Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface
Abstract
:1. Introduction
2. Materials and Methods
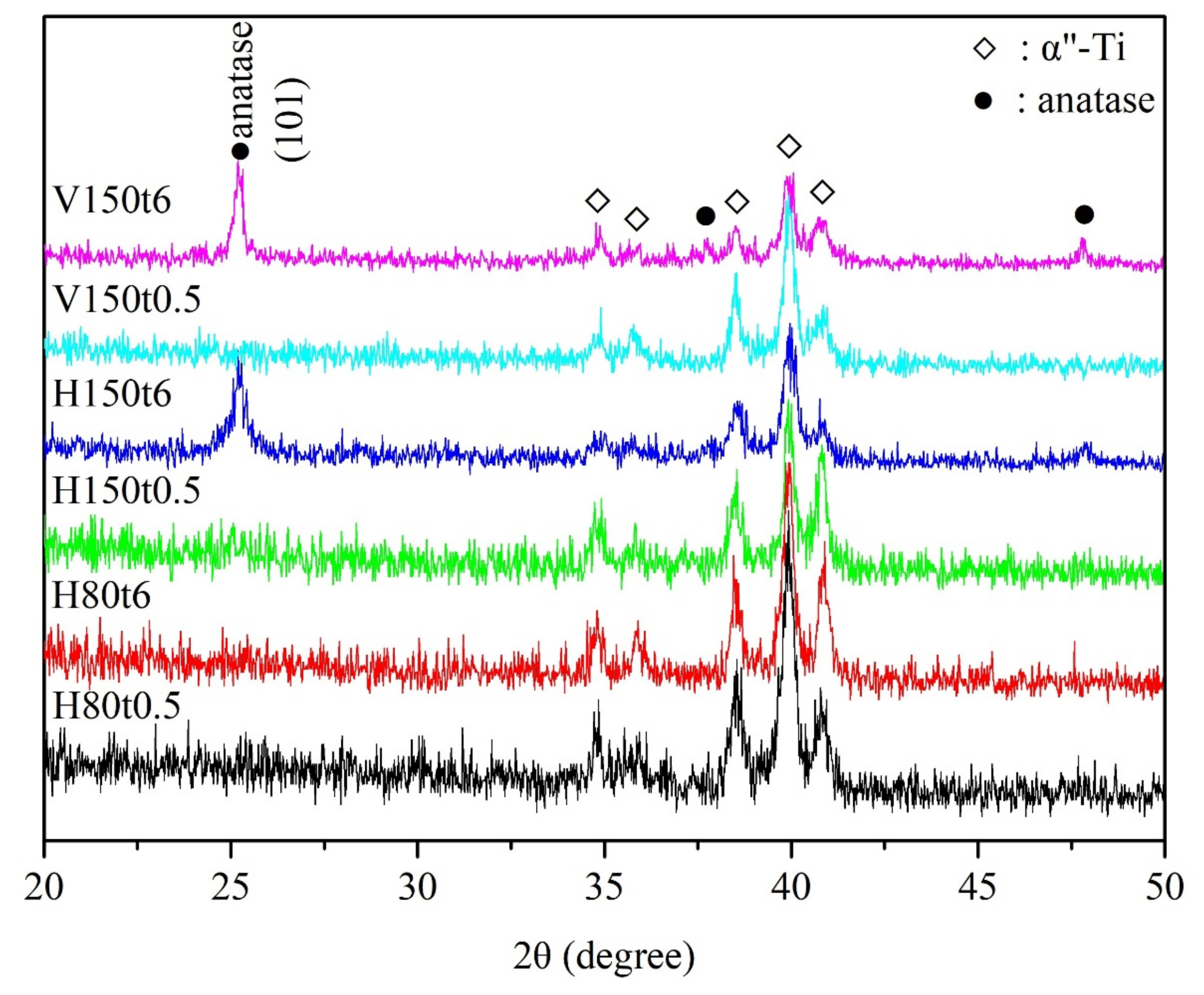
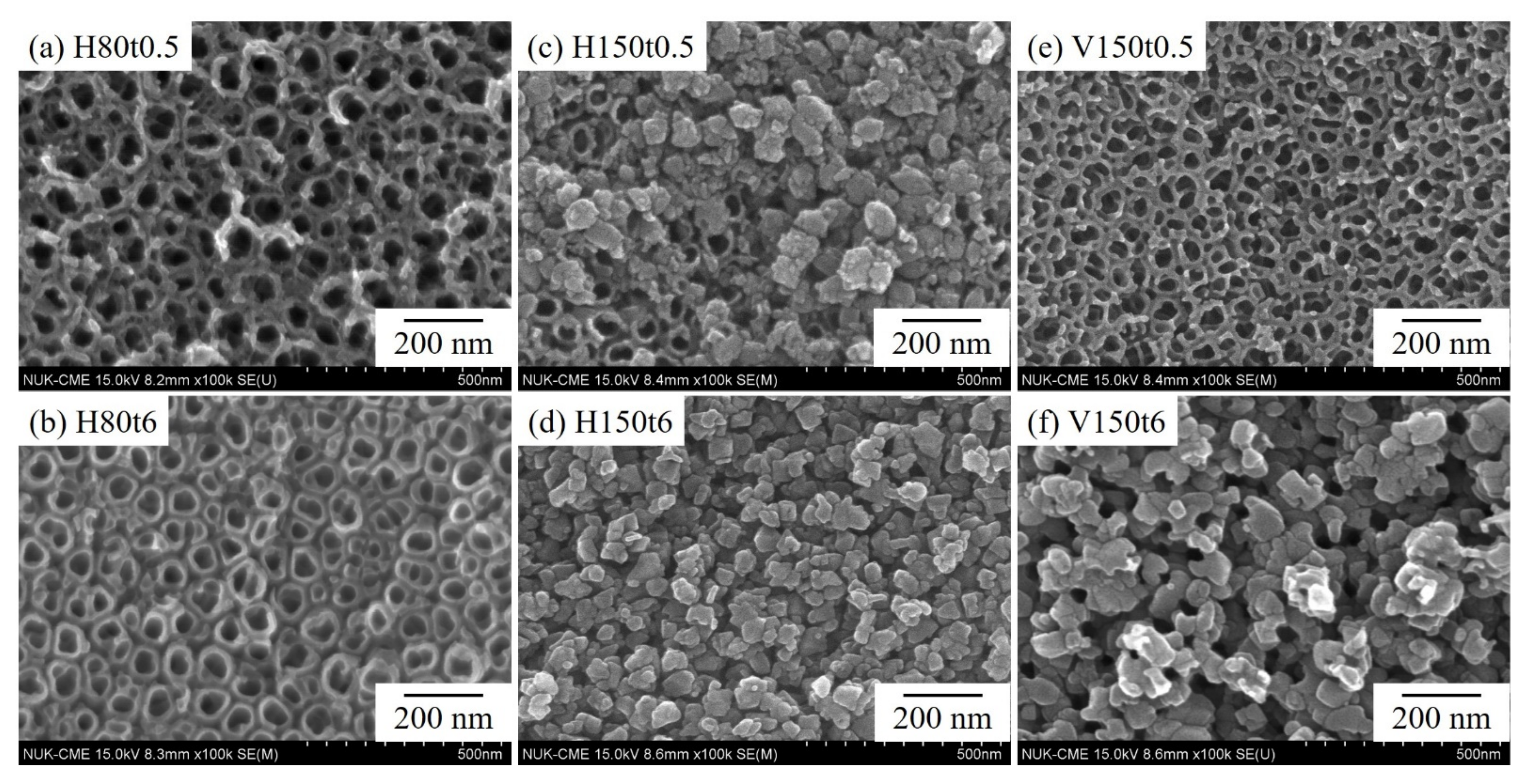
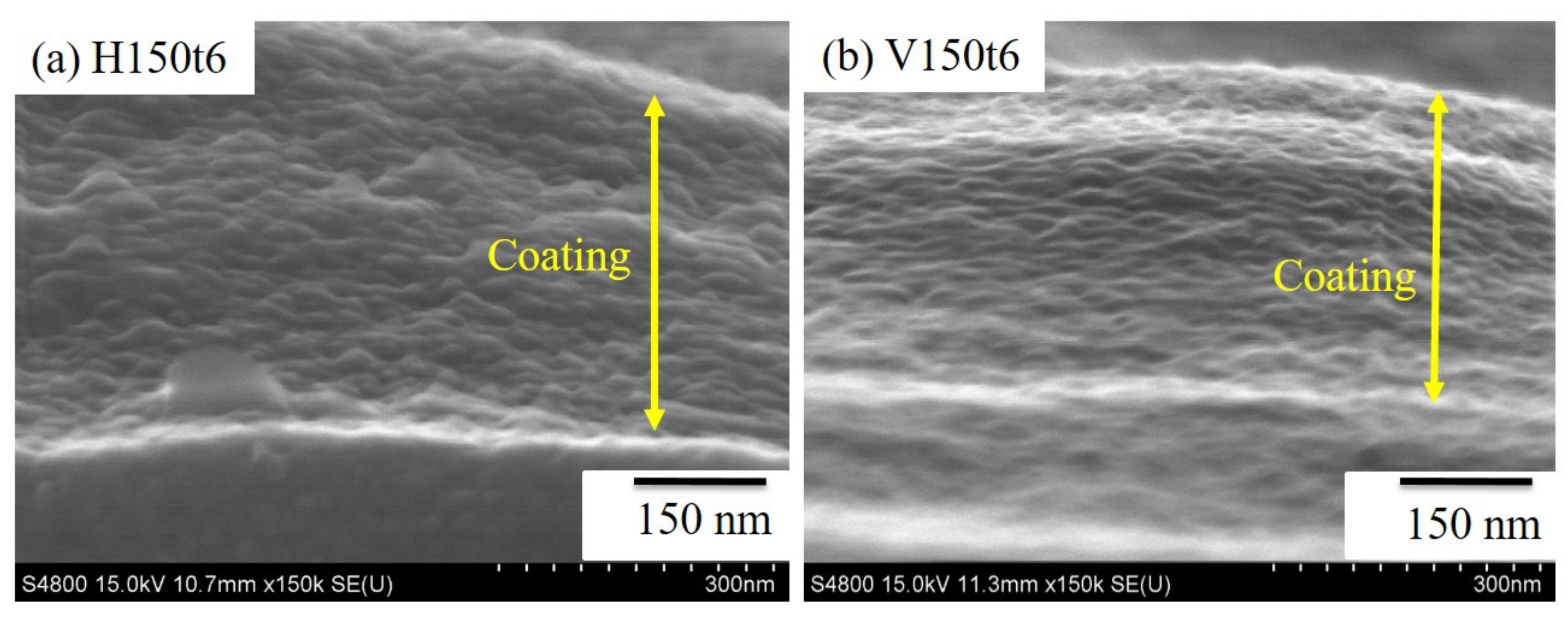
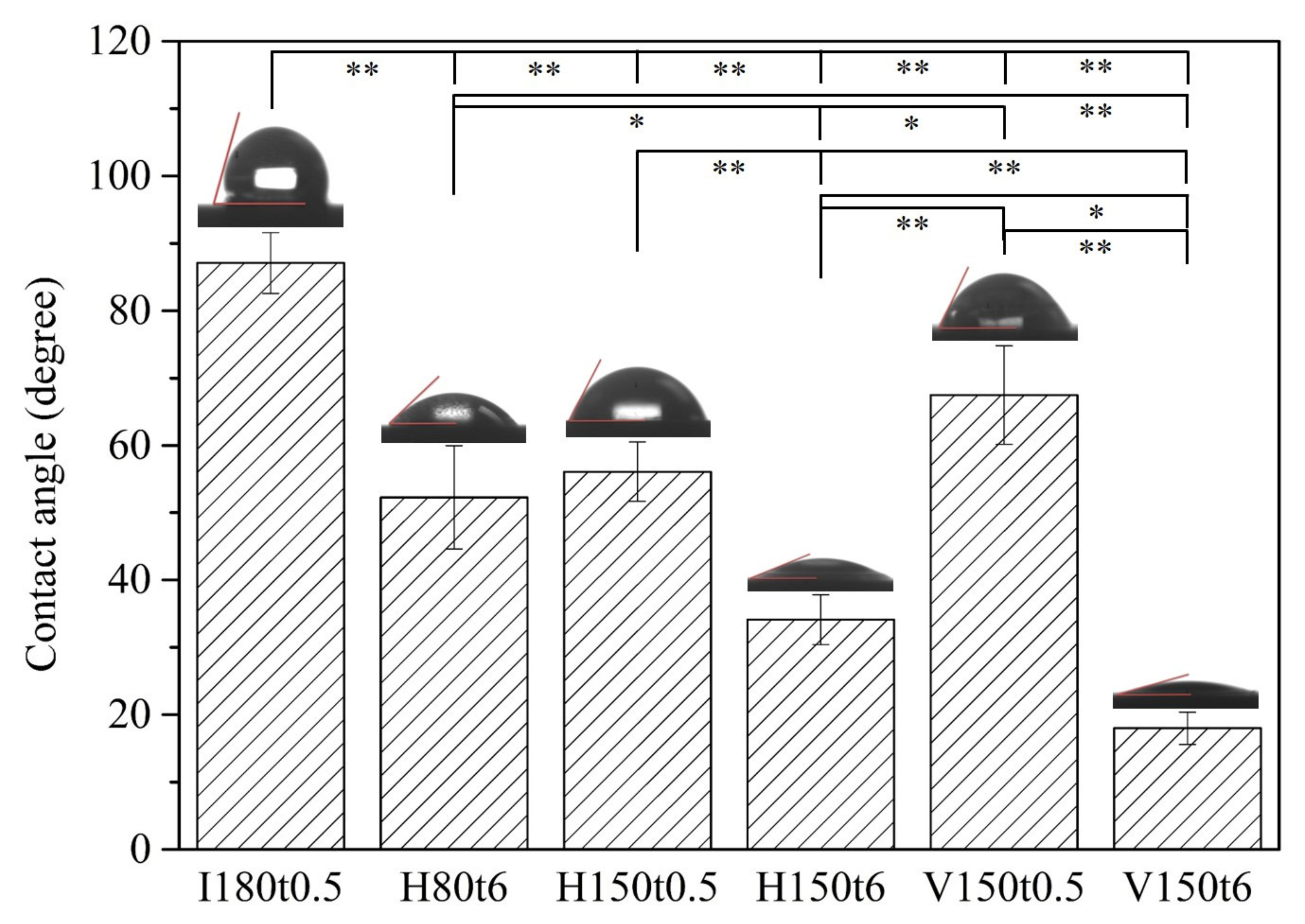
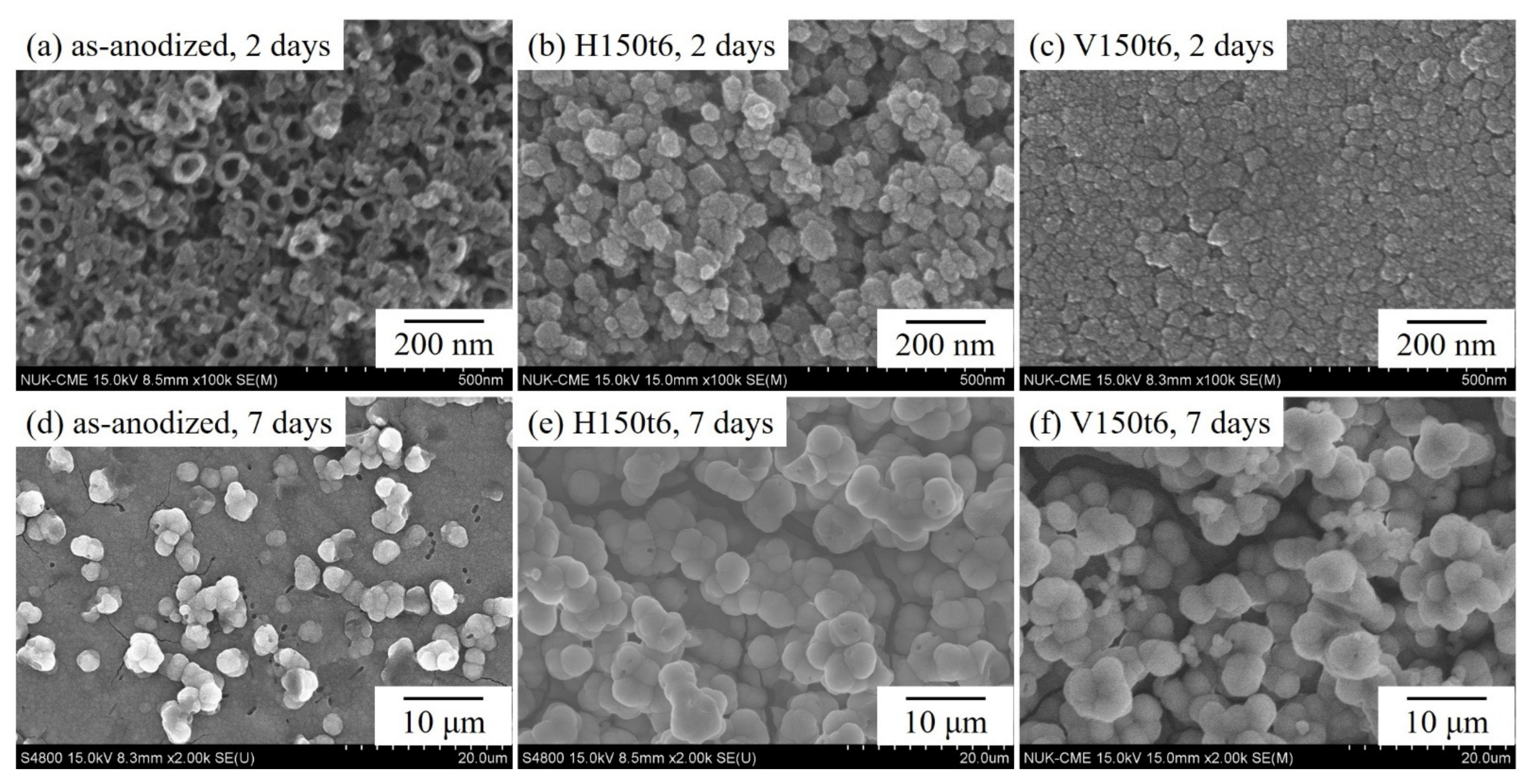
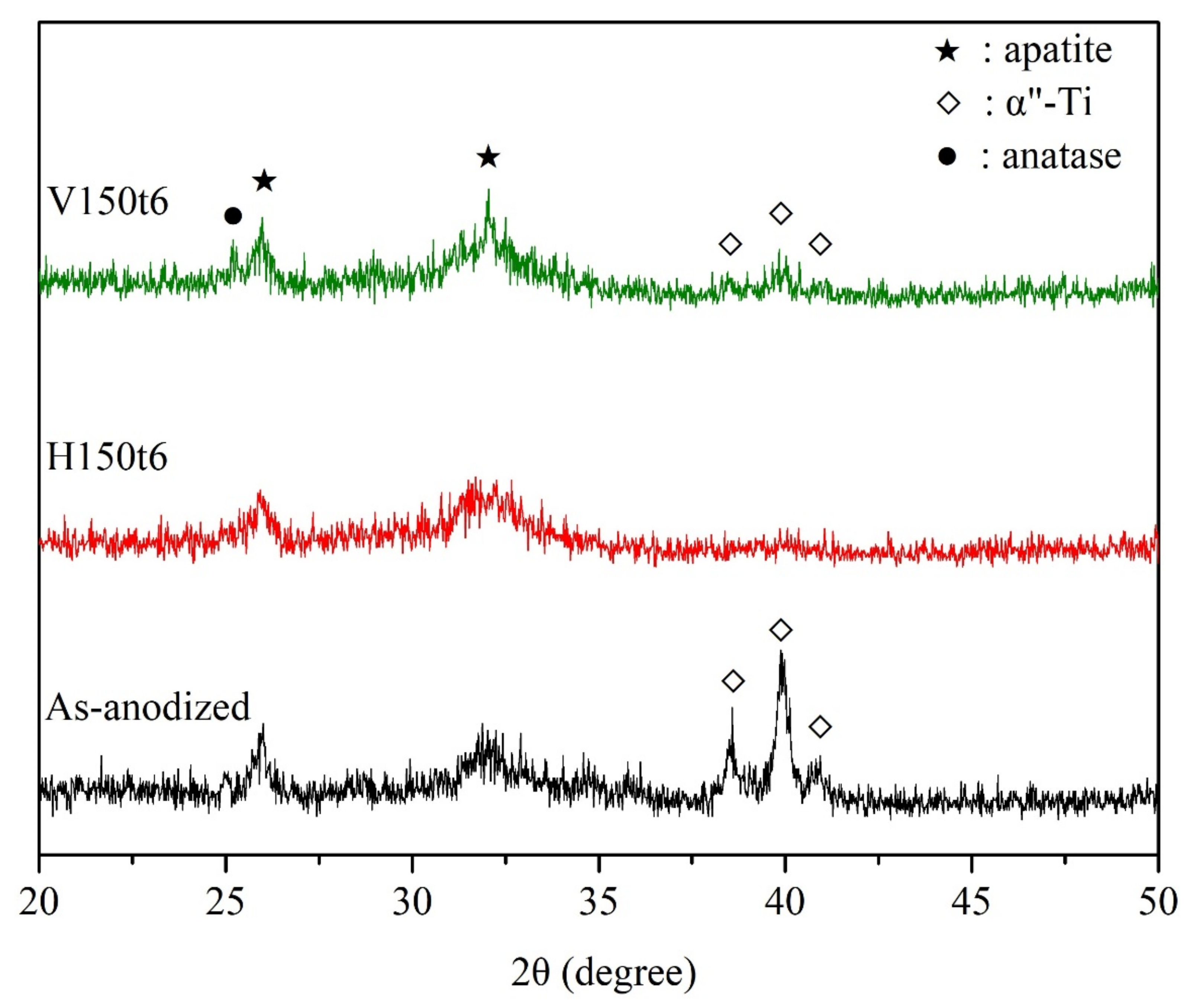
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bittredge, O.; Hassanin, H.; El-Sayed, M.A.; Eldessouky, H.M.; Alsaleh, N.A.; Alrasheedi, N.H.; Essa, K.; Ahmadein, M. Fabrication and Optimisation of Ti-6Al-4V Lattice-Structured Total Shoulder Implants Using Laser Additive Manufacturing. Materials 2022, 15, 3095. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Todai, M.; Nakano, T. Beta titanium single crystal with bone-like elastic modulus and large crystallographic elastic anisotropy. Mater. Sci. Eng. A 2019, 782, 667–671. [Google Scholar] [CrossRef]
- Fouda, N.; Mostafa, R.; Saker, A. Numerical study of stress shielding reduction at fractured bone using metallic and composite bone-plate models. Ain Shams Eng. J. 2019, 10, 481–488. [Google Scholar] [CrossRef]
- Luo, C.; Liu, Y.; Peng, B.; Chen, M.; Liu, Z.; Li, Z.; Kuang, H.; Gong, B.; Li, Z.; Sun, H. PEEK for Oral Applications: Recent Advances in Mechanical and Adhesive Properties. Polymers 2023, 15, 386. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Hariharan, A.; Günther, F.; Zimmermann, M.; Gebert, A. Influence of isothermal omega precipitation aging on deformation mechanisms and mechanical properties of a β-type Ti-Nb alloy. J. Alloys Compd. 2023, 930, 167309. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K.; Kao, W.H.; Ho, W.F. Structure and mechanical properties of as-cast Ti–5Nb-based alloy with Mo addition. Mater. Sci. Eng. A 2013, 579, 86–91. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Luo, D.M. Corrosion behavior of Ti–Mo alloys cold rolled and heat treated. J. Alloys Compd. 2011, 509, 6267–6272. [Google Scholar] [CrossRef]
- Wong, K.K.; Hsu, H.C.; Wu, S.C.; Ho, W.F. Structure and properties of Ti-rich Ti-Zr-Nb-Mo medium-entropy alloys. J. Alloys Compd. 2021, 868, 159137. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K. Multifunctional Coatings on Implant Materials—A Systematic Review of the Current Scenario. Coatings 2023, 13, 69. [Google Scholar] [CrossRef]
- Şimşek, M.; Aldemir, S.D.; Gümüşderelioğlu, M. Anticellular PEO coatings on titanium surfaces by sequential electrospinning and crosslinking processes. Emerg. Mater. 2019, 2, 169–179. [Google Scholar] [CrossRef]
- Shanmugapriya; Sivamaran, V.; Padma Rao, A.; Senthil Kumar, P.; Selvamani, S.T.; Mandal, T.K. Sol–gel derived Al2O3/Gr/HAP nanocomposite coatings on Ti–6Al–4V alloy for enhancing tribo-mech properties and antibacterial activity for bone implants. Appl. Phys. A 2022, 128, 635. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, M.T.; Chang, Y.Y.; Lin, Y.J.; Hsu, J.T. Fabrication of a novel Ta(Zn)O thin film on titanium by magnetron sputtering and plasma electrolytic oxidation for cell biocompatibilities and antibacterial applications. Metals 2020, 10, 649. [Google Scholar] [CrossRef]
- Kumari, R.; Yadav, K.B.; Barole, S.; Archana, K.; Besra, L.D. Microstructural characterisation and wettability behaviour of nano-HA coating on Ti-6Al-4V alloy by electrophoretic deposition method (EPD). Adv. Mater. Process. Technol. 2022, 8, 611–618. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Nadaraia, K.V.; Plekhova, N.G.; Imshinetskiy, I.M.; Piatkova, M.A.; Pleshkova, A.I.; Kislova, S.E.; Sinebryukhov, S.L.; Gnedenkov, S.V. Antibacterial Ca/P-coatings formed on Mg alloy using plasma electrolytic oxidation and antibiotic impregnation. Mater. Lett. 2022, 317, 132099. [Google Scholar] [CrossRef]
- Imshinetskiy, I.; Kashepa, V.; Nadaraia, K.; Mashtalyar, D.; Suchkov, S.; Zadorozhny, P.; Ustinov, A.; Sinebryukhov, S.; Gnedenkov, S. PEO Coatings Modified with Halloysite Nanotubes: Composition, Properties, and Release Performance. Int. J. Mol. Sci. 2023, 24, 305. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.C. Synchronized Improvements in the Protective and Bioactive Properties of Plasma-Electrolyzed Layers via Cellulose Microcrystalline. ACS Biomater. Sci. Eng. 2023, 9, 197–210. [Google Scholar] [CrossRef]
- Ocampo, R.A.; Echeverria, F.E. Effect of the anodization parameters on TiO2 nanotubes characteristics produced in aqueous electrolytes with CMC. Appl. Surf. Sci. 2019, 469, 994–1006. [Google Scholar] [CrossRef]
- Pishkar, N.; Ghoranneviss, M.; Ghorannevis, Z.; Akbari, H. Study of the highly ordered TiO2 nanotubes physical properties prepared with two-step anodization. Results Phys. 2018, 9, 1246–1249. [Google Scholar] [CrossRef]
- Khrunyk, Y.Y.; Belikov, S.V.; Tsurkan, M.V.; Vyalykh, I.V.; Markaryan, A.Y.; Karabanalov, M.S.; Popov, A.A.; Wysokowski, M. Surface-Dependent Osteoblasts Response to TiO2 Nanotubes of Different Crystallinity. Nanomaterials 2020, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Savitha, R.; Raghunathan, R.; Chetty, R. Enhanced visible light sensitized photoreaction by mixed phase titania nanotubes. Appl. Surf. Sci. 2023, 609, 155252. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jin, G.C.; Kim, Y.K.; Kao, W.H.; Park, I.S. Effect of alkali and heat treatments for bioactivity of TiO2 nanotubes. Appl. Surf. Sci. 2014, 321, 412–419. [Google Scholar] [CrossRef]
- Shimagami, K.; Ito, T.; Toda, Y.; Yumoto, A.; Yamabe-Mitarai, Y. Effects of Zr and Si addition on high-temperature mechanical properties and microstructure in Ti–10Al–2Nb-based alloys. Mat. Sci. Eng. A 2019, 756, 46–53. [Google Scholar] [CrossRef]
- Kim, S.P.; Kaseem, M.; Choe, H.C. Plasma electrolytic oxidation of Ti–25Nb–xTa alloys in solution containing Ca and P ions. Surf. Coat. Technol. 2020, 395, 125916. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Cheng, B. Effect of crystallization methods on morphology and photocatalytic activity of anodized TiO2 nanotube array films. J. Phys. Chem. C 2010, 114, 19378–19385. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Yu, J.; Wang, B. Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays. Appl. Catal. B 2010, 94, 295–302. [Google Scholar] [CrossRef]
- Fischer, K.; Gläser, R.; Schulze, A. Nanoneedle and nanotubular titanium dioxide–PES mixed matrix membrane for photocatalysis. Appl. Catal. B 2014, 160, 456–464. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Wei, D.; Qing, W.F.; Zhou, R.; Li, B.; Wang, Y.; Zhou, Y.; Jia, D. Titania nanotube/nano-brushite composited bioactive coating with micro/nanotopography on titanium formed by anodic oxidation and hydrothermal treatment. Ceram. Int. 2015, 41, 13115–13125. [Google Scholar] [CrossRef]
- Getz, M.N.; Chatzitakis, A.; Liu, X.; Carvalho, P.A.; Bjorheim, T.S.; Norby, T. Voids in walls of mesoporous TiO2 anatase nanotubes by controlled formation and annihilation of protonated titanium vacancies. Mater. Chem. Phys. 2020, 239, 121953. [Google Scholar] [CrossRef]
- Aeimbhu, A. Effect of calcination temperature on morphology, wettability and anatase/rutile phase ratio of titanium dioxide nanotube arrays. Mater. Today Proc. 2018, 5, 14950–14954. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kamonsuangkasem, K.; Kidkhunthod, P.; Chirawatkul, P.; Saiyasombat, C.; Chanlek, N.; Wantala, K. Influence of nitrogen content levels on structural properties and photocatalytic activities of nanorice-like N-doped TiO2 with various calcination temperatures. Mater. Res. Bull. 2018, 105, 265–276. [Google Scholar] [CrossRef]
- Ding, K.L.; Miao, Z.J.; Hu, B.J.; An, G.M.; Sun, Z.Y.; Han, B.X.; Liu, Z.M. Study on the anatase to rutile phase transformation and controlled synthesis of rutile nanocrystals with the assistance of ionic liquid. Langmuir 2010, 26, 10294–10302. [Google Scholar] [CrossRef]
- Lima, G.G.; Souza, G.B.; Lepienski, C.M.; Kuromoto, N.K. Mechanical properties of anodic titanium films containing ions of Ca and P submitted to heat and hydrothermal treatment. J. Mech. Behav. Biomed. Mater. 2016, 64, 18–30. [Google Scholar] [CrossRef]
- Roguska, A.; Pisarek, M.; Belcarz, A.; Marconc, L.; Holdynski, M.; Andrzejczuk, M.; Janik-Czachora, M. Improvement of the bio-functional properties of TiO2 nanotubes. Appl. Sur. Sci. 2016, 388, 775–885. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 579, 551–560. [Google Scholar] [CrossRef]
- Xiao, X.; Liang, J.; Tang, H.; Yang, X.; Liu, R.; Chen, Y. Preparation and bioactivity of TiO2 nanotube arrays containing calcium and phosphorus. Appl. Surf. Sci. 2013, 15, 312–319. [Google Scholar] [CrossRef]
- Burant, B.; Robak, J.; Leniart, A.; Piwonski, I.; Batory, D. The effect of concentration and source of calcium ions on anticorrosion properties of Ca-doped TiO2 bioactive sol-gel coatings. Ceram. Int. 2017, 43, 13735–13742. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Müller, L.; Kunze, J.; Müller, F.; Greil, P.; Virtanen, S.; Schmuki, P. Hydroxyapatite growth on anodic TiO2 nanotubes. J. Biomed. Mater. Res. 2006, 77, 534–541. [Google Scholar] [CrossRef]

| Sample Code | Hydrothermal | Vapor Thermal |
|---|---|---|
| H80t0.5 | 80 °C, 0.5 h | |
| H80t6 | 80 °C, 6 h | |
| H150t0.5 | 150 °C, 0.5 h | |
| H150t6 | 150 °C, 6 h | |
| V150t0.5 | 150 °C, 0.5 h | |
| V150t6 | 150 °C, 6 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, K.-H.; Hsu, H.-C.; Wu, S.-C.; Shih, Y.-C.; Yang, H.-W.; Ho, W.-F. Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface. Nanomaterials 2023, 13, 1296. https://doi.org/10.3390/nano13081296
Hsieh K-H, Hsu H-C, Wu S-C, Shih Y-C, Yang H-W, Ho W-F. Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface. Nanomaterials. 2023; 13(8):1296. https://doi.org/10.3390/nano13081296
Chicago/Turabian StyleHsieh, Kuan-Hsiang, Hsueh-Chuan Hsu, Shih-Ching Wu, Yi-Cheng Shih, Hsiang-Wei Yang, and Wen-Fu Ho. 2023. "Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface" Nanomaterials 13, no. 8: 1296. https://doi.org/10.3390/nano13081296
APA StyleHsieh, K.-H., Hsu, H.-C., Wu, S.-C., Shih, Y.-C., Yang, H.-W., & Ho, W.-F. (2023). Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface. Nanomaterials, 13(8), 1296. https://doi.org/10.3390/nano13081296








