Facile Synthesis of Heterogeneous Indium Nanoparticles for Formate Production via CO2 Electroreduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis Procedures
2.2.1. In NPs Synthesis
2.2.2. Inx/Cy Synthesis
2.2.3. Catalytic ink and Working Electrodes Preparation
2.3. Physicochemical Characterization of Materials
2.4. Electrochemical Characterization
2.5. Products Analysis
3. Results and Discussion
3.1. Materials Characterization
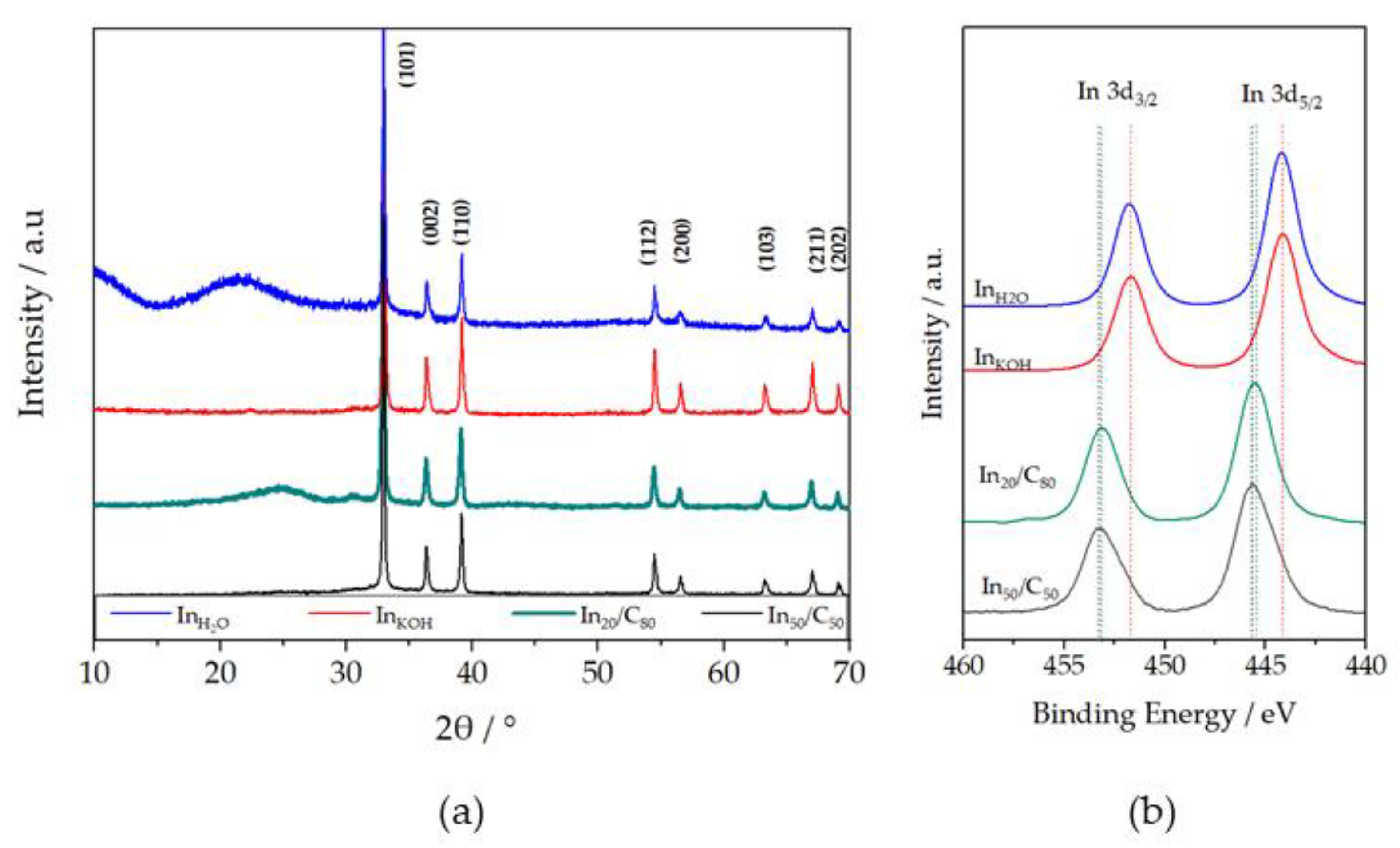
3.1.1. XRD Diffraction Patterns and XPS Spectra
3.1.2. SEM
3.1.3. TEM
3.2. Electrochemical Characterization
3.2.1. Effect of the Washing Agents
3.2.2. Effect of the Carbon Support
3.2.3. Effect of the Applied Potential
3.2.4. Electrodes Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Köne, A.I.; Büke, T. Forecasting of CO2 Emissions from Fuel Combustion Using Trend Analysis. Renew. Sustain. Energy Rev. 2010, 14, 2906–2915. [Google Scholar] [CrossRef]
- Florides, G.A.; Christodoulides, P. Global Warming and Carbon Dioxide through Sciences. Environ. Int. 2009, 35, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Inamuddin, R.B.; Ahamed, M.I.; Khan, A. (Eds.) Carbon Dioxide Utilization to Sustainable Energy and Fuels. In Advances in Science, Technology & Innovation; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-72876-2. [Google Scholar]
- Wickramasinghe, S.; Wang, J.; Morsi, B.; Li, B. Carbon Dioxide Conversion to Nanomaterials: Methods, Applications, and Challenges. Energy Fuels 2021, 35, 11820–11834. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wei, T.; Sun, J.; Liu, Q.; Ma, D.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. Atomically Dispersed Metal-Based Catalysts for Zn–CO 2 Batteries. Small Struct. 2022, 3, 2200086. [Google Scholar] [CrossRef]
- Liu, W.; Feng, J.; Wei, T.; Liu, Q.; Zhang, S.; Luo, Y.; Luo, J.; Liu, X. Active-Site and Interface Engineering of Cathode Materials for Aqueous Zn—Gas Batteries. Nano Res. 2022, 16, 2325–2346. [Google Scholar] [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 Reduction: Electrocatalyst, Reaction Mechanism, and Process Engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Leung, D.Y.C.; Wang, H.; Leung, M.K.H.; Xuan, J. Electrochemical Reduction of Carbon Dioxide to Formic Acid. ChemElectroChem 2014, 1, 836–849. [Google Scholar] [CrossRef]
- Burke, L.; Healy, J.; Burke, L.D.; Roche, M.C.B.; Electroanal Chem, J.; Roche, M.B.; Scannel, R.; Ahern, M.J.G.; McCarthy, M.M.; Borodzinski, J.; et al. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK, 1992; Volume 139. [Google Scholar]
- Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Understanding the Roadmap for Electrochemical Reduction of CO2 to Multi-Carbon Oxygenates and Hydrocarbons on Copper-Based Catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659. [Google Scholar] [CrossRef]
- Fan, L.; Xia, C.; Yang, F.; Wang, J.; Wang, H.; Lu, Y. Strategies in Catalysts and Electrolyzer Design for Electrochemical CO2 Reduction toward C2+ Products. Sci. Adv. 2020, 6, eaay3111. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Zhang, S.; Liu, Q.; Qiu, Y.; Luo, J.; Liu, X. Oxygen Vacancy-Rich Amorphous Copper Oxide Enables Highly Selective Electroreduction of Carbon Dioxide to Ethylene. Wuli Huaxue Xuebao Acta Physico—Chimica Sinica 2023, 39, 2207026. [Google Scholar] [CrossRef]
- Müller, K.; Brooks, K.; Autrey, T. Hydrogen Storage in Formic Acid: A Comparison of Process Options. Energy Fuels 2017, 31, 12603–12611. [Google Scholar] [CrossRef]
- Yang, W.; Dastafkan, K.; Jia, C.; Zhao, C. Design of Electrocatalysts and Electrochemical Cells for Carbon Dioxide Reduction Reactions. Adv. Mater. Technol. 2018, 3, 1700377. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and Homogeneous Approaches to Conversion of CO2 to Liquid Fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef]
- Hou, X.; Ding, J.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. Asymmetric Coordination Environment Engineering of Atomic Catalysts for CO2 Reduction. Nanomaterials 2023, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, C.; Yamauchi, Y. Nanostructured Nonprecious Metal Catalysts for Electrochemical Reduction of Carbon Dioxide. Nano Today 2016, 11, 373–391. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Suzuki, S. Cathodic Reduction of Carbon Dioxide for Energy Storage. J. Res. Inst. Catalysis Hokkaido Univ. 1983, 30, 81–82. [Google Scholar]
- Li, J.; Zhu, M.; Han, Y.F. Recent Advances in Electrochemical CO2 Reduction on Indium-Based Catalysts. ChemCatChem 2021, 13, 514–531. [Google Scholar] [CrossRef]
- White, J.L.; Bocarsly, A.B. Enhanced Carbon Dioxide Reduction Activity on Indium-Based Nanoparticles. J. Electrochem. Soc. 2016, 163, H410–H416. [Google Scholar] [CrossRef]
- Ding, C.; Li, A.; Lu, S.M.; Zhang, H.; Li, C. In Situ Electrodeposited Indium Nanocrystals for Efficient CO2 Reduction to CO with Low Overpotential. ACS Catal. 2016, 6, 6438–6443. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, X.; Zhou, R.; Zhang, T.; Zhou, R.; Ouyang, B.; Kan, E.; Cullen, P.J.; Ostrikov, K.K.; Tu, X. Facile Synthesis of High-Performance Indium Nanocrystals for Selective CO2-to-Formate Electroreduction. Energy Convers. Manag. 2021, 231, 113847. [Google Scholar] [CrossRef]
- Zhang, A.; Liang, Y.; Li, H.; Zhao, X.; Chen, Y.; Zhang, B.; Zhu, W.; Zeng, J. Harmonizing the Electronic Structures of the Adsorbate and Catalysts for Efficient CO2 Reduction. Nano Lett. 2019, 19, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Lee, J.; Kim, J.H.; Lee, B.J.; Lee, J.S. One-Dimensional CuIn Alloy Nanowires as a Robust and Efficient Electrocatalyst for Selective CO2-to-CO Conversion. J. Power Sources 2018, 378, 412–417. [Google Scholar] [CrossRef]
- Rabiee, A.; Nematollahi, D. Electrochemical Reduction of CO2 to Formate Ion Using Nanocubic Mesoporous In(OH)3/Carbon Black System. Mater. Chem. Phys. 2017, 193, 109–116. [Google Scholar] [CrossRef]
- Hoffman, Z.B.; Gray, T.S.; Moraveck, K.B.; Gunnoe, T.B.; Zangari, G. Electrochemical Reduction of Carbon Dioxide to Syngas and Formate at Dendritic Copper-Indium Electrocatalysts. ACS Catal. 2017, 7, 5381–5390. [Google Scholar] [CrossRef]
- Xia, Z.; Freeman, M.; Zhang, D.; Yang, B.; Lei, L.; Li, Z.; Hou, Y. Highly Selective Electrochemical Conversion of CO2 to HCOOH on Dendritic Indium Foams. ChemElectroChem 2018, 5, 253–259. [Google Scholar] [CrossRef]
- Wang, M.; Ren, X.; Yuan, G.; Niu, X.; Xu, Q.; Gao, W.; Zhu, S.; Wang, Q. Selective Electroreduction of CO2 to CO over Co-Electrodeposited Dendritic Core-Shell Indium-Doped Cu@Cu2O Catalyst. J. CO2 Util. 2020, 37, 204–212. [Google Scholar] [CrossRef]
- Hu, S.; Jin, L.; Si, W.; Wang, B.; Zhu, M. Sulfur Vacancies Enriched 2D ZnIn2 S4 Nanosheets for Improving Photoelectrochemical Performance. Catalysts 2022, 12, 400. [Google Scholar] [CrossRef]
- Gao, S.; Sun, Z.; Liu, W.; Jiao, X.; Zu, X.; Hu, Q.; Sun, Y.; Yao, T.; Zhang, W.; Wei, S.; et al. Atomic Layer Confined Vacancies for Atomic-Level Insights into Carbon Dioxide Electroreduction. Nat. Commun. 2017, 8, 14503. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yin, R.; Shao, Q.; Zhu, T.; Huang, X. Oxygen Vacancies in Amorphous InO x Nanoribbons Enhance CO2 Adsorption and Activation for CO2 Electroreduction. Angew. Chem. 2019, 131, 5665–5669. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wu, H.; Liu, F.; Ren, X.; Wang, Y.; Cao, Y.; Lu, Q.; Zheng, X.; Han, X.; et al. Correlating the Crystal Structure and Facet of Indium Oxides with Their Activities for CO2 Electroreduction. Fundam. Res. 2022; in press. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, Y.; Chen, S.; Ren, W.; Chen, X.; Jia, C.; Su, Z.; Wang, Y.; Zhao, C. Defective Indium/Indium Oxide Heterostructures for Highly Selective Carbon Dioxide Electrocatalysis. Inorg. Chem. 2020, 59, 12437–12444. [Google Scholar] [CrossRef]
- Bin Li, W.; Yu, C.; Tan, X.Y.; Cui, S.; Zhang, Y.F.; Qiu, J.S. Recent Advances in the Electroreduction of Carbon Dioxide to Formic Acid over Carbon-Based Materials. Xinxing Tan. Cailiao/New. Carbon. Mater. 2022, 37, 277–289. [Google Scholar]
- Minh Trung, H.; Duy Thien, N.; Thi Lien, D.; Ngoc Long, N.; van Vu, L. Synthesis and Characterization of Indium Nanoparticles. VNU J. Sci. Math. Phys. 2011, 27, 3. [Google Scholar]
- Bitar, Z.; Fecant, A.; Trela-Baudot, E.; Chardon-Noblat, S.; Pasquier, D. Electrocatalytic Reduction of Carbon Dioxide on Indium Coated Gas Diffusion Electrodes-Comparison with Indium Foil. Appl. Catal. B 2016, 189, 172–180. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Cook, D.; de Groot, C.H.; Hector, A.L.; Huang, R.; Jolleys, A.; Kissling, G.P.; Levason, W.; Pearce, S.J.; Reid, G. Non-Aqueous Electrodeposition of p-Block Metals and Metalloids from Halometallate Salts. RSC Adv. 2013, 3, 15645–15654. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Dang, H. A Novel Solution Route for Preparing Indium Nanoparticles. J. Phys. Chem. B 2003, 107, 7574–7576. [Google Scholar] [CrossRef]
- Wang, Y.; Nguyen, T.S.; Liu, X.; Wang, X. Novel Palladium-Lead (Pd-Pb/C) Bimetallic Catalysts for Electrooxidation of Ethanol in Alkaline Media. J. Power Sources 2010, 195, 2619–2622. [Google Scholar] [CrossRef]
- Gu, L.; Dong, Y.; Zhang, Y.; Wang, B.; Yuan, Q.; Du, H.; Zhao, J. Insights into the Role of an Fe-N Active Site in the Oxygen Reduction Reaction on Carbon-Supported Supramolecular Catalysts. RSC Adv. 2020, 10, 8709–8716. [Google Scholar] [CrossRef] [Green Version]
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the “Debye-Scherrer Equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy AReference Book of Standard Spectra for Identification and Interpretation of XPS Data; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Hewitt, R.W.; Winograd, N. Oxidation of Polycrystalline Indium Studied by X-Ray Photoelectron Spectroscopy and Static Secondary Ion Mass Spectroscopy. J. Appl. Phys. 1980, 51, 2620–2624. [Google Scholar] [CrossRef]
- McGuire, G.E.; Schweitzer, G.K.; Thomas, A.C. Study of Core Electron Binding Energies in Some Group IlIa, Vb, and VIb Compounds. Inorg. Chem. 1973, 12, 2450–2453. [Google Scholar] [CrossRef]
- Wagner, C.D. Chemical Shifts of Auger Lines, and the Auger Parameter. Faraday Discuss. Chem. Soc. 1975, 60, 291–300. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-Ray Photoelectron Spectroscopy: Towards Reliable Binding Energy Referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A Review on C1s XPS-Spectra for Some Kinds of Carbon Materials. Fuller. Nanotub. Carbon. Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Yokota, S.; Okumura, K.; Niwa, M. Support Effect of Metal Oxide on Rh Catalysts in the CH4—CO2 Reforming Reaction. Catal. Lett. 2002, 84, 131–134. [Google Scholar] [CrossRef]
- Whang, H.S.; Lim, J.; Choi, M.S.; Lee, J.; Lee, H. Heterogeneous Catalysts for Catalytic CO2 Conversion into Value-Added Chemicals. BMC Chem. Eng. 2019, 1, 9. [Google Scholar] [CrossRef]
- Chou, N.H.; Ke, X.; Schiffer, P.; Schaak, R.E. Room-Temperature Chemical Synthesis of Shape-Controlled Indium Nanoparticles. J. Am. Chem. Soc. 2008, 130, 8140–8141. [Google Scholar] [CrossRef]
- Banerjee, S.; Loza, K.; Meyer-Zaika, W.; Prymak, O.; Epple, M. Structural Evolution of Silver Nanoparticles during Wet-Chemical Synthesis. Chem. Mater. 2014, 26, 951–957. [Google Scholar] [CrossRef]
- Lu, X.; Yin, L. Porous Indium Oxide Nanorods: Synthesis, Characterization and Gas Sensing Properties. J. Mater. Sci. Technol. 2011, 27, 680–684. [Google Scholar] [CrossRef]
- Das, R.; Soni, R.K. Synthesis and Surface-Enhanced Raman Scattering of Indium Nanotriangles and Nanowires. RSC Adv. 2017, 7, 32255–32263. [Google Scholar] [CrossRef] [Green Version]
- Aghazadeh Meshgi, M.; Kriechbaum, M.; Biswas, S.; Holmes, J.D.; Marschner, C. Synthesis of Indium Nanoparticles at Ambient Temperature; Simultaneous Phase Transfer and Ripening. J. Nanopart. Res. 2016, 18, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, A.; Choudhary, H.K.; Satpati, B.; Mandal, S. Synthesis, Characterization and Optical Properties of Ligand-Protected Indium Nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 7109–7113. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Wang, Y.; Luo, G. Preparation of In(OH)3 and In2O3 Nanorods through a Novel Hydrothermal Method and the Effect of Sn Dopant on Crystal Structures. Ind. Eng. Chem. Res. 2018, 57, 2882–2889. [Google Scholar] [CrossRef]
- Detweiler, Z.M.; White, J.L.; Bernasek, S.L.; Bocarsly, A.B. Anodized Indium Metal Electrodes for Enhanced Carbon Dioxide Reduction in Aqueous Electrolyte. Langmuir 2014, 30, 7593–7600. [Google Scholar] [CrossRef] [PubMed]
- Markovac, V.; Ovrecek, B. Studies of the Electrochemical Kinetics of Indium III. Systems In + InCI3and In + Combined Sulfate-Chloride Electrolyte. J. Electrochem. Soc. 1996, 113, 838. [Google Scholar]
- Piercy, R.; Hampson, N.A. The Indium Electrode in Chloride Electrolytes a Kinetic Study. J. Electroanal. Chem. Interfacial Electrochem 1975, 59, 261–271. [Google Scholar]
- Chung, Y.-H.; Lee, C.-W. Electrochemical Behaviors of Indium. J. Electrochem. Sci. Technol. 2012, 3, 1–13. [Google Scholar] [CrossRef]
- Ávila-Bolívar, B.; Montiel, V.; Solla-Gullón, J. Electrochemical Reduction of CO2 to Formate on Nanoparticulated Bi−Sn−Sb Electrodes. ChemElectroChem 2022, 9, e202200272. [Google Scholar] [CrossRef]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially Oxidized Atomic Cobalt Layers for Carbon Dioxide Electroreduction to Liquid Fuel. Nature 2016, 529, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xie, W.; Li, M.; Zhang, J.; Züttel, A. 3D Hierarchical Porous Indium Catalyst for Highly Efficient Electroreduction of CO2. J. Mater. Chem. A Mater. 2019, 7, 4505–4515. [Google Scholar] [CrossRef]
- Mou, K.; Chen, Z.; Yao, S.; Liu, L. Enhanced Electrochemical Reduction of Carbon Dioxide to Formate with In-Situ Grown Indium-Based Catalysts in an Aqueous Electrolyte. Electrochim. Acta 2018, 289, 65–71. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Wang, J.; Ning, S.; Luo, M.; Xiang, D.; Chen, W.; Kang, X.; Jiang, Z.; Chen, S. In-Sn alloy core-shell nanoparticles: In-doped SnOx shell enables high stability and activity towards selective formate production from electrochemical reduction of CO2. Appl. Catal. B Environ. 2021, 288, 119979. [Google Scholar] [CrossRef]
- Li, C.W.; Ciston, J.; Kanan, M.W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 2014, 508, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; De Luna, P.; García de Arquer, F.P.; Zhang, B.; Becknell, N.; Ross, M.B.; Li, Y.; Banis, M.N.; Li, Y.; Liu, M.; et al. Sulfur-Modulated Tin Sites Enable Highly Selective Electrochemical Reduction of CO2 to Formate. Joule 2017, 1, 794–805. [Google Scholar] [CrossRef] [Green Version]










| Catalyst | Particle Size (nm) | Current Density at −1.8 V vs. Ag/AgCl (mA·cmgeo−2) | Onset Potential of CO2RR (V) vs. Ag/AgCl |
|---|---|---|---|
| In foil | - | −10.71 | −1.25 |
| InH2O | 35.95 | −12.08 | −1.35 |
| InKOH | 38.39 | −15.20 | −1.42 |
| In20/C80 | 30.67 | −33.20 | −1.20 |
| In50/C50 | 36.47 | −37.98 | −1.20 |
| Electrocatalyst | Electrolyte | E (V) | j (mA. cm−2) | FE HCOOH | Ref. |
|---|---|---|---|---|---|
| In50/C50 | 0.5 M KHCO3 +0.45 M KCl | −1.6 V vs. Ag/AgCl (−0.96 V vs. RHE) | −10.6 | ~97% | This work |
| In/C (mp-in) | 0.1 M KHCO3 | −0.95 V vs. RHE | −29.6 | ~90% | [24] |
| In2O3@C | 0.5 M KHCO3 | −0.9 V vs. RHE | −29.5 | ~88% | [66] |
| In(OH)3/C | 0.5 M K2SO4 | −1.1 V vs. RHE | −5,2 | ~77% | [27] |
| In/C | 0.1 M KHCO3 | −1.0 V vs. RHE | −1 | 87.8% | [7] |
| In | 0.1 M KHCO3 | −1.55 V vs. RHE | 5.0 | 94.9% | [67] |
| In NPs | 0.5 M K2SO4 | −1.5 V vs. Ag/AgCl | ~6.0 | ~90% | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Sequera, A.C.; Diaz-Perez, M.A.; Lara Angulo, M.A.; Holgado, J.P.; Serrano-Ruiz, J.C. Facile Synthesis of Heterogeneous Indium Nanoparticles for Formate Production via CO2 Electroreduction. Nanomaterials 2023, 13, 1304. https://doi.org/10.3390/nano13081304
Pérez-Sequera AC, Diaz-Perez MA, Lara Angulo MA, Holgado JP, Serrano-Ruiz JC. Facile Synthesis of Heterogeneous Indium Nanoparticles for Formate Production via CO2 Electroreduction. Nanomaterials. 2023; 13(8):1304. https://doi.org/10.3390/nano13081304
Chicago/Turabian StylePérez-Sequera, Ana Cristina, Manuel Antonio Diaz-Perez, Mayra Anabel Lara Angulo, Juan P. Holgado, and Juan Carlos Serrano-Ruiz. 2023. "Facile Synthesis of Heterogeneous Indium Nanoparticles for Formate Production via CO2 Electroreduction" Nanomaterials 13, no. 8: 1304. https://doi.org/10.3390/nano13081304
APA StylePérez-Sequera, A. C., Diaz-Perez, M. A., Lara Angulo, M. A., Holgado, J. P., & Serrano-Ruiz, J. C. (2023). Facile Synthesis of Heterogeneous Indium Nanoparticles for Formate Production via CO2 Electroreduction. Nanomaterials, 13(8), 1304. https://doi.org/10.3390/nano13081304








