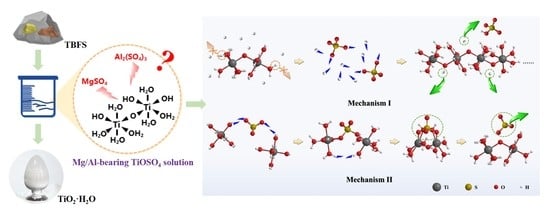
Preparation of Hydrated TiO2 Particles by Hydrothermal Hydrolysis of Mg/Al-Bearing TiOSO4 Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Hydrated TiO2
2.2. Characterization
3. Results and Discussion
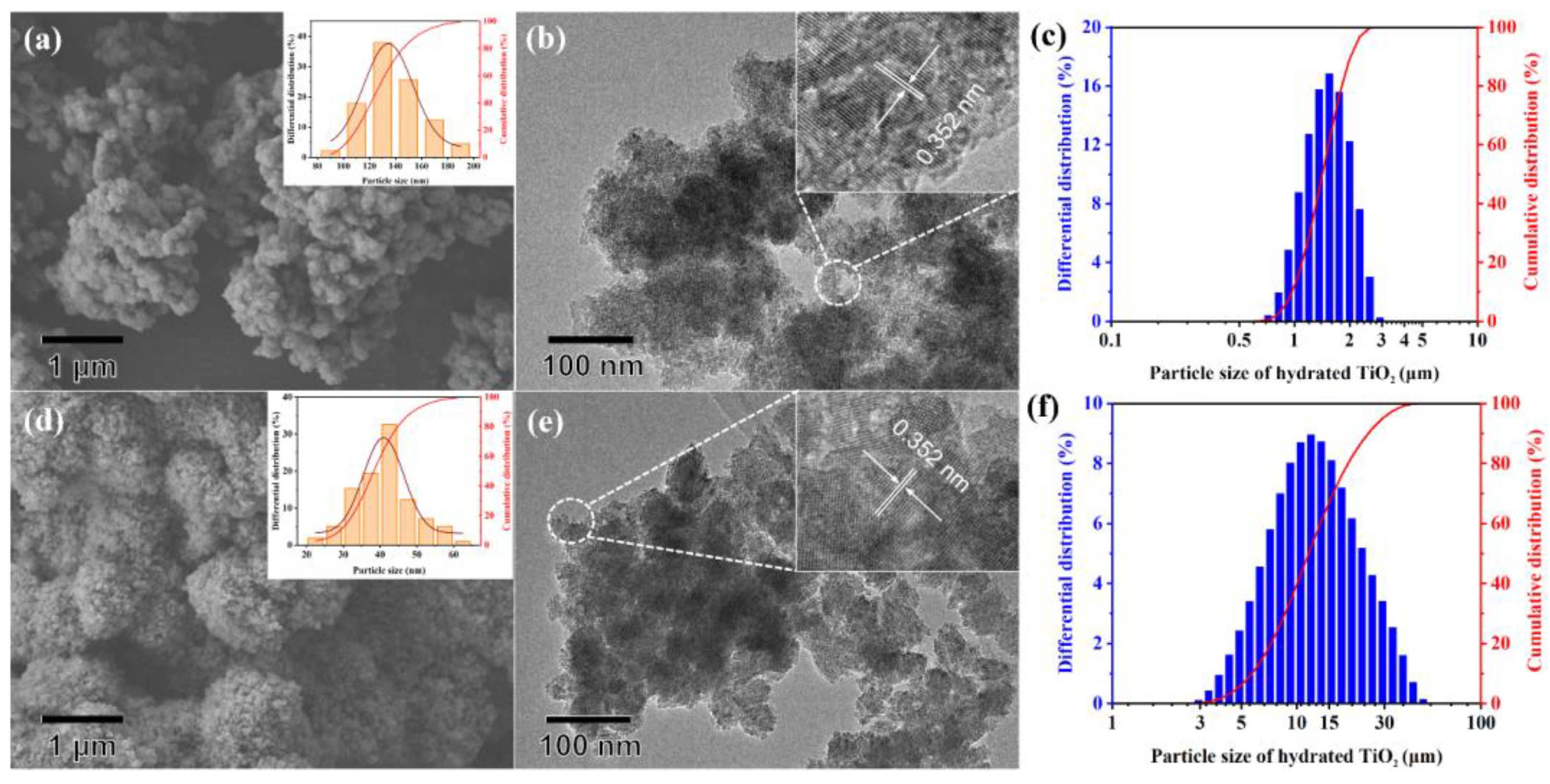
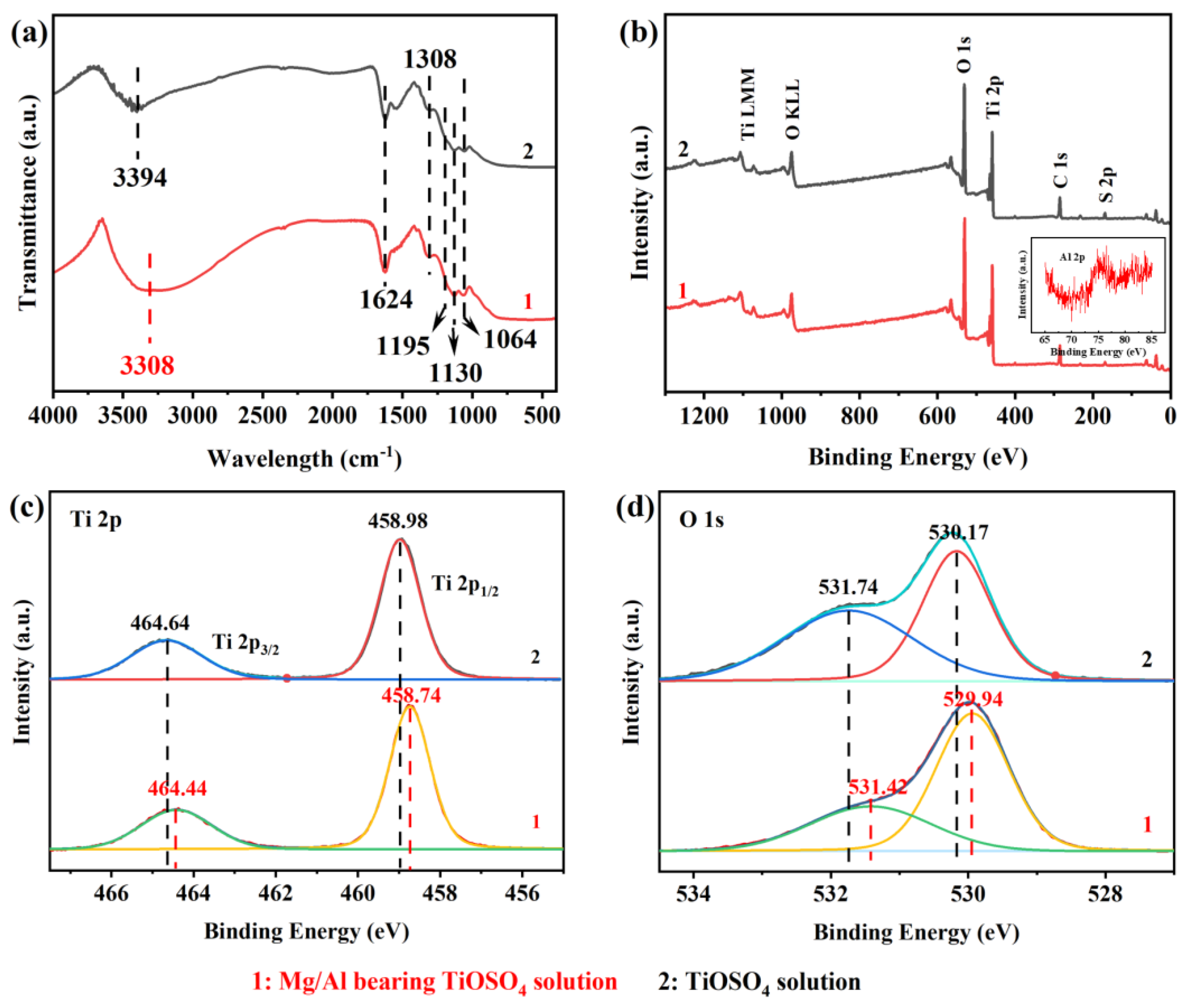
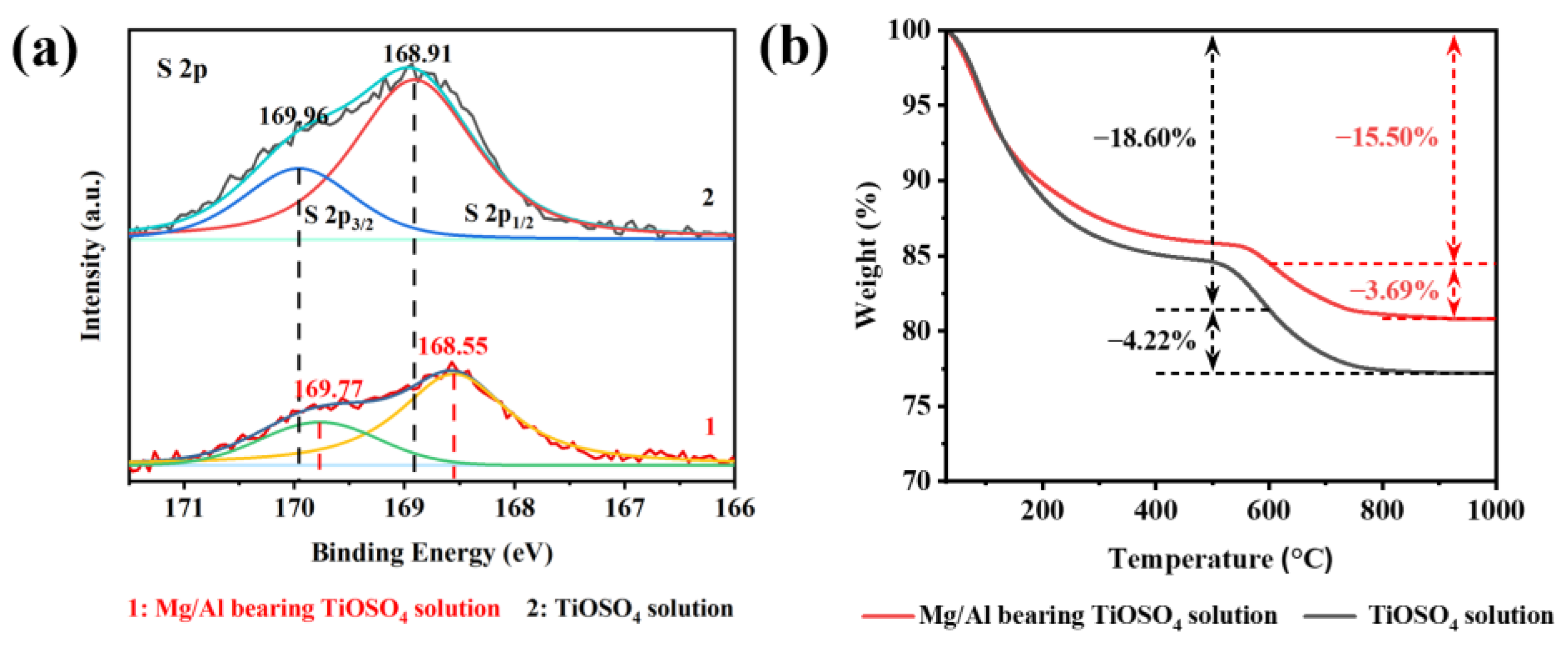
3.1. Characterization of Hydrated TiO2
3.2. Effect of MgSO4 and Al2(SO4)3 on the Hydrolysis Rate
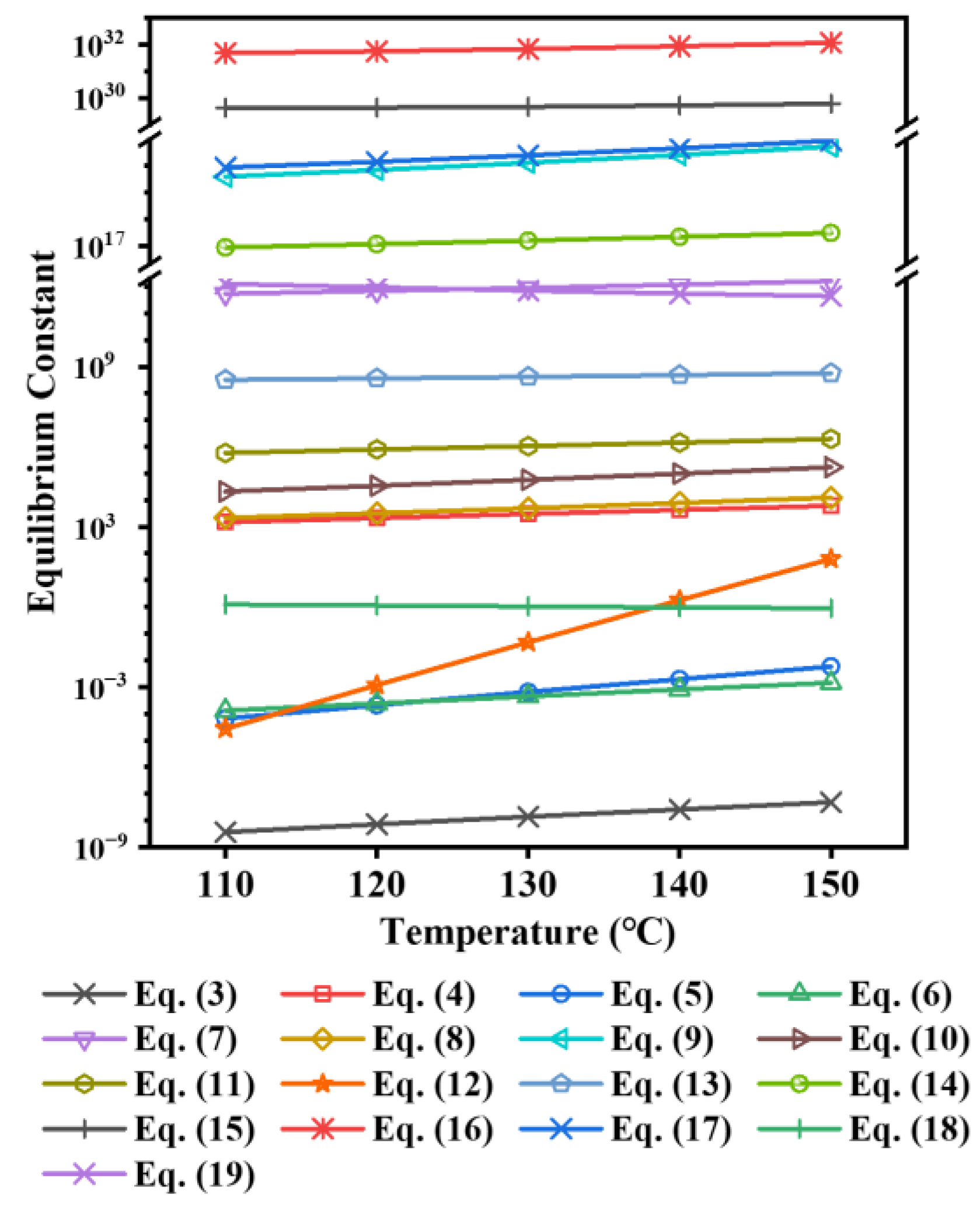
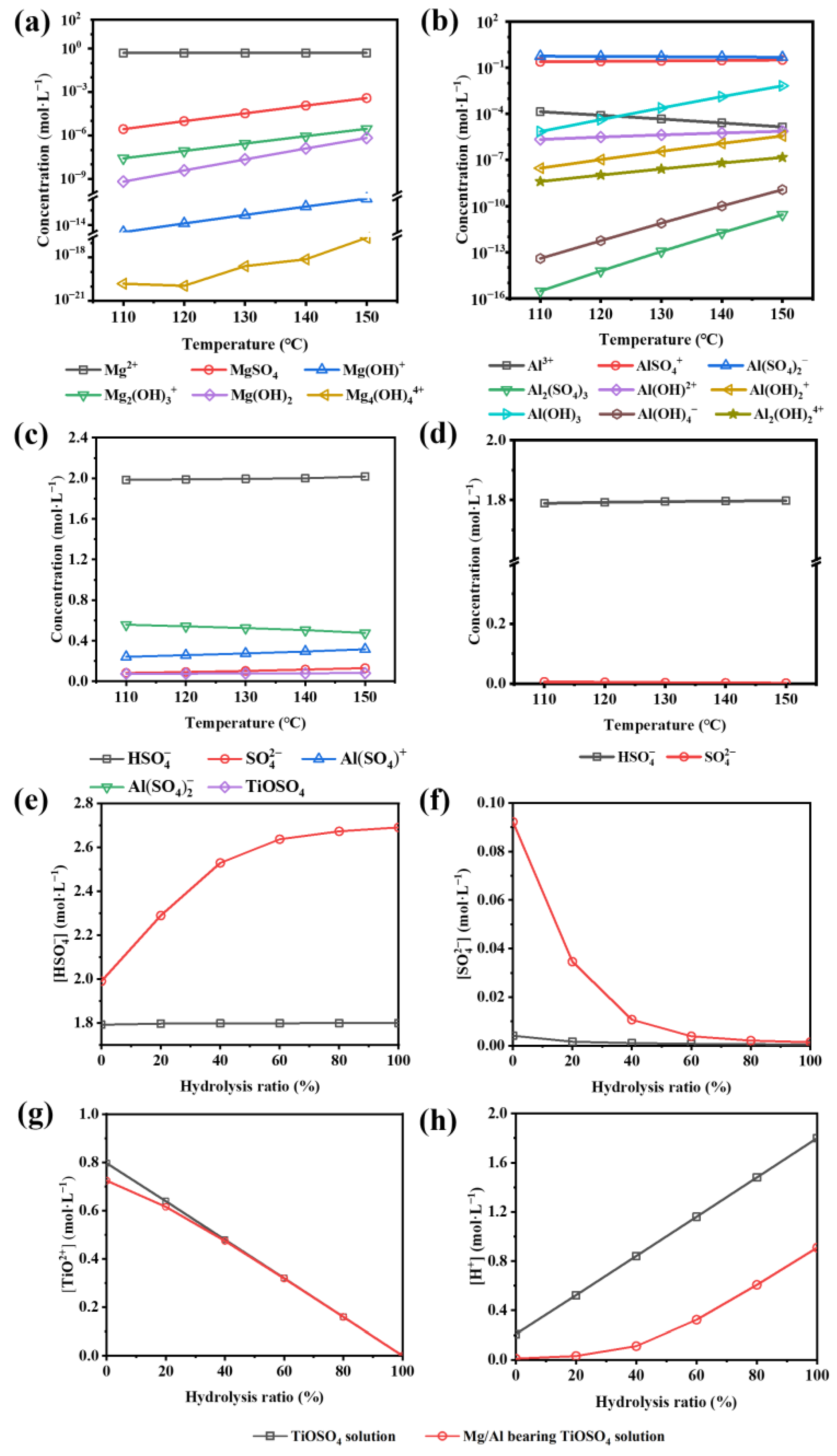
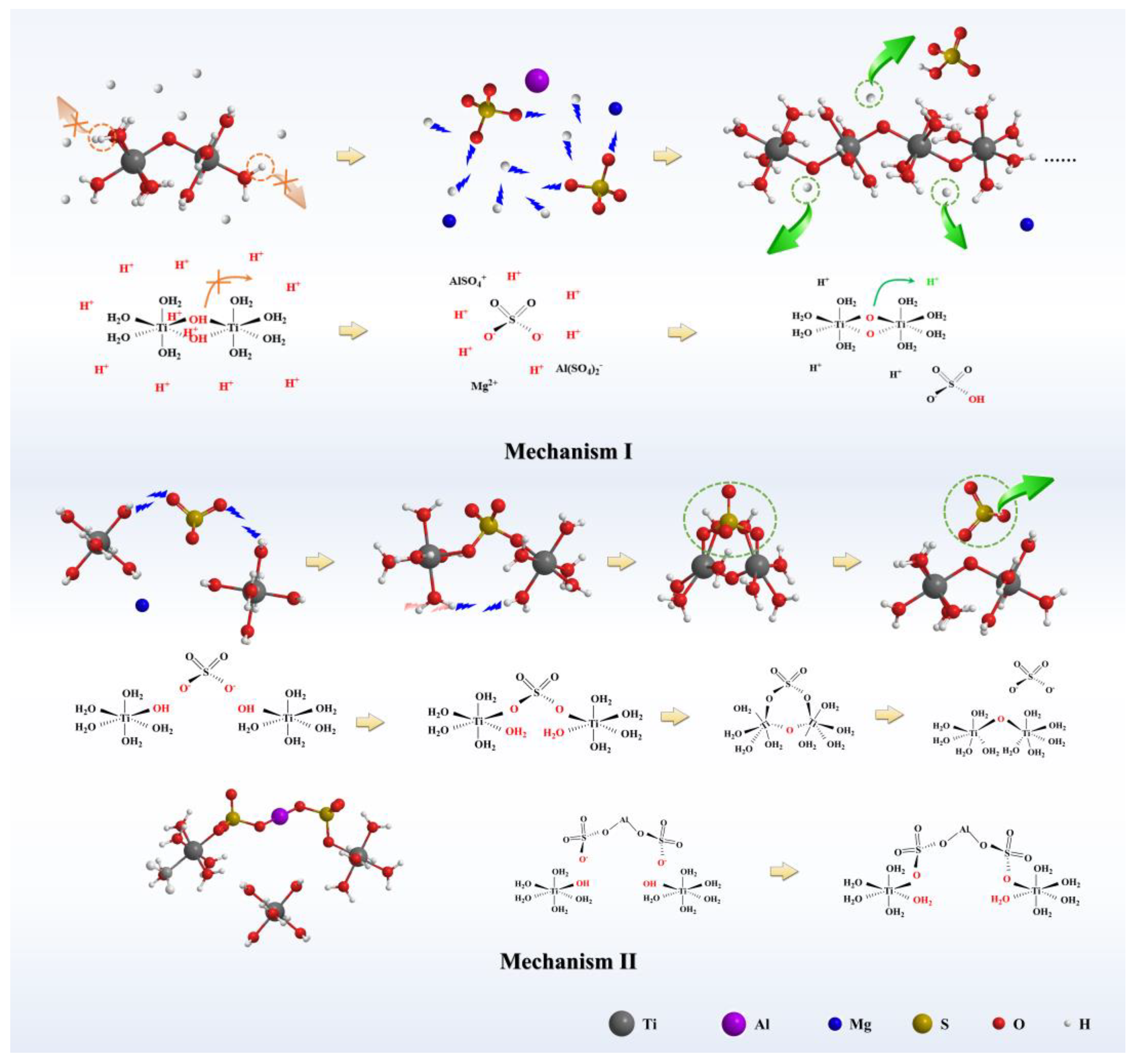
3.3. Thermodynamic Calculation
3.3.1. Reaction Equation for TiOSO4 Hydrothermal Hydrolysis System
3.3.2. Comparison of the Hydrolysis Tendency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, G.; Dang, J.; Lv, X.; Hu, M. Effect of basicity on the crystallization behavior of TiO2-CaO-SiO2 ternary system slag. Crystengcomm 2018, 20, 5422–5431. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Hu, J.; Liu, Q.; Yue, H.; Liang, B.; Zhang, G.; Luo, D.; Xie, H.; Li, C. Indirect mineral carbonation of titanium-bearing blast furnace slag coupled with recovery of TiO2 and Al2O3. Chin. J. Chem. Eng. 2018, 26, 583–592. [Google Scholar] [CrossRef]
- Fan, G.Q.; Wang, M.; Dang, J.; Zhang, R.; Lv, Z.P.; He, W.C.; Lv, X.W. A novel recycling approach for efficient extraction of titanium from high-titanium-bearing blast furnace slag. Waste Manag. 2021, 120, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, K.; Sun, Q. Effect of calcium carbonate on the preparation of glass ceramic foams from water-quenched titanium-bearing blast furnace slag and waste glass. Adv. Appl. Ceram. 2018, 117, 312–318. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, F.; Yi, M.; Xiang, L. Estimation of Reaction Heat in Ti-Bearing Blast Furnace Slag—Sulfuric Acid System Based on Mechanical Mixture Model. Min. Metall. Explor. 2021, 38, 1247–1252. [Google Scholar] [CrossRef]
- Zhou, H.-L.; Feng, K.-Q.; Chen, C.-H.; Yan, Z.-D. Influence of CeO2 addition on the preparation of foamed glass-ceramics from high-titanium blast furnace slag. Int. J. Miner. Met. Mater. 2018, 25, 689–695. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T., Jr. Surface science studies of the photoactivation of TiO2 new photochemical processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef]
- Zhong, B.; Xue, T.; Zhao, L.; Zhao, H.; Qi, T.; Chen, W. Preparation of Ti-enriched slag from V-bearing titanomagnetite by two-stage hydrochloric acid leaching route. Sep. Purif. Technol. 2014, 137, 59–65. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Z.C.; Li, G. Preparation of nano-titanium dioxide from ilmenite using sulfuric acid-decomposition by liquid phase method. Powder Technol. 2016, 287, 256–263. [Google Scholar] [CrossRef]
- Zhen, Y.-L.; Zhang, G.-H.; Chou, K.-C. Carbothermic Reduction of Titanium-Bearing Blast Furnace Slag. High Temp. Mater. Process. 2016, 35, 309–319. [Google Scholar] [CrossRef]
- Jalava, J.P. Precipitation and Properties of Titania Pigments in the Sulfate Process. 1. Preparation of the Liquor and Effects of Iron(II) in Isoviscous Liquor. Ind. Eng. Chem. Res. 1992, 31, 608–611. [Google Scholar] [CrossRef]
- Grzmil, B.U.; Grela, D.; Kic, B. Hydrolysis of titanium sulphate compounds. Chem. Pap. 2008, 62, 18–25. [Google Scholar] [CrossRef]
- Grzmil, B.; Grela, D.; Kic, B. Formation of hydrated titanium dioxide from seeded titanyl sulphate solution. Chem. Pap. 2009, 63, 217–225. [Google Scholar] [CrossRef]
- Tian, C.X. Effects of Structural Factors of Hydrated TiO2 on Rutile TiO2 Pigment Preparation via Short Sulfate Process. Sci. Rep. 2020, 10, 7999. [Google Scholar] [CrossRef]
- Tian, C.X.; Huang, S.H.; Yang, Y. Anatase TiO2 white pigment production from unenriched industrial titanyl sulfate solution via short sulfate process. Dye. Pigment. 2013, 96, 609–613. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, C.R.; Yuan, Z.C. Precipitation and growth behaviour of metatitanic acid particles from titanium sulfate solution. Powder Technol. 2017, 315, 31–36. [Google Scholar] [CrossRef]
- Xin, W.H.; Zhao, Z.D.; Yang, X.H.; Cheng, Y.Z.; Sun, F.Z.; Feng, L. A Facile Preparation Strategy for TiO2 Spheres by Direct Hydrolysis. Chemnanomat 2019, 5, 1263–1266. [Google Scholar] [CrossRef]
- Tian, M.; Liu, Y.H.; Wang, W.J.; Zhao, W.; Chen, D.S.; Wang, L.N.; Zhao, H.X.; Meng, F.C.; Zhen, Y.L.; Hu, Z.Y.; et al. Mechanism of synthesis of anatase TiO2 pigment from low concentration of titanyl sulfuric-chloric acid solution under hydrothermal hydrolysis. J. Chin. Chem. Soc. Taip. 2020, 67, 277–287. [Google Scholar] [CrossRef]
- Wan, X.B.; Shi, J.J.; Qiu, Y.C.; Chen, M.; Li, J.Z.; Liu, C.S.; Taskinen, P.; Jokilaakso, A. The effect of 15 wt% Al2O3 addition on the equilibrium phase relations of CaO-SiO2-TiO2 system at 1400 °C in air. Ceram. Int. 2021, 47, 24802–24808. [Google Scholar] [CrossRef]
- Ma, R.M.; Chen, Y.H.; Yang, Y.; Wu, Z.X.; Bao, Y.J.; Li, N.; Li, J.P. Facile hydrothermal deposition of octahedral-shaped Cu2O crystallites on bamboo veneer for efficient degradation of organic aqueous solution. Vacuum 2021, 185, 110038. [Google Scholar] [CrossRef]
- Alli, U.; McCarthy, K.; Baragau, I.-A.; Power, N.P.; Morgan, D.J.; Dunn, S.; Killian, S.; Kennedy, T.; Kellici, S. In-situ continuous hydrothermal synthesis of TiO2 nanoparticles on conductive N-doped MXene nanosheets for binder-free Li-ion battery anodes. Chem. Eng. J. 2022, 430, 132976. [Google Scholar] [CrossRef]
- Arlina, A.; Ter, T.P.; Ameram, N.; Najwa, M.N.; Norhafifi, A.; Norsyazleen, M.; Shaari, A. Effect of heating times on the structural and optical properties of Al-doped ZnO via hydrothermal method. Mater. Today Proc. 2022, 51, 1444–1447. [Google Scholar] [CrossRef]
- Nakamoto, K.; Fujita, J.; Tanaka, S.; Kobayashi, M. Infrared Spectra of Metallic Complexes. IV. Comparison of the Infrared Spectra of Unidentate and Bidentate Metallic Complexes. J. Am. Chem. Soc. 1957, 79, 4904–4908. [Google Scholar] [CrossRef]
- Navarrete, J.; Lopez, T.; Gomez, R.; Figueras, F. Surface Acidity of Sulfated TiO2-SiO2 Sol-Gels. Langmuir 1996, 12, 4385–4390. [Google Scholar] [CrossRef]
- Li, H.X.; Li, G.S.; Zhu, J.; Wan, Y. Preparation of an active SO42−/TiO2 photocatalyst for phenol degradation under supercritical conditions. J. Mol. Catal. A Chem. 2005, 226, 93–100. [Google Scholar] [CrossRef]
- Speight, J.G. Lange’s Handbook of Chemistry; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Bond, G.C.; Flamerz, S. Structure and reactivity of titania-supported oxides: IV. Characterisation of dried vanadia/titania catalyst precursors. Appl. Catal. 1989, 46, 89–102. [Google Scholar] [CrossRef]
- Stypula, B.; Stoch, J. The characterization of passive films on chromium electrodes by XPS. Corros. Sci. 1994, 36, 2159–2167. [Google Scholar] [CrossRef]
- Bierla, A.; Fromentin, G.; Minfray, C.; Martin, J.-M.; Le Mogne, T.; Genet, N. Mechanical and physico-chemical study of sulfur additives effect in milling of high strength steel. Wear 2012, 286, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Liu, Y.H.; Wang, L.N.; Chen, D.S.; Zhao, H.X.; Meng, F.C.; Zhen, Y.L.; Qi, T. Role of aluminum salt on thermal hydrolysis of titanyl sulfuric-chloric mixture acid solution. J. Mater. Res. Technol. 2021, 14, 2486–2496. [Google Scholar] [CrossRef]

| TBFS | Component | TiO2 | SiO2 | CaO | Al2O3 | MgO | Fe |
| Content (wt%) | 21~25 | 22~26 | 22~29 | 16~19 | 7~9 | 0.2~0.4 | |
| Mg/Al-bearing TiOSO4 solution | Component | TiOSO4 | MgSO4 | Al2(SO4)3 | H2SO4 | ||
| Concentration (mol·L−1) | 0.8 | 0.5 | 0.4 | 1.0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Yang, F.; Yang, Z.; Wang, J.; Xiang, L. Preparation of Hydrated TiO2 Particles by Hydrothermal Hydrolysis of Mg/Al-Bearing TiOSO4 Solution. Nanomaterials 2023, 13, 1179. https://doi.org/10.3390/nano13071179
Lin S, Yang F, Yang Z, Wang J, Xiang L. Preparation of Hydrated TiO2 Particles by Hydrothermal Hydrolysis of Mg/Al-Bearing TiOSO4 Solution. Nanomaterials. 2023; 13(7):1179. https://doi.org/10.3390/nano13071179
Chicago/Turabian StyleLin, Shuyu, Fan Yang, Zhuoying Yang, Jing Wang, and Lan Xiang. 2023. "Preparation of Hydrated TiO2 Particles by Hydrothermal Hydrolysis of Mg/Al-Bearing TiOSO4 Solution" Nanomaterials 13, no. 7: 1179. https://doi.org/10.3390/nano13071179