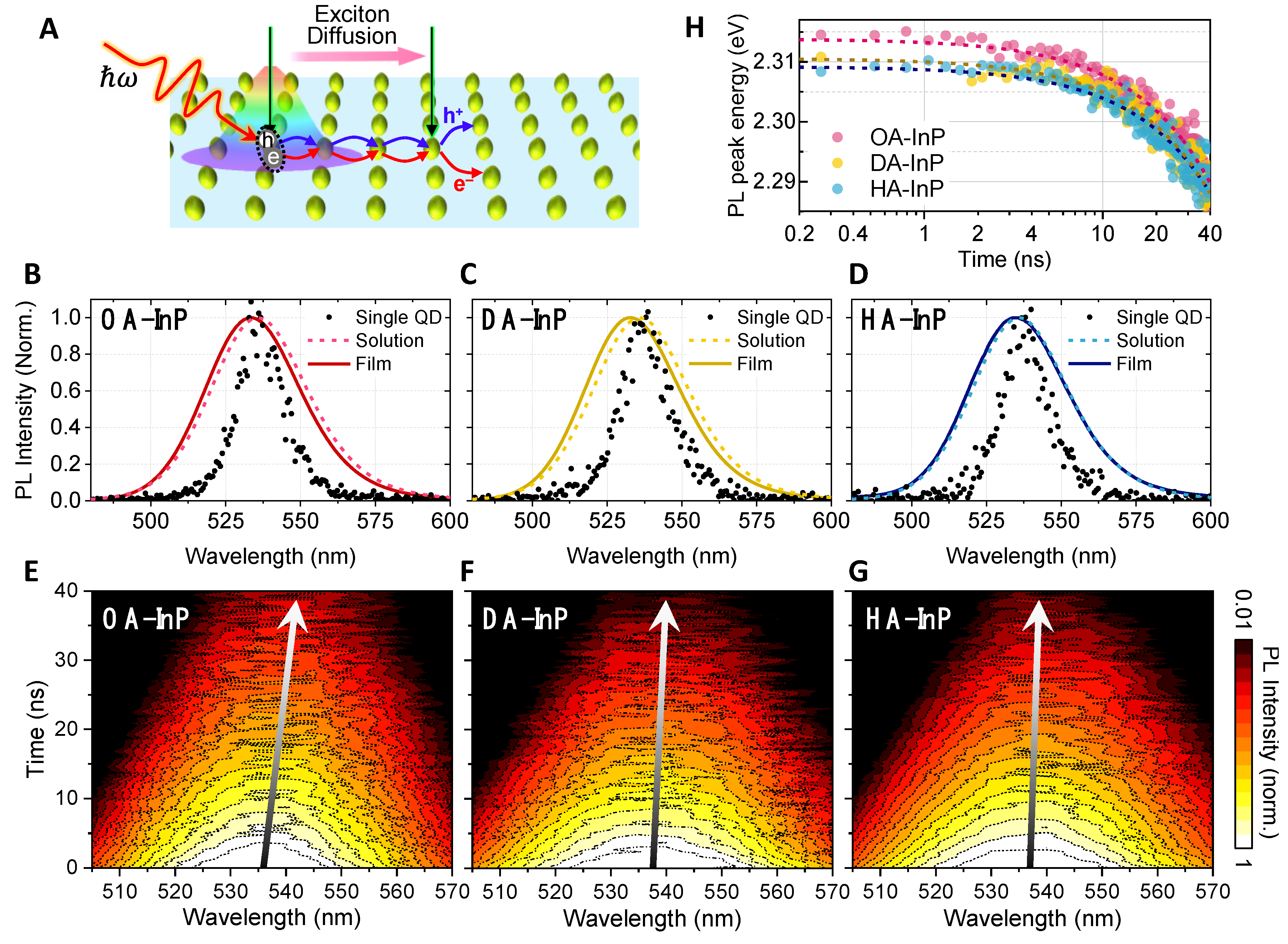
3.2. Time-Resolved Photoluminescence of Quantum Dot Solids
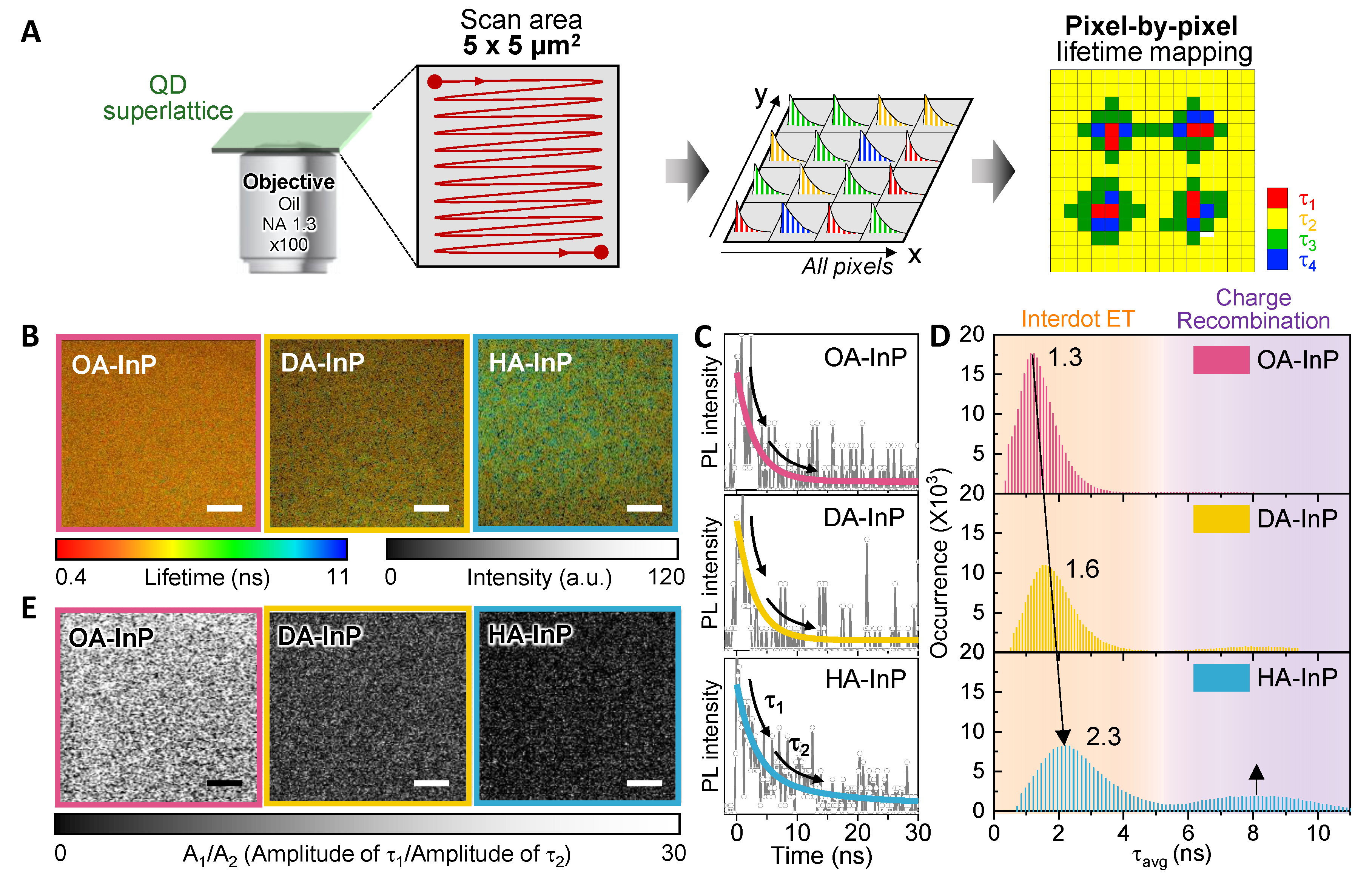
3.3. Photoluminescence Lifetime Imaging Microscopic Measurements
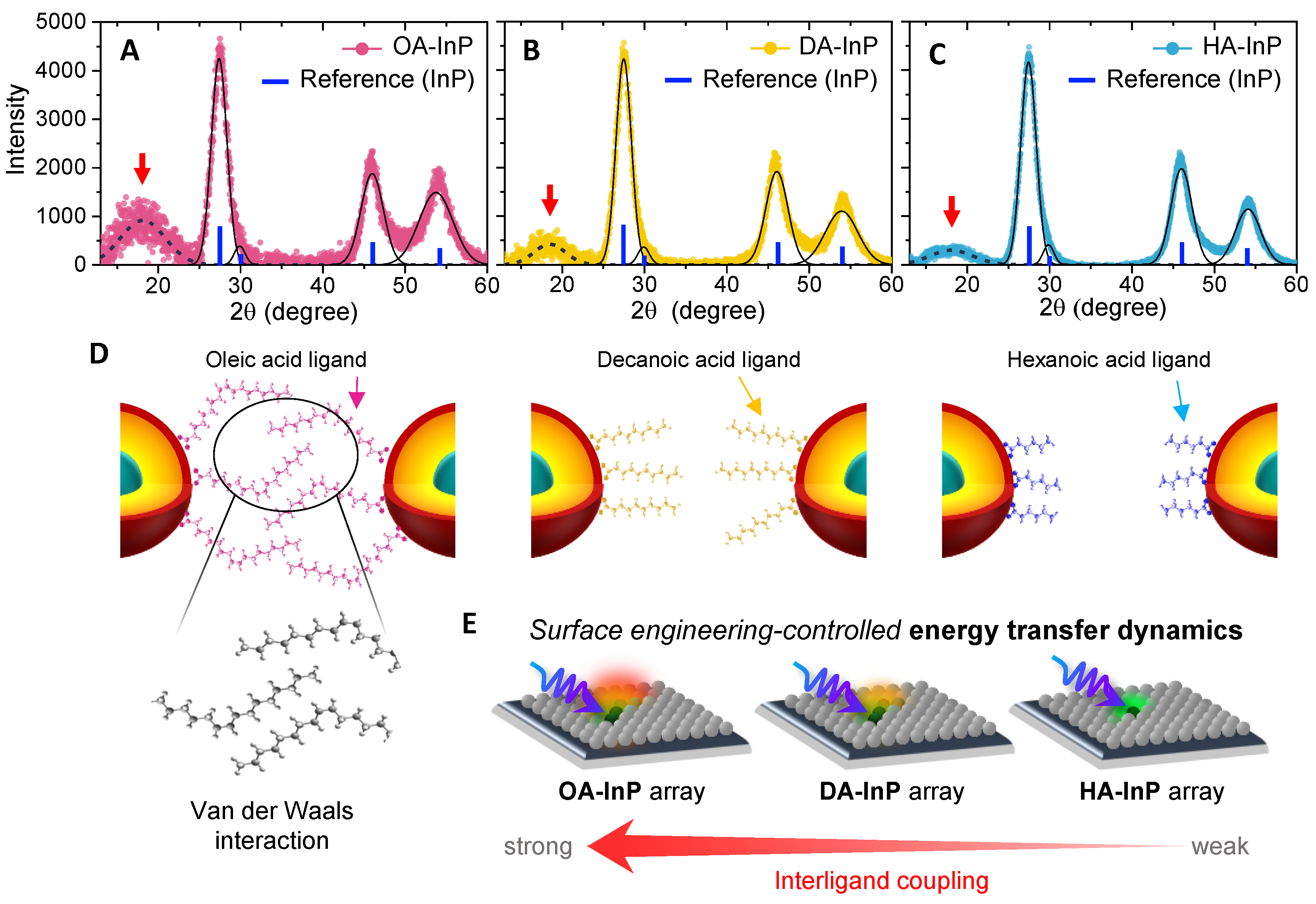
3.4. X-ray Diffraction Spectra and Ligand Ordering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, E.; Kim, Y.; Won, Y.H.; Jang, H.; Choi, S.M. Environmentally Friendly InP-Based Quantum Dots for Efficient Wide Color Gamut Displays. ACS Energy Lett. 2020, 5, 1316–1327. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.H.; Jo, D.Y.; Lee, S.H.; Yoon, S.Y.; Lim, H.B.; Lee, B.J.; Do, Y.R.; Yang, H. InP-Based Quantum Dots Having an InP Core, Composition-Gradient ZnSeS Inner Shell, and ZnS Outer Shell with Sharp, Bright Emissivity, and Blue Absorptivity for Display Devices. ACS Appl. Nano Mater. 2020, 3, 1972–1980. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Dai, X.; Yao, Z.; Lv, L.; Jin, Y.; Peng, X. Stoichiometry-Controlled InP-Based Quantum Dots: Synthesis, Photoluminescence, and Electroluminescence. J. Am. Chem. Soc. 2019, 141, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Jo, D.Y.; Choi, S.W.; Lee, S.H.; Kim, H.M.; Yoon, S.Y.; Kim, Y.; Han, J.N.; Yang, H. Highly Bright, Narrow Emissivity of InP Quantum Dots Synthesized by Aminophosphine: Effects of Double Shelling Scheme and Ga Treatment. Adv. Opt. Mater. 2021, 9, 2100427. [Google Scholar] [CrossRef]
- Taylor, D.A.; Teku, J.A.; Cho, S.; Chae, W.S.; Jeong, S.J.; Lee, J.S. Importance of Surface Functionalization and Purification for Narrow FWHM and Bright Green-Emitting InP Core-Multishell Quantum Dots via a Two-Step Growth Process. Chem. Mater. 2021, 33, 4399–4407. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, F.; Wang, S.; Wang, Y.; Sun, Z.; Feng, J.; Liu, Y.; Wang, L.; Cao, Q.; Li, Y.; et al. Quasi-Shell-Growth Strategy Achieves Stable and Efficient Green InP Quantum Dot Light-Emitting Diodes. Adv. Sci. 2022, 9, 2200959. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Wu, Q.; Wang, L.; Jia, G.; Chen, T.; Zhang, C.; Yang, X. Efficient and Bright Green InP Quantum Dot Light-Emitting Diodes Enabled by a Self-Assembled Dipole Interface Monolayer. Nanoscale 2023, 15, 2837–2842. [Google Scholar] [CrossRef]
- Park, S.A.; Jung, W.H.; Yoo, J.Y.; Lee, C.W.; Kim, J.S.; Kim, J.G.; Chin, B.D. Electrical Resonant Effects of Ligands on the Luminescent Properties of InP/ZnSeS/ZnS Quantum Dots and Devices Configured Therefrom. Org. Electron. 2020, 87, 105955. [Google Scholar] [CrossRef]
- Chao, W.C.; Chiang, T.H.; Liu, Y.C.; Huang, Z.X.; Liao, C.C.; Chu, C.H.; Wang, C.H.; Tseng, H.W.; Hung, W.Y.; Chou, P.T. High Efficiency Green InP Quantum Dot Light-Emitting Diodes by Balancing Electron and Hole Mobility. Commun. Mater. 2021, 2, 96. [Google Scholar] [CrossRef]
- Shin, D.H.; Lampande, R.; Kim, S.J.; Jung, Y.H.; Kwon, J.H. Understanding the Origin of Degradation of InP-Quantum Dot Light-Emitting Diodes. Adv. Electron. Mater. 2022, 8, 2200256. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Motomura, G.; Ogura, K.; Tsuzuki, T. Efficient Green InP Quantum Dot Light-Emitting Diodes Using Suitable Organic Electron-Transporting Materials. Appl. Phys. Lett. 2020, 117, 3–7. [Google Scholar] [CrossRef]
- Jiang, Y.; Cui, M.; Li, S.; Sun, C.; Huang, Y.; Wei, J.; Zhang, L.; Lv, M.; Qin, C.; Liu, Y.; et al. Reducing the Impact of Auger Recombination in Quasi-2D Perovskite Light-Emitting Diodes. Nat. Commun. 2021, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [Green Version]
- Kirkwood, N.; Monchen, J.O.V.; Crisp, R.W.; Grimaldi, G.; Bergstein, H.A.C.; Du Fossé, I.; Van Der Stam, W.; Infante, I.; Houtepen, A.J. Finding and Fixing Traps in II-VI and III-V Colloidal Quantum Dots: The Importance of Z-Type Ligand Passivation. J. Am. Chem. Soc. 2018, 140, 15712–15723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lv, Y.; Lin, J.; Zhao, J.; Liu, X.; Zeng, R.; Wang, X.; Zou, B. Surface Organic Ligand-Passivated Quantum Dots: Toward High-Performance Light-Emitting Diodes with Long Lifetimes. J. Mater. Chem. C 2021, 9, 2483–2490. [Google Scholar] [CrossRef]
- Ding, S.; Hao, M.; Lin, T.; Bai, Y.; Wang, L. Ligand Engineering of Perovskite Quantum Dots for Efficient and Stable Solar Cells. J. Energy Chem. 2022, 69, 626–648. [Google Scholar] [CrossRef]
- Shen, H.; Cao, W.; Shewmon, N.T.; Yang, C.; Li, L.S.; Xue, J. High-Efficiency, Low Turn-on Voltage Blue-Violet Quantum-Dot-Based Light-Emitting Diodes. Nano Lett. 2015, 15, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Y.B.; Fan, F.; Levina, L.; Liu, M.; Quintero-Bermudez, R.; Gong, X.; Quan, L.N.; Fan, J.; Yang, Z.; et al. Bright Colloidal Quantum Dot Light-Emitting Diodes Enabled by Efficient Chlorination. Nat. Photonics 2018, 12, 159–164. [Google Scholar] [CrossRef]
- Won, Y.-H.; Cho, O.; Kim, T.; Chung, D.-Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly Efficient and Stable InP/ZnSe/ZnS Quantum Dot Light-Emitting Diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, Y.; Wu, L.; Zhao, Y.; Chen, K.; Chen, W. Influence of Surface States of CuInS2 Quantum Dots in Quantum Dots Sensitized Photo-Electrodes. Appl. Surf. Sci. 2016, 388, 437–443. [Google Scholar] [CrossRef]
- Zeng, S.; Li, Z.; Tan, W.; Si, J.; Huang, Z.; Hou, X. Ultrafast Electron Transfer Dynamics Affected by Ligand Chain Length in InP/ZnS Core/Shell Quantum Dots. J. Phys. Chem. C 2022, 126, 9091–9098. [Google Scholar] [CrossRef]
- Yu, S.; Fan, X.B.; Wang, X.; Li, J.; Zhang, Q.; Xia, A.; Wei, S.; Wu, L.Z.; Zhou, Y.; Patzke, G.R. Efficient Photocatalytic Hydrogen Evolution with Ligand Engineered All-Inorganic InP and InP/ZnS Colloidal Quantum Dots. Nat. Commun. 2018, 9, 4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.G.; Jain, P.K. The Ligand Shell as an Energy Barrier in Surface Reactions on Transition Metal Nanoparticles. J. Am. Chem. Soc. 2016, 138, 6765–6773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietryga, J.M.; Park, Y.S.; Lim, J.; Fidler, A.F.; Bae, W.K.; Brovelli, S.; Klimov, V.I. Spectroscopic and Device Aspects of Nanocrystal Quantum Dots. Chem. Rev. 2016, 116, 10513–10622. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, M.; Wu, W.; Yang, X.; Sun, X.W.; Zhang, J.; Wang, H.C.; Liu, R.S.; Han, C.Y.; Yang, H.; et al. Recent Advances in Quantum Dot-Based Light-Emitting Devices: Challenges and Possible Solutions. Mater. Today 2019, 24, 69–93. [Google Scholar] [CrossRef]
- Hahm, D.; Chang, J.H.; Jeong, B.G.; Park, P.; Kim, J.; Lee, S.; Choi, J.; Kim, W.D.; Rhee, S.; Lim, J.; et al. Design Principle for Bright, Robust, and Color-Pure InP/ZnSexS1−x/ZnS Heterostructures. Chem. Mater. 2019, 31, 3476–3484. [Google Scholar] [CrossRef]
- Yoon, S.J.; Guo, Z.; Dos Santos Claro, P.C.; Shevchenko, E.V.; Huang, L. Direct Imaging of Long-Range Exciton Transport in Quantum Dot Superlattices by Ultrafast Microscopy. ACS Nano 2016, 10, 7208–7215. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Talapin, D.V.; Shevchenko, E.V.; Murray, C.B.; Titov, A.V.; Kral, P. Dipole-Dipole Interactions in Nanoparticle Superlattices. Nano Lett. 2007, 7, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Kroupa, D.M.; Vörös, M.; Brawand, N.P.; McNichols, B.W.; Miller, E.M.; Gu, J.; Nozik, A.J.; Sellinger, A.; Galli, G.; Beard, M.C. Tuning Colloidal Quantum Dot Band Edge Positions through Solution-Phase Surface Chemistry Modification. Nat. Commun. 2017, 8, 15257. [Google Scholar] [CrossRef] [Green Version]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The Surface Science of Nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Li, Y.; Ye, M.; Fan, L.; Ding, Y.; Li, Y. Ligand-Tuned Shape Control, Oriented Assembly, and Electrochemical Characterization of Colloidal Znte Nanocrystals. Chem. Mater. 2010, 22, 4632–4641. [Google Scholar] [CrossRef]
- Ashokan, A.; Mulvaney, P. Spectroelectrochemistry of Colloidal CdSe Quantum Dots. Chem. Mater. 2021, 33, 1353–1362. [Google Scholar] [CrossRef]
- Liu, Y.; Gibbs, M.; Ihly, R.; Law, M.; Tolentino, J. The Photothermal Stability of PbS Quantum Dot Solids. ACS Nano 2011, 5, 8175–8186. [Google Scholar]
- Kagan, C.R.; Murray, C.B.; Bawendi, M.G. Long-Range Resonance Transfer of Electronic Excitations in Close-Packed CdSe Quantum-Dot Solids. Phys. Rev. B 1996, 54, 8633–8643. [Google Scholar] [CrossRef] [PubMed]
- Kholmicheva, N.; Moroz, P.; Bastola, E.; Razgoniaeva, N.; Bocanegra, J.; Shaughnessy, M.; Porach, Z.; Khon, D.; Zamkov, M. Mapping the Exciton Diffusion in Semiconductor Nanocrystal Solids. ACS Nano 2015, 9, 2926–2937. [Google Scholar] [CrossRef]
- Kholmicheva, N.; Moroz, P.; Eckard, H.; Jensen, G.; Zamkov, M. Energy Transfer in Quantum Dot Solids. ACS Energy Lett. 2017, 2, 154–160. [Google Scholar] [CrossRef]
- Moroz, P.; Romero, L.R.; Zamkov, M. Colloidal Semiconductor Nanocrystals in Energy Transfer Reactions. Chem. Commun. 2019, 55, 3033–3048. [Google Scholar] [CrossRef]
- Gellen, T.A.; Lem, J.; Turner, D.B. Probing Homogeneous Line Broadening in CdSe Nanocrystals Using Multidimensional Electronic Spectroscopy. Nano Lett. 2017, 17, 2809–2815. [Google Scholar] [CrossRef]
- Cui, J.; Beyler, A.P.; Coropceanu, I.; Cleary, L.; Avila, T.R.; Chen, Y.; Cordero, J.M.; Heathcote, S.L.; Harris, D.K.; Chen, O.; et al. Evolution of the Single-Nanocrystal Photoluminescence Linewidth with Size and Shell: Implications for Exciton-Phonon Coupling and the Optimization of Spectral Linewidths. Nano Lett. 2016, 16, 289–296. [Google Scholar] [CrossRef]
- Mićić, O.I.; Jones, K.M.; Cahill, A.; Nozik, A.J. Optical, Electronic, and Structural Properties of Uncoupled and Close-Packed Arrays of InP Quantum Dots. J. Phys. Chem. B 1998, 102, 9791–9796. [Google Scholar] [CrossRef]
- Crooker, S.A.; Hollingsworth, J.A.; Tretiak, S.; Klimov, V.I. Spectrally Resolved Dynamics of Energy Transfer in Quantum-Dot Assemblies: Towards Engineered Energy Flows in Artificial Materials. Phys. Rev. Lett. 2002, 89, 18–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranov, D.; Fieramosca, A.; Yang, R.X.; Polimeno, L.; Lerario, G.; Toso, S.; Giansante, C.; De Giorgi, M.; Tan, L.Z.; Sanvitto, D.; et al. Aging of Self-Assembled Lead Halide Perovskite Nanocrystal Superlattices: Effects on Photoluminescence and Energy Transfer. ACS Nano 2021, 15, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Dancik, Y.; Prow, T.W.; Thorling, C.A.; Lin, L.L.; Grice, J.E.; Robertson, T.A.; König, K.; Becker, W. Non-Invasive Imaging of Skin Physiology and Percutaneous Penetration Using Fluorescence Spectral and Lifetime Imaging with Multiphoton and Confocal Microscopy. Eur. J. Pharm. Biopharm. 2011, 77, 469–488. [Google Scholar] [CrossRef]
- Zheng, K.; Žídek, K.; Abdellah, M.; Zhu, N.; Chábera, P.; Lenngren, N.; Chi, Q.; Pullerits, T. Directed Energy Transfer in Films of CdSe Quantum Dots: Beyond the Point Dipole Approximation. J. Am. Chem. Soc. 2014, 136, 6259–6268. [Google Scholar] [CrossRef]
- Calvin, J.J.; Sedlak, A.B.; Crook, M.F.; Alivisatos, A.P. Observation of Ordered Organic Capping Ligands on Semiconducting Quantum Dots via Powder X-Ray Diffraction. Nat. Commun. 2021, 12, 2663. [Google Scholar] [CrossRef]
- Kapadia, R.; Yu, Z.H.; Wang, H.H.; Zheng, M.; Battaglia, C.; Hettick, M.; Kiriya, D.; Takei, K.; Lobaccaro, P.; Beeman, J.W.; et al. A Direct Thin-Film Path towards Low-Cost Large-Area III-V Photovoltaics. Sci. Rep. 2013, 3, 2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patzke, G.R.; Kontic, R.; Shiolashvili, Z.; Makhatadze, N.; Jishiashvili, D. Hydrazine-Assisted Formation of Indium Phosphide (InP)-Based Nanowires and Core-Shell Composites. Materials 2013, 6, 85–100. [Google Scholar] [CrossRef]
- Makarov, N.S.; Guo, S.; Isaienko, O.; Liu, W.; Robel, I.; Klimov, V.I. Spectral and Dynamical Properties of Single Excitons, Biexcitons, and Trions in Cesium-Lead-Halide Perovskite Quantum Dots. Nano Lett. 2016, 16, 2349–2362. [Google Scholar] [CrossRef]
- Lakovicz, J.R. Principles of fluorescence spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, T.; Kim, D. Charge Injection and Energy Transfer of Surface-Engineered InP/ZnSe/ZnS Quantum Dots. Nanomaterials 2023, 13, 1159. https://doi.org/10.3390/nano13071159
Park J, Kim T, Kim D. Charge Injection and Energy Transfer of Surface-Engineered InP/ZnSe/ZnS Quantum Dots. Nanomaterials. 2023; 13(7):1159. https://doi.org/10.3390/nano13071159
Chicago/Turabian StylePark, Jumi, Taehee Kim, and Dongho Kim. 2023. "Charge Injection and Energy Transfer of Surface-Engineered InP/ZnSe/ZnS Quantum Dots" Nanomaterials 13, no. 7: 1159. https://doi.org/10.3390/nano13071159
APA StylePark, J., Kim, T., & Kim, D. (2023). Charge Injection and Energy Transfer of Surface-Engineered InP/ZnSe/ZnS Quantum Dots. Nanomaterials, 13(7), 1159. https://doi.org/10.3390/nano13071159







