Iron Oxide@Mesoporous Silica Core-Shell Nanoparticles as Multimodal Platforms for Magnetic Resonance Imaging, Magnetic Hyperthermia, Near-Infrared Light Photothermia, and Drug Delivery
Abstract
:1. Introduction
2. Iron Oxide Core@Silica Shell NPs
2.1. Iron Oxide Generalities
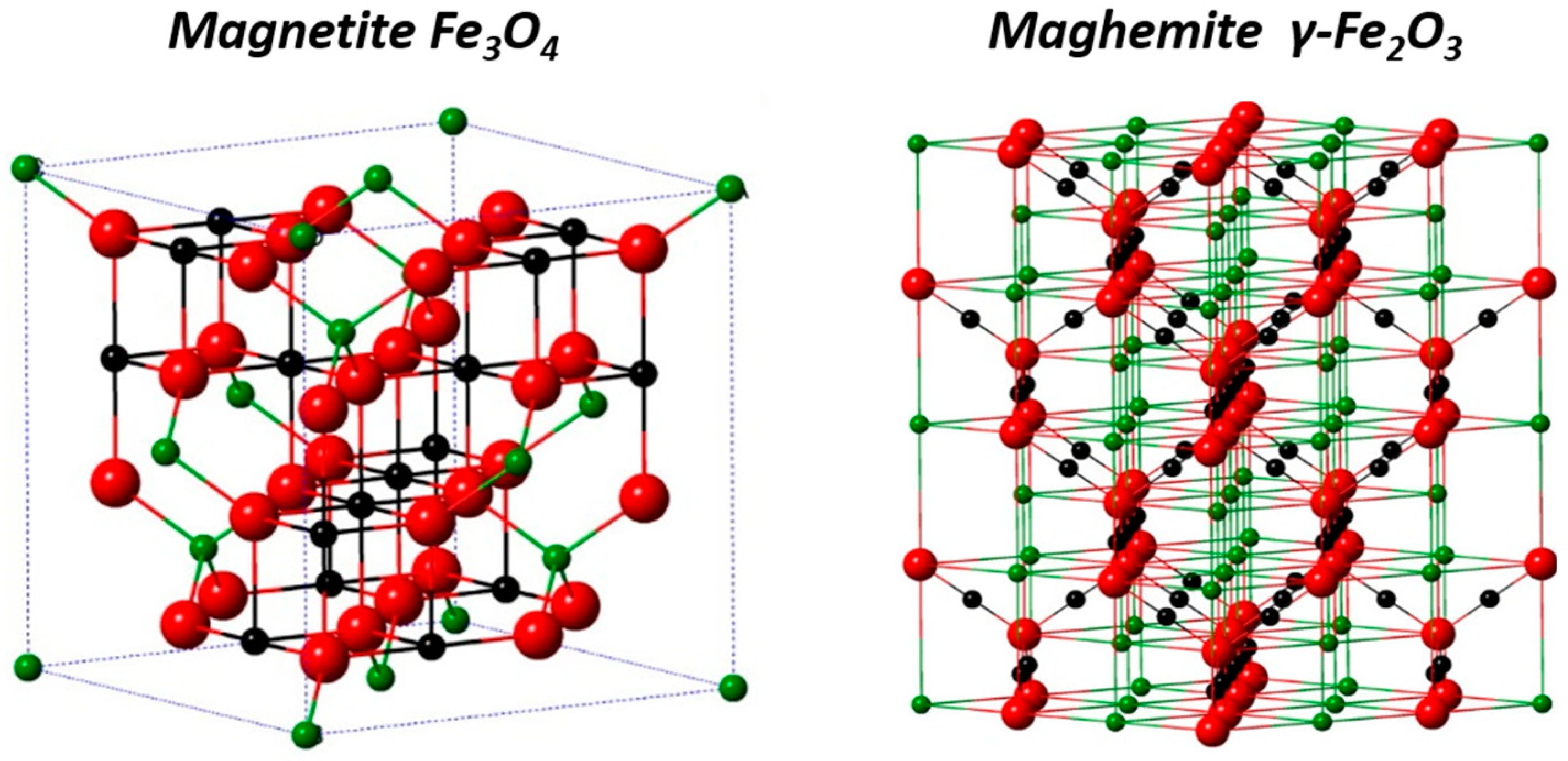
2.1.1. Crystal Structure
2.1.2. Superparamagnetism
2.1.3. Iron Oxide Synthesis
Co-Precipitation Method
Polyol Method
Hydrothermal Method
Thermal Decomposition
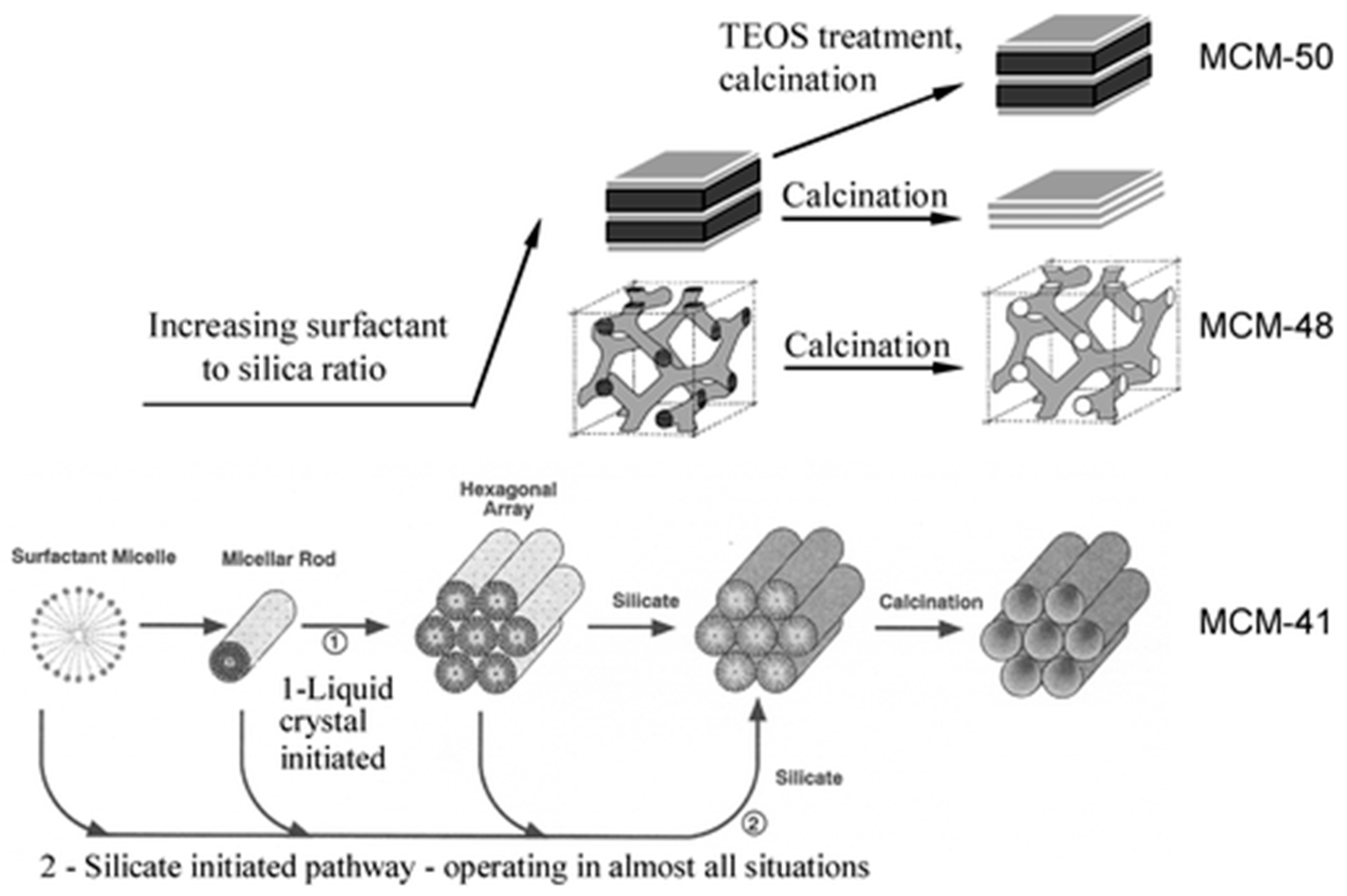
2.2. Silica and Mesoporous Silica Nanomaterials
2.3. Non-Porous Silica Shell Coating around Iron Oxide
2.3.1. Coating by Stöber Sol-Gel Method
2.3.2. Non-Porous Silica Coating by Reverse Microemulsion Process
2.4. Porous Silica Shell Coating around Iron Oxide
2.4.1. Mesoporous Silica on IO NPs via Direct Templating
2.4.2. Mesoporous Silica Coating on IO NPs through Direct Emulsion
3. Physical Properties of IO@MS as Theranostic Agents
3.1. IO@MS as MRI Contrast Agents
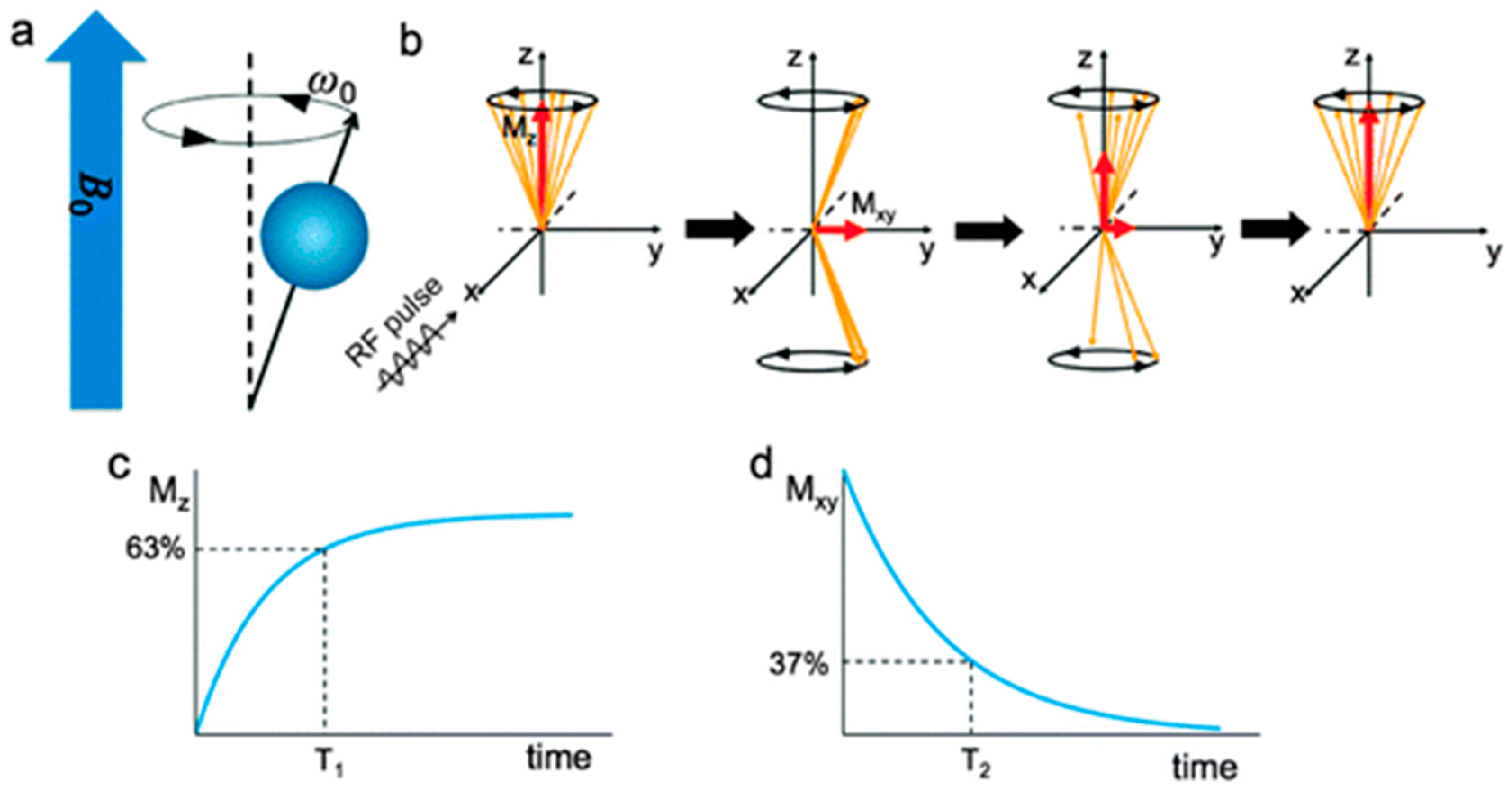
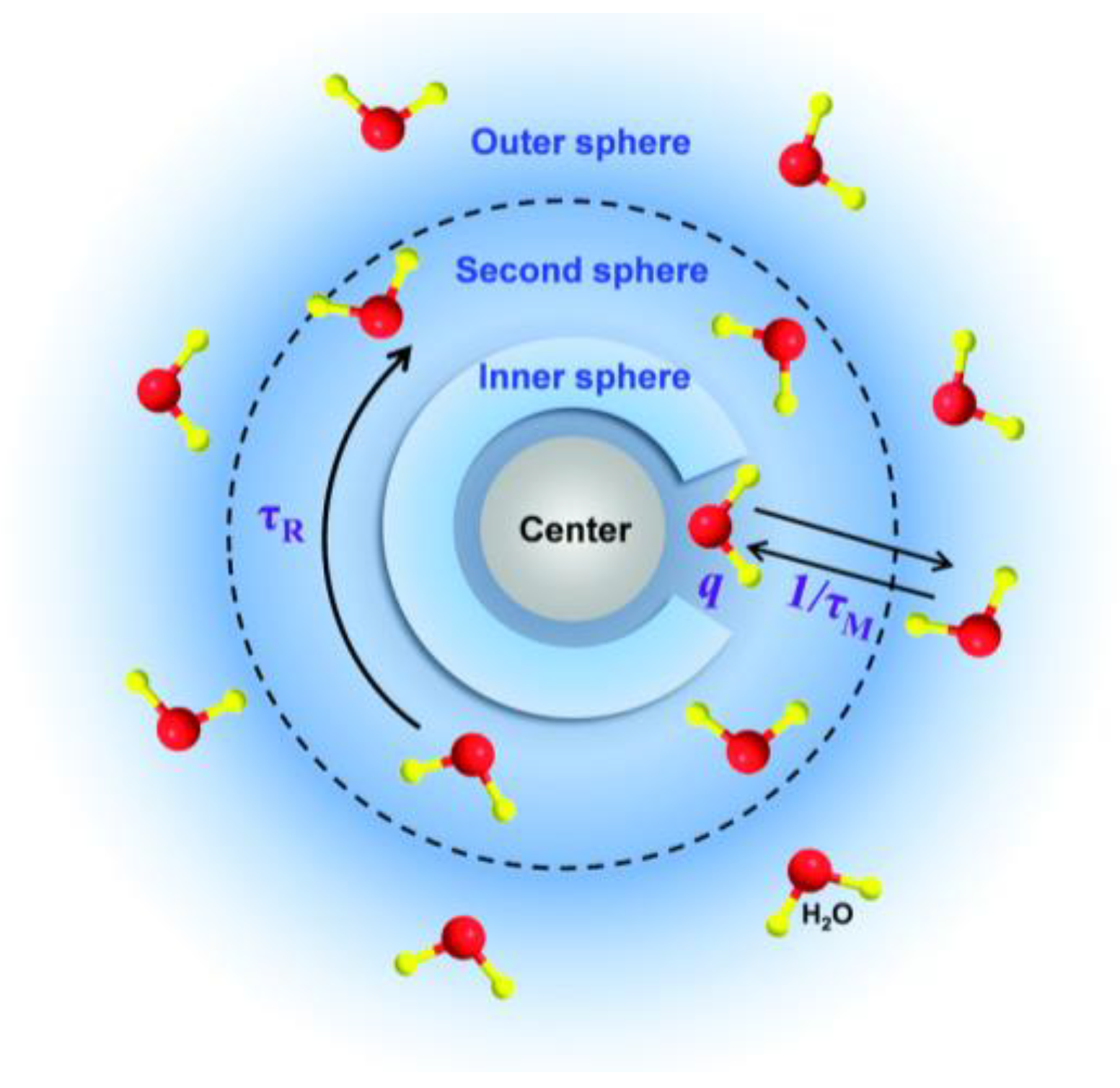
3.1.1. MRI and Contrast Agent Principles
3.1.2. IO@MS as MRI Contrast Agents
3.2. Design of IO@MS for Magnetic Hyperthermia
3.2.1. Magnetic Hyperthermia Principles and Mechanisms
3.2.2. Main Parameters Influencing MHT Potential
Extrinsic Parameters
Intrinsic Parameters
3.2.3. Core-Shell IO@MS NPs for Magnetic Hyperthermia
3.3. Design of IO@MS for Photothermal Therapy
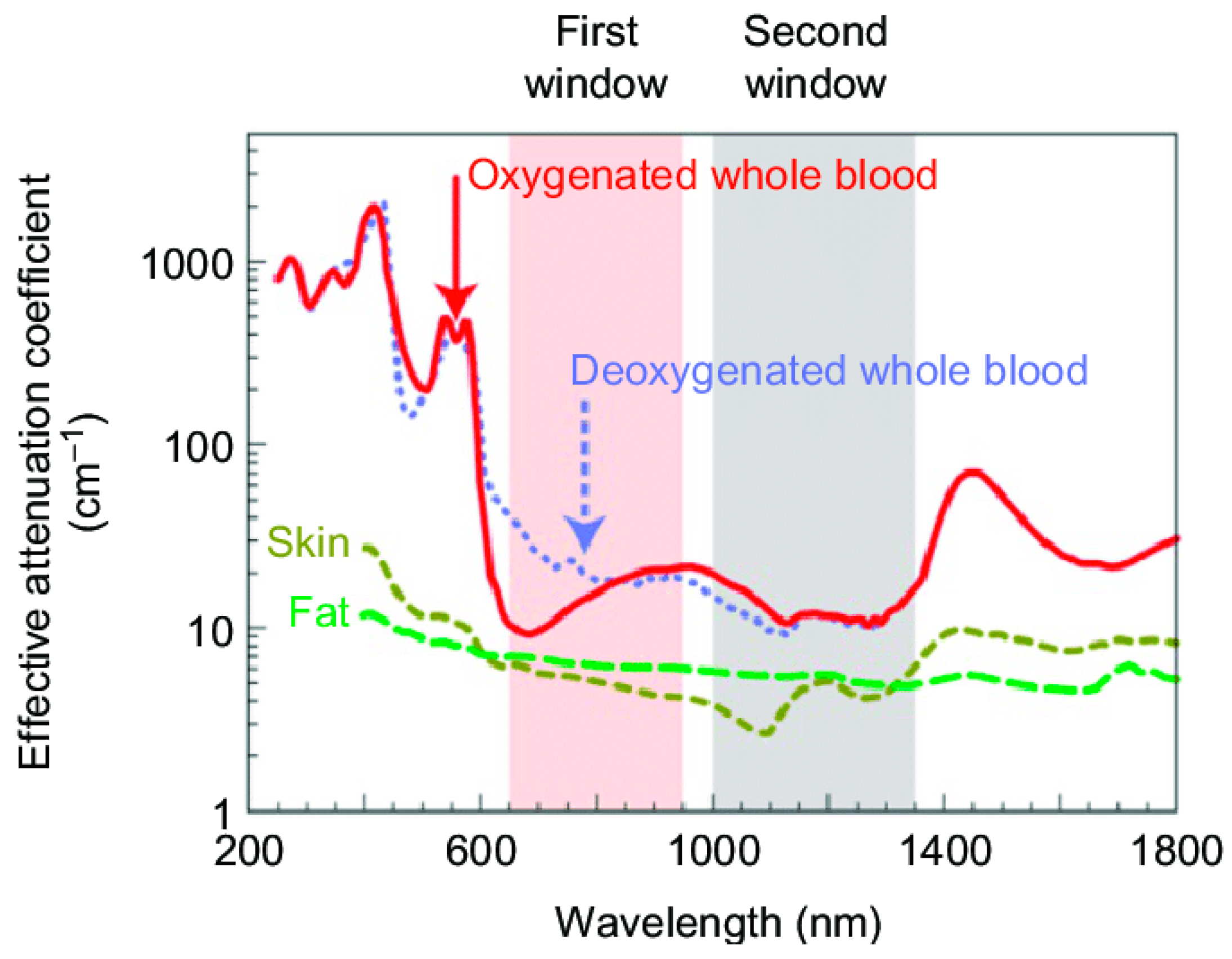
3.3.1. Nanomaterials for Photothermal Therapy
3.3.2. Parameters Influencing Photothermal Effect
3.3.3. Photothermal Therapy with IO@MS NPs
3.4. Design of IO@MS as a Carrier for Drug Delivery
3.5. Nanothermometry
4. Biological Applications of IO@MS Core-Shell NPs
4.1. IO@MS NPs—In Vitro/In Vivo Cancer Therapy Applications
4.1.1. Interactions of NPs with Living Systems
4.1.2. Various Applications of IO@MS Core-Shell NPs for Cancer Therapy
| Application | Nanocomposite | Functionalization | Active Molecule | Reference |
|---|---|---|---|---|
| Dual drug delivery | Fe3O4@MS | Polyethylenimine + 2-methacryloyloxyethyl phosphorylcholine | siRNA and daunorubicin | [250] |
| Drug delivery combined with MHT | Fe3O4@MS | Chitosan-g-N-isopropylacrylamide | DOX | [251] |
| Drug delivery, dual MRI +cell targeting | Fe3O4@SiO2@mSiO2 | Gd-DTPA | peptide RGERPPR and DOX | [137] |
| MHT + radiosensitizer +cell targeting | multicore Fe3O4@SiO2 | / | L-selenocystine + Folic acid | [252] |
| MHT + antibody- targeting | multicore Fe3O4@SiO2 | glutaraldehyde | Anti-αvβ6 mouse monoclonal antibody | [253] |
| Targeted drug delivery | Fe3O4@MS | polyethyleneimine | Folic acid and erlotinib | [255] |
| BBB crossing + drug delivery | Fe3O4@MS | APTES + Pluronic F-127 | DOX and transferrin | [257] |
| Gene therapy under AMF | Fe3O4 nanoclusters@large pore MS | APTES + Tannic acid | siRNA | [258] |
| Immunotherapy | Fe3O4@MS | APTES+PEG | CpG ODN | [259] |
4.2. Smart Scaffolds Using AMF and/or NIR Light as Trigger
5. Conclusions
- (1)
- The first topic that should be explored in the future with such core-shell is to investigate their biodegradability in different biological mimicking fluids, cells, and their biological fate in vivo. Iron oxides are reported to be rapidly degradable once internalized by cells, but the degradation fate of the silica shell requires specific investigations according to its intrinsic features: thickness, morphology/size of the pore, Si-O-Si crosslinking, aggregation state, and surface functionalization but also other extrinsic parameters, such as temperature, local pH, flow dynamics, and NP concentration in the buffer or the tissue.
- (2)
- Another topic of interest is the control of the photothermal or the magnetothermal dose delivered by the IO@MS core-shell NPs as a function of the core material and of the silica shell features. Indeed, the silica shell may have a critical role either as a thermally insulating or conductive layer to adjust the effects of treatments and to avoid thermal denaturation of potentially fragile loaded therapeutics, such as siRNA or therapeutic proteins. Designing engineered silica shells with various shell features for local thermal dose control and understanding the influence of this silica shell on the thermal transfer through physical modeling studies are important investigations to conduct. This would allow us to improve multimodal treatments that may be achieved by these nanoplatforms.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic Nanoparticles in Clinical Trials and Translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Elmi, G.R.; Saleem, K.; Baig, M.M.F.A.; Aamir, M.N.; Wang, M.; Gao, X.; Abbas, M.; Rehman, M.U. Recent Advances of Magnetic Gold Hybrids and Nanocomposites, and Their Potential Biological Applications. Magnetochemistry 2022, 8, 38. [Google Scholar] [CrossRef]
- Długosz, O.; Matyjasik, W.; Hodacka, G.; Szostak, K.; Matysik, J.; Krawczyk, P.; Piasek, A.; Pulit-Prociak, J.; Banach, M. Inorganic Nanomaterials Used in Anti-Cancer Therapies:Further Developments. Nanomaterials 2023, 13, 1130. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Shivanna, A.T.; Dash, B.S.; Chen, J.-P. Functionalized Magnetic Nanoparticles for Alternating Magnetic Field- or Near Infrared Light-Induced Cancer Therapies. Micromachines 2022, 13, 1279. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Arcentales-Vera, B.; Chichande-Proaño, E.; Bucio, E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers 2022, 14, 752. [Google Scholar] [CrossRef]
- Xue, W.; Liu, Y.; Zhang, N.; Yao, Y.; Ma, P.; Wen, H.; Huang, S.; Luo, Y.; Fan, H. Effects of Core Size and PEG Coating Layer of Iron Oxide Nanoparticles on the Distribution and Metabolism in Mice. Int. J. Nanomed. 2018, 13, 5719–5731. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Mignani, S.; Shen, M.; Shi, X. Dendrimer-Based Magnetic Iron Oxide Nanoparticles: Their Synthesis and Biomedical Applications. Drug Discov. Today 2016, 21, 1873–1885. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Tosi, D.; Balmassov, D.; Schena, E.; Saccomandi, P.; Inglezakis, V. Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials 2019, 9, 1195. [Google Scholar] [CrossRef] [Green Version]
- Jordan, A.; Maier-Hauff, K. Magnetic Nanoparticles for Intracranial Thermotherapy. J. Nanosci. Nanotechnol. 2007, 7, 4604–4606. [Google Scholar] [CrossRef]
- Van der Zee, J. Heating the Patient: A Promising Approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
- Overgaard, J. Effect of Hyperthermia on Malignant Cells in Vivo: A Review and a Hypothesis. Cancer 1977, 39, 2637–2646. [Google Scholar] [CrossRef]
- Vergnaud, F.; Kesse, X.; Jacobs, A.; Perton, F.; Begin-Colin, S.; Mertz, D.; Descamps, S.; Vichery, C.; Nedelec, J.-M. Magnetic Bioactive Glass Nano-Heterostructures: A Deeper Insight into Magnetic Hyperthermia Properties in the Scope of Bone Cancer Treatment. Biomater. Sci. 2022, 10, 3993–4007. [Google Scholar] [CrossRef]
- Vichery, C.; Nedelec, J.-M. Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 Core/Shell Nanoparticles: The Silica Coating Regulations with a Single Core for Different Core Sizes and Shell Thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Ménard, M.; Meyer, F.; Affolter-Zbaraszczuk, C.; Rabineau, M.; Adam, A.; Ramirez, P.D.; Bégin-Colin, S.; Mertz, D. Design of Hybrid Protein-Coated Magnetic Core-Mesoporous Silica Shell Nanocomposites for MRI and Drug Release Assessed in a 3D Tumor Cell Model. Nanotechnology 2019, 30, 174001. [Google Scholar] [CrossRef]
- Adam, A.; Parkhomenko, K.; Duenas-Ramirez, P.; Nadal, C.; Cotin, G.; Zorn, P.-E.; Choquet, P.; Bégin-Colin, S.; Mertz, D. Orienting the Pore Morphology of Core-Shell Magnetic Mesoporous Silica with the Sol-Gel Temperature. Influence on MRI and Magnetic Hyperthermia Properties. Molecules 2021, 26, 971. [Google Scholar] [CrossRef]
- Yang, J.; Shen, D.; Wei, Y.; Li, W.; Zhang, F.; Kong, B.; Zhang, S.; Teng, W.; Fan, J.; Zhang, W.; et al. Monodisperse Core-Shell Structured Magnetic Mesoporous Aluminosilicate Nanospheres with Large Dendritic Mesochannels. Nano Res. 2015, 8, 2503–2514. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 978-3-527-30274-1. [Google Scholar]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent Progress on Magnetic Iron Oxide Nanoparticles: Synthesis, Surface Functional Strategies and Biomedical Applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Lekha, G.M.; George, S. Colloidal Magnetic Metal Oxide Nanocrystals and Their Applications. In Colloidal Metal Oxide Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 289–335. ISBN 978-0-12-813357-6. [Google Scholar]
- Nigam, A.; Pawar, S.J. Structural, Magnetic, and Antimicrobial Properties of Zinc Doped Magnesium Ferrite for Drug Delivery Applications. Ceram. Int. 2020, 46, 4058–4064. [Google Scholar] [CrossRef]
- Abdellatif, M.H.; El-Komy, G.M.; Azab, A.A. Magnetic Characterization of Rare Earth Doped Spinel Ferrite. J. Magn. Magn. Mater. 2017, 442, 445–452. [Google Scholar] [CrossRef]
- Jaison, D.; Gangwar, A.; Kishore, P.N.; Chandrasekaran, G.; Mothilal, M. Effect of Gd3+ Substitution on Proton Relaxation and Magnetic Hyperthermia Efficiency of Cobalt Ferrite Nanoparticles. Mater. Res. Express 2020, 7, 064009. [Google Scholar] [CrossRef]
- Somvanshi, S.B.; Jadhav, S.A.; Khedkar, M.V.; Kharat, P.B.; More, S.D.; Jadhav, K.M. Structural, Thermal, Spectral, Optical and Surface Analysis of Rare Earth Metal Ion (Gd3+) Doped Mixed Zn–Mg Nano-Spinel Ferrites. Ceram. Int. 2020, 46, 13170–13179. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Hu, P.; Sun, S.; Shi, L.; Sun, L. Facile Synthesis of Er3+/Tm3+ Co-Doped Magnetic/Luminescent Nanosystems for Possible Bioimaging and Therapy Applications. J. Rare Earths 2022, 40, 11–19. [Google Scholar] [CrossRef]
- Debnath, S.; Das, R. Study of the Optical Properties of Zn Doped Mn Spinel Ferrite Nanocrystals Shows Multiple Emission Peaks in the Visible Range –a Promising Soft Ferrite Nanomaterial for Deep Blue LED. J. Mol. Struct. 2020, 1199, 127044. [Google Scholar] [CrossRef]
- Chakraborty, I.; Majumder, D.; Rakshit, R.; Alam, M.; Mukherjee, S.; Gorai, A.; Mandal, K. Magnetic Field-Dependent Photoluminescence of Tartrate-Functionalized Gadolinium-Doped Manganese Ferrite Nanoparticles: A Potential Therapeutic Agent for Hyperbilirubinemia Treatment. ACS Appl. Nano Mater. 2021, 4, 4379–4387. [Google Scholar] [CrossRef]
- Javanbakht, T.; Laurent, S.; Stanicki, D.; Raphael, W.; Tavares, J.R. Charge Effect of Superparamagnetic Iron Oxide Nanoparticles on Their Surface Functionalization by Photo-Initiated Chemical Vapour Deposition. J. Nanoparticle Res. 2015, 17, 462. [Google Scholar] [CrossRef] [Green Version]
- Abdulwahid, F.S.; Haider, A.J.; Al-Musawi, S. Folate Decorated Dextran-Coated Magnetic Nanoparticles for Targeted Delivery of Ellipticine in Cervical Cancer Cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2023, 14, 015001. [Google Scholar] [CrossRef]
- Duque, J.S.; Madrigal, B.M.; Riascos, H.; Avila, Y.P. Colloidal Metal Oxide Nanoparticles Prepared by Laser Ablation Technique and Their Antibacterial Test. Colloids Interfaces 2019, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Ismail, R.A.; Sulaiman, G.M.; Abdulrahman, S.A.; Marzoog, T.R. Antibacterial Activity of Magnetic Iron Oxide Nanoparticles Synthesized by Laser Ablation in Liquid. Mater. Sci. Eng. C 2015, 53, 286–297. [Google Scholar] [CrossRef]
- Fazio, E.; Santoro, M.; Lentini, G.; Franco, D.; Guglielmino, S.P.P.; Neri, F. Iron Oxide Nanoparticles Prepared by Laser Ablation: Synthesis, Structural Properties and Antimicrobial Activity. Colloids Surf. Physicochem. Eng. Asp. 2016, 490, 98–103. [Google Scholar] [CrossRef]
- Lungu, I.I.; Nistorescu, S.; Badea, M.A.; Petre, A.-M.; Udrea, A.-M.; Banici, A.-M.; Fleacă, C.; Andronescu, E.; Dinischiotu, A.; Dumitrache, F.; et al. Doxorubicin-Conjugated Iron Oxide Nanoparticles Synthesized by Laser Pyrolysis: In Vitro Study on Human Breast Cancer Cells. Polymers 2020, 12, 2799. [Google Scholar] [CrossRef]
- Carmen Bautista, M.; Bomati-Miguel, O.; del Puerto Morales, M.; Serna, C.J.; Veintemillas-Verdaguer, S. Surface Characterisation of Dextran-Coated Iron Oxide Nanoparticles Prepared by Laser Pyrolysis and Coprecipitation. J. Magn. Magn. Mater. 2005, 293, 20–27. [Google Scholar] [CrossRef]
- Calderón Bedoya, P.A.; Botta, P.M.; Bercoff, P.G.; Fanovich, M.A. Influence of the Milling Materials on the Mechanochemical Synthesis of Magnetic Iron Oxide Nanoparticles. J. Alloys Compd. 2023, 939, 168720. [Google Scholar] [CrossRef]
- Seyedi, M.; Haratian, S.; Khaki, J.V. Mechanochemical Synthesis of Fe2O3 Nanoparticles. Procedia Mater. Sci. 2015, 11, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Hajiali, S.; Daneshjou, S.; Daneshjoo, S. Biomimetic Synthesis of Iron Oxide Nanoparticles from Bacillus Megaterium to Be Used in Hyperthermia Therapy. AMB Express 2022, 12, 145. [Google Scholar] [CrossRef]
- Alphandéry, E. Bio-Synthesized Iron Oxide Nanoparticles for Cancer Treatment. Int. J. Pharm. 2020, 586, 119472. [Google Scholar] [CrossRef]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Kim, D.K.; Mikhaylova, M.; Zhang, Y.; Muhammed, M. Protective Coating of Superparamagnetic Iron Oxide Nanoparticles. Chem. Mater. 2003, 15, 1617–1627. [Google Scholar] [CrossRef]
- Aziz, T.; Masum, S.M.; Qadir, M.R.; Gafur, A.; Huq, D. Physicochemical Characterization of Iron Oxide Nanoparticle Coated with Chitosan for Biomedical Application. Int. Res. J. Pure Appl. Chem. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Hasany, S.F.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic Review of the Preparation Techniques of Iron Oxide Magnetic Nanoparticles. Nanosci. Nanotechnol. 2012, 2, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Fazel-Rezai, R. Biomedical Engineering: Frontiers and Challenges; BoD—Books on Demand: Norderstedt, Germany, 2011; ISBN 978-953-307-309-5. [Google Scholar]
- Jolivet, J.-P.; Henry, M.; Livage, J. Metal Oxide Chemistry and Synthesis: From Solution to Solid State; John Wiley: Chichester, UK; New York, NY, USA, 2000; ISBN 978-0-471-97056-9. [Google Scholar]
- Karaagac, O.; Kockar, H.; Beyaz, S.; Tanrisever, T. A Simple Way to Synthesize Superparamagnetic Iron Oxide Nanoparticles in Air Atmosphere: Iron Ion Concentration Effect. IEEE Trans. Magn. 2010, 46, 3978–3983. [Google Scholar] [CrossRef]
- Karaagac, O.; Kockar, H. Effect of Synthesis Parameters on the Properties of Superparamagnetic Iron Oxide Nanoparticles. J. Supercond. Nov. Magn. 2012, 25, 2777–2781. [Google Scholar] [CrossRef]
- Jolivet, J.-P.; Froidefond, C.; Pottier, A.; Chanéac, C.; Cassaignon, S.; Tronc, E.; Euzen, P. Size Tailoring of Oxide Nanoparticles by Precipitation in Aqueous Medium. A Semi-Quantitative Modelling. J. Mater. Chem. 2004, 14, 3281–3288. [Google Scholar] [CrossRef]
- Jolivet, J.-P.; Chanéac, C.; Tronc, E. Iron Oxide Chemistry. From Molecular Clusters to Extended Solid Networks. Chem. Commun. 2004, 481–483. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.P.; Blin, B.; Beaudoin, B.; Figlarz, M. Homogeneous and Heterogeneous Nucleations in the Polyol Process for the Preparation of Micron and Submicron Size Metal Particles. Solid State Ion. 1989, 32–33, 198–205. [Google Scholar] [CrossRef]
- Joseyphus, R.J.; Shinoda, K.; Kodama, D.; Jeyadevan, B. Size Controlled Fe Nanoparticles through Polyol Process and Their Magnetic Properties. Mater. Chem. Phys. 2010, 123, 487–493. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Joy, P.A.; Khollam, Y.B.; Potdar, H.S.; Deshpande, S.B. Synthesis of Nanosized MgFe2O4 Powders by Microwave Hydrothermal Method. Mater. Lett. 2004, 58, 1092–1095. [Google Scholar] [CrossRef]
- Khollam, Y.B.; Dhage, S.R.; Potdar, H.S.; Deshpande, S.B.; Bakare, P.P.; Kulkarni, S.D.; Date, S.K. Microwave Hydrothermal Preparation of Submicron-Sized Spherical Magnetite (Fe3O4) Powders. Mater. Lett. 2002, 56, 571–577. [Google Scholar] [CrossRef]
- Hao, Y.; Teja, A.S. Continuous Hydrothermal Crystallization of α–Fe2O3 and Co3O4 Nanoparticles. J. Mater. Res. 2003, 18, 415–422. [Google Scholar] [CrossRef]
- Gul, S.; Khan, S.B.; Rehman, I.U.; Khan, M.A.; Khan, M.I. A Comprehensive Review of Magnetic Nanomaterials Modern Day Theranostics. Front. Mater. 2019, 6, 179. [Google Scholar] [CrossRef] [Green Version]
- Patsula, V.; Kosinová, L.; Lovrić, M.; Ferhatovic Hamzić, L.; Rabyk, M.; Konefal, R.; Paruzel, A.; Šlouf, M.; Herynek, V.; Gajović, S.; et al. Superparamagnetic Fe3O4 Nanoparticles: Synthesis by Thermal Decomposition of Iron(III) Glucuronate and Application in Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2016, 8, 7238–7247. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Shipway, A.N.; Katz, E.; Willner, I. Nanoparticle Arrays on Surfaces for Electronic, Optical, and Sensor Applications. ChemPhysChem 2000, 1, 18–52. [Google Scholar] [CrossRef]
- Herman, D.A.J.; Cheong-Tilley, S.; McGrath, A.J.; McVey, B.F.P.; Lein, M.; Tilley, R.D. How to Choose a Precursor for Decomposition Solution-Phase Synthesis: The Case of Iron Nanoparticles. Nanoscale 2015, 7, 5951–5954. [Google Scholar] [CrossRef]
- Cotin, G.; Perton, F.; Petit, C.; Sall, S.; Kiefer, C.; Begin, V.; Pichon, B.; Lefevre, C.; Mertz, D.; Greneche, J.-M.; et al. Harnessing Composition of Iron Oxide Nanoparticle: Impact of Solvent-Mediated Ligand–Ligand Interaction and Competition between Oxidation and Growth Kinetics. Chem. Mater. 2020, 32, 9245–9259. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of Iron Oxide-Based Nanoparticles for MRI and Magnetic Hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Inagaki, S.; Fukushima, Y.; Kuroda, K. Synthesis of Highly Ordered Mesoporous Materials from a Layered Polysilicate. J. Chem. Soc. Chem. Commun. 1993, 680–682. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The Preparation of Alkyltrimethylammonium–Kanemite Complexes and Their Conversion to Microporous Materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef] [Green Version]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Kresge, C.T.; Roth, W.J. The Discovery of Mesoporous Molecular Sieves from the Twenty Year Perspective. Chem. Soc. Rev. 2013, 42, 3663–3670. [Google Scholar] [CrossRef]
- Firouzi, A.; Kumar, D.; Bull, L.M.; Besier, T.; Sieger, P.; Huo, Q.; Walker, S.A.; Zasadzinski, J.A.; Glinka, C.; Nicol, J.; et al. Cooperative Organization of Inorganic-Surfactant and Biomimetic Assemblies. Science 1995, 267, 1138–1143. [Google Scholar] [CrossRef] [Green Version]
- Fowler, C.E.; Khushalani, D.; Lebeau, B.; Mann, S. Nanoscale Materials with Mesostructured Interiors. Adv. Mater. 2001, 13, 649–652. [Google Scholar] [CrossRef]
- Nooney, R.I.; Thirunavukkarasu, D.; Chen, Y.; Josephs, R.; Ostafin, A.E. Synthesis of Nanoscale Mesoporous Silica Spheres with Controlled Particle Size. Chem. Mater. 2002, 14, 4721–4728. [Google Scholar] [CrossRef]
- Cai, Q.; Luo, Z.-S.; Pang, W.-Q.; Fan, Y.-W.; Chen, X.-H.; Cui, F.-Z. Dilute Solution Routes to Various Controllable Morphologies of MCM-41 Silica with a Basic Medium. Chem. Mater. 2001, 13, 258–263. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Durand, J.-O. Large Pore Mesoporous Silica Nanomaterials for Application in Delivery of Biomolecules. Nanoscale 2015, 7, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; He, J. Fine-Tuning of Silica Nanosphere Structure by Simple Regulation of the Volume Ratio of Cosolvents. Langmuir 2010, 26, 10057–10062. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-G.; Wu, C.-W.; Chen, K.; Lin, V.S.-Y. Fine-Tuning Mesochannel Orientation of Organically Functionalized Mesoporous Silica Nanoparticles. Chem.—Asian J. 2009, 4, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shi, R.; Ivanisevic, A.; Borgens, R.B. A Mesoporous Silica Nanosphere-Based Drug Delivery System Using an Electrically Conducting Polymer. Nanotechnology 2009, 20, 275102. [Google Scholar] [CrossRef]
- Wu, L.; Jiao, Z.; Wu, M.; Song, T.; Zhang, H. Formation of Mesoporous Silica Nanoparticles with Tunable Pore Structure as Promising Nanoreactor and Drug Delivery Vehicle. RSC Adv. 2016, 6, 13303–13311. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, L.-L.; Jiang, J.-G.; Calin, N.; Lam, K.-F.; Zhang, S.-J.; Wu, H.-H.; Wu, G.-D.; Albela, B.; Bonneviot, L.; et al. Facile Large-Scale Synthesis of Monodisperse Mesoporous Silica Nanospheres with Tunable Pore Structure. J. Am. Chem. Soc. 2013, 135, 2427–2430. [Google Scholar] [CrossRef]
- Ménard, M.; Meyer, F.; Parkhomenko, K.; Leuvrey, C.; Francius, G.; Bégin-Colin, S.; Mertz, D. Mesoporous Silica Templated-Albumin Nanoparticles with High Doxorubicin Payload for Drug Delivery Assessed with a 3-D Tumor Cell Model. Biochim. Biophys. Acta BBA—Gen. Subj. 2019, 1863, 332–341. [Google Scholar] [CrossRef]
- Perton, F.; Harlepp, S.; Follain, G.; Parkhomenko, K.; Goetz, J.G.; Bégin-Colin, S.; Mertz, D. Wrapped Stellate Silica Nanocomposites as Biocompatible Luminescent Nanoplatforms Assessed in Vivo. J. Colloid Interface Sci. 2019, 542, 469–482. [Google Scholar] [CrossRef]
- Duenas-Ramirez, P.; Bertagnolli, C.; Müller, R.; Sartori, K.; Boos, A.; Elhabiri, M.; Bégin-Colin, S.; Mertz, D. Highly Chelating Stellate Mesoporous Silica Nanoparticles for Specific Iron Removal from Biological Media. J. Colloid Interface Sci. 2020, 579, 140–151. [Google Scholar] [CrossRef]
- Mertz, D.; Affolter-Zbaraszczuk, C.; Barthès, J.; Cui, J.; Caruso, F.; Baumert, T.F.; Voegel, J.-C.; Ogier, J.; Meyer, F. Templated Assembly of Albumin-Based Nanoparticles for Simultaneous Gene Silencing and Magnetic Resonance Imaging. Nanoscale 2014, 6, 11676–11680. [Google Scholar] [CrossRef]
- Wang, J.-G.; Zhou, H.-J.; Sun, P.-C.; Ding, D.-T.; Chen, T.-H. Hollow Carved Single-Crystal Mesoporous Silica Templated by Mesomorphous Polyelectrolyte−Surfactant Complexes. Chem. Mater. 2010, 22, 3829–3831. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Krämer, E.; Förster, S.; Göltner, C.; Antonietti, M. Synthesis of Nanoporous Silica with New Pore Morphologies by Templating the Assemblies of Ionic Block Copolymers. Langmuir 1998, 14, 2027–2031. [Google Scholar] [CrossRef]
- Göltner, C.G.; Berton, B.; Krämer, E.; Antonietti, M. Nanoporous Silica from Amphiphilic Block Copolymer (ABC) Aggregates: Control over Correlation and Architecture of Cylindrical Pores. Chem. Commun. 1998, 2287–2288. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, S.; Gu, W.; Russell, T.P. Highly Ordered Nanoporous Template from Triblock Copolymer. ACS Nano 2011, 5, 1207–1214. [Google Scholar] [CrossRef]
- Bloch, E.; Phan, T.; Bertin, D.; Llewellyn, P.; Hornebecq, V. Direct Synthesis of Mesoporous Silica Presenting Large and Tunable Pores Using BAB Triblock Copolymers: Influence of Each Copolymer Block on the Porous Structure. Microporous Mesoporous Mater. 2008, 112, 612–620. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and Surface Functionalization of Silica Nanoparticles for Nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Panwar, N.; Tng, D.J.H.; Tjin, S.C.; Wang, K.; Yong, K.-T. The Application of Mesoporous Silica Nanoparticle Family in Cancer Theranostics. Coord. Chem. Rev. 2016, 319, 86–109. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Zhong, Q.; Wang, Y.; Hu, P.; Zhong, W.; Huang, C.-B.; Yu, Z.-Q.; Ding, C.-D.; Liu, H.; Fu, J. Chemically Engineered Mesoporous Silica Nanoparticles-Based Intelligent Delivery Systems for Theranostic Applications in Multiple Cancerous/Non-Cancerous Diseases. Coord. Chem. Rev. 2022, 452, 214309. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Walsh, L.J.; Xu, C. Biomedical Application of Mesoporous Silica Nanoparticles as Delivery Systems: A Biological Safety Perspective. J. Mater. Chem. B 2020, 8, 9863–9876. [Google Scholar] [CrossRef] [PubMed]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic Nanoparticles for Theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal Clearance of Quantum Dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.-Y. A Mesoporous Silica Nanosphere-Based Carrier System with Chemically Removable CdS Nanoparticle Caps for Stimuli-Responsive Controlled Release of Neurotransmitters and Drug Molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef]
- Schlossbauer, A.; Kecht, J.; Bein, T. Biotin–Avidin as a Protease-Responsive Cap System for Controlled Guest Release from Colloidal Mesoporous Silica. Angew. Chem. Int. Ed. 2009, 48, 3092–3095. [Google Scholar] [CrossRef]
- Mertz, D.; Sandre, O.; Bégin-Colin, S. Drug Releasing Nanoplatforms Activated by Alternating Magnetic Fields. Biochim. Biophys. Acta BBA—Gen. Subj. 2017, 1861, 1617–1641. [Google Scholar] [CrossRef]
- Hernandez, R.; Tseng, H.-R.; Wong, J.W.; Stoddart, J.F.; Zink, J.I. An Operational Supramolecular Nanovalve. J. Am. Chem. Soc. 2004, 126, 3370–3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canton, I.; Battaglia, G. Endocytosis at the Nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; Rotin, D. Acid PH in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar] [PubMed]
- Barnakov, Y.A.; Yu, M.H.; Rosenzweig, Z. Manipulation of the Magnetic Properties of Magnetite−Silica Nanocomposite Materials by Controlled Stober Synthesis. Langmuir 2005, 21, 7524–7527. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Andrade, A.L.; Souza, D.M.; Pereira, M.C.; Fabris, J.D.; Domingues, R.Z. Synthesis and Characterization of Magnetic Nanoparticles Coated with Silica through a Sol-Gel Approach. Cerâmica 2009, 55, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Im, S.H.; Herricks, T.; Lee, Y.T.; Xia, Y. Synthesis and Characterization of Monodisperse Silica Colloids Loaded with Superparamagnetic Iron Oxide Nanoparticles. Chem. Phys. Lett. 2005, 401, 19–23. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Z.; Teng, Z.; Liang, J.; Guo, Z.; Wang, D.; Han, M.-Y.; Yang, W. Unraveling the Growth Mechanism of Silica Particles in the Stöber Method: In Situ Seeded Growth Model. Langmuir 2017, 33, 5879–5890. [Google Scholar] [CrossRef]
- Deng, Y.-H.; Wang, C.-C.; Hu, J.-H.; Yang, W.-L.; Fu, S.-K. Investigation of Formation of Silica-Coated Magnetite Nanoparticles via Sol–Gel Approach. Colloids Surf. Physicochem. Eng. Asp. 2005, 262, 87–93. [Google Scholar] [CrossRef]
- Vogt, C.; Toprak, M.S.; Muhammed, M.; Laurent, S.; Bridot, J.-L.; Müller, R.N. High Quality and Tuneable Silica Shell–Magnetic Core Nanoparticles. J. Nanoparticle Res. 2010, 12, 1137–1147. [Google Scholar] [CrossRef]
- Liu, J.; Detrembleur, C.; Pauw-Gillet, M.-C.D.; Mornet, S.; Vander Elst, L.; Laurent, S.; Jérôme, C.; Duguet, E. Heat-Triggered Drug Release Systems Based on Mesoporous Silica Nanoparticles Filled with a Maghemite Core and Phase-Change Molecules as Gatekeepers. J. Mater. Chem. B 2014, 2, 59–70. [Google Scholar] [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic High-Magnetization Microspheres with an Fe3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Laurent, S.; Fornara, A.; Astolfi, L.; Qin, J.; Roch, A.; Martini, A.; Toprak, M.S.; Muller, R.N.; Muhammed, M. Uniform Mesoporous Silica Coated Iron Oxide Nanoparticles as a Highly Efficient, Nontoxic MRI T2 Contrast Agent with Tunable Proton Relaxivities. Contrast Media Mol. Imaging 2012, 7, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.E.; Lee, J.; Yu, J.H.; Kim, B.C.; An, K.; Hwang, Y.; Shin, C.-H.; Park, J.-G.; Kim, J.; et al. Magnetic Fluorescent Delivery Vehicle Using Uniform Mesoporous Silica Spheres Embedded with Monodisperse Magnetic and Semiconductor Nanocrystals. J. Am. Chem. Soc. 2006, 128, 688–689. [Google Scholar] [CrossRef]
- Nyalosaso, J.L.; Rascol, E.; Pisani, C.; Dorandeu, C.; Dumail, X.; Maynadier, M.; Gary-Bobo, M.; Him, J.L.K.; Bron, P.; Garcia, M.; et al. Synthesis, Decoration, and Cellular Effects of Magnetic Mesoporous Silica Nanoparticles. RSC Adv. 2016, 6, 57275–57283. [Google Scholar] [CrossRef]
- Rho, W.-Y.; Kim, H.-M.; Kyeong, S.; Kang, Y.-L.; Kim, D.-H.; Kang, H.; Jeong, C.; Kim, D.-E.; Lee, Y.-S.; Jun, B.-H. Facile Synthesis of Monodispersed Silica-Coated Magnetic Nanoparticles. J. Ind. Eng. Chem. 2014, 20, 2646–2649. [Google Scholar] [CrossRef]
- Fan, H.; Leve, E.; Gabaldon, J.; Wright, A.; Haddad, R.E.; Brinker, C.J. Ordered Two- and Three-Dimensional Arrays Self-Assembled from Water-Soluble Nanocrystal-Micelles. Adv. Mater. 2005, 17, 2587–2590. [Google Scholar] [CrossRef]
- Croissant, J.; Maynadier, M.; Mongin, O.; Hugues, V.; Blanchard-Desce, M.; Chaix, A.; Cattoën, X.; Wong Chi Man, M.; Gallud, A.; Gary-Bobo, M.; et al. Enhanced Two-Photon Fluorescence Imaging and Therapy of Cancer Cells via Gold@Bridged Silsesquioxane Nanoparticles. Small 2015, 11, 295–299. [Google Scholar] [CrossRef]
- Croissant, J.; Salles, D.; Maynadier, M.; Mongin, O.; Hugues, V.; Blanchard-Desce, M.; Cattoën, X.; Wong Chi Man, M.; Gallud, A.; Garcia, M.; et al. Mixed Periodic Mesoporous Organosilica Nanoparticles and Core–Shell Systems, Application to in Vitro Two-Photon Imaging, Therapy, and Drug Delivery. Chem. Mater. 2014, 26, 7214–7220. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoën, X.; Wong Chi Man, M.; Durand, J.-O.; Khashab, N.M. Syntheses and Applications of Periodic Mesoporous Organosilica Nanoparticles. Nanoscale 2015, 7, 20318–20334. [Google Scholar] [CrossRef]
- Lu, N.; Tian, Y.; Tian, W.; Huang, P.; Liu, Y.; Tang, Y.; Wang, C.; Wang, S.; Su, Y.; Zhang, Y.; et al. Smart Cancer Cell Targeting Imaging and Drug Delivery System by Systematically Engineering Periodic Mesoporous Organosilica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Kumar Kankala, R.; Busa, P.; Lee, C.-H. Hydrophobicity-Tuned Periodic Mesoporous Organo-Silica Nanoparticles for Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21, 2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggini, L.; Cabrera, I.; Ruiz-Carretero, A.; Prasetyanto, E.A.; Robinet, E.; De Cola, L. Breakable Mesoporous Silica Nanoparticles for Targeted Drug Delivery. Nanoscale 2016, 8, 7240–7247. [Google Scholar] [CrossRef] [PubMed]
- Prasetyanto, E.A.; Bertucci, A.; Septiadi, D.; Corradini, R.; Castro-Hartmann, P.; DeCola, L. Breakable Hybrid Organosilica Nanocapsules for Protein Delivery. Angew. Chem. 2016, 128, 3384–3388. [Google Scholar] [CrossRef]
- Croissant, J.; Cattoën, X.; Man, M.W.C.; Gallud, A.; Raehm, L.; Trens, P.; Maynadier, M.; Durand, J.-O. Biodegradable Ethylene-Bis(Propyl)Disulfide-Based Periodic Mesoporous Organosilica Nanorods and Nanospheres for Efficient In-Vitro Drug Delivery. Adv. Mater. 2014, 26, 6174–6180. [Google Scholar] [CrossRef]
- Van Geuns, R.-J.M.; Wielopolski, P.A.; de Bruin, H.G.; Rensing, B.J.; van Ooijen, P.M.A.; Hulshoff, M.; Oudkerk, M.; de Feyter, P.J. Basic Principles of Magnetic Resonance Imaging. Prog. Cardiovasc. Dis. 1999, 42, 149–156. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed Synthesis of Uniformly Sized Iron Oxide Nanoparticles for Efficient Magnetic Resonance Imaging Contrast Agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Ni, D.; Bu, W.; Ehlerding, E.B.; Cai, W.; Shi, J. Engineering of Inorganic Nanoparticles as Magnetic Resonance Imaging Contrast Agents. Chem. Soc. Rev. 2017, 46, 7438–7468. [Google Scholar] [CrossRef]
- Pinho, S.L.C.; Pereira, G.A.; Voisin, P.; Kassem, J.; Bouchaud, V.; Etienne, L.; Peters, J.A.; Carlos, L.; Mornet, S.; Geraldes, C.F.G.C.; et al. Fine Tuning of the Relaxometry of γ-Fe2O3@SiO2 Nanoparticles by Tweaking the Silica Coating Thickness. ACS Nano 2010, 4, 5339–5349. [Google Scholar] [CrossRef]
- Pinho, S.L.C.; Laurent, S.; Rocha, J.; Roch, A.; Delville, M.-H.; Mornet, S.; Carlos, L.D.; Vander Elst, L.; Muller, R.N.; Geraldes, C.F.G.C. Relaxometric Studies of γ-Fe2O3@SiO2 Core Shell Nanoparticles: When the Coating Matters. J. Phys. Chem. C 2012, 116, 2285–2291. [Google Scholar] [CrossRef]
- Zhang, C.; Wängler, B.; Morgenstern, B.; Zentgraf, H.; Eisenhut, M.; Untenecker, H.; Krüger, R.; Huss, R.; Seliger, C.; Semmler, W.; et al. Silica- and Alkoxysilane-Coated Ultrasmall Superparamagnetic Iron Oxide Particles: A Promising Tool To Label Cells for Magnetic Resonance Imaging. Langmuir 2007, 23, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Perton, F.; Tasso, M.; Muñoz Medina, G.A.; Ménard, M.; Blanco-Andujar, C.; Portiansky, E.; van Raap, M.B.F.; Bégin, D.; Meyer, F.; Begin-Colin, S.; et al. Fluorescent and Magnetic Stellate Mesoporous Silica for Bimodal Imaging and Magnetic Hyperthermia. Appl. Mater. Today 2019, 16, 301–314. [Google Scholar] [CrossRef]
- Gao, L.; Yu, J.; Liu, Y.; Zhou, J.; Sun, L.; Wang, J.; Zhu, J.; Peng, H.; Lu, W.; Yu, L.; et al. Tumor-Penetrating Peptide Conjugated and Doxorubicin Loaded T 1 -T 2 Dual Mode MRI Contrast Agents Nanoparticles for Tumor Theranostics. Theranostics 2018, 8, 92–108. [Google Scholar] [CrossRef]
- Cai, J.; Gu, B.; Cao, F.; Liu, S. A Transferrin-Target Magnetic/Fluorescent Dual-Mode Probe Significantly Enhances the Diagnosis of Non-Small Cell Lung Cancer. Oncotarget 2016, 7, 40047–40059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Han, L.; Wang, J.; Wan, J.; Song, G.; Rao, J. Engineering of Magnetic Nanoparticles as Magnetic Particle Imaging Tracers. Chem. Soc. Rev. 2021, 50, 8102–8146. [Google Scholar] [CrossRef]
- Kim Duong, H.T.; Abdibastami, A.; Gloag, L.; Barrera, L.; Justin Gooding, J.; Tilley, R.D. A Guide to the Design of Magnetic Particle Imaging Tracers for Biomedical Applications. Nanoscale 2022, 14, 13890–13914. [Google Scholar] [CrossRef]
- Moor, L.; Scheibler, S.; Gerken, L.; Scheffler, K.; Thieben, F.; Knopp, T.; Herrmann, I.K.; Starsich, F.H.L. Particle Interactions and Their Effect on Magnetic Particle Spectroscopy and Imaging. Nanoscale 2022, 14, 7163–7173. [Google Scholar] [CrossRef] [PubMed]
- Habash, R.W.Y.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal Therapy, Part 1: An Introduction to Thermal Therapy. Crit. Rev. Biomed. Eng. 2006, 34. [Google Scholar] [CrossRef] [Green Version]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic Fluid Hyperthermia: Focus on Superparamagnetic Iron Oxide Nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and Advances in Magnetic Hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ring, H.L.; Hurley, K.R.; Shao, Q.; Carlson, C.S.; Idiyatullin, D.; Manuchehrabadi, N.; Hoopes, P.J.; Haynes, C.L.; Bischof, J.C.; et al. Quantification and Biodistribution of Iron Oxide Nanoparticles in the Primary Clearance Organs of Mice Using T1 Contrast for Heating. Magn. Reson. Med. 2017, 78, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Glöckl, G.; Hergt, R.; Zeisberger, M.; Dutz, S.; Nagel, S.; Weitschies, W. The Effect of Field Parameters, Nanoparticle Properties and Immobilization on the Specific Heating Power in Magnetic Particle Hyperthermia. J. Phys. Condens. Matter 2006, 18, S2935–S2949. [Google Scholar] [CrossRef]
- Garaio, E.; Sandre, O.; Collantes, J.-M.; Garcia, J.A.; Mornet, S.; Plazaola, F. Specific Absorption Rate Dependence on Temperature in Magnetic Field Hyperthermia Measured by Dynamic Hysteresis Losses (Ac Magnetometry). Nanotechnology 2014, 26, 015704. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wang, S.-Y.; Gupta, A.; Borca-Tasciuc, D.-A.; Salon, S.J. On the Measurement Technique for Specific Absorption Rate of Nanoparticles in an Alternating Electromagnetic Field. Meas. Sci. Technol. 2012, 23, 035701. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic Particle Hyperthermia: Nanoparticle Magnetism and Materials Development for Cancer Therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Suriyanto; Ng, E.Y.K.; Kumar, S.D. Physical Mechanism and Modeling of Heat Generation and Transfer in Magnetic Fluid Hyperthermia through Néelian and Brownian Relaxation: A Review. Biomed. Eng. Online 2017, 16, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Presa, P.; Luengo, Y.; Multigner, M.; Costo, R.; Morales, M.P.; Rivero, G.; Hernando, A. Study of Heating Efficiency as a Function of Concentration, Size, and Applied Field in γ-Fe2O3 Nanoparticles. J. Phys. Chem. C 2012, 116, 25602–25610. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic Particle Hyperthermia—Biophysical Limitations of a Visionary Tumour Therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Hugounenq, P.; Levy, M.; Alloyeau, D.; Lartigue, L.; Dubois, E.; Cabuil, V.; Ricolleau, C.; Roux, S.; Wilhelm, C.; Gazeau, F.; et al. Iron Oxide Monocrystalline Nanoflowers for Highly Efficient Magnetic Hyperthermia. J. Phys. Chem. C 2012, 116, 15702–15712. [Google Scholar] [CrossRef]
- Atkinson, W.J.; Brezovich, I.A.; Chakraborty, D.P. Usable Frequencies in Hyperthermia with Thermal Seeds. IEEE Trans. Biomed. Eng. 1984, 31, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Rheinländer, T.; Waldöfner, N.; Scholz, R. Increase of the Specific Absorption Rate (SAR) by Magnetic Fractionation of Magnetic Fluids. J. Nanoparticle Res. 2003, 5, 597–600. [Google Scholar] [CrossRef]
- Basly, B.; Popa, G.; Fleutot, S.; Pichon, B.P.; Garofalo, A.; Ghobril, C.; Billotey, C.; Berniard, A.; Bonazza, P.; Martinez, H.; et al. Effect of the Nanoparticle Synthesis Method on Dendronized Iron Oxides as MRI Contrast Agents. Dalton Trans. 2013, 42, 2146–2157. [Google Scholar] [CrossRef]
- Kossatz, S.; Ludwig, R.; Dähring, H.; Ettelt, V.; Rimkus, G.; Marciello, M.; Salas, G.; Patel, V.; Teran, F.J.; Hilger, I. High Therapeutic Efficiency of Magnetic Hyperthermia in Xenograft Models Achieved with Moderate Temperature Dosages in the Tumor Area. Pharm. Res. 2014, 31, 3274–3288. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.; Seo, J.; Jang, J.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S.; et al. Artificially Engineered Magnetic Nanoparticles for Ultra-Sensitive Molecular Imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, M.A.; Torres, T.E.; Andrés-Vergés, M.; Costo, R.; de la Presa, P.; Serna, C.J.; Morales, M.P.; Marquina, C.; Ibarra, M.R.; Goya, G.F. Magnetic Nanoparticles for Power Absorption: Optimizing Size, Shape and Magnetic Properties. J. Solid State Chem. 2009, 182, 2779–2784. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Ansari, L.; Malaekeh-Nikouei, B. Magnetic Silica Nanocomposites for Magnetic Hyperthermia Applications. Int. J. Hyperthermia 2017, 33, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Tao, C.; Zhu, Y. Magnetic Mesoporous Silica Nanoparticles for Potential Delivery of Chemotherapeutic Drugs and Hyperthermia. Dalton Trans. 2014, 43, 15482–15490. [Google Scholar] [CrossRef] [PubMed]
- Larumbe, S.; Gómez-Polo, C.; Pérez-Landazábal, J.I.; Pastor, J.M. Effect of a SiO2 Coating on the Magnetic Properties of Fe3O4 Nanoparticles. J. Phys. Condens. Matter 2012, 24, 266007. [Google Scholar] [CrossRef]
- Arcos, D.; Fal-Miyar, V.; Ruiz-Hernández, E.; Garcia-Hernández, M.; Luisa Ruiz-González, M.; González-Calbet, J.; Vallet-Regí, M. Supramolecular Mechanisms in the Synthesis of Mesoporous Magnetic Nanospheres for Hyperthermia. J. Mater. Chem. 2012, 22, 64–72. [Google Scholar] [CrossRef]
- Hurley, K.R.; Ring, H.L.; Etheridge, M.; Zhang, J.; Gao, Z.; Shao, Q.; Klein, N.D.; Szlag, V.M.; Chung, C.; Reineke, T.M.; et al. Predictable Heating and Positive MRI Contrast from a Mesoporous Silica-Coated Iron Oxide Nanoparticle. Mol. Pharm. 2016, 13, 2172–2183. [Google Scholar] [CrossRef]
- Jiang, F.; Fu, Y.; Zhu, Y.; Tang, Z.; Sheng, P. Fabrication of Iron Oxide/Silica Core–Shell Nanoparticles and Their Magnetic Characteristics. J. Alloys Compd. 2012, 543, 43–48. [Google Scholar] [CrossRef]
- García-Acevedo, P.; González-Gómez, M.A.; Arnosa-Prieto, Á.; de Castro-Alves, L.; Piñeiro, Y.; Rivas, J. Role of Dipolar Interactions on the Determination of the Effective Magnetic Anisotropy in Iron Oxide Nanoparticles. Adv. Sci. 2023, 10, 2203397. [Google Scholar] [CrossRef]
- Serantes, D.; Baldomir, D.; Martinez-Boubeta, C.; Simeonidis, K.; Angelakeris, M.; Natividad, E.; Castro, M.; Mediano, A.; Chen, D.-X.; Sanchez, A.; et al. Influence of Dipolar Interactions on Hyperthermia Properties of Ferromagnetic Particles. J. Appl. Phys. 2010, 108, 073918. [Google Scholar] [CrossRef]
- Nitica, S.; Fizesan, I.; Dudric, R.; Barbu-Tudoran, L.; Pop, A.; Loghin, F.; Vedeanu, N.; Lucaciu, C.M.; Iacovita, C. A Fast, Reliable Oil-In-Water Microemulsion Procedure for Silica Coating of Ferromagnetic Zn Ferrite Nanoparticles Capable of Inducing Cancer Cell Death In Vitro. Biomedicines 2022, 10, 1647. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Li, K.; Mi, Y.; Li, Z.; Wu, D.; Sun, F.; He, S.; Zeng, H. Tuning Dipolar Effects on Magnetic Hyperthermia of Zn0.3Fe2.7O4/SiO2 Nanoparticles by Silica Shell. J. Magn. Magn. Mater. 2021, 521, 167483. [Google Scholar] [CrossRef]
- Iacoviță, C.; Fizeșan, I.; Nitica, S.; Florea, A.; Barbu-Tudoran, L.; Dudric, R.; Pop, A.; Vedeanu, N.; Crisan, O.; Tetean, R.; et al. Silica Coating of Ferromagnetic Iron Oxide Magnetic Nanoparticles Significantly Enhances Their Hyperthermia Performances for Efficiently Inducing Cancer Cells Death In Vitro. Pharmaceutics 2021, 13, 2026. [Google Scholar] [CrossRef]
- Andrade, A.L.; Fabris, J.D.; Pereira, M.C.; Domingues, R.Z.; Ardisson, J.D. Preparation of Composite with Silica-Coated Nanoparticles of Iron Oxide Spinels for Applications Based on Magnetically Induced Hyperthermia. Hyperfine Interact. 2013, 218, 71–82. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second Window for in Vivo Imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Y.X.; Hodgson, S.N.B.; Weglinski, B.; Gaworska, D. Investigations into Sol-Gel Silica and Silica Hybrid Coatings for Dielectromagnetic Soft Magnetic Composite Applications. J. Mater. Sci. 2006, 41, 5926–5936. [Google Scholar] [CrossRef]
- Henderson, T.A.; Morries, L.D. Near-Infrared Photonic Energy Penetration: Can Infrared Phototherapy Effectively Reach the Human Brain? Neuropsychiatr. Dis. Treat. 2015, 11, 2191–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional Mesoporous Silica-Coated Graphene Nanosheet Used for Chemo-Photothermal Synergistic Targeted Therapy of Glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef]
- Choi, W.I.; Sahu, A.; Kim, Y.H.; Tae, G. Photothermal Cancer Therapy and Imaging Based on Gold Nanorods. Ann. Biomed. Eng. 2012, 40, 534–546. [Google Scholar] [CrossRef]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold Nanorod Assisted Near-Infrared Plasmonic Photothermal Therapy (PPTT) of Squamous Cell Carcinoma in Mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Chitgupi, U.; Qin, Y.; Lovell, J.F. Targeted Nanomaterials for Phototherapy. Nanotheranostics 2017, 1, 38–58. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Chan, K.K.; Anderson, T.; Kong, K.V.; Ng, B.K.; Yong, K.-T. Advanced Near-Infrared Light-Responsive Nanomaterials as Therapeutic Platforms for Cancer Therapy. Adv. Ther. 2019, 2, 1800090. [Google Scholar] [CrossRef]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the Fundamental Mechanisms of Cell Death Triggered by Photothermal Therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Shrivastava, N.; Rossi, F.; Tung, L.D.; Thanh, N.T.K. Nanoparticles-Based Magnetic and Photo Induced Hyperthermia for Cancer Treatment. Nano Today 2019, 29, 100795. [Google Scholar] [CrossRef]
- Nanospectra | Leveraging Nanoshells in the First True Focal Therapy. Available online: https://nanospectra.com/ (accessed on 19 November 2021).
- Sadat, M.E.; Kaveh Baghbador, M.; Dunn, A.W.; Wagner, H.P.; Ewing, R.C.; Zhang, J.; Xu, H.; Pauletti, G.M.; Mast, D.B.; Shi, D. Photoluminescence and Photothermal Effect of Fe3O4 Nanoparticles for Medical Imaging and Therapy. Appl. Phys. Lett. 2014, 105, 091903. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic Nanoparticle Clusters for Photothermal Therapy with Near-Infrared Irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Sangnier, A.P.; Curcio, A.; Silva, A.K.A.; Corato, R.D.; Neveu, S.; Pellegrino, T.; Liz-Marzán, L.M.; et al. Magnetic (Hyper)Thermia or Photothermia? Progressive Comparison of Iron Oxide and Gold Nanoparticles Heating in Water, in Cells, and In Vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Chen, C. Near-Infrared Light-Mediated Nanoplatforms for Cancer Thermo-Chemotherapy and Optical Imaging. Adv. Mater. 2013, 25, 3869–3880. [Google Scholar] [CrossRef] [PubMed]
- Pązik, R.; Zachanowicz, E.; Pożniak, B.; Małecka, M.; Zięcina, A.; Marciniak, Ł. Non-Contact Mn1−xNixFe2O4 Ferrite Nano-Heaters for Biological Applications—Heat Energy Generated by NIR Irradiation. RSC Adv. 2017, 7, 18162–18171. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Liu, X.; Ji, J. More Efficient NIR Photothermal Therapeutic Effect from Intracellular Heating Modality than Extracellular Heating Modality: An in Vitro Study. J. Nanoparticle Res. 2012, 14, 1128. [Google Scholar] [CrossRef]
- Gormley, A.J.; Larson, N.; Banisadr, A.; Robinson, R.; Frazier, N.; Ray, A.; Ghandehari, H. Plasmonic Photothermal Therapy Increases the Tumor Mass Penetration of HPMA Copolymers. J. Control. Release 2013, 166, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Fay, B.L.; Melamed, J.R.; Day, E.S. Nanoshell-Mediated Photothermal Therapy Can Enhance Chemotherapy in Inflammatory Breast Cancer Cells. Int. J. Nanomed. 2015, 10, 6931–6941. [Google Scholar] [CrossRef] [Green Version]
- Adam, A.; Harlepp, S.; Ghilini, F.; Cotin, G.; Freis, B.; Goetz, J.; Bégin, S.; Tasso, M.; Mertz, D. Core-Shell Iron Oxide@stellate Mesoporous Silica for Combined near-Infrared Photothermia and Drug Delivery: Influence of PH and Surface Chemistry. Colloids Surf. Physicochem. Eng. Asp. 2022, 640, 128407. [Google Scholar] [CrossRef]
- Nemec, S.; Kralj, S.; Wilhelm, C.; Abou-Hassan, A.; Rols, M.-P.; Kolosnjaj-Tabi, J. Comparison of Iron Oxide Nanoparticles in Photothermia and Magnetic Hyperthermia: Effects of Clustering and Silica Encapsulation on Nanoparticles’ Heating Yield. Appl. Sci. 2020, 10, 7322. [Google Scholar] [CrossRef]
- Arranz, D.; Weigand, R.; de la Presa, P. Towards the Standardization of Photothermal Measurements of Iron Oxide Nanoparticles in Two Biological Windows. Nanomaterials 2023, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Kralj, S.; Griseti, E.; Nemec, S.; Wilhelm, C.; Plan Sangnier, A.; Bellard, E.; Fourquaux, I.; Golzio, M.; Rols, M.-P. Magnetic Silica-Coated Iron Oxide Nanochains as Photothermal Agents, Disrupting the Extracellular Matrix, and Eradicating Cancer Cells. Cancers 2019, 11, 2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Shao, R.; Elliott, A.M.; Stafford, R.J.; Esparza-Coss, E.; Bankson, J.A.; Liang, G.; Luo, Z.-P.; Park, K.; Markert, J.T.; et al. Bifunctional Gold Nanoshells with a Superparamagnetic Iron Oxide−Silica Core Suitable for Both MR Imaging and Photothermal Therapy. J. Phys. Chem. C 2007, 111, 6245–6251. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-K.; Su, C.-H.; Chen, J.-J.; Chang, C.-T.; Tsai, Y.-H.; Syu, S.-F.; Tseng, T.-T.; Yeh, C.-S. Fabrication of Silica-Coated Hollow Carbon Nanospheres Encapsulating Fe3O4 Cluster for Magnetical and MR Imaging Guided NIR Light Triggering Hyperthermia and Ultrasound Imaging. ACS Appl. Mater. Interfaces 2016, 8, 14470–14480. [Google Scholar] [CrossRef]
- Yougbaré, S.; Chou, H.-L.; Yang, C.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.-R. Facet-Dependent Gold Nanocrystals for Effective Photothermal Killing of Bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-Based Photothermal Therapy and Its Potentials in Antibacterial Treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Xu, J.-W.; Yao, K.; Xu, Z.-K. Nanomaterials with a Photothermal Effect for Antibacterial Activities: An Overview. Nanoscale 2019, 11, 8680–8691. [Google Scholar] [CrossRef]
- Ke, X.; Tang, S.; Dong, Z.; Wang, H.; Xu, X.; Qiu, R.; Yang, J.; Luo, J.; Li, J. A Silk Fibroin Based Bioadhesive with Synergistic Photothermal-Reinforced Antibacterial Activity. Int. J. Biol. Macromol. 2022, 209, 608–617. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Manivasagan, P.; Jeong, M.S.; Choi, M.; Jang, E.-S.; Priya, V.V.; Wang, M.-H. Photothermally Responsive Chitosan-Coated Iron Oxide Nanoparticles for Enhanced Eradication of Bacterial Biofilms. Biomater. Adv. 2022, 141, 213129. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.J.; Sharker, S.M.; In, I.; Park, S.Y. Iron Oxide@PEDOT-Based Recyclable Photothermal Nanoparticles with Poly(Vinylpyrrolidone) Sulfobetaines for Rapid and Effective Antibacterial Activity. ACS Appl. Mater. Interfaces 2015, 7, 9469–9478. [Google Scholar] [CrossRef]
- Lv, X.; Fang, Z.; Sun, Y.; Yang, Y.; Wang, X.; Chen, Y.; Qin, Y.; Li, N.; Li, C.; Xu, J.; et al. Interfacial Preparation of Multi-Branched Magneto-Plasmonic Fe3O4@Au Core@shell Nanocomposites as Efficient Photothermal Agents for Antibacterial Application. J. Alloys Compd. 2023, 932, 167712. [Google Scholar] [CrossRef]
- Yu, T.-J.; Li, P.-H.; Tseng, T.-W.; Chen, Y.-C. Multifunctional Fe3O4/Alumina Core/Shell MNPs as Photothermal Agents for Targeted Hyperthermia of Nosocomial and Antibiotic-Resistant Bacteria. Nanomed. 2011, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- García, A.; González, B.; Harvey, C.; Izquierdo-Barba, I.; Vallet-Regí, M. Effective Reduction of Biofilm through Photothermal Therapy by Gold Core@shell Based Mesoporous Silica Nanoparticles. Microporous Mesoporous Mater. 2021, 328, 111489. [Google Scholar] [CrossRef]
- Wu, S.; Li, A.; Zhao, X.; Zhang, C.; Yu, B.; Zhao, N.; Xu, F.-J. Silica-Coated Gold–Silver Nanocages as Photothermal Antibacterial Agents for Combined Anti-Infective Therapy. ACS Appl. Mater. Interfaces 2019, 11, 17177–17183. [Google Scholar] [CrossRef]
- Hernandes, E.P.; Bini, R.D.; Endo, K.M.; de Oliveira Junior, V.A.; de Almeida, I.V.; Dias, G.S.; dos Santos, I.A.; de Oliveira, P.N.; Vicentini, V.E.P.; Cotica, L.F. Doxorubicin-Loaded Magnetic Nanoparticles: Enhancement of Doxorubicin’s Effect on Breast Cancer Cells (MCF-7). Magnetochemistry 2022, 8, 114. [Google Scholar] [CrossRef]
- Popova, V.; Poletaeva, Y.; Chubarov, A.; Pyshnyi, D.; Dmitrienko, E. Doxorubicin-Loaded Silica Nanocomposites for Cancer Treatment. Coatings 2023, 13, 324. [Google Scholar] [CrossRef]
- Demin, A.M.; Vakhrushev, A.V.; Pershina, A.G.; Valova, M.S.; Efimova, L.V.; Syomchina, A.A.; Uimin, M.A.; Minin, A.S.; Levit, G.L.; Krasnov, V.P.; et al. Magnetic-Responsive Doxorubicin-Containing Materials Based on Fe3O4 Nanoparticles with a SiO2/PEG Shell and Study of Their Effects on Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 9093. [Google Scholar] [CrossRef]
- Shao, D.; Wang, Z.; Dong, W.; Zhang, X.; Zheng, X.; Xiao, X.; Wang, Y.; Zhao, X.; Zhang, M.; Li, J.; et al. Facile Synthesis of Core–Shell Magnetic Mesoporous Silica Nanoparticles for PH-Sensitive Anticancer Drug Delivery. Chem. Biol. Drug Des. 2015, 86, 1548–1553. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Yue, X.; Cao, Z.; Dai, Z. One-Pot Construction of Doxorubicin Conjugated Magnetic Silica Nanoparticles. New J. Chem. 2009, 33, 2414–2418. [Google Scholar] [CrossRef]
- Waters, M.; Hopf, J.; Tam, E.; Wallace, S.; Chang, J.; Bennett, Z.; Aquino, H.; Roeder, R.K.; Helquist, P.; Stack, M.S.; et al. Biocompatible, Multi-Mode, Fluorescent, T2 MRI Contrast Magnetoelectric-Silica Nanoparticles (MagSiNs), for On-Demand Doxorubicin Delivery to Metastatic Cancer Cells. Pharmaceuticals 2022, 15, 1216. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.E.; Jadhav, S.A.; Magnacca, G.; Scalarone, D.; Mártire, D.O.; Parolo, M.E.; Carlos, L. Synthesis and in Vitro Testing of Thermoresponsive Polymer-Grafted Core-Shell Magnetic Mesoporous Silica Nanoparticles for Efficient Controlled and Targeted Drug Delivery. J. Colloid Interface Sci. 2019, 544, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, Q.; Wang, L.; Jiang, W.; Zhang, W.; Song, X. Multifunctional Triple-Porous Fe3O4@SiO2 Superparamagnetic Microspheres for Potential Hyperthermia and Controlled Drug Release. RSC Adv. 2017, 7, 32049–32057. [Google Scholar] [CrossRef] [Green Version]
- Saint-Cricq, P.; Deshayes, S.; Zink, J.I.; Kasko, A.M. Magnetic Field Activated Drug Delivery Using Thermodegradable Azo-Functionalised PEG-Coated Core–Shell Mesoporous Silica Nanoparticles. Nanoscale 2015, 7, 13168–13172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Tao, C. DNA-Capped Fe3O4/SiO2 Magnetic Mesoporous Silica Nanoparticles for Potential Controlled Drug Release and Hyperthermia. RSC Adv. 2015, 5, 22365–22372. [Google Scholar] [CrossRef]
- Guisasola, E.; Asín, L.; Beola, L.; de la Fuente, J.M.; Baeza, A.; Vallet-Regí, M. Beyond Traditional Hyperthermia: In Vivo Cancer Treatment with Magnetic-Responsive Mesoporous Silica Nanocarriers. ACS Appl. Mater. Interfaces 2018, 10, 12518–12525. [Google Scholar] [CrossRef] [Green Version]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Moros, M.; de la Fuente, J.M.; Vallet-Regí, M. Magnetic-Responsive Release Controlled by Hot Spot Effect. Langmuir 2015, 31, 12777–12782. [Google Scholar] [CrossRef]
- Patil-Sen, Y.; Torino, E.; Sarno, F.D.; Ponsiglione, A.M.; Chhabria, V.; Ahmed, W.; Mercer, T. Biocompatible Superparamagnetic Core-Shell Nanoparticles for Potential Use in Hyperthermia-Enabled Drug Release and as an Enhanced Contrast Agent. Nanotechnology 2020, 31, 375102. [Google Scholar] [CrossRef]
- Lu, F.; Popa, A.; Zhou, S.; Zhu, J.-J.; Samia, A.C.S. Iron Oxide-Loaded Hollow Mesoporous Silica Nanocapsules for Controlled Drug Release and Hyperthermia. Chem. Commun. 2013, 49, 11436–11438. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, X.; Ruan, Z.; Zhu, M.; Zhu, Y.; Hanagata, N. Magnetic Mesoporous Silica Nanoparticles Coated with Thermo-Responsive Copolymer for Potential Chemo- and Magnetic Hyperthermia Therapy. Microporous Mesoporous Mater. 2018, 256, 1–9. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Kaskel, S. A Smart Magnetic Nanosystem with Controllable Drug Release and Hyperthermia for Potential Cancer Therapy. RSC Adv. 2015, 5, 99875–99883. [Google Scholar] [CrossRef]
- Horny, M.-C.; Gamby, J.; Dupuis, V.; Siaugue, J.-M. Magnetic Hyperthermia on γ-Fe2O3@SiO2 Core-Shell Nanoparticles for Mi-RNA 122 Detection. Nanomaterials 2021, 11, 149. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Subramanian, B.; Panchanathan, M.; Mondal, S.; Kim, H.; Lee, K.D.; Oh, J. Fucoidan-Coated Core–Shell Magnetic Mesoporous Silica Nanoparticles for Chemotherapy and Magnetic Hyperthermia-Based Thermal Therapy Applications. New J. Chem. 2017, 41, 15334–15346. [Google Scholar] [CrossRef]
- Cazares-Cortes, E.; Cabana, S.; Boitard, C.; Nehlig, E.; Griffete, N.; Fresnais, J.; Wilhelm, C.; Abou-Hassan, A.; Ménager, C. Recent Insights in Magnetic Hyperthermia: From the “Hot-Spot” Effect for Local Delivery to Combined Magneto-Photo-Thermia Using Magneto-Plasmonic Hybrids. Adv. Drug Deliv. Rev. 2019, 138, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, W.; Cui, L.; An, N.; Zhang, T.; Guo, G.; Lin, H.; Qu, F. Fe3O4@mSiO2 Core–Shell Nanocomposite Capped with Disulfide Gatekeepers for Enzyme-Sensitive Controlled Release of Anti-Cancer Drugs. J. Mater. Chem. B 2015, 3, 1010–1019. [Google Scholar] [CrossRef]
- Baki, A.; Remmo, A.; Löwa, N.; Wiekhorst, F.; Bleul, R. Albumin-Coated Single-Core Iron Oxide Nanoparticles for Enhanced Molecular Magnetic Imaging (MRI/MPI). Int. J. Mol. Sci. 2021, 22, 6235. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Malinovskaya, J.; Salami, R.; Valikhov, M.; Vadekhina, V.; Semyonkin, A.; Semkina, A.; Abakumov, M.; Harel, Y.; Levy, E.; Levin, T.; et al. Supermagnetic Human Serum Albumin (HSA) Nanoparticles and PLGA-Based Doxorubicin Nanoformulation: A Duet for Selective Nanotherapy. Int. J. Mol. Sci. 2023, 24, 627. [Google Scholar] [CrossRef]
- Huang, H.; Delikanli, S.; Zeng, H.; Ferkey, D.M.; Pralle, A. Remote Control of Ion Channels and Neurons through Magnetic-Field Heating of Nanoparticles. Nat. Nanotechnol. 2010, 5, 602–606. [Google Scholar] [CrossRef]
- Rühle, B.; Datz, S.; Argyo, C.; Bein, T.; Zink, J.I. A Molecular Nanocap Activated by Superparamagnetic Heating for Externally Stimulated Cargo Release. Chem. Commun. 2016, 52, 1843–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polo-Corrales, L.; Rinaldi, C. Monitoring Iron Oxide Nanoparticle Surface Temperature in an Alternating Magnetic Field Using Thermoresponsive Fluorescent Polymers. J. Appl. Phys. 2012, 111, 07B334. [Google Scholar] [CrossRef]
- Griffete, N.; Fresnais, J.; Espinosa, A.; Taverna, D.; Wilhelm, C.; Ménager, C. Thermal Polymerization on the Surface of Iron Oxide Nanoparticles Mediated by Magnetic Hyperthermia: Implications for Multishell Grafting and Environmental Applications. ACS Appl. Nano Mater. 2018, 1, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Ortgies, D.H.; Teran, F.J.; Rocha, U.; de la Cueva, L.; Salas, G.; Cabrera, D.; Vanetsev, A.S.; Rähn, M.; Sammelselg, V.; Orlovskii, Y.V.; et al. Optomagnetic Nanoplatforms for In Situ Controlled Hyperthermia. Adv. Funct. Mater. 2018, 28, 1704434. [Google Scholar] [CrossRef] [Green Version]
- Jaque, D.; Vetrone, F. Luminescence Nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zink, J.I. Taking the Temperature of the Interiors of Magnetically Heated Nanoparticles. ACS Nano 2014, 8, 5199–5207. [Google Scholar] [CrossRef]
- Riedinger, A.; Guardia, P.; Curcio, A.; Garcia, M.A.; Cingolani, R.; Manna, L.; Pellegrino, T. Subnanometer Local Temperature Probing and Remotely Controlled Drug Release Based on Azo-Functionalized Iron Oxide Nanoparticles. Nano Lett. 2013, 13, 2399–2406. [Google Scholar] [CrossRef]
- Kalyane, D.; Kumar, N.; Anup, N.; Rajpoot, K.; Maheshwari, R.; Sengupta, P.; Kalia, K.; Tekade, R.K. Recent Advancements and Future Submissions of Silica Core-Shell Nanoparticles. Int. J. Pharm. 2021, 609, 121173. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In Vivo Delivery, Pharmacokinetics, Biodistribution and Toxicity of Iron Oxide Nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [Green Version]
- Ling, D.; Hyeon, T. Chemical Design of Biocompatible Iron Oxide Nanoparticles for Medical Applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Liu, Y.; Zhong, R.; Yu, X.; Liu, Q.; Zhang, L.; Lv, C.; Mao, K.; Tang, P. The Cell Uptake Properties and Hyperthermia Performance of Zn0.5Fe2.5O4/SiO2 Nanoparticles as Magnetic Hyperthermia Agents. R. Soc. Open Sci. 2020, 7, 191139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesse, X.; Adam, A.; Begin-Colin, S.; Mertz, D.; Larquet, E.; Gacoin, T.; Maurin, I.; Vichery, C.; Nedelec, J.-M. Elaboration of Superparamagnetic and Bioactive Multicore–Shell Nanoparticles (γ-Fe2O3@SiO2-CaO): A Promising Material for Bone Cancer Treatment. ACS Appl. Mater. Interfaces 2020, 12, 47820–47830. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.W.-W.; Hui, Y.Y.; Tsai, P.-C.; Chang, H.-C. Fluorescent Nanodiamond: A Versatile Tool for Long-Term Cell Tracking, Super-Resolution Imaging, and Nanoscale Temperature Sensing. Acc. Chem. Res. 2016, 49, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Hartono, S.B.; Yu, M.; Gu, W.; Yang, J.; Strounina, E.; Wang, X.; Qiao, S.; Yu, C. Synthesis of Multi-Functional Large Pore Mesoporous Silica Nanoparticles as Gene Carriers. Nanotechnology 2014, 25, 055701. [Google Scholar] [CrossRef]
- Sanchez-Salcedo, S.; Vallet-Regí, M.; Shahin, S.A.; Glackin, C.A.; Zink, J.I. Mesoporous Core-Shell Silica Nanoparticles with Anti-Fouling Properties for Ovarian Cancer Therapy. Chem. Eng. J. 2018, 340, 114–124. [Google Scholar] [CrossRef]
- Pon-On, W.; Tithito, T.; Maneeprakorn, W.; Phenrat, T.; Tang, I.-M. Investigation of Magnetic Silica with Thermoresponsive Chitosan Coating for Drug Controlled Release and Magnetic Hyperthermia Application. Mater. Sci. Eng. C 2019, 97, 23–30. [Google Scholar] [CrossRef]
- Lin, H.; Yin, L.; Chen, B.; Ji, Y. Design of Functionalized Magnetic Silica Multi-Core Composite Nanoparticles for Synergistic Magnetic Hyperthermia/Radiotherapy in Cancer Cells. Colloids Surf. B-Biointerfaces 2022, 219, 112814. [Google Scholar] [CrossRef]
- Legge, C.J.; Colley, H.E.; Lawson, M.A.; Rawlings, A.E. Targeted Magnetic Nanoparticle Hyperthermia for the Treatment of Oral Cancer. J. Oral Pathol. Med. 2019, 48, 803–809. [Google Scholar] [CrossRef]
- Martín-Saavedra, F.M.; Ruíz-Hernández, E.; Boré, A.; Arcos, D.; Vallet-Regí, M.; Vilaboa, N. Magnetic Mesoporous Silica Spheres for Hyperthermia Therapy. Acta Biomater. 2010, 6, 4522–4531. [Google Scholar] [CrossRef]
- Avedian, N.; Zaaeri, F.; Daryasari, M.P.; Akbari Javar, H.; Khoobi, M. PH-Sensitive Biocompatible Mesoporous Magnetic Nanoparticles Labeled with Folic Acid as an Efficient Carrier for Controlled Anticancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2018, 44, 323–332. [Google Scholar] [CrossRef]
- Naz, S.; Shamoon, M.; Wang, R.; Zhang, L.; Zhou, J.; Chen, J. Advances in Therapeutic Implications of Inorganic Drug Delivery Nano-Platforms for Cancer. Int. J. Mol. Sci. 2019, 20, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heggannavar, G.B.; Hiremath, C.G.; Achari, D.D.; Pangarkar, V.G.; Kariduraganavar, M.Y. Development of Doxorubicin-Loaded Magnetic Silica–Pluronic F-127 Nanocarriers Conjugated with Transferrin for Treating Glioblastoma across the Blood–Brain Barrier Using an in Vitro Model. ACS Omega 2018, 3, 8017–8026. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Bi, J.; Tang, Y.; Qiao, S.-Z. Magnetic Core–Shell Silica Nanoparticles with Large Radial Mesopores for SiRNA Delivery. Small 2016, 12, 4735–4742. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wen, S.; Zhang, Y.; Sun, Z. Organosilane and Polyethylene Glycol Functionalized Magnetic Mesoporous Silica Nanoparticles as Carriers for CpG Immunotherapy In Vitro and In Vivo. PLoS ONE 2015, 10, e0140265. [Google Scholar] [CrossRef]
- Bizeau, J.; Mertz, D. Design and Applications of Protein Delivery Systems in Nanomedicine and Tissue Engineering. Adv. Colloid Interface Sci. 2021, 287, 102334. [Google Scholar] [CrossRef]
- Mertz, D.; Harlepp, S.; Goetz, J.; Bégin, D.; Schlatter, G.; Bégin-Colin, S.; Hébraud, A. Nanocomposite Polymer Scaffolds Responding under External Stimuli for Drug Delivery and Tissue Engineering Applications. Adv. Ther. 2020, 3, 1900143. [Google Scholar] [CrossRef]
- Satarkar, N.S.; Hilt, J.Z. Magnetic Hydrogel Nanocomposites for Remote Controlled Pulsatile Drug Release. J. Control. Release 2008, 130, 246–251. [Google Scholar] [CrossRef]
- Campbell, S.B.; Patenaude, M.; Hoare, T. Injectable Superparamagnets: Highly Elastic and Degradable Poly(N-Isopropylacrylamide)–Superparamagnetic Iron Oxide Nanoparticle (SPION) Composite Hydrogels. Biomacromolecules 2013, 14, 644–653. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A Smart Hyperthermia Nanofiber with Switchable Drug Release for Inducing Cancer Apoptosis. Adv. Funct. Mater. 2013, 23, 5753–5761. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Q.; Guo, Z.; Wang, D.; Gao, F.; Wang, X.; Wei, Y.; Zhao, L. Injectable and Self-Healing Thermosensitive Magnetic Hydrogel for Asynchronous Control Release of Doxorubicin and Docetaxel to Treat Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 33660–33673. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, A.; Mertz, D. Iron Oxide@Mesoporous Silica Core-Shell Nanoparticles as Multimodal Platforms for Magnetic Resonance Imaging, Magnetic Hyperthermia, Near-Infrared Light Photothermia, and Drug Delivery. Nanomaterials 2023, 13, 1342. https://doi.org/10.3390/nano13081342
Adam A, Mertz D. Iron Oxide@Mesoporous Silica Core-Shell Nanoparticles as Multimodal Platforms for Magnetic Resonance Imaging, Magnetic Hyperthermia, Near-Infrared Light Photothermia, and Drug Delivery. Nanomaterials. 2023; 13(8):1342. https://doi.org/10.3390/nano13081342
Chicago/Turabian StyleAdam, Alexandre, and Damien Mertz. 2023. "Iron Oxide@Mesoporous Silica Core-Shell Nanoparticles as Multimodal Platforms for Magnetic Resonance Imaging, Magnetic Hyperthermia, Near-Infrared Light Photothermia, and Drug Delivery" Nanomaterials 13, no. 8: 1342. https://doi.org/10.3390/nano13081342
APA StyleAdam, A., & Mertz, D. (2023). Iron Oxide@Mesoporous Silica Core-Shell Nanoparticles as Multimodal Platforms for Magnetic Resonance Imaging, Magnetic Hyperthermia, Near-Infrared Light Photothermia, and Drug Delivery. Nanomaterials, 13(8), 1342. https://doi.org/10.3390/nano13081342







