Paper-Based Humidity Sensors as Promising Flexible Devices, State of the Art, Part 2: Humidity-Sensor Performances
Abstract
:1. Introduction
2. Capacitance-Based Humidity Sensors
2.1. Capacitive Humidity Sensors with Paper as the Sensing Material
2.2. Humidity Sensors with Solid-State Sensing Elements
3. Conductometric (Resistive) Humidity Sensors
3.1. Resistive Humidity Sensors with Paper as the Sensing Material
3.2. Resistive Humidity Sensors with Carbon-Based Sensing Materials
3.3. Paper-Based Resistive Humidity Sensors with Solid-State Sensing Materials
3.4. Paper-Based Resistive Humidity Sensors with Polymer Sensing Layer
4. Impedance Paper-Based Humidity Sensors
5. Mass-Sensitive, Microwave and Fiber-Optic Paper-Based Humidity Sensors
5.1. Mass-Sensitive PB Humidity Sensors
5.2. Fiber-Optic PB Humidity Sensors
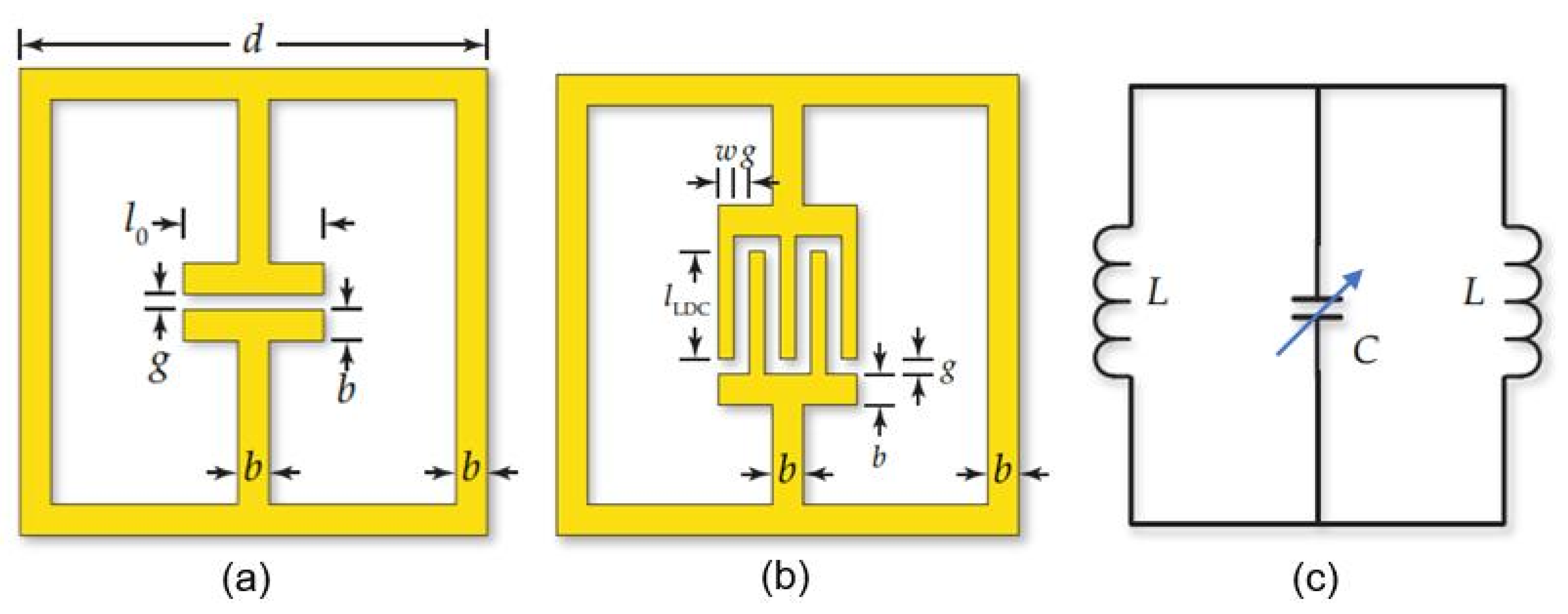
5.3. Microwave PB Humidity Sensors
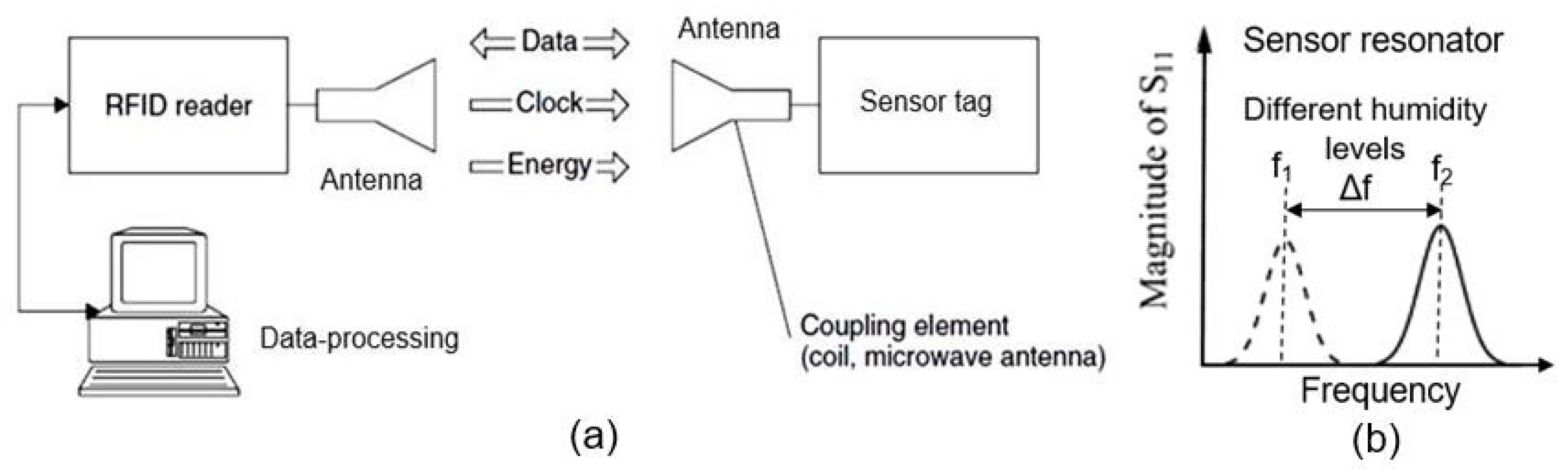
6. RFID (Radio-Frequency Identification) Humidity Sensor
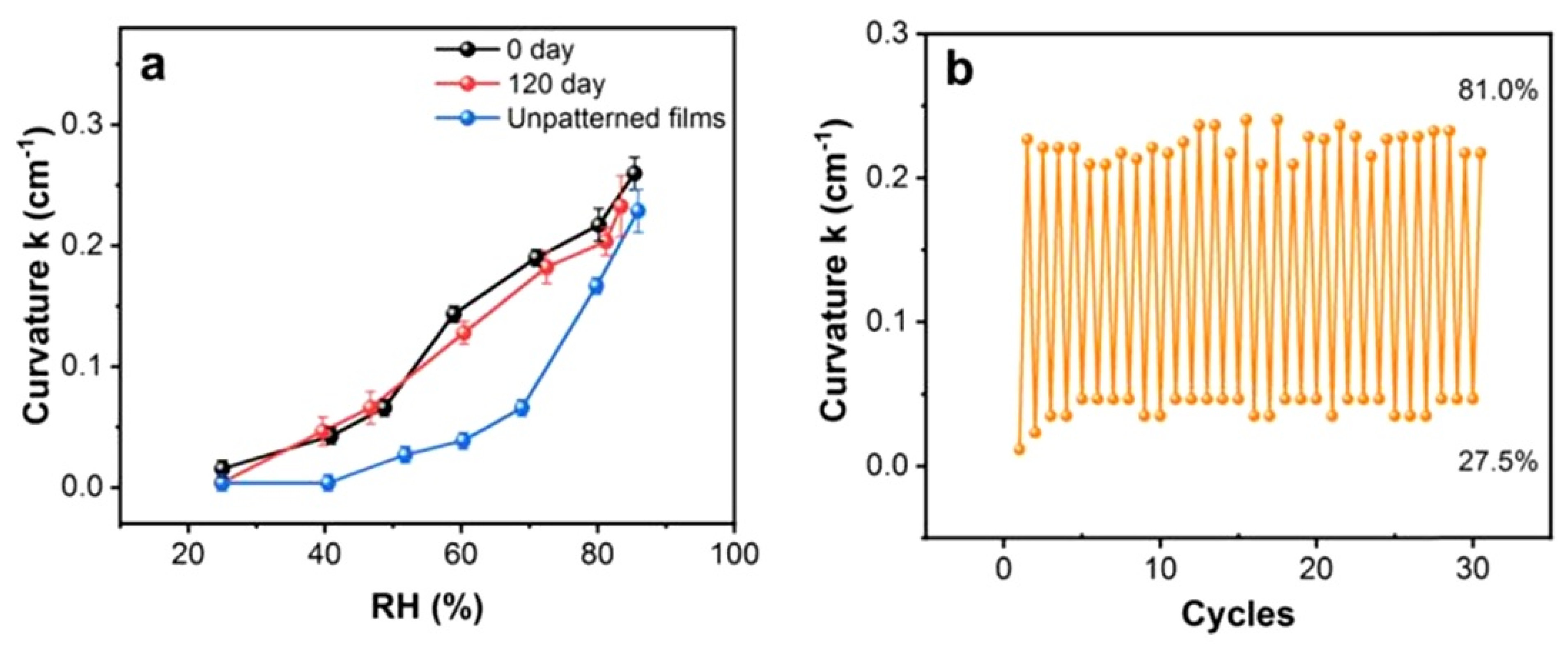
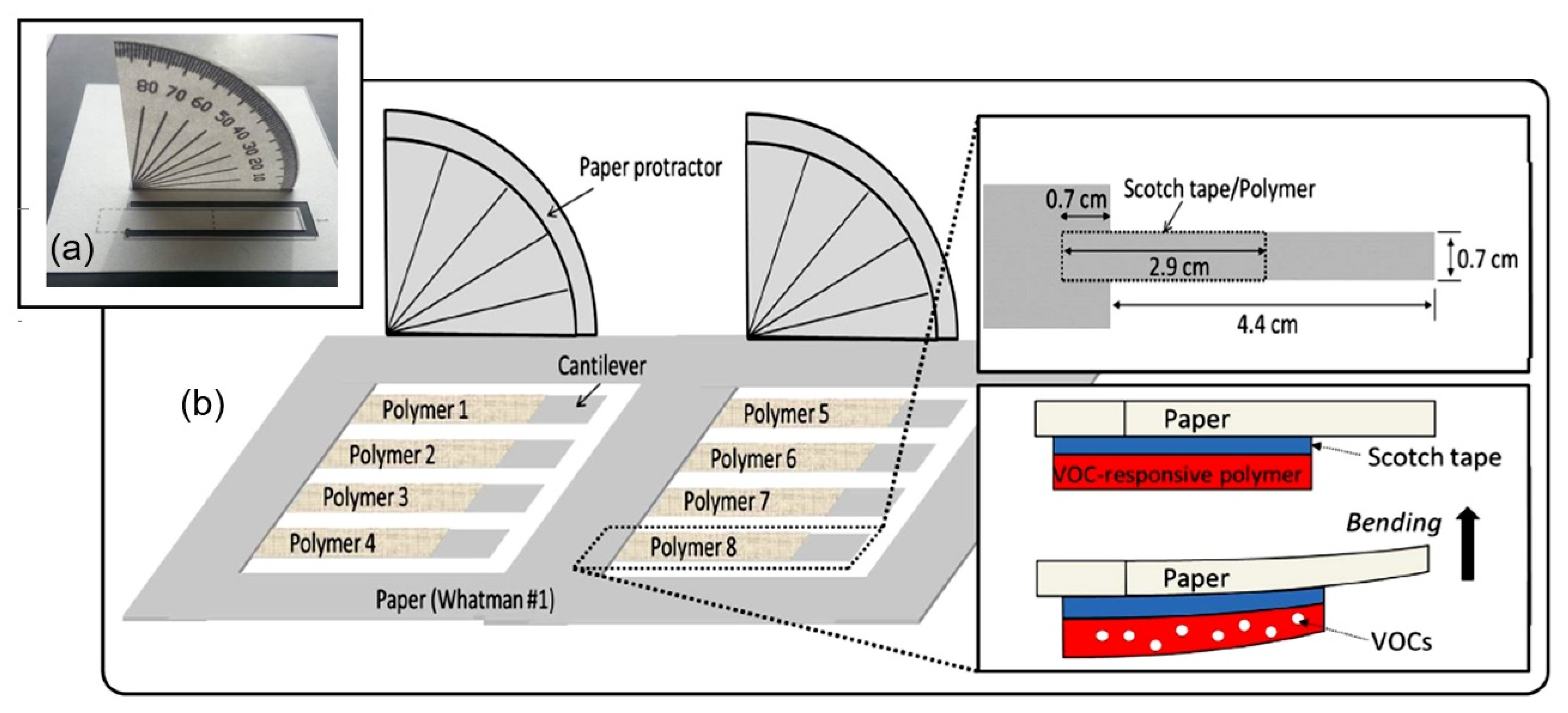
7. PB Humidity Sensors Based on Shape Deformation and Luminescence
7.1. Shape-Deformation-Based PB Humidity Sensors
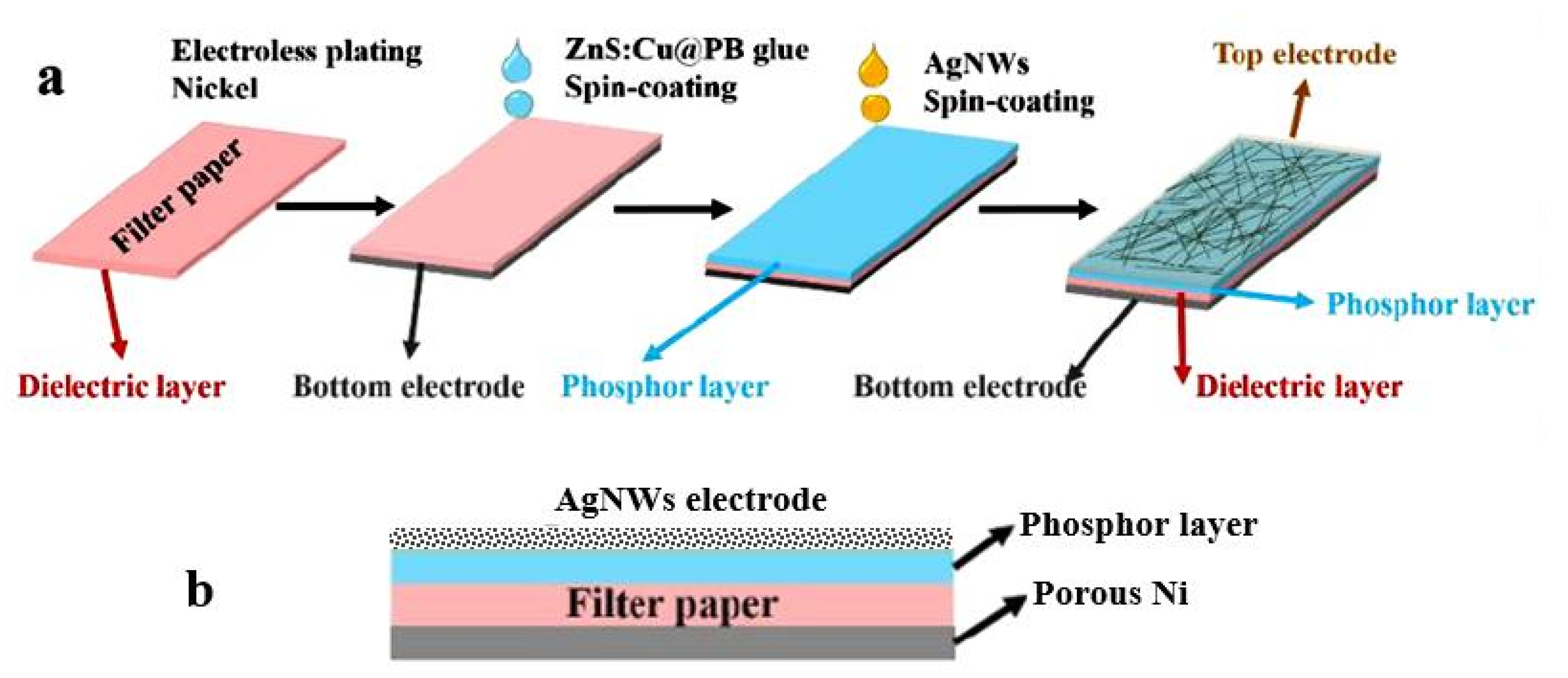
7.2. Electroluminescent Devices as PB Humidity Sensors
8. PB Humidity Indicators
9. Challenges in Paper-Based Humidity Sensors and Trends in Development
10. Summary
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korotcenkov, G. Paper-based humidity sensors as promising flexible devices: State of the art. Part 1. General consideration. Nanomaterials 2023, 13, 1110. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N.; Shimizu, Y. Humidity sensors: Principles and applications. Sens. Actuators 1986, 10, 379–398. [Google Scholar] [CrossRef]
- Rittersma, Z.M. Recent achievements in miniaturised humidity sensors-a review of transduction techniques. Sens. Actuators B Chem. 2002, 96, 196–210. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lee, G.-B. Humidity sensors: A review. Sens. Lett. 2005, 3, 1–15. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent advances in graphene-based humidity sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Humidity Measurements, Vol. 1: Spectroscopic Methods of Humidity Measurement; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Korotcenkov, G. Handbook of Humidity Measurement, Vol. 2: Electronic and Electrical Humidity Sensors; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Korotcenkov, G. Handbook of Humidity Measurements, Vol. 3: Sensing Materials and Technologies; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Liang, R.; Luo, A.; Zhang, Z.; Li, Z.; Han, C.; Wu, W. Research progress of graphene-based flexible humidity sensor. Sensors 2020, 20, 5601. [Google Scholar] [CrossRef]
- Kano, S.; Jarulertwathana, N.; Mohd-Noor, S.; Hyun, J.K.; Asahara, R.; Mekaru, H. Respiratory monitoring by ultrafast humidity sensors with nanomaterials: A review. Sensors 2022, 22, 1251. [Google Scholar] [CrossRef]
- Sajid, M.; Khattak, Z.J.; Rahman, K.; Hassan, G.; Choi, K.H. Progress and future of relative humidity sensors: A review from materials perspective. Bull. Mater. Sci. 2022, 45, 238. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, G.; Bapna, K.; Shivagan, D.D. Semiconductor-metal-oxide-based nano-composites for humidity sensing applications. Mater. Res. Bull. 2023, 158, 112053. [Google Scholar] [CrossRef]
- Courbat, J.; Kim, Y.B.; Briand, D.; de Rooi, N.F. Inkjet printing on paper for the realization of humidity and temperature sensors. In Proceedings of the 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 1356–1359. [Google Scholar] [CrossRef]
- Mraovic, M.; Muck, T.; Pivar, M.; Trontelj, J.; Pletersek, A. Humidity sensors printed on recycled paper and cardboard. Sensors 2014, 14, 13628–13643. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Manuilskiy, A.; Gao, J.; Lidenmark, C.; Sidén, J.; Forsberg, S.; Unander, T.; Nilsson, H.-E. Investigation of humidity sensor effect in silver nanoparticle ink sensors printed on paper. IEEE Sens. J. 2014, 14, 623–628. [Google Scholar] [CrossRef]
- Balde, M.; Vena, A.; Sorli, B. Fabrication of porous anodic aluminium oxide layers on paper for humidity sensors. Sens. Actuators B 2015, 220, 829–839. [Google Scholar] [CrossRef]
- Wawrzynek, E.; Baumbauer, C.; Arias, A.C. Characterization and comparison of biodegradable printed capacitive humidity sensors. Sensors 2021, 21, 6557. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Zulfiqar, M.H.; Khan, M.A.; Ali, M.; Zubair, M.; Mehmood, M.Q.; Massoud, Y. Garage-fabricated, ultrasensitive capacitive humidity sensor based on tissue paper. Sensors 2022, 22, 7885. [Google Scholar] [CrossRef]
- Gaspar, C.; Olkkonen, J.; Passoja, S.; Smolander, M. Paper as active layer in inkjet-printed capacitive humidity sensors. Sensors 2017, 17, 1464. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Paschoal, C.W.A.; Arias, A.C. Printed and flexible biosensor for antioxidants using interdigitated ink-jetted electrodes and gravure-deposited active layer. Biosens. Bioelectron. 2015, 67, 553–559. [Google Scholar] [CrossRef]
- Rahimi, R.; Ochoa, M.; Ziaie, B. A comparison of direct and indirect laser ablation of metallized paper for inexpensive paper-based sensors. ACS Appl. Mater. Interfaces 2018, 10, 36332–36341. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Badhulika, S. Solvent-free fabrication of paper based all-carbon disposable multifunctional sensors and passive electronic circuits. RSC Adv. 2016, 6, 95574. [Google Scholar] [CrossRef]
- Kanaparthi, S. Pencil-drawn paper-based non-invasive and wearable capacitive respiration sensor. Electroanalysis 2017, 29, 2680–2684. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Yang, Q.; Atashbar, M.Z. Rapid, highly sensitive, and highly repeatable printed porous paper humidity sensor. Chem. Eng. J. 2022, 433, 133751. [Google Scholar] [CrossRef]
- Nassar, J.M.; Cordero, M.D.; Kutbee, A.T.; Karimi, M.A.; Sevilla, G.A.T.; Hussain, A.M.; Shamim, A.; Hussain, M.M. Paper skin multisensory platform for simultaneous environmental monitoring. Adv. Mater. Technol. 2016, 1, 1600004. [Google Scholar] [CrossRef]
- Lim, W.Y.; Goh, C.H.; Yap, K.Z.; Ramakrishnan, N. One-step fabrication of paper-based inkjet-printed graphene for breath monitor sensors. Biosensors 2023, 13, 209. [Google Scholar] [CrossRef]
- Qian, Z.; Li, T.; Sakthivelpathi, V.; Goodman, S.M.; Dichiara, A.B.; Mamishev, A.V.; Chung, J.-H. Humidity response of a capacitive sensor based on auxeticity of carbon nanotube-paper composites. Nano Express 2022, 3, 025001. [Google Scholar] [CrossRef]
- Thiyagarajan, K.; Rajini, G.; Maji, D. Flexible, highly sensitive paper-based screen printed MWCNT/PDMS composite breath sensor for human respiration monitoring. IEEE Sens. J. 2020, 21, 13985–13995. [Google Scholar] [CrossRef] [PubMed]
- Alrammouz, R.; Podlecki, J.; Vena, A.; Garcia, R.; Abboud, P.; Habchi, R.; Sorli, B. Highly porous and flexible capacitive humidity sensor based on self-assembled graphene oxide sheets on a paper substrate. Sens. Actuators B 2019, 298, 126892. [Google Scholar] [CrossRef]
- Song, Z.; Li, S.; Hou, B.; Cheng, Z.; Xue, Y.; Chen, B. High-sensitivity paper-based capacitive humidity sensors for respiratory monitoring. IEEE Sens. J. 2023, 23, 2291–2302. [Google Scholar] [CrossRef]
- Kafy, A.; Akther, A.; Shishir, M.I.R.; Kim, H.C.; Yun, Y.; Kim, J. Cellulose nanocrystal/graphene oxide composite film as humidity sensor. Sens. Actuators A 2016, 247, 221–226. [Google Scholar] [CrossRef]
- Jalkanen, T.; Määttänen, A.; Mäkilä, E.; Tuura, J.; Kaasalainen, M.; Lehto, V.-P.; Ihalainen, P.; Peltonen, J.; Salonen, J. Fabrication of porous silicon-based humidity sensing elements on paper. J. Sens. 2015, 2015, 927396. [Google Scholar] [CrossRef]
- Jalkanen, T.; Mäkilä, E.; Määttänen, A.; Tuura, J.; Kaasalainen, M.; Lehto, V.-P.; Ihalainen, P.; Peeltonen, J.; Salonen, J. Porous silicon micro- and nanoparticles for printed humidity sensors. Appl. Phys. Lett. 2012, 101, 263110. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, flexible, cost-saving, and environment-friendly paper-based humidity sensor for multifunctional applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, S.; Zou, P.; Li, R.; Fan, Y. Paper-based humidity sensor for respiratory monitoring. Materials 2022, 15, 6447. [Google Scholar] [CrossRef] [PubMed]
- Steffens, C.; Manzoli, A.; Paschoalin, R.T.; Tiggemann, L.; Steffens, J.; Teixeira, E.; de Paula Herrmann, P.S. Tracing paper substrate used for development of interdigitated graphite electrode and its application as humidity sensor. Synth. Met. 2013, 183, 36–39. [Google Scholar] [CrossRef]
- Guder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.J.; Whitesides, G.M. Paper-based electrical respiration sensor. Angew. Chem. Int. Ed. 2016, 55, 5727–5732. [Google Scholar] [CrossRef]
- Niarchos, G.; Dubourg, G.; Afroudakis, G.; Tsouti, V.; Makarona, E.; Matovic, J.; Crnojevic-Bengin, V.; Tsamis, C. Paper-based humidity sensor coated with ZnO nanoparticles: The influence of ZnO. Proc. Eng. 2016, 168, 325–328. [Google Scholar] [CrossRef]
- Barmpakos, D.; Segkos, A.; Tsamis, C.; Kaltsas, G. A disposable flexible humidity sensor directly printed on paper for medical applications. J. Phys. Conf. Ser. 2017, 931, 012003. [Google Scholar] [CrossRef]
- Barmpakos, D.; Tsamis, C.; Kaltsas, G. Multi-parameter paper sensor fabricated by inkjet-printed silver nanoparticle ink and PEDOT:PSS. Microelectron. Eng. 2020, 225, 111266. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Huang, Q.; Wang, S.; Wang, Y.; Pan, H.; Zhao, Q.; Xie, G.; Du, X.; Tai, H. Paper and carbon ink enabled low-cost, eco-friendly, flexible, multifunctional pressure and humidity sensors. Smart Mater. Struct. 2021, 30, 055012. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Kasuga, T.; Uetani, K.; Nogi, M.; Koga, H. All-cellulose-derived humidity sensor prepared via direct laser writing of conductive and moisture-stable electrodes on TEMPO-oxidized cellulose paper. J. Mater. Chem. C 2022, 10, 3712–3719. [Google Scholar] [CrossRef]
- Mansoori, A.; Ahmad, S.; Bansal, S.; Vashishath, M. Flexible graphite-based humidity sensor using green technology. ECS Sens. Plus 2023, 1, 044401. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhou, J.; Lu, A. Flexible and transparent cellulose based ionic film as humidity sensor. ACS Appl. Mater. Interfaces 2020, 12, 7631–7638. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishiyama, Y.; Putaux, J.L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Langford, M. Basic Photography; Focal Press: Oxford, UK, 2000. [Google Scholar]
- Andersson, H.; Manuilskiy, A.; Unander, T.; Lidenmark, C.; Forsberg, S.; Nilsson, H.-E. Inkjet printed silver nanoparticle humidity sensor with memory effect on paper. IEEE Sens. J. 2012, 12, 1901–1905. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, T.; Qi, R.; Dai, J.; Liu, S.; Fei, T. Drawn on paper: A reproducible humidity sensitive device by handwriting. ACS Appl. Mater. Interfaces 2017, 9, 28002–28009. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, Z.; Zou, H.; Ma, M. Drawn a facile sensor: A fast response humidity sensor based on pencil-trace. Sens. Actuators B 2018, 261, 345–353. [Google Scholar] [CrossRef]
- Lou, Z.; Shen, G. Flexible photodetectors based on 1D inorganic nanostructures. Adv. Sci. 2016, 3, 1500287. [Google Scholar] [CrossRef]
- Zhu, P.; Ou, H.; Kuang, Y.; Hao, L.; Diao, J.; Chen, G. Cellulose nanofiber/carbon nanotube dual network-enabled humidity sensor with high sensitivity and durability. ACS Appl. Mater. Interfaces 2020, 12, 33229–33238. [Google Scholar] [CrossRef]
- Zhu, P.; Kuang, Y.; Wei, Y.; Li, F.; Ou, H.; Jiang, F.; Chen, G. Electrostatic self- assembly enabled flexible paper-based humidity sensor with high sensitivity and superior durability. Chem. Eng. J. 2021, 404, 127105. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, Y.; Fang, Z.; Kuang, Y.; Zhang, Y.; Peng, C.; Chen, G. Flexible and highly sensitive humidity sensor based on cellulose nanofibers and carbon nanotube composite film. Langmuir 2019, 35, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Schulz, B.; Vad, T.; Liu, J.; Mader, E.; Seide, G.; Gries, T. Novel carbon nanotube/cellulose composite fibers as multifunctional materials. ACS Appl. Mater. Interfaces 2015, 7, 22404–22412. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, J.; Gao, S.; Mader, E. Multifunctional films composed of carbon nanotubes and cellulose regenerated from alkaline–urea solution. J. Mater. Chem. A 2013, 1, 2161–2168. [Google Scholar] [CrossRef]
- Xie, L.; Feng, Y.; Mäntysalo, M.; Chen, Q.; Zheng, L.-R. Integration of f-MWCNT sensor and printed circuits on paper substrate. IEEE Sens. J. 2013, 13, 3948–3956. [Google Scholar] [CrossRef]
- Han, J.-W.; Kim, B.; Li, J.; Meyyappan, M. Carbon nanotube-based humidity sensor on cellulose paper. J. Phys. Chem. C 2012, 116, 22094–22097. [Google Scholar] [CrossRef]
- Khalifa, M.; Wuzella, G.; Lammer, H.; Mahendran, A.R. Smart paper from graphene coated cellulose for high-performance humidity and piezoresistive force sensor. Synth. Met. 2020, 266, 116420. [Google Scholar] [CrossRef]
- Yoshida, A.; Wang, Y.-F.; Tachibana, S.; Hasegawa, A.; Sekine, T.; Takeda, Y.; Hong, J.; Kumaki, D.; Shiba, T.; Tokito, S. Printed, all-carbon-based flexible humidity sensor using a cellulose nanofiber/graphene nanoplatelet composite. Carbon Trends 2022, 7, 100166. [Google Scholar] [CrossRef]
- Beniwal, A.; Ganguly, P.; Aliyana, A.K.; Khandelwal, G.; Dahiy, R. Screen-printed graphene-carbon ink based disposable humidity sensor with wireless communication. Sens. Actuators B 2023, 374, 132731. [Google Scholar] [CrossRef]
- Balakrishnan, V.; Dinh, T.; Foisal, A.R.M.; Nguyen, T.; Phan, H.-P.; Dao, D.V.; Nguyen, N.-T. Paper-based electronics using graphite and silver nanoparticles for respiration monitoring. IEEE Sens. J. 2019, 19, 11784–11790. [Google Scholar] [CrossRef]
- Choo, T.F.; Kok, K.Y.; Saidin, N.U.; Zali, N.M. Effect of chemical treatment and intrinsic resistance on the humidity sensitivity of pencil graphite sensing material coated on paper substrate. Sens. Actuators A Phys. 2012, 332, 113085. [Google Scholar] [CrossRef]
- Shobin, L.R.; Manivannan, S. Carbon nanotubes on paper: Flexible and disposable chemiresistors. Sens. Actuators B 2015, 220, 1178–1185. [Google Scholar] [CrossRef]
- Chitnis, G.; Ziaie, B. Waterproof active paper via laser surface micropatterning of magnetic nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 4435–4439. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Tang, J.; Li, J.; Xiao, H. Plasma-induced polymerization for enhancing paper hydrophobicity. Carnohydr. Polym. 2013, 92, 928–933. [Google Scholar] [CrossRef]
- Stanssens, D.; Van den Abbeele, H.; Vonck, L.; Schoukens, G.; Deconinck, M.; Samyn, P. Creating water-repellent and super-hydrophobic cellulose substrates by deposition of organic nanoparticle. Mater. Lett. 2011, 65, 1781–1784. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Okagaki, J.; Saji, T. Simple method for preparing superhydrophobic paper: Spray-deposited hydrophobic silica nanoparticle coatings exhibit high water-repellency and transparency. Langmuir 2012, 28, 4605–4608. [Google Scholar] [CrossRef] [PubMed]
- Bollstrom, R.; Pettersson, F.; Dolietis, P.; Preston, J.; Osterbacka, R.; Toivakka, M. Impact of humidity on functionality of on-paper printed electronics. Nanotechnology 2014, 25, 094003. [Google Scholar] [CrossRef]
- Kano, S.; Fujii, M. All-painting process to produce respiration sensor using humidity-sensitive nanoparticle film and graphite trace. ACS Sustain. Chem. Eng. 2018, 6, 12217–12223. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Bandyopadhyay, D. Mechanisms of humidity sensing on a CdS nanoparticle coated paper sensor. Sens. Actuators A Phys. 2019, 285, 241–247. [Google Scholar] [CrossRef]
- Niarchos, G.; Dubourg, G.; Afroudakis, G.; Georgopoulos, M.; Tsouti, V.; Makarona, E.; Crnojevic-Bengin, V.; Tsamis, C. Humidity sensing properties of paper substrates and their passivation with ZnO nanoparticles for sensor applications. Sensors 2017, 17, 516. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Nemade, H.B.; Bandyopadhyay, D. Nano-enabled paper humidity sensor for mobile based point-of-care lung function monitoring. Biosens. Bioelectron. 2017, 94, 544–551. [Google Scholar] [CrossRef]
- Reilly, J.M. Basic strategy for acetate film preservation. Microform Digit. Rev. 2007, 31, 117–130. [Google Scholar] [CrossRef]
- Gomes, T.C.; Constantino, C.J.L.; Lopes, E.M.; Job, A.E.; Alves, N. Thermal inkjet printing of polyaniline on Paper. Thin Solid Films 2012, 520, 7200–7204. [Google Scholar] [CrossRef]
- Shukla, S.K. Synthesis of polyaniline grafted cellulose suitable for humidity sensing. Ind. J. Eng. Mater. Sci. 2012, 19, 417–420. [Google Scholar]
- Ragazzini, I.; Castagnoli, R.; Gualandi, I.; Cassani, M.C.; Nanni, D.; Gambassi, F.; Scavetta, E.; Bernardi, E.; Ballarin, B. A resistive sensor for humidity detection based on cellulose/polyaniline. RSC Adv. 2022, 12, 28217–28226. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, J.; Wuzella, G.; Lammer, H.; Khalifa, M. A smart functional surfactant activated conductive polymer coated on paper with ultra-sensitive humidity sensing characteristics. Mater. Adv. 2022, 3, 1804–1815. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Zhao, X.; Wang, N.; Li, D. Fabrication and humidity sensing of reduced graphene oxide/polyaniline composite film on flexible paper substrate. Sens. Mater. 2020, 34, 2065–2074. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Kumar, S.; Augustine, S.; Saha, U.; Arora, K.; Bayan, S.; Ray, S.K.; Puri, N.K.; Malhotra, B.D. Nanoengineered conductive polyaniline enabled sensor for sensitive humidity detection. IEEE Sens. J. 2020, 20, 12574–12581. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Liu, R.; Liu, J.; Li, Z.; Liu, X. Humidity sensor fabricated by inkjet-printing photosensitive conductive inks PEDOT: PVMA on a paper substrate. RSC Adv. 2016, 6, 47498–47508. [Google Scholar] [CrossRef]
- Santhiago, M.; da Costa, P.G.; Pereira, M.P.; Correa, C.C.; de Morais, V.B.; Bufon, C.C.B. Versatile and robust integrated sensors to locally assess humidity changes in fully enclosed paper-based devices. ACS Appl. Mater. Interfaces 2018, 10, 35631–35638. [Google Scholar] [CrossRef]
- Jain, S.; Chakane, S.; Samui, A.; Krishnamurthy, V.N.; Bhoraskar, S.V. Humidity sensing with weak acid-doped polyaniline and its composites. Sens. Actuators B 2003, 96, 124–129. [Google Scholar] [CrossRef]
- Zeng, F.-W.; Liu, X.-X.; Diamond, D.; Lau, K.T. Humidity sensors based on polyaniline nanofibers. Sens. Actuators B Chem. 2010, 143, 530–534. [Google Scholar] [CrossRef]
- Anisimov, Y.A.; Evitts, R.W.; Cree, D.E.; Wilson, L.D. Polyaniline/biopolymer composite systems for humidity sensor applications: A review. Polymers 2021, 13, 2722. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.F.; Hong, J.D. Humidity sensing properties of transferable polyaniline thin films formed at the air–water interface. RSC Adv. 2016, 6, 96935–96941. [Google Scholar] [CrossRef]
- Morais, R.M.; Klem, M.D.S.; Nogueira, G.L.; Gomes, T.C.; Alves, N. Low cost humidity sensor based on PANI/PEDOT:PSS printed on paper. IEEE Sens. J. 2018, 18, 2647–2651. [Google Scholar] [CrossRef]
- Duncan, B.C.; Broughton, W.R. Absorption and Diffusion of Moisture in Polymeric Materials; National Physical Laboratory: Middlesex, UK, 2007. [Google Scholar]
- Cavallo, P.; Acevedo, D.F.; Fuertes, M.C.; Soler-Illia, G.J.A.A.; Barbero, C.A. Understanding the sensing mechanism of polyaniline resistive sensors. Effect of humidity on sensing of organic volatiles. Sens. Actuators B Chem. 2015, 210, 574–580. [Google Scholar] [CrossRef]
- Liao, G.; Li, Q.; Xu, Z. The chemical modification of polyaniline with enhanced properties: A review. Prog. Org. Coat. 2019, 126, 35–43. [Google Scholar] [CrossRef]
- Mo, Z.-L.; Zhao, Z.-L.; Chen, H.; Niu, G.-P.; Shi, H.-F. Heterogeneous preparation of cellulose–polyaniline conductive composites with cellulose activated by acids and its electrical properties. Carbohydr. Polym. 2009, 75, 660–664. [Google Scholar] [CrossRef]
- Imali, D.E.; Perera, E.C.J.; Kaumal, M.N.; Dissanayake, D.P. Fabrication and characterization of a flexible and disposable impedance-type humidity sensor based on polyaniline (PAni). RSC Adv. 2023, 13, 6396–6411. [Google Scholar] [CrossRef]
- Kotresh, S.; Ravikiran, Y.T.; Raj Prakash, H.G.; Ramana, C.V.V.; Vijayakumari, S.C.; Thomas, S. Humidity sensing performance of spin coated polyaniline– carboxymethyl cellulose composite at room temperature. Cellulose 2016, 23, 3177–3186. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, X.; Qi, R.; Dai, J.; Liu, S.; Feng, T.; Zhang, T. A composite structure of in situ cross-linked poly (ionic liquid)s and paper for humidity-monitoring applications. IEEE Sens. J. 2019, 19, 833–837. [Google Scholar] [CrossRef]
- Falco, A.; Marín-Sánchez, A.; Loghin, F.C.; Castillo, E.; Salinas-Castillo, A.; Salmerón, J.F.; Rivadeneyra, A. Paper and salt: Biodegradable NaCl-based humidity sensors for sustainable electronics. Front. Electron. 2022, 3, 838472. [Google Scholar] [CrossRef]
- Guan, X.; Hou, Z.; Wu, K.; Zhao, H.; Liu, S.; Fei, T.; Zhang, T. Flexible humidity sensor based on modified cellulose paper. Sens. Actuators B 2021, 339, 129879. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Marín-Sanchez, A.; Wicklein, B.; Salmeron, J.F.; Castillo, E.; Bobinger, M.; Salinas-Castillo, A. Cellulose nanofibers as substrate for flexible and biodegradable moisture sensors. Compos. Sci. Technol. 2021, 208, 108738. [Google Scholar] [CrossRef]
- Sahoo, K.; Mohanty, B.; Biswas, A.; Nayak, J. Role of hexamethylenetetramine in ZnO-cellulose nanocomposite enabled UV and humidity sensor. Mater. Sci. Semicond. Proces. 2020, 105, 104699. [Google Scholar] [CrossRef]
- Syrový, T.; Maronová, S.; Kuberský, P.; Ehman, N.V.; Vallejos, M.E.; Pretl, S.; Felissia, F.E.; Area, M.C.; Chinga-Carrasco, G. Wide range humidity sensors printed on biocomposite films of cellulose nanofibril and poly(ethylene glycol). J. Appl. Polym. Sci. 2019, 136, 47920. [Google Scholar] [CrossRef]
- Nash, C.; Spiesschaert, Y.; Amarandei, G.; Stoeva, Z.; Tomov, R.I.; Tonchev, D.; van Driessche, I.; Glowacki, B.A. A comparative study on the conductive properties of coated and printed silver layers on a paper substrate. J. Electron. Mater. 2015, 44, 497–510. [Google Scholar] [CrossRef]
- Farrell, M.J.; Ormond, R.B.; Gabler, W.J. Quantitative analysis of trimethyl amine in cotton fabrics cationized with 3-chloro-2-hydroxypropyltrimethylammonium chloride. Cellulose 2015, 22, 3435–3439. [Google Scholar] [CrossRef]
- Correia, J.; Rainert, K.T.; Oliveira, F.R.; de Ca’ssia Siqueira, R.; Valle, C.; Valle, J.A.B. Cationization of cotton fiber: An integrated view of cationic agents, processes variables, properties, market and future prospects. Cellulose 2020, 27, 8527–8550. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Xu, Y.; Shen, M.; Duan, C.; Dai, L.; Ni, Y. Green and sustainable cellulose-derived humidity sensors: A review. Carbohydr. Polym. 2021, 270, 118385. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, X.-H.; Zhang, B.-Y.; Zhang, Z.; Hou, D.; Zhou, Z.-K. Facile fabrication of high sensitivity cellulose nanocrystals based QCM humidity sensors with asymmetric electrode structure. Sens. Actuators B Chem. 2020, 302, 127192. [Google Scholar] [CrossRef]
- Chen, W.X.; Chen, B.; Lv, R.X.; Wu, M.L.; Zhou, J. Fabrication of quartz crystal microbalance humidity sensors based on super-hydrophilic cellulose nanocrystals. Cellulose 2021, 28, 3409–3421. [Google Scholar] [CrossRef]
- Tang, L.; Chen, W.; Chen, B.; Lv, R.; Zheng, X.; Rong, C.; Lu, B.; Huang, B. Sensitive and renewable quartz crystal microbalance humidity sensor based on nitrocellulose nanocrystals. Sens. Actuators B Chem. 2021, 327, 128944. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, X.H.; Chen, Q.; Zhang, Z.; Ling, W.W. High sensitivity and high stability QCM humidity sensors based on polydopamine coated cellulose nanocrystals/graphene oxide nanocomposite. Nanomaterials 2020, 10, 2210. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Guo, Y.J.; Li, D.J.; Long, G.D.; Tang, Q.B. Bacterial cellulose coated ST-cut quartz surface acoustic wave humidity sensor with high sensitivity, fast response and recovery. Smart Mater. Struct. 2020, 29, 045037. [Google Scholar] [CrossRef]
- Zheng, Z.; Tang, C.X.; Yeow, J.T.W. A high-performance CMUT humidity sensor based on cellulose nanocrystal sensing film. Sens. Actuators B Chem. 2020, 320, 128596. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Zhou, B.; Liu, L.; Ding, B.; Wang, H. Highly stable and sensitive humidity sensors based on quartz crystal microbalance coated with bacterial cellulose membrane. Sens. Actuators B Chem. 2011, 159, 301–306. [Google Scholar] [CrossRef]
- Pawar, D.; Kale, S.N. A review on nanomaterial-modified optical fiber sensors for gases, vapors and ions. Microchim. Acta 2019, 186, 253. [Google Scholar] [CrossRef]
- Lokman, A.; Nodehi, S.; Batumalay, M.; Ahmad, H.A.; Harun, S.W. Optical fiber humidity sensor based on a tapered fiber with hydroxyethylcellulose/polyvinylidenefluoride composite. Microw. Opt. Technol. Let. 2013, 56, 380–382. [Google Scholar] [CrossRef]
- Muhammad, M.Z.; Lokman, A.; Batumalay, M.; Arof, H.; Ahmad, H.; Harun, W. Tapered fiber coated with hydroxyethyl cellulose/polyvinylidene fluoride composite for relative humidity sensor. Adv. Mater. Sci. Eng. 2013, 2013, 624314. [Google Scholar] [CrossRef]
- Ma, Q.; Ni, K.; Huang, R. A carboxy-methyl cellulose coated humidity sensor based on Mach-Zehnder interferometer with waist-enlarged bi-tapers. Opt. Fiber Technol. 2017, 33, 60–63. [Google Scholar] [CrossRef]
- Hu, S.; Wu, S.; Li, C.; Chen, R.; Forsberg, E.; He, S. SNR-enhanced temperature-insensitive microfiber humidity sensor based on upconversion nanoparticles and cellulose liquid crystal coating. Sens. Actuators B Chem. 2020, 305, 127517. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Sun, H.; Yang, Y.; Ye, Y.; Cui, J.; He, W.; Yong, X.; Xie, Y. An optical fiber sensor based on carboxymethyl cellulose/carbon nanotubes composite film for simultaneous measurement of relative humidity and temperature. Opt. Commun. 2020, 467, 125740. [Google Scholar] [CrossRef]
- Xu, W.; Huang, W.-B.; Huang, X.-G.; Yu, C.-Y. A simple fiber-optic humidity sensor based on extrinsic Fabry–Perot cavity constructed by cellulose acetate butyrate film. Opt. Fiber Technol. 2013, 19, 583–586. [Google Scholar] [CrossRef]
- Hokkanen, A.; Kapulainen, M.; Jaiswal, A.K.; Hynninen, V.; Nonappa; Ikkala, O.; Orelm, H. Cellulose optical fiber for sensing applications. Proc. SPIE 2022, 11953, 1195302. [Google Scholar] [CrossRef]
- Eyebe, G.A.; Bideau, B.; Boubekeur, N.; Loranger, E.; Domingue, F. Environmentally-friendly cellulose nanofibre sheets for humidity sensing in microwave frequencies. Sens. Actuators B Chem. 2017, 245, 484–492. [Google Scholar] [CrossRef]
- Eyebe, G.A.; Bideau, B.; Boubekeur, N.; Loranger, E.; Domingue, F. TEMPO-oxidized cellulose nanofibre (TOCN) films and composites with PVOH as sensitive dielectrics for microwave humidity sensing. Sens. Actuators B Chem. 2019, 291, 385–393. [Google Scholar] [CrossRef]
- Tanguy, N.R.; Moradpour, M.; Jain, M.C.; Ning Yan, N.; Zarifi, M.H. Exploring the Potential of Cellulose Nanofibrils for Humidity Sensing Using an Organic Microwave Resonator. In Proceedings of the 2021 IEEE Sensors Conference, Sydney, Australia, 31 October–3 November 2021. [Google Scholar] [CrossRef]
- Antti, R.; Hanhikorpi, M.; Bertuccelli, F.; Colonna, A.; Malik, W.; Ranasinghe, D.; Lopéz, T.S.; Yan, N.; Tavilampi, M. Sensor-Enabled RFID Tag Handbook; European Commission: Munich, Germany, 2008.
- Gao, J.; Sidén, J.; Nilsson, H.-E.; Gulliksson, M. Printed humidity sensor with memory functionality for passive RFID tags. IEEE Sens. J. 2013, 13, 1824–1834. [Google Scholar] [CrossRef]
- Kerry, J.; Butler, P. Smart Packaging Technologies for Fast Moving Consumer Goods; John Wiley and Sons, Ltd.: Chichester, UK, 2008. [Google Scholar]
- Yang, L.; Zhang, R.; Staiculescu, D.; Wong, C.; Tentzeris, M. A novel conformal RFID-enabled module utilizing inkjet-printed antennas and carbon nanotubes for gas-detection applications. IEEE Antennas Wirel. Propag. Lett. 2009, 8, 653–656. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Nagraj, N.; Tang, Z.; Mondello, F.J.; Surman, C.; Morris, W. Battery-free radio frequency identification (RFID) sensors for food quality and safety. J. Agric. Food Chem. 2012, 60, 8535–8543. [Google Scholar] [CrossRef]
- Yang, L.; Rida, A.; Vyas, R.; Tentzeris, M.M. RFID tag and RF structures on a paper substrate using inkjet-printing technology. IEEE Trans. Microw. Theory Tech. 2007, 55, 2894–2901. [Google Scholar] [CrossRef]
- Shao, B.; Amin, Y.; Chen, Q.; Liu, R.; Zheng, L.-R. Directly-printed packaging paper based chipless RFID tag with coplanar LC resonator. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 325–328. [Google Scholar] [CrossRef]
- Virtanen, J.; Ukkonen, L.; Bjorninen, T.; Sydanheimo, L. Printed humidity sensor for UHF RFID systems. In Proceedings of the 2010 IEEE Sensors Applications Symposium (SAS), Limerick, Ireland, 23–25 February 2010; pp. 269–272. [Google Scholar] [CrossRef]
- Virtanen, J.; Ukkonen, L.; Bjorninen, T.; Elsherbeni, A.Z.; Sydänheimo, L. Inkjet-printed humidity sensor for passive UHF RFID systems. IEEE Trans. Instrum. Meas. 2011, 60, 2768–2777. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, L.; Chen, Q.; Zheng, L.R. Low-cost printed chipless RFID humidity sensor tag for intelligent packaging. IEEE Sens. J. 2015, 15, 3201–3208. [Google Scholar] [CrossRef]
- Withayachumnankul, W.; Fumeaux, C.; Abbott, D. Compact electric-LC resonators for metamaterials. Opt. Express 2010, 18, 25912. [Google Scholar] [CrossRef]
- Quddious, A.; Yang, S.; Khan, M.M.; Tahir, F.A.; Shamim, A.; Salama, K.N.; Cheema, H.M. Disposable, Paper-based, inkjet-printed humidity and H2S gas sensor for passive sensing applications. Sensors 2016, 16, 2073. [Google Scholar] [CrossRef]
- Xie, M.-Z.; Wang, L.-F.; Dong, L.; Deng, W.-J.; Huang, Q.-A. Low cost paper-based LC wireless humidity sensors and distance-insensitive readout system. IEEE Sens. J. 2019, 19, 4717–4725. [Google Scholar] [CrossRef]
- Tan, E.L.; Ng, W.N.; Shao, R.; Pereles, B.D.; Ong, K.G. A wireless, passive sensor for quantifying packaged food quality. Sensors 2007, 7, 1747–1756. [Google Scholar] [CrossRef]
- Fraiwan, A.; Lee, H.; Choi, S. A paper-based cantilever array sensor: Monitoring volatile organic compounds with naked eye. Talanta 2016, 158, 57–62. [Google Scholar] [CrossRef]
- Tian, P.; Gao, X.; Wen, G.; Zhong, L.; Wang, Z.; Guo, Z. Novel fabrication of polymer/carbon nanotube composite coated Janus paper for humidity stress sensor. J. Colloid. Interface Sci. 2018, 532, 517–526. [Google Scholar] [CrossRef]
- Sansinena, J.M.; Gao, J.; Wang, H.L. High-performance, monolithic polyaniline electrochemical actuators. Adv. Funct. Mater. 2003, 13, 703–709. [Google Scholar] [CrossRef]
- Wang, M.; Tian, X.; Ras, R.H.A.; Ikkala, O. Sensitive humidity-driven reversible and bidirectional bending of nanocellulose thin films as bio-inspired actuation. Adv. Mater. Interfaces 2015, 2, 1500080. [Google Scholar] [CrossRef]
- Geissler, A.; Loyal, F.; Biesalski, M.; Zhang, K. Thermo-responsive superhydrophobic paper using nanostructured cellulose stearoyl ester. Cellulose 2014, 21, 357–366. [Google Scholar] [CrossRef]
- Zhang, K.; Geissler, A.; Standhardt, M.; Mehlhase, S.; Gallei, M.; Chen, L.; Thiele, C.M. Moisture-responsive films of cellulose stearoyl esters showing reversible shape transitions. Sci. Rep. 2015, 5, 11011. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Bi, W.; Zhong, C.; Huang, M.; Ni, Y.; He, L.; Wu, C. Moisture-triggered actuator and detector with high-performance: Interface engineering of graphene oxide/ethyl cellulose. Sci. China Mater. 2018, 61, 1291–1296. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Li, J.; Ye, S.; Wei, J.; Guo, J. A bio-inspired cellulose nanocrystal-based nanocomposite photonic film with hyper-reflection and humidity-responsive actuator properties. J. Mater. Chem. C 2016, 4, 9687–9696. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Dai, L.; Sun, X.; An, M.; Duan, C.; Li, J.; Ni, Y. Asymmetrically patterned cellulose nanofibers/graphene oxide composite film for humidity sensing and moist-induced electricity generation. ACS Appl. Mater. Interfaces 2020, 12, 55205–55214. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, L.; Yao, L.; Weng, M.; Zhang, W. Humidity- and light-driven actuators based on carbon nanotube-coated paper and polymer composite. Nanoscale 2018, 10, 8422–8427. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, Z.; Song, Q.; Chen, K.; Su, G.; Zhou, T.; Zheng, Z.; Lu, C.; Zhang, X. Ultrarobust Ti3C2Tx MXene-based soft actuators via bamboo-inspired mesoscale assembly of hybrid nanostructures. ACS Nano 2020, 14, 7055–7065. [Google Scholar] [CrossRef]
- Wu, M.; Sukyai, P.; Lv, D.; Zhang, F.; Wang, P.; Liu, C.; Li, B. Water and humidity-induced shape memory cellulose nanopaper with quick response, excellent wet strength and folding resistance. Chem. Eng. J. 2020, 392, 123673. [Google Scholar] [CrossRef]
- He, Y.; Zhang, M.; Zhang, N.; Zhu, D.; Huang, C.; Kang, L.; Zhou, X.; Hu, M.; Zhang, J. Paper-based ZnS:Cu alternating current electroluminescent devices for current humidity sensors with high–linearity and flexibility. Sensors 2019, 19, 4607. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, S.H.; Jeong, T.; Bae, M.J.; Song, S.; Lee, J.; Han, I.T.; Jung, D.; Yu, S. Paper as a substrate for inorganic powder electroluminescence devices. IEEE Trans. Electron. Dev. 2010, 57, 1470–1474. [Google Scholar] [CrossRef]
- Blinn, B.G. Properties and uses of color change humidity indicators. In Humidity and Moisture: Principles and Methods of Measuring Humidity in Gases; Wexler, A., Ruskin, R.E., Eds.; Reinhold Publishing Corporation: New York, NY, USA, 1965; pp. 602–605. [Google Scholar]
- Katzin, L.I.; Ferraro, J.R. The system cobaltous chloride—Water—Acetone at 25°. J. Am. Chem. Soc. 1952, 74, 2752–2754. [Google Scholar] [CrossRef]
- Balköse, D.; Köktürk, U.; Yilmaz, H. A study of cobaltous chloride dispersion on the surface of the silica gel. Appl. Surf. Sci. 1999, 147, 77–84. [Google Scholar] [CrossRef]
- Boltinghouse, F.; Abel, K. Development of an optical relative humidity sensor. Cobalt chloride optical absorbency sensor study. Anal. Chem. 1989, 61, 1863–1866. [Google Scholar] [CrossRef]
- Spomer, L.A.; Tibbits, T.W. Humidity. In Plant Growth Chamber Handbook; Langhans, R.W., Tibbits, T.W., Eds.; Iowa State University of Science and Technology: Ames, IA, USA, 1997; pp. 43–64. [Google Scholar]
- Moreton, S. Silica gel impregnated with iron(III) salts: A safe humidity indicator. Mater. Res. Innov. 2002, 5, 226–229. [Google Scholar] [CrossRef]
- Matsushima, R.; Nishimura, N.; Goto, K.; Kohno, Y. Vapochromism of ionic dyes in thin films of sugar gels. J. Bull. Chem. Soc. Jpn. 2003, 76, 1279–1283. [Google Scholar] [CrossRef]
- Matsushima, R.; Atsushi, O.; Fujimoto, S. Thermochromic properties of flavylium salts in agar-gel matrix. Chem. Lett. 2000, 6, 590–591. [Google Scholar] [CrossRef]
- Matsushima, R.; Ogiue, A.; Kohno, Y. Humidity-sensitive color changes of ionic dyes in solid thin film of sugar gel. Chem. Lett. 2002, 4, 436–437. [Google Scholar] [CrossRef]
- Carmona, N.; Herrero, E.; Llopis, J.; Villegas, M.A. Chemical sol-gel-based sensors for evaluation of environmental humidity. Sens. Actuators B Chem. 2006, 126, 455–460. [Google Scholar] [CrossRef]
- Fueda, Y.; Matsumoto, J.; Shiragami, T.; Nobuhara, K.; Yasuda, M. Porphyrin/MgCl2/silica gel composite as a cobalt-free humidity indicator. Chem. Lett. 2007, 36, 1246–1247. [Google Scholar] [CrossRef]
- Mackenzie, K.; O’Leary, B. Inorganic polymers (geopolymers) containing acid-base indicators as possible colour-change humidity indicators. Mater. Lett. 2008, 63, 230–232. [Google Scholar] [CrossRef]
- Mills, A.; Grosshans, P.; Hazafy, D. A novel reversible relative-humidity indicator ink based on methylene blue and urea. Analyst 2010, 135, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Hawthorne, D.; Burns, L.; Hazafy, D. Novel temperature-activated humidity-sensitive optical sensor. Sens. Actuators B Chem. 2017, 240, 1009–1015. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Chodavarapu, V.P.; Kirk, A.G.; Andrews, M.P. Structured color humidity indicator from reversible pitch tuning in self-assembled nanocrystalline cellulose films. Sens. Actuators B Chem. 2013, 176, 692–697. [Google Scholar] [CrossRef]
- Kunzelman, J.; Crenshaw, B.R.; Weder, C. Self-assembly of chromogenic dyes—A new mechanism for humidity sensors. J. Mater. Chem. 2007, 17, 2989–2991. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Zhai, S.; Sugiyama, J.; Pan, M.; Shi, J.; Lu, H. Dual response of photonic films with chiral nematic cellulose nanocrystals: Humidity and formaldehyde. ACS Appl. Mater. Interfaces 2020, 12, 17833–17844. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Lee, W.; Chung, U.; Ko, S. Study of humidity-responsive behavior in chiral nematic cellulose nanocrystal films for colorimetric response. Cellulose 2018, 25, 305–317. [Google Scholar] [CrossRef]
- Yao, K.; Meng, Q.; Bulone, V.; Zhou, Q. Flexible and responsive chiral nematic cellulose nanocrystal/poly(ethylene glycol) composite films with uniform and tunable structural color. Adv. Mater. 2017, 29, 1701323. [Google Scholar] [CrossRef]
- Chen, H.; Hoi, A.; Zheng, C.; Tang, J.; Xie, K.; Gao, A. Light- and humidity-responsive chiral nematic photonic crystal films based on cellulose nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 24505–24511. [Google Scholar] [CrossRef]
- He, Y.-D.; Zhang, Z.-L.; Xue, J.; Wang, X.-H.; Song, F.; Wang, X.-L.; Zhu, L.-L.; Wang, Y.-Z. Biomimetic optical cellulose nanocrystal films with controllable iridescent color and environmental stimuli-responsive chromism. ACS Appl. Mater. Interfaces 2018, 10, 5805–5811. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Ma, C.; Yu, H.; Wu, Y.; Wang, Y.; Chen, Z.; Li, J.; Liu, S. Multifunctional chiral nematic cellulose nanocrystals/glycerol structural colored nanocomposites for intelligent responsive films, photonic inks and iridescent coatings. J. Mater. Chem. C 2018, 6, 5391–5400. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, Y.; Ji, H.; Chen, J.; He, Z.; Long, Z.; Dong, C. Fabrication of environmental humidity-responsive iridescent films with cellulose nanocrystal/polyols. Carbohydr. Polym. 2020, 240, 116281. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Pan, H.; Ma, J.; Li, Y.; Bokhari, S.W.; Jiang, X.; Zhu, S.; Zhang, D. Cellulose nanocrystals/polyacrylamide composites of high sensitivity and cycling performance to gauge humidity. ACS Appl. Mater. Interfaces 2017, 9, 18231–18237. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhu, D.; Jia, H.; Lei, K.; Zheng, Z.; Wang, X. Humidity and heat dual response cellulose nanocrystals/poly(N-Isopropylacrylamide) composite films with cyclic performance. ACS Appl. Mater. Interfaces 2019, 11, 39192–39200. [Google Scholar] [CrossRef]
- Sui, Y.; Li, X.; Chang, W.; Wan, H.; Li, W.; Yang, F.; Yu, Z.-Z. Multi-responsive nanocomposite membranes of cellulose nanocrystals and poly(N-isopropyl acrylamide) with tunable chiral nematic structures. Carbohydr. Polym. 2020, 232, 115778. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Kwon, S.; Lee, W.; Ko, S. Optical response of photonic cellulose nanocrystal film for a novel humidity indicator. Intern. J. Biol. Macromol. 2019, 140, 91–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Z.; Fu, Y.; Wang, Z.; Qin, M.; Yuan, Z. Responsive and patterned cellulose nanocrystal films modified by N-methylmorpholine-N-oxide. Carbohydr. Polym. 2020, 228, 115387. [Google Scholar] [CrossRef]
- Koroleva, M.S.; Tracey, C.; Sidunets, Y.A.; Torlopov, M.A.; Mikhaylov, V.I.; Krivoshapkin, P.V.; Martakov, I.S.; Krivoshapkina, E.F. Environmentally friendly Au@CNC hybrid systems as prospective humidity sensors. RSC Adv. 2020, 10, 35031–35038. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, N.; Wang, Y.; Wu, J.; Gan, Q.; Li, W. Photo-patternable nanolayered polymeric films with fast tunable color responses triggered by humidity. Adv. Mater. 2019, 29, 1904453. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible electronics based on micro/nanostructured paper. Adv. Mater. 2018, 30, 1801588. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-based sensors for gas, humidity, and strain detections: A review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, G.; Shen, Y.; Yang, H.; Xu, K. Multifunctional flexible humidity sensor systems towards noncontact wearable electronics. Nano-Micro Lett. 2022, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, G.; Chen, C.; Gong, Q.; Xie, G.; Yao, M.; Tai, H.; Jiang, Y.; Chen, J. Self-powered respiration monitoring enabled by a triboelectric nanogenerator. Adv. Mater. 2021, 33, 2101262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, C.; Feng, G.; Fang, J. Hospitals and laboratories on paper-based sensors: A mini review. Sensors 2021, 21, 5998. [Google Scholar] [CrossRef]
- Barhoum, A.; Samyn, P.; Öhlund, T.; Dufresne, A. Review of recent research on flexible multifunctional nanopapers. Nanoscale 2017, 9, 15181–15205. [Google Scholar] [CrossRef]
- Zhu, H.; Parvinian, S.; Preston, C.; Vaaland, O.; Ruan, Z.; Hu, L. Transparent nanopaper with tailored optical properties. Nanoscale 2013, 5, 3787–3792. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, H.; Zao, W.; Ling, M.; Zhao, Y. Flexible superhydrophobic paper with a large and stable floating capacity. RSC Adv. 2014, 4, 48443–48448. [Google Scholar] [CrossRef]
- Knight, C.C.; Ip, F.; Zeng, C.; Zhang, C.; Wang, B. A highly efficient fire-retardant nanomaterial based on carbon nanotubes and magnesium hydroxide. Fire Mater. 2013, 37, 91–99. [Google Scholar] [CrossRef]
- Liu, F.; Song, S.; Xue, D.; Zhang, H. Folded structured graphene paper for high performance electrode materials. Adv. Mater. 2012, 24, 1089–1094. [Google Scholar] [CrossRef]
- Liu, H.; Qing, H.; Li, Z.; Han, Y.L.; Lin, M.; Yang, H.; Lie, A.; Lu, T.; Li, F.; Xu, F. Paper: A promising material for human-friendly functional wearable electronics. Mater. Sci. Eng. R. 2017, 112, 1–22. [Google Scholar] [CrossRef]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent advances in paper-based sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Coatsworth, P.; Shi, X.; Zhi, J.; Hu, L.; Yan, R.; Guder, F.; Yu, H.-D. Paper-based sensors for diagnostics, human activity monitoring, food safety and environmental detection. Sens. Diagn. 2022, 1, 312–342. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Fisenko, N.A.; Fedorov, F.S.; Simonenko, T.L.; Mokrushin, A.S.; Simonenko, E.P.; Korotcenkov, G.; Sysoev, V.V.; Sevastyanov, V.G.; Kuznetsov, N.T. Printing technologies as an emerging approach in gas sensors: Survey of literature. Sensors 2022, 22, 3473. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Yuan, Z.; Jiang, Y.; Yuan, L.; Tai, H. Amorphous carbon material of daily carbon ink: Emerging applications in pressure, strain, and humidity sensors. J. Mater. Chem. C, 2023; in press. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindstrom, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Afra, E.; Yousefi, H.; Hadilam, M.M.; Nishino, T. Comparative effect of mechanical beating and nanofibrillation of cellulose on paper properties made from bagasse and softwood pulps. Carbohydr. Polym. 2013, 97, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Faezipour, M.; Hedjazi, S.; Mousavi, M.M.; Azusa, Y.; Heidari, A.H. Comparative study of paper and nanopaper properties prepared from bacterial cellulose nanofibers and fibers/ground cellulose nanofibers of canola straw. Ind. Crops Prod. 2013, 43, 732–737. [Google Scholar] [CrossRef]
- Yousefi, H.; Azad, S.; Mashkour, M.; Khazaeian, A. Cellulose nanofiber board. Carbohydr. Polym. 2018, 187, 133–139. [Google Scholar] [CrossRef]
- Ostlund, S.; Niskanen, K. (Eds.) Mechanics of Paper Products; De. Gruyter: Berlin, Germany, 2011. [Google Scholar] [CrossRef]
- Zauscher, S.; Caulfield, D.F.; Nissan, A.H. The influence of water on the elastic modulus of paper. Tappi J. 1996, 79, 178–182. [Google Scholar]
- Korotcenkov, G. Black phosphorus–new nanostructured material for humidity sensors: Achievements and limitations. Sensors 2019, 19, 1010. [Google Scholar] [CrossRef]
- Feng, Y.; Gong, S.; Du, E.; Yu, K.; Ren, J.; Wang, Z.; Zhu, Z. TaS2 nanosheet-based ultrafast response and flexible humidity sensor for multifunctional applications. J. Mater. Chem. C 2019, 7, 9284–9292. [Google Scholar] [CrossRef]
- Zavabeti, A.; Jannat, A.; Zhong, L.; Haidry, A.A.; Yao, Z.; Ou, J.Z. Two-dimensional materials in large-areas: Synthesis, properties and applications. Nano-Micro Lett. 2020, 12, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent advances in two-dimensional materials-based sensing technology towards health and environmental applications. Nanoscale 2020, 12, 3535–3559. [Google Scholar] [CrossRef] [PubMed]
- Simonenko, E.P.; Simonenko, N.P.; Mokrushin, A.S.; Simonenko, T.L.; Gorobtsov, P.Y.; Nagornov, I.A.; Korotcenkov, G.; Sysoev, V.V.; Kuznetsov, N.T. Application of titanium carbide MXenes in chemiresistive gas sensors. Nanomaterials 2023, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- Nassar, J.M.; Mishra, K.; Lau, K.; Aguirre-Pablo, A.A.; Hussain, M.M. Recyclable nonfunctionalized paper-based ultralow-cost wearable health monitoring system. Adv. Mater. Technol. 2017, 2, 1600228. [Google Scholar] [CrossRef]
- Cinti, S.; Moscone, D.; Arduini, F. Preparation of paper-based devices for reagentless electrochemical (bio)sensor strips. Nat. Protoc. 2019, 14, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Shi, X.; Gao, E.; Xu, Z.; Tang, H.; Tong, K.; Pei, Q.; Liang, J.; Chen, Y. Rollerball-pen-drawing technology for extremely foldable paper-based electronics. Adv. Electron. Mater. 2017, 3, 1700098. [Google Scholar] [CrossRef]
- Huang, G.-W.; Xiao, H.-M.; Fu, S.-Y. Paper-based silver-nanowire electronic circuits with outstanding electrical conductivity and extreme bending stability. Nanoscale 2014, 6, 8495–8502. [Google Scholar] [CrossRef]
- Hyun, W.J.; Park, O.O.; Chin, B.D. Foldable graphene electronic circuits based on paper substrates. Adv. Mater. 2013, 25, 4729–4734. [Google Scholar] [CrossRef]
- Kan, H.; Li, M.; Luo, J.; Zhang, B.; Liu, J.; Hu, Z.; Zhang, G.; Jiang, S.; Liu, H. PbS nanowires-on-paper sensors for room-temperature gas detection. IEEE Sens. J. 2018, 19, 846–851. [Google Scholar] [CrossRef]
- Guo, R.; Chen, J.; Yang, B.; Liu, L.; Su, L.; Shen, B.; Yan, X. In-plane micro-supercapacitors for an integrated device on one piece of paper. Adv. Funct. Mater. 2017, 27, 1702394. [Google Scholar] [CrossRef]
- Liu, H.; Xiang, H.; Wang, Y.; Li, Z.; Qian, L.; Li, P.; Ma, Y.; Zhou, H.; Huang, W. A flexible multimodal sensor that detects strain, humidity, temperature, and pressure with carbon black and reduced graphene oxide hierarchical composite on paper. ACS Appl. Mater. Interfaces 2019, 11, 40613–40619. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Yang, X.; Li, Z.; Yang, B.-R.; Liu, C.; Qiu, L.; Wang, X. Multifunctional and high-sensitive sensor capable of detecting humidity, temperature, and flow stimuli using an integrated microheater. ACS Appl. Mater. Interfaces 2019, 11, 43383. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Phan, H.-P.; Dao, D.V.; Woodfield, P.; Qamar, A.; Nguyen, N.-T. Graphite on paper as material for sensitive thermoresistive sensors. J. Mater. Chem. C 2015, 3, 8776–8779. [Google Scholar] [CrossRef]
- Gomathi, P.T.; Sahatiya, P.; Badhulika, S. Large-area, flexible broadband photodetector based on ZnS-MoS2 hybrid on paper substrate. Adv. Funct. Mater. 2017, 27, 1701611. [Google Scholar] [CrossRef]
- Tao, X.; Jia, H.; He, Y.; Liao, S.; Wang, Y. Ultrafast paper thermometers based on a green sensing ink. ACS Sens. 2017, 2, 449–454. [Google Scholar] [CrossRef]
- Bihar, E.; Wustoni, S.; Pappa, A.M.; Salama, K.N.; Baran, D.; Inal, S. A fully inkjet-printed disposable glucose sensor on paper. NPJ Flex. Electron. 2018, 2, 30. [Google Scholar] [CrossRef]
- Ding, R.; Krikstolaityte, V.; Lisak, G. Inorganic salt modified paper substrates utilized in paper based microfluidic sampling for potentiometric determination of heavy metals. Sens. Actuators B Chem. 2019, 290, 347–356. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Ha, M.; Cho, S.; Ko, H. Micro/nanostructured surfaces for self-powered and multifunctional electronic skins. J. Mater. Chem. B 2016, 4, 2999–3018. [Google Scholar] [CrossRef]
- Islam, T.; Uddin, Z.; Gangopadhyay, A. Temperature effect on capacitive humidity sensors and its compensation using artificial neural networks. Sens. Transducers 2015, 191, 126–134. [Google Scholar]
- Carvajal, S.A.; Sánchez, C.A. Temperature effect in the calibration of capacitive humidity sensors. Int. J. Metrol. Qual. Eng. 2018, 9, 9. [Google Scholar] [CrossRef]
- Nenov, T.; Ivanov, S. Linearization of characteristic of relative humidity sensor and compensation of temperature impact. Sens. Mater. 2007, 19, 95–106. [Google Scholar]
- Korotcenkov, G.; Cho, B.K. Engineering approaches to improvement operating characteristics of conductometric gas sensors. Part 2: Decrease of dissipated (consumable) power and improvement stability and reliability. Sens. Actuators B Chem. 2014, 198, 316–341. [Google Scholar] [CrossRef]
- Yang, W.; Li, W.; Lu, H.; Liu, J.; Zhang, T. Dynamic compensation method for humidity sensors based on temperature and humidity decoupling. Sensors 2022, 22, 7229. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Pramanik, C.; Saha, H. Modelling, simulation and temperature compensation of porous polysilicon capacitive humidity sensor characteristic of relative humidity sensor using ANN techniques. Microelectron. Reliab. 2005, 45, 697–703. [Google Scholar] [CrossRef]
- Rivera, J.; Carrillo, M.; Chacón, M.; Herrera, G.; Bojorquez, G. Self-calibration and optimal response in intelligent sensors design based on artificial neural networks. Sensors 2007, 7, 1509–1529. [Google Scholar] [CrossRef]
- Khan, S.M.; Qaiser, N.; Hussain, M.M. In do-it-yourself (DIY) based flexible paper sensor based electronic system for pill health monitoring. In Proceedings of the 2018 International Flexible Electronics Technology Conference (IFETC), Ottawa, ON, Canada, 7−9 August, 2018; pp. 1–2. [Google Scholar] [CrossRef]
- Khan, S.M.; Nassar, J.M.; Hussain, M.M. Paper as a substrate and an active material in paper electronics. ACS Appl. Electron. Mater. 2021, 3, 30–52. [Google Scholar] [CrossRef]
- Khan, S.M.; Qaiser, N.; Shaikh, S.F.; Ding, L.J.; Hussain, M.M. Do-it-yourself integration of a paper sensor in a smart lid for medication adherence. Flexible Print. Electron. 2019, 4, 025001. [Google Scholar] [CrossRef]
- Valadares Romanholo, P.V.; Sgobbi, L.F.; Carrilho, E. Chapter One—Exploring paper as a substrate for electrochemical micro-devices. Comprehen. Anal. Chem. 2020, 89, 1–29. [Google Scholar] [CrossRef]
- Grau, G.; Kitsomboonloha, R.; Swisher, S.L.; Kang, H.; Subramanian, V. Printed transistors on paper: Towards smart consumer product packaging. Adv. Funct. Mater. 2014, 24, 5067–5074. [Google Scholar] [CrossRef]
- Liao, X.; Liao, Q.; Yan, X.; Liang, Q.; Si, H.; Li, M.; Wu, H.; Cao, S.; Zhang, Y. Flexible and highly sensitive strain sensors fabricated by pencil drawn for wearable monitor. Adv. Funct. Mater. 2015, 25, 2395–2401. [Google Scholar] [CrossRef]
- He, X.; Zi, Y.; Yu, H.; Zhang, S.L.; Wang, J.; Ding, W.; Zou, H.; Zhang, W.; Wang, Z.L.; Wang, Z.L. An ultrathin paper-based self-powered system for portable electronics and wireless human-machine interaction. Nano Energy 2017, 39, 328–336. [Google Scholar] [CrossRef]
- Liu, A.; Kovacik, P.; Peard, N.; Tian, W.; Goktas, H.; Lau, J.; Dunn, B.; Gleason, K.K. Monolithic flexible supercapacitors integrated into single sheets of paper and membrane via vapor printing. Adv. Mater. 2017, 29, 1606091. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.T.; Araujo, A.; Mendes, M.J.; Nunes, D.; Oliveira, M.J.; Sanchez-Sobrado, O.; Ferreira, M.P.; Agus, H.; Fortunato, E.; Martins, R. Multifunctional cellulose-paper for light harvesting and smart sensing applications. J. Mater. Chem. C 2018, 6, 3143–3181. [Google Scholar] [CrossRef]
- Carneiro, M.C.C.G.; Rodrigues, L.R.; Moreira, F.T.C.; Sales, M.G.F. Colorimetric paper-based sensors against cancer biomarkers. Sensors 2022, 22, 3221. [Google Scholar] [CrossRef]
- Liu, X.Y.; Mwangi, M.; Li, X.J.; O’Brien, M.; Whitesides, G.M. Paper-based piezoresistive MEMS sensors. Lab Chip 2011, 11, 2189–2196. [Google Scholar] [CrossRef]
- Ahmed, S.; Bui, M.-P.N.; Abbas, A. Paper-based chemical and biological sensors: Engineering aspects. Biosens. Bioelectron. 2016, 77, 249–263. [Google Scholar] [CrossRef]
- Casula, G.; Lai, S.; Matino, L.; Santoro, F.; Bonfiglio, A.; Cosseddu, P. Printed, low voltage, all organic transistors and complimentary circuits on paper substrate. Adv. Electron. Mater. 2010, 6, 1901027. [Google Scholar] [CrossRef]
- Deroco, P.B.; Wachholz, D., Jr.; Kubota, L.T. Paper-based wearable electrochemical sensors: A new generation of analytical devices. Electroanalysis 2023, 35, e202200177. [Google Scholar] [CrossRef]
- Ghasemi, F.; Fahimi-Kashani, N.; Bigdeli, A.; Alshatteri, A.; Abbasi-Moayed, S.; Al-Jaf, S.H.; Merry, M.Y.; Omer, K.M.; Hormozi-Nezhad, M.R. Paper-based optical nanosensors–A review. Anal. Chim. Acta 2023, 1238, 340640. [Google Scholar] [CrossRef]
- Song, Y.; Mukasa, D.; Zhang, H.; Gao, W. Self-powered wearable biosensors. Acc. Mater. Res. 2021, 2, 184–197. [Google Scholar] [CrossRef]
- Xu, C.; Song, Y.; Han, M.; Zhang, H. Portable and wearable self-powered systems based on emerging energy harvesting technology. Microsyst. Nanoeng. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhang, J.; Kou, T.; Song, Y.; Liu, T.; Li, Y. Paper-based electrodes for flexible energy storage devices. Adv. Sci. 2017, 4, 1700107. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Yang, R.; Li, W.; Tao, Y.; Chen, C.; Tai, H.; Su, Y.; Wang, Y.; Jiang, Y.; Li, W. Self-powered multifunctional flexible sensor for wearable biomonitoring. Sens. Actuators B Chem. 2023, 377, 132996. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, H.; Zhang, Z.; Jiang, L.; Qu, L. Direct power generation from a graphene oxide film under moisture. Adv. Mater. 2015, 27, 4351–4357. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, H.; Ward, J.E.; Liu, X.; Yin, B.; Fu, T.; Chen, J.; Lovley, D.R.; Yao, J. Power generation from ambient humidity using protein nanowires. Nature 2020, 578, 550–554. [Google Scholar] [CrossRef]
- Komazaki, Y.; Kanazawa, K.; Nobeshima, T.; Hirama, H.; Watanabe, Y.; Suemori, K.; Uemura, S. Energy harvesting by ambient humidity variation with continuous milliampere current output and energy storage. Sustain. Energy Fuels 2021, 5, 3570–3577. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Liang, X.; Wang, H.; Lu, H.; Zhu, M.; Wang, H.; Mingchao Zhang, M.; Qiu, X.; Song, Y.; et al. Humidity-sensitive chemoelectric flexible sensors based on metal-air redox reaction for health management. Nat. Commun. 2022, 13, 5416. [Google Scholar] [CrossRef]
- Kan, Y.; Meng, J.; Guo, Y.; Li, X.; Gao, D. Humidity sensor based on cobalt chloride/cellulose filter-paper for respiration monitoring. J. Electroanal. Chem. 2021, 895, 115423. [Google Scholar] [CrossRef]
- Li, X.; Meng, J.; Guo, Y.; Li, X.; Li, M.; Gao, D. Preparation and performance of polypyrrole modified filter paper humidity sensor by in situ polymerization. Text. Res. J. 2023, 93, 1389–1400. [Google Scholar] [CrossRef]
- Fraiwan, A.; Choi, S. A stackable, two-chambered, paper-based microbial fuel cell. Biosens. Bioelectron. 2016, 83, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Thom, N.K.; Yeung, K.; Pillion, M.B.; Phillips, S.T. “Fluidic batteries” as low-cost sources of power in paper-based microfluidic devices. Lab Chip 2012, 12, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.P.; Conchouso, D.; Arevalo, A.; Singh, D.; Foulds, I.G.; Hussain, M.M. Paper-based origami flexible and foldable thermoelectric nanogenerator. Nano Energy 2017, 31, 296–301. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; Cui, Y. Printed energy storage devices by integration of electrodes and separators into single sheets of paper. Appl. Phys. Lett. 2010, 96, 183502. [Google Scholar] [CrossRef]
- Yuan, L.; Xiao, X.; Ding, T.; Zhong, J.; Zhang, X.; Shen, Y.; Hu, B.; Huang, Y.; Zhou, J.; Wang, Z.L. Paper-based supercapacitors for self-powered nanosystems. Angew. Chem. Intern. Ed. 2012, 51, 4934–4938. [Google Scholar] [CrossRef]
- He, X.; Zou, H.; Geng, Z.; Wang, X.; Ding, W.; Hu, F.; Zi, Y.; Xu, C.; Zhang, S.L.; Yu, H.; et al. A hierarchically nanostructured cellulose fiber-based triboelectric nanogenerator for self-powered healthcare products. Adv. Fanct. Mater. 2018, 28, 1805540. [Google Scholar] [CrossRef]
- Shen, D.; Xiao, M.; Zou, G.; Liu, L.; Duley, W.W.; Zhou, Y.N. Self-powered wearable electronics based on moisture enabled electricity generation. Adv. Mater. 2018, 30, 1705925. [Google Scholar] [CrossRef]
- Shen, D.; Duley, W.W.; Peng, P.; Xiao, M.; Feng, J.; Liu, L.; Zou, G.; Zhou, Y.N. Moisture-enabled electricity generation: From physics and materials to self-powered applications. Adv. Mater. 2020, 32, 2003722. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Y.; Duan, Z.; Yuan, Z.; Zha, J.; Wu, Z.; Huang, Q.; Zhou, Z.; Li, H.; He, F.; et al. A Nb2CTx/sodium alginate-based composite film with neuron-like network for self-powered humidity sensing. Chem. Eng. J. 2022, 438, 135588. [Google Scholar] [CrossRef]
- Duan, Z.; Yuan, Z.; Jiang, Y.; Zhao, Q.; Huang, Q.; Zhang, Y.; Liu, B.; Tai, H. Power generation humidity sensor based on primary battery structure. Chem. Eng. J. 2022, 446, 136910. [Google Scholar] [CrossRef]
- Jiang, Y.; Duan, Z.; Fan, Z.; Yao, P.; Yuan, Z.; Jiang, Y.; Cao, Y.; Tai, H. Power generation humidity sensor based on NaCl/halloysite nanotubes for respiratory patterns monitoring. Chemical 2023, 380, 133396. [Google Scholar] [CrossRef]
- Hu, L.; Choi, J.W.; Yang, Y.; Jeong, S.; La Mantia, F.; Cui, L.-F.; Cui, Y. Highly conductive paper for energy-storage devices. Proc. Natl. Acad. Sci. USA 2009, 106, 21490–21494. [Google Scholar] [CrossRef] [PubMed]


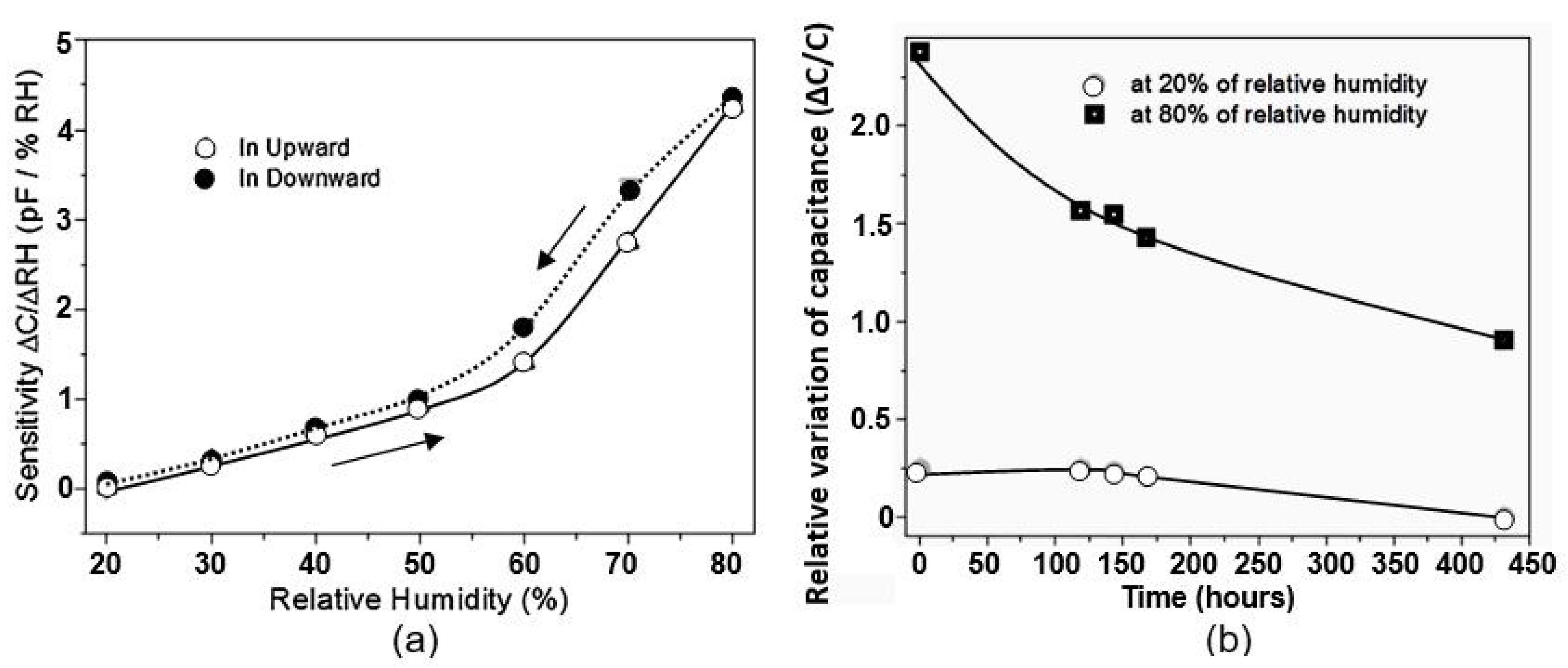

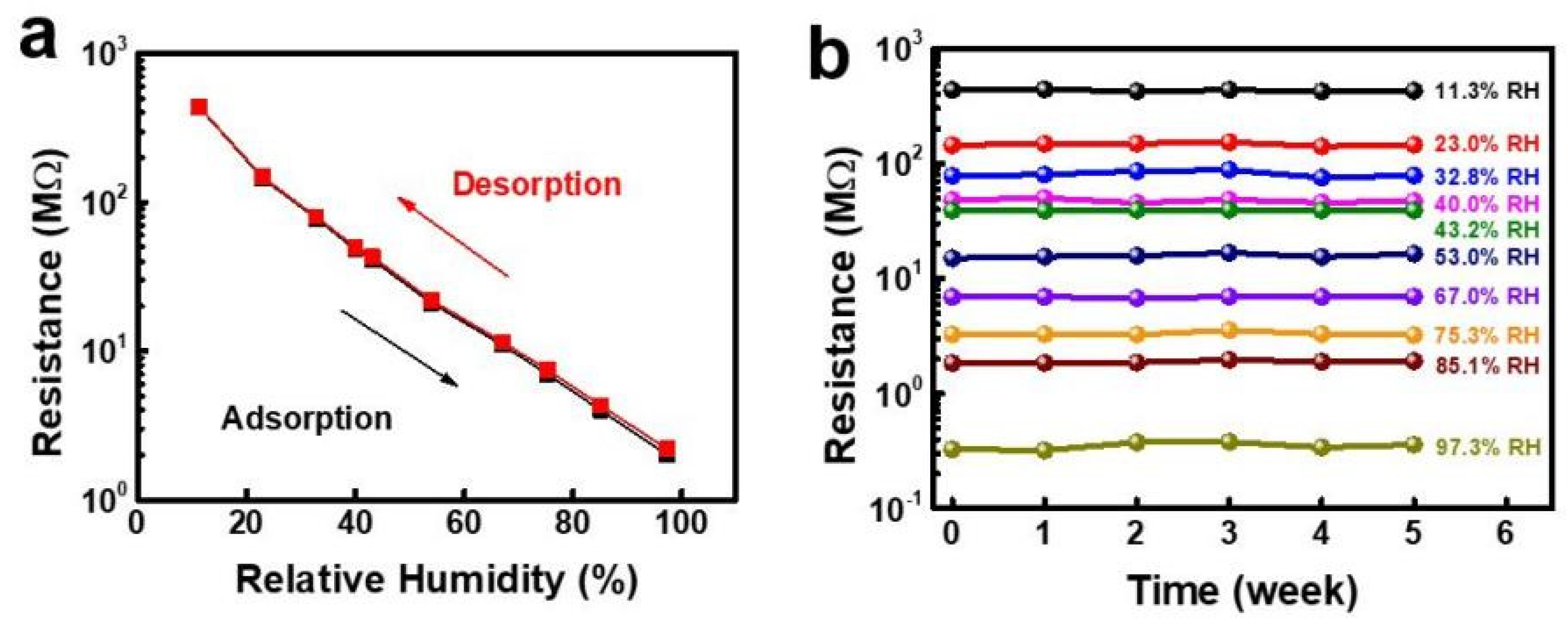
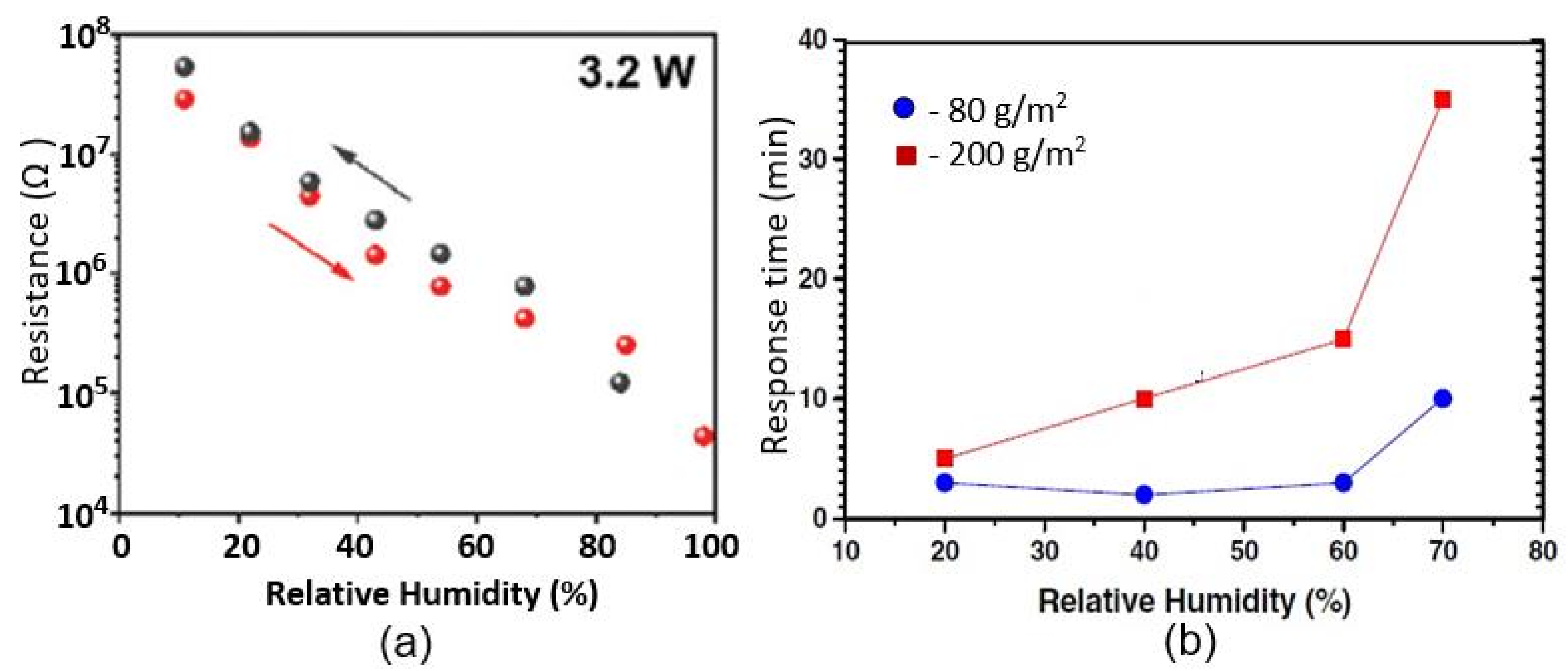
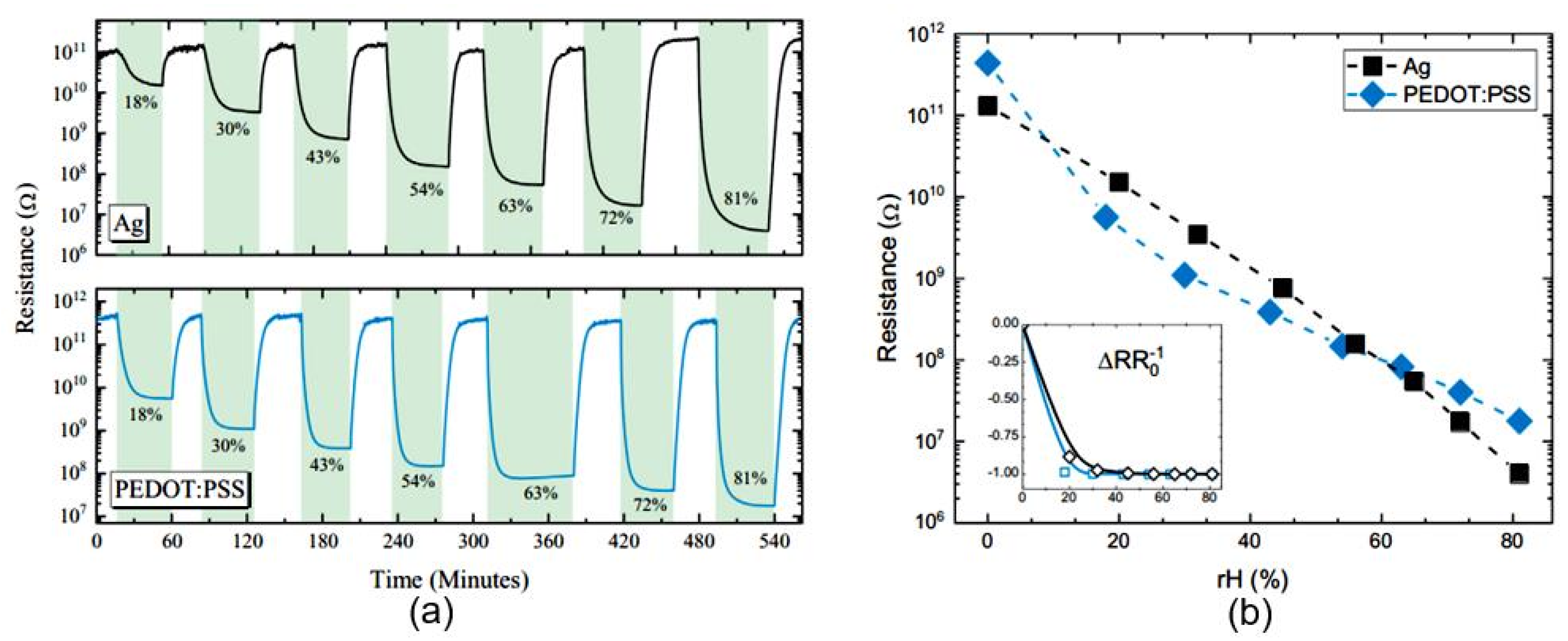


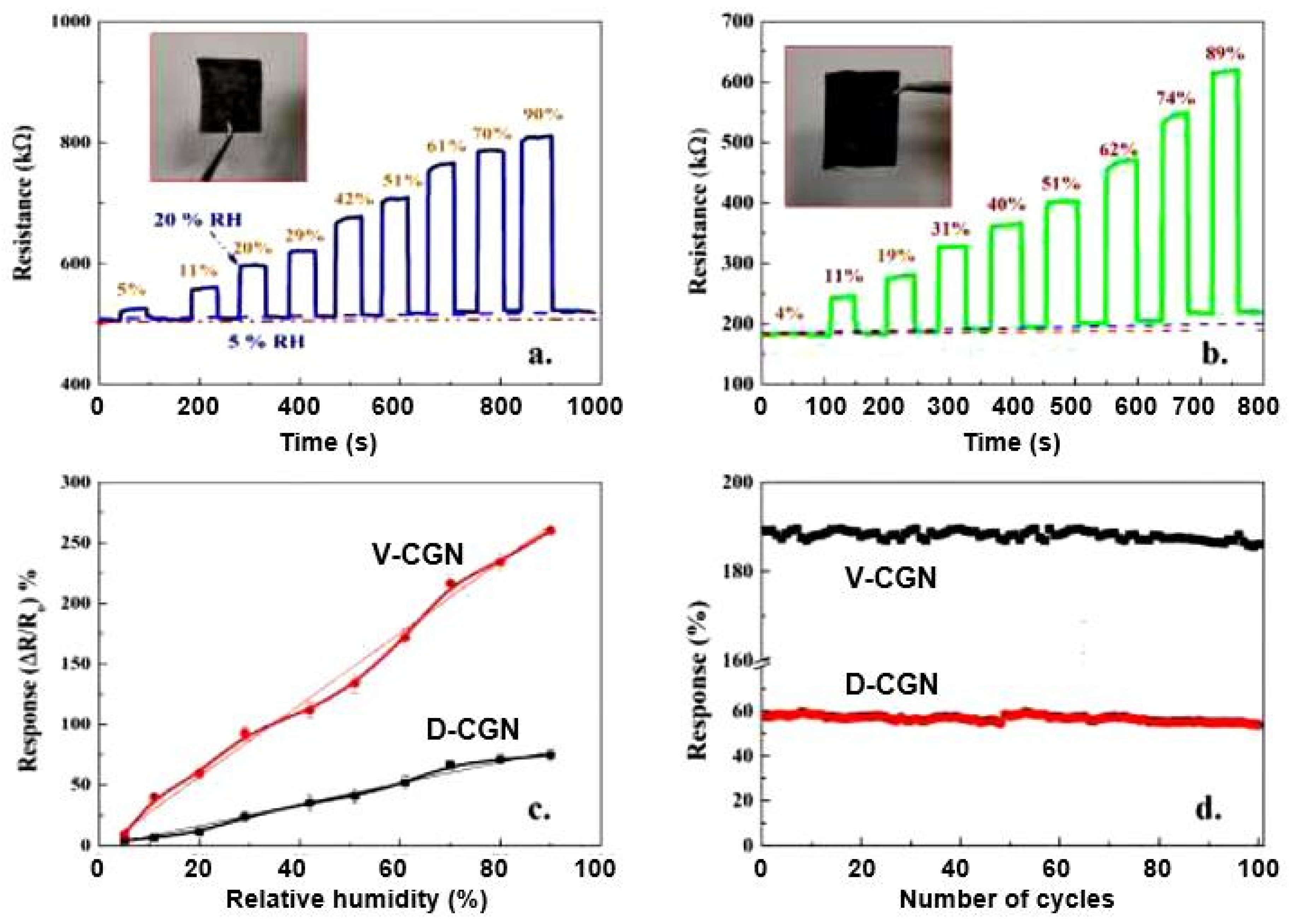
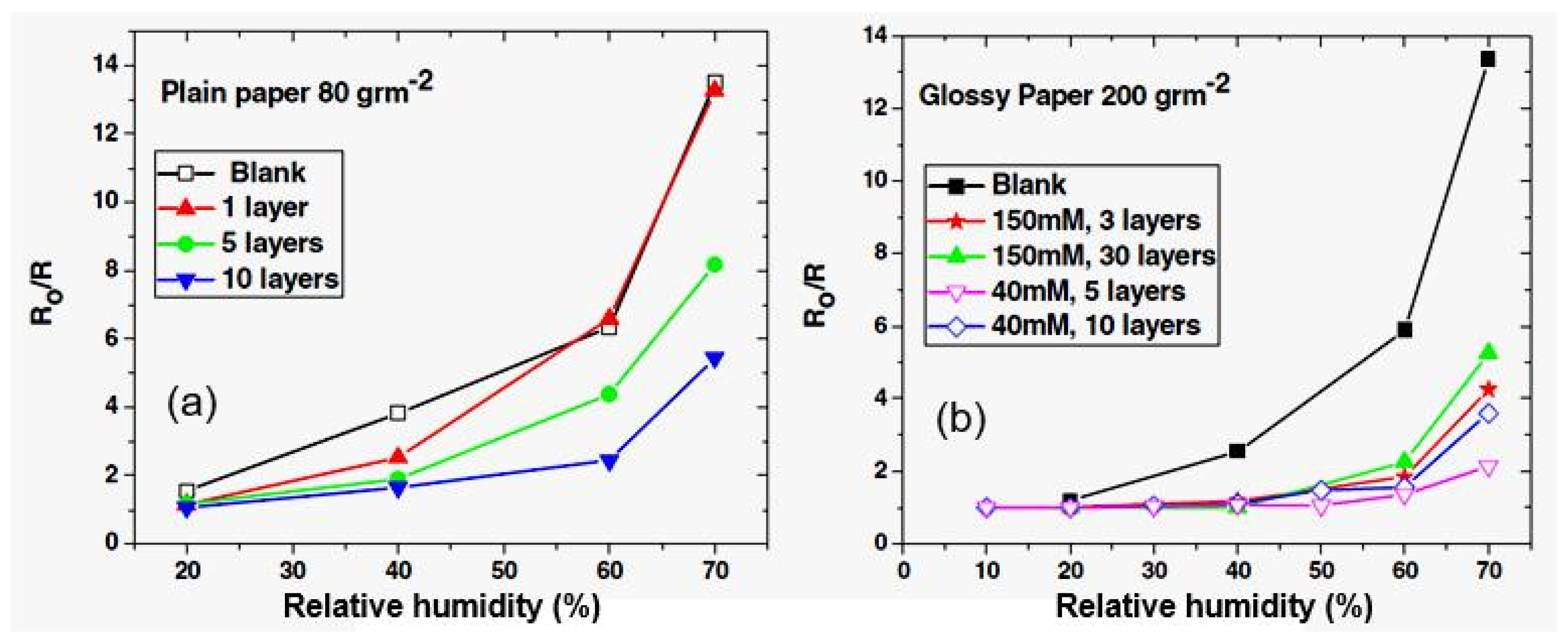
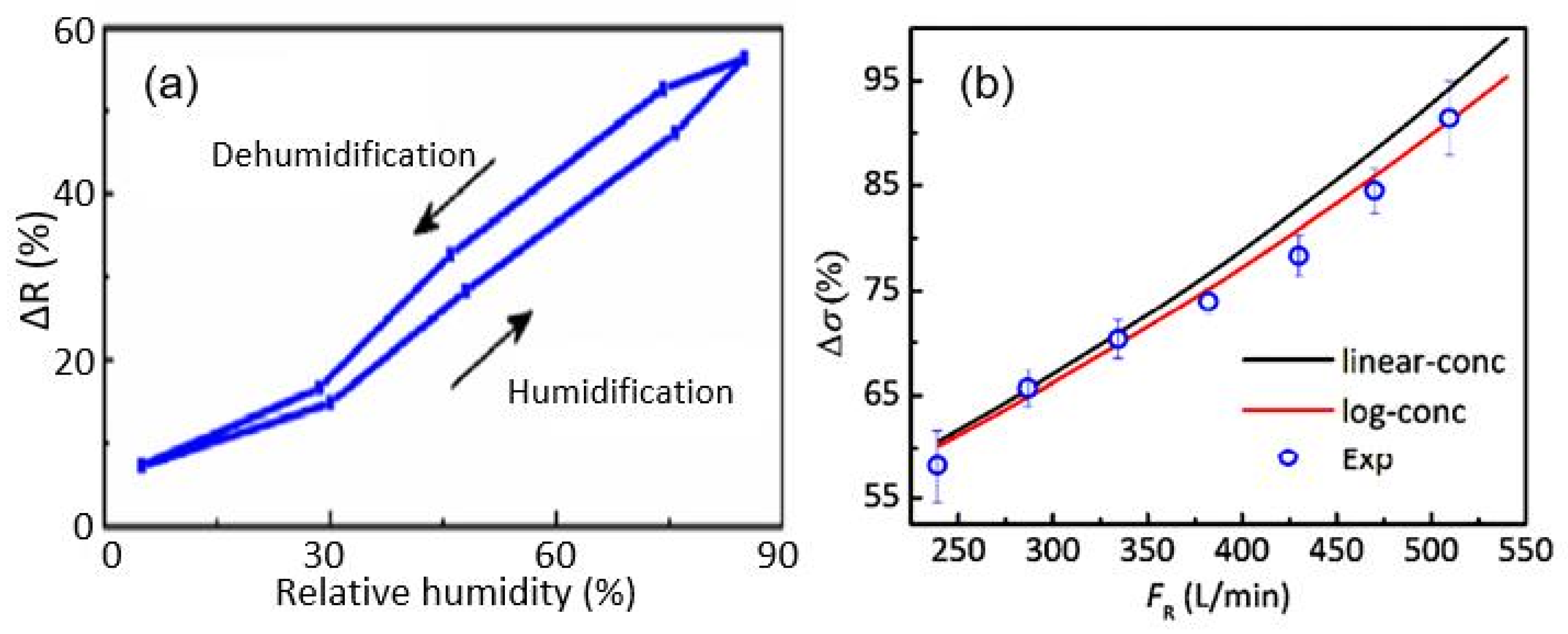
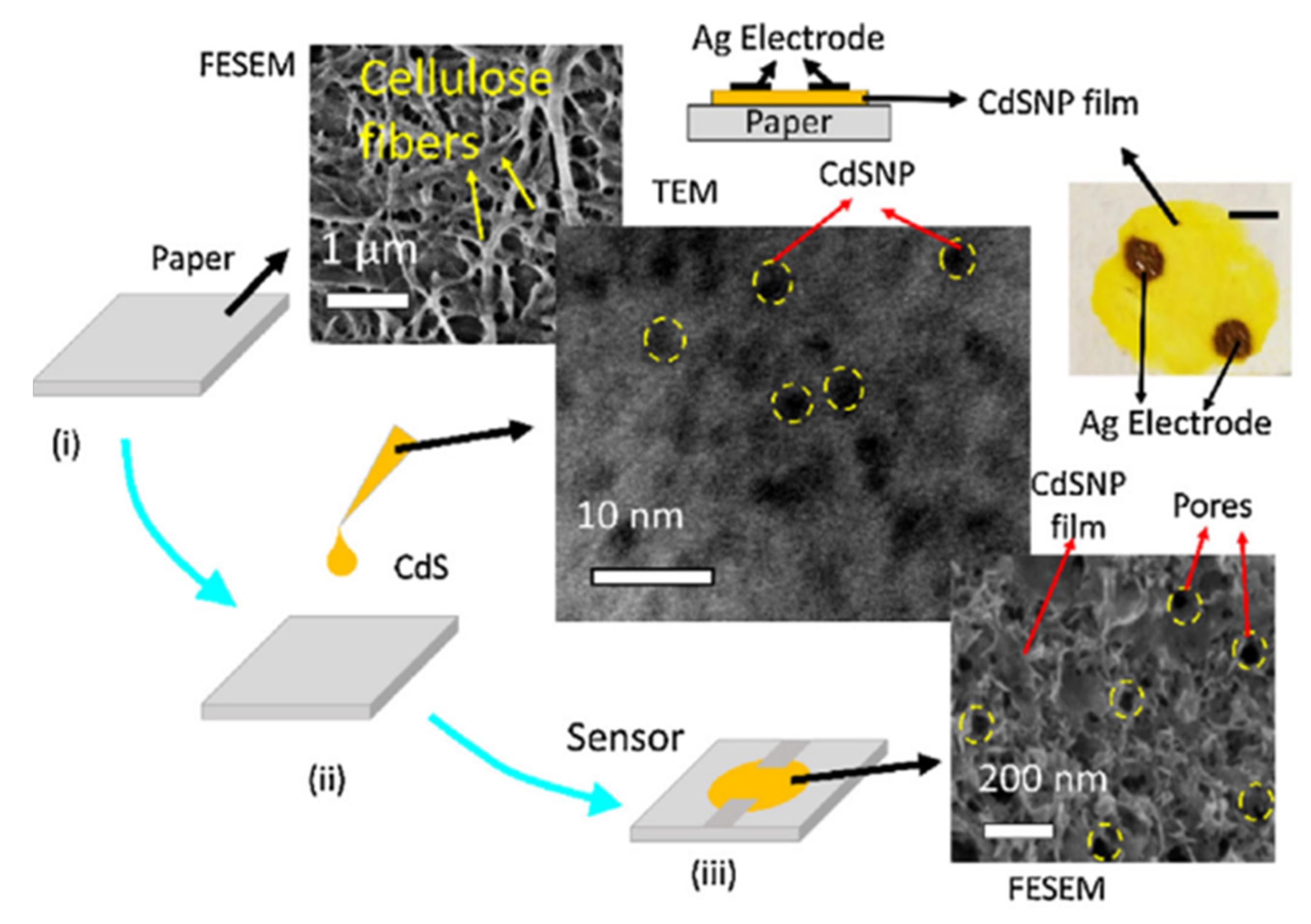
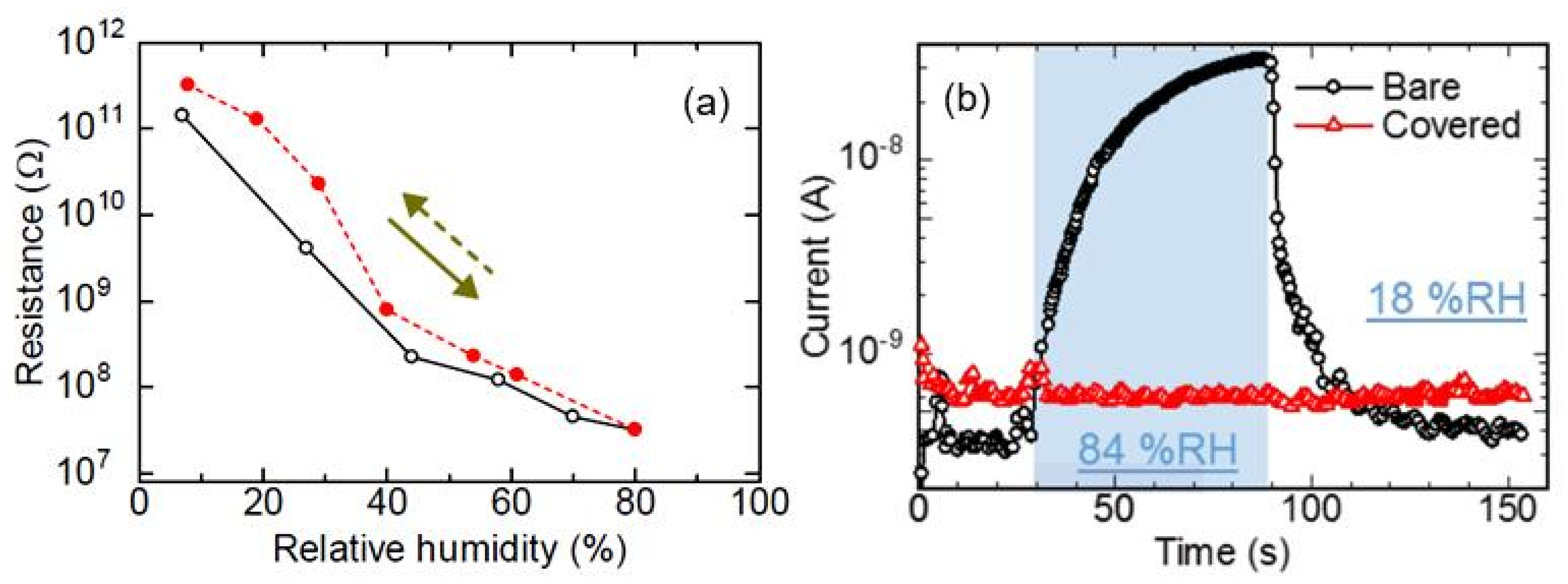
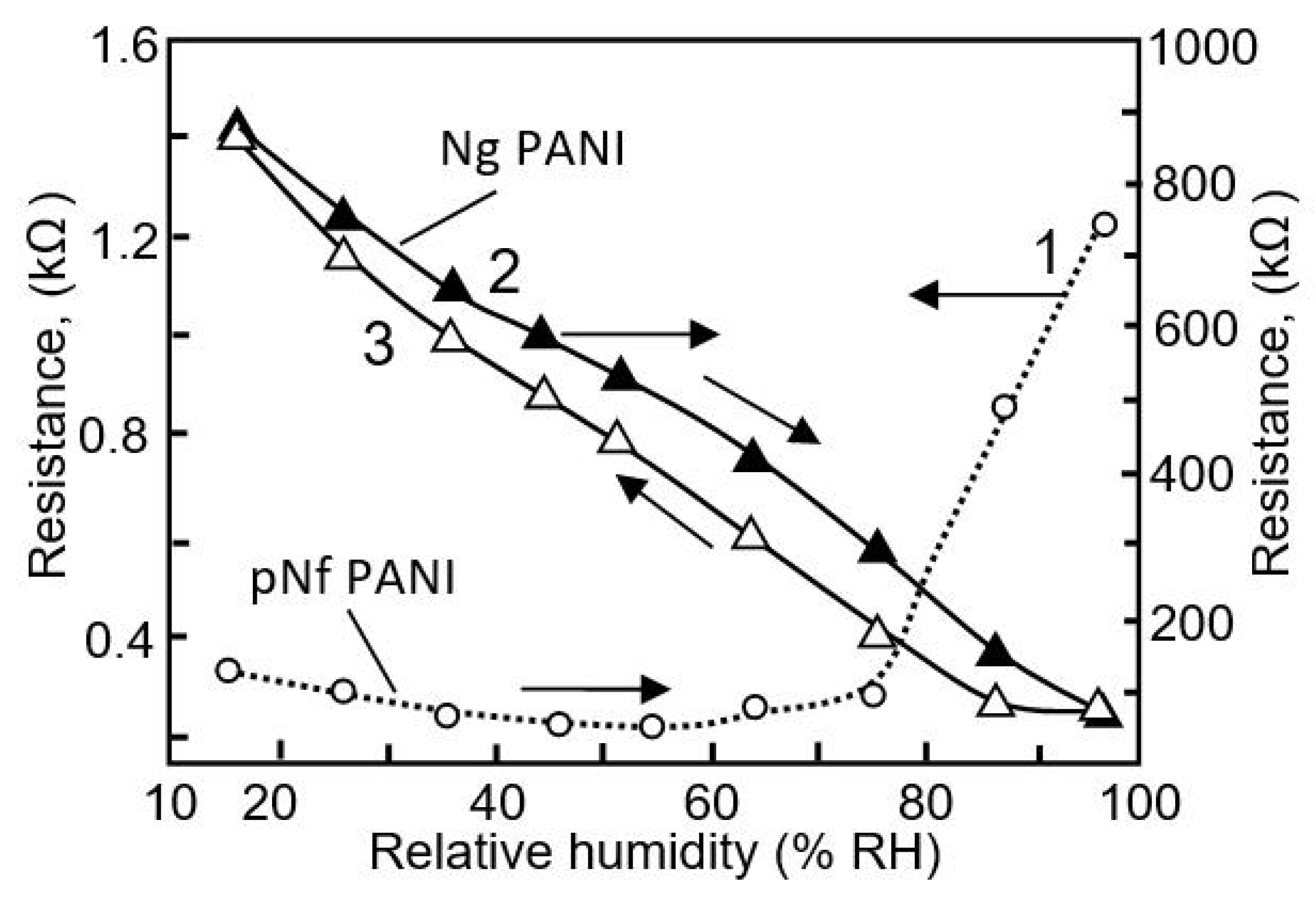
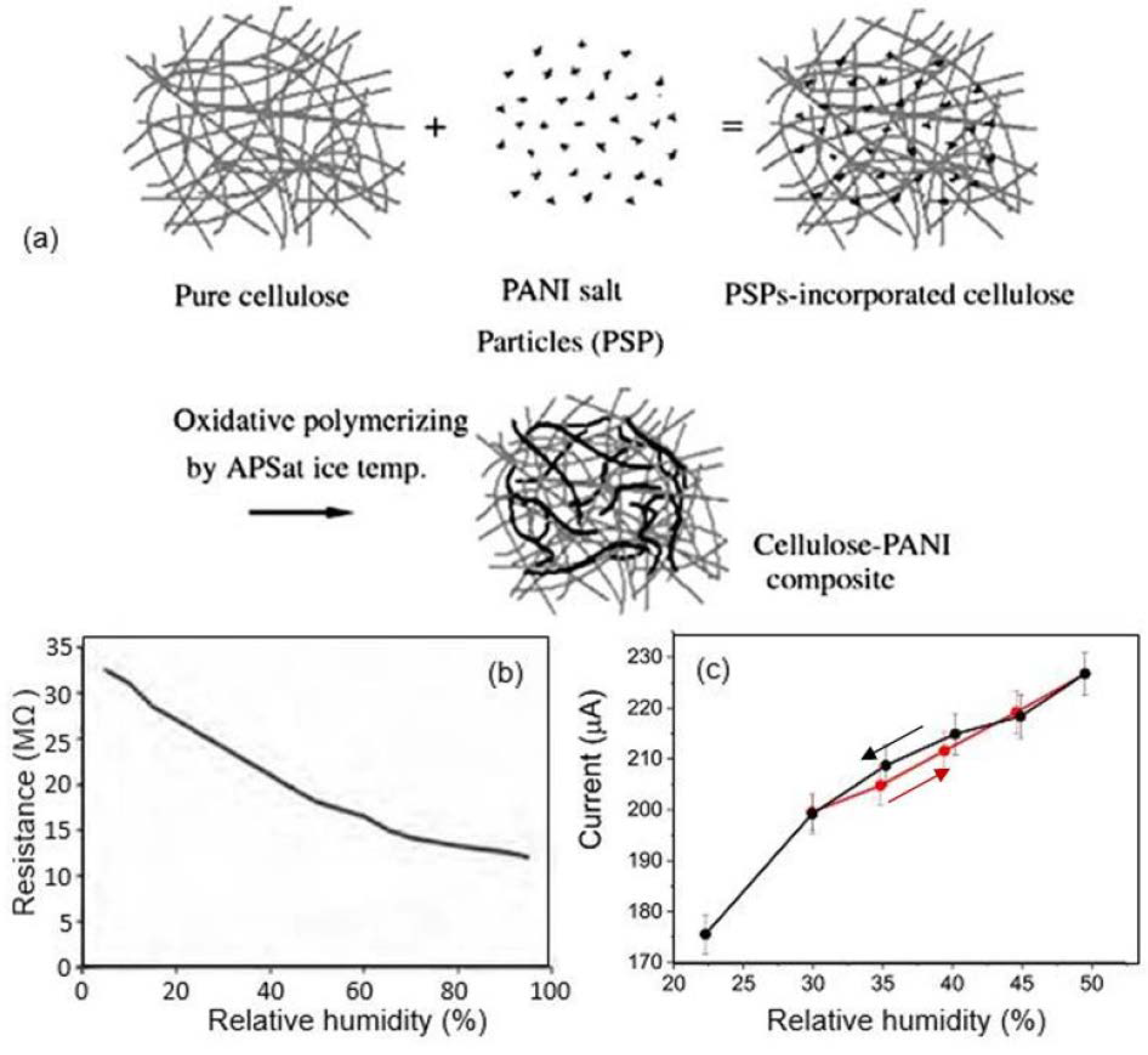
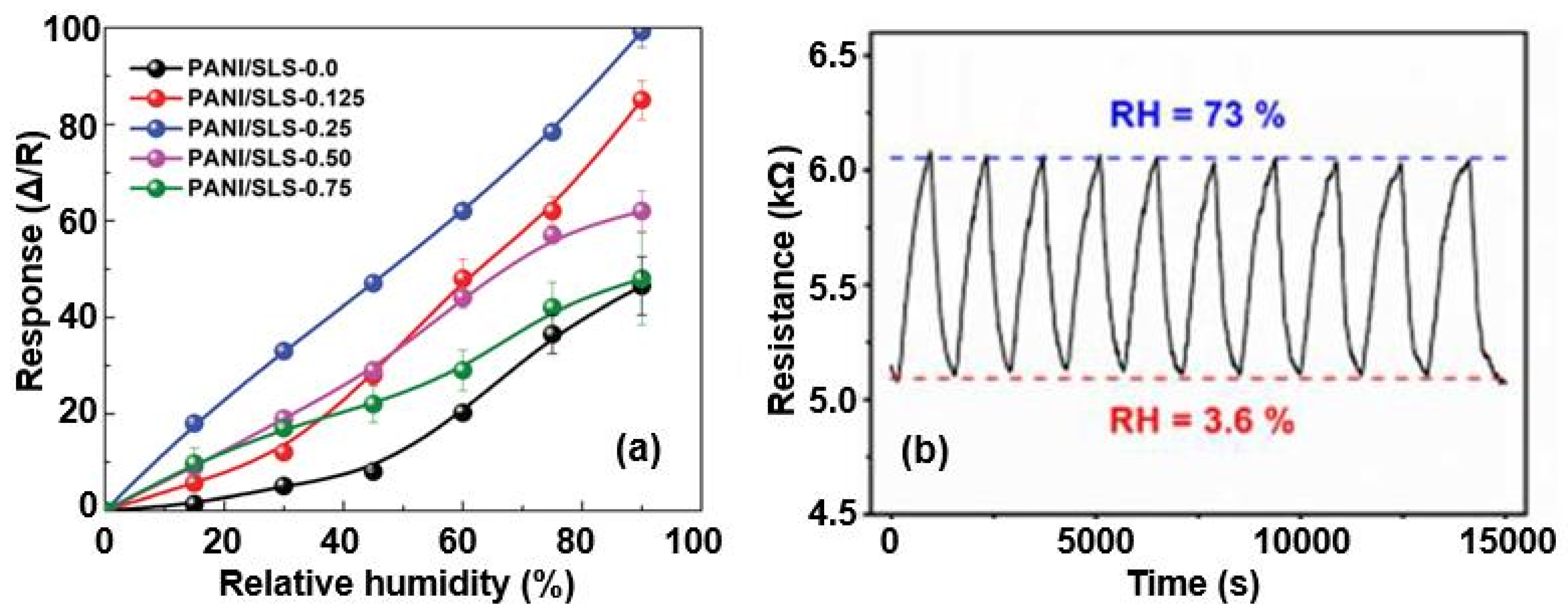






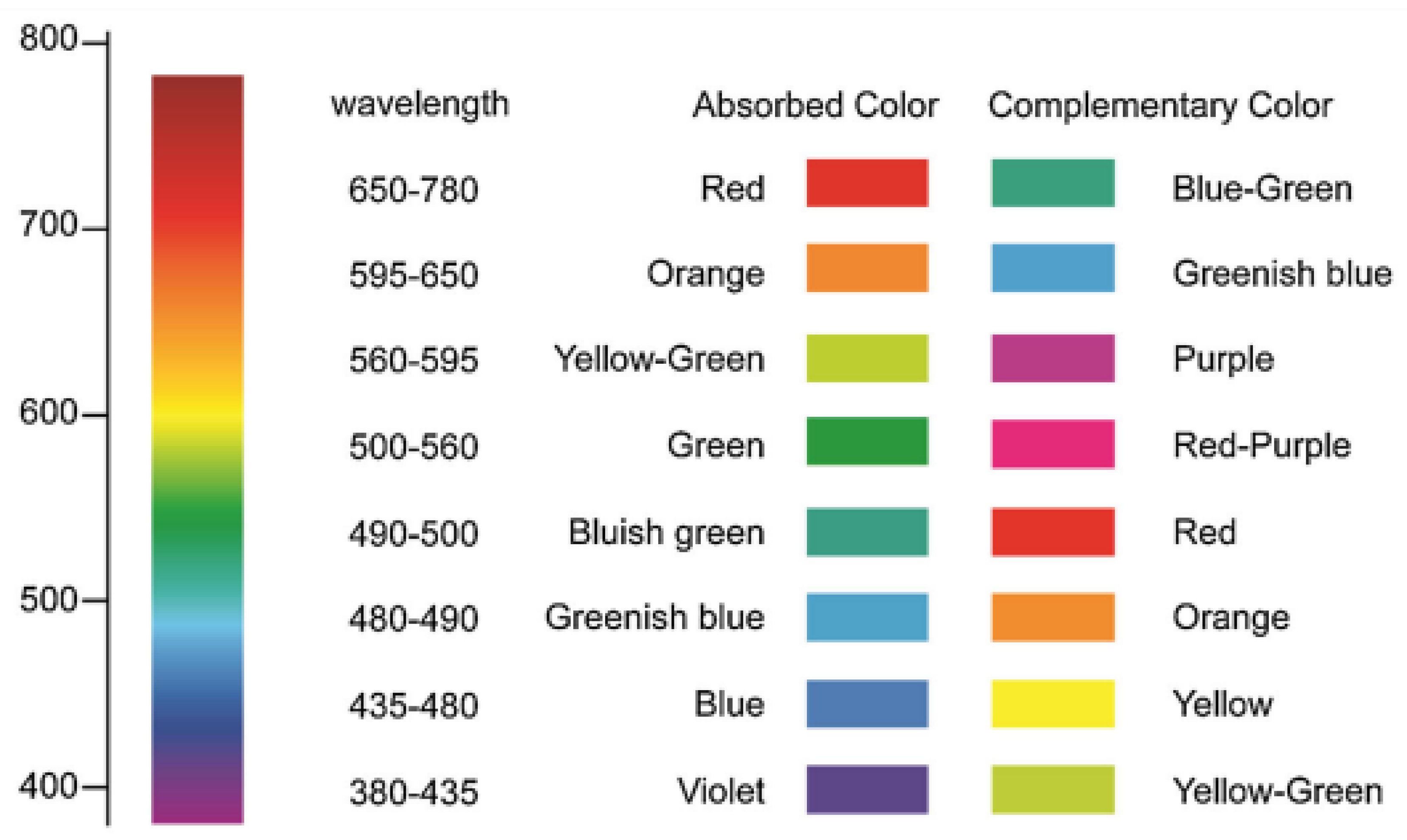
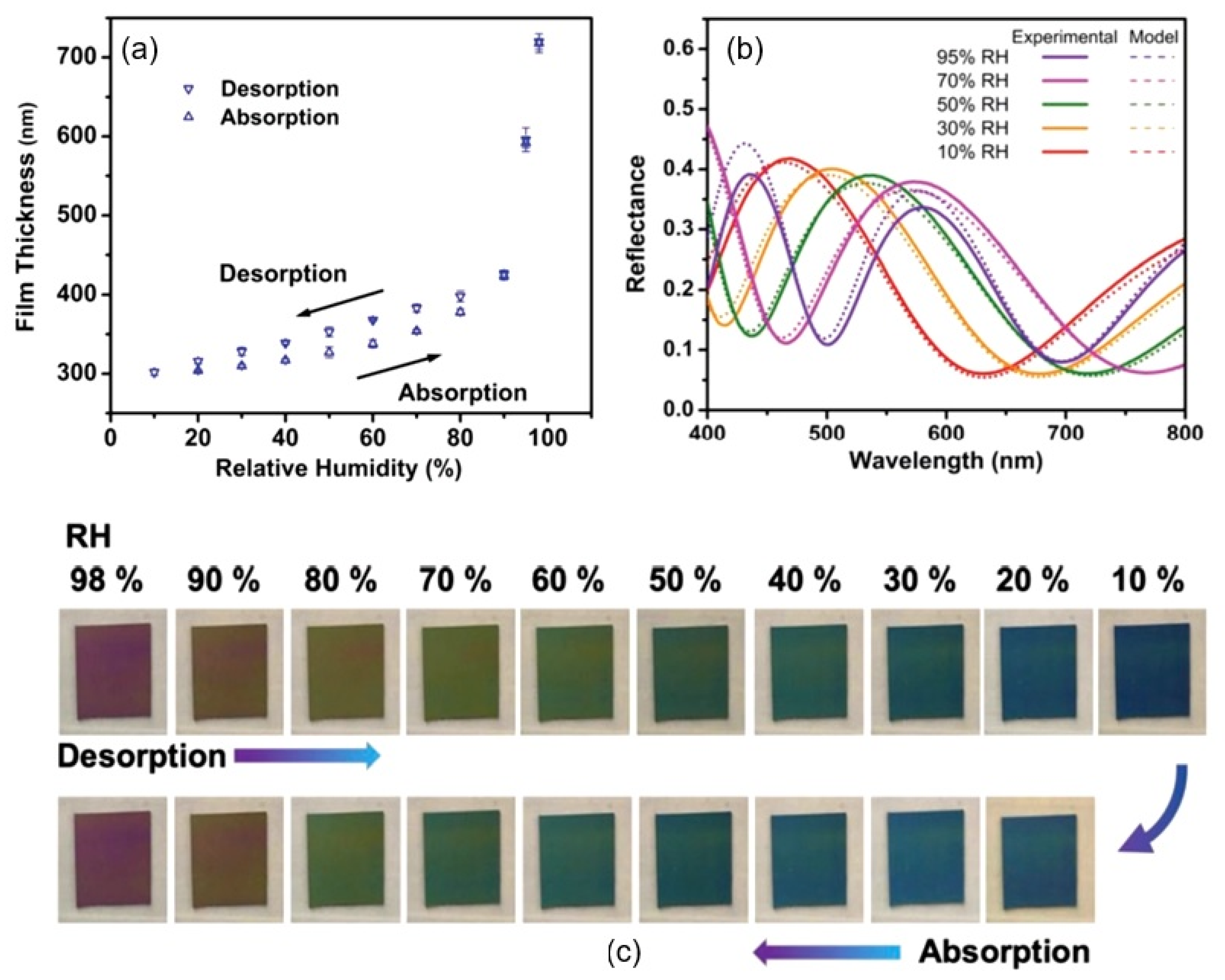

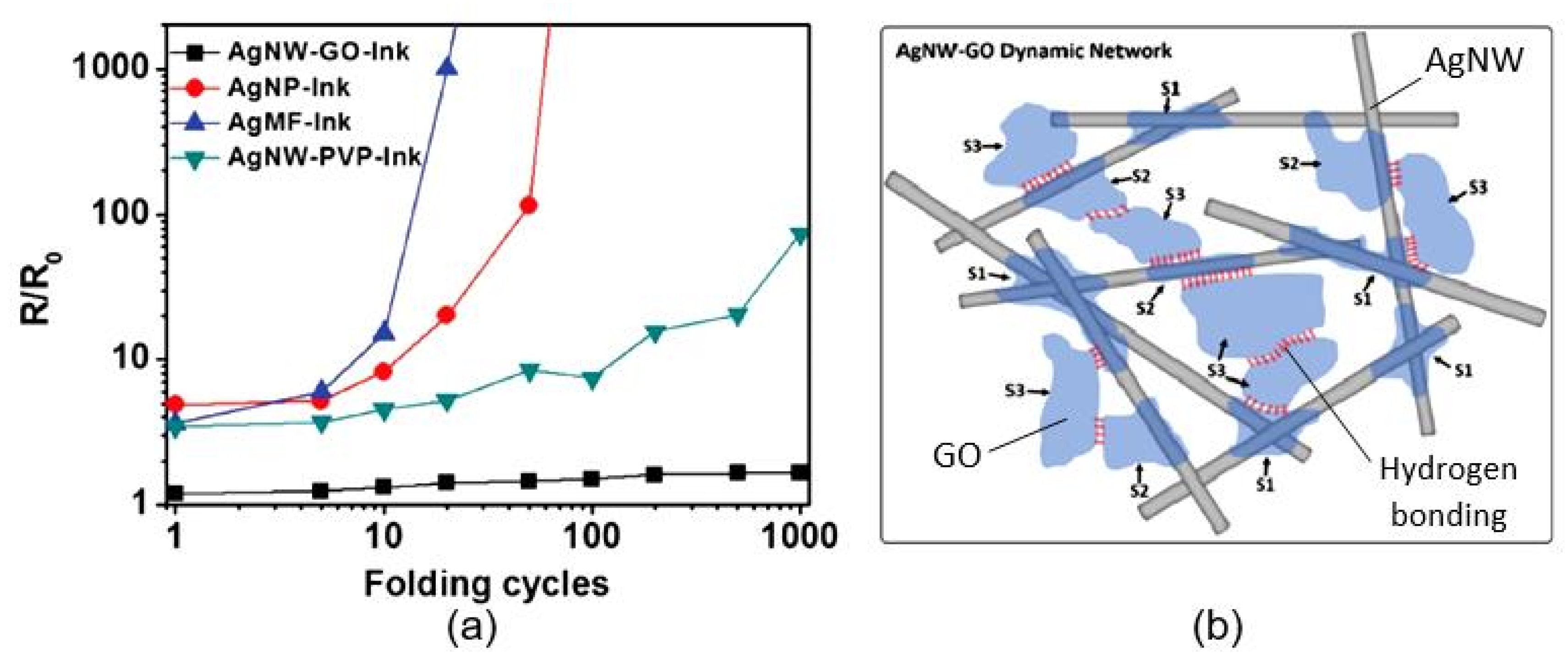
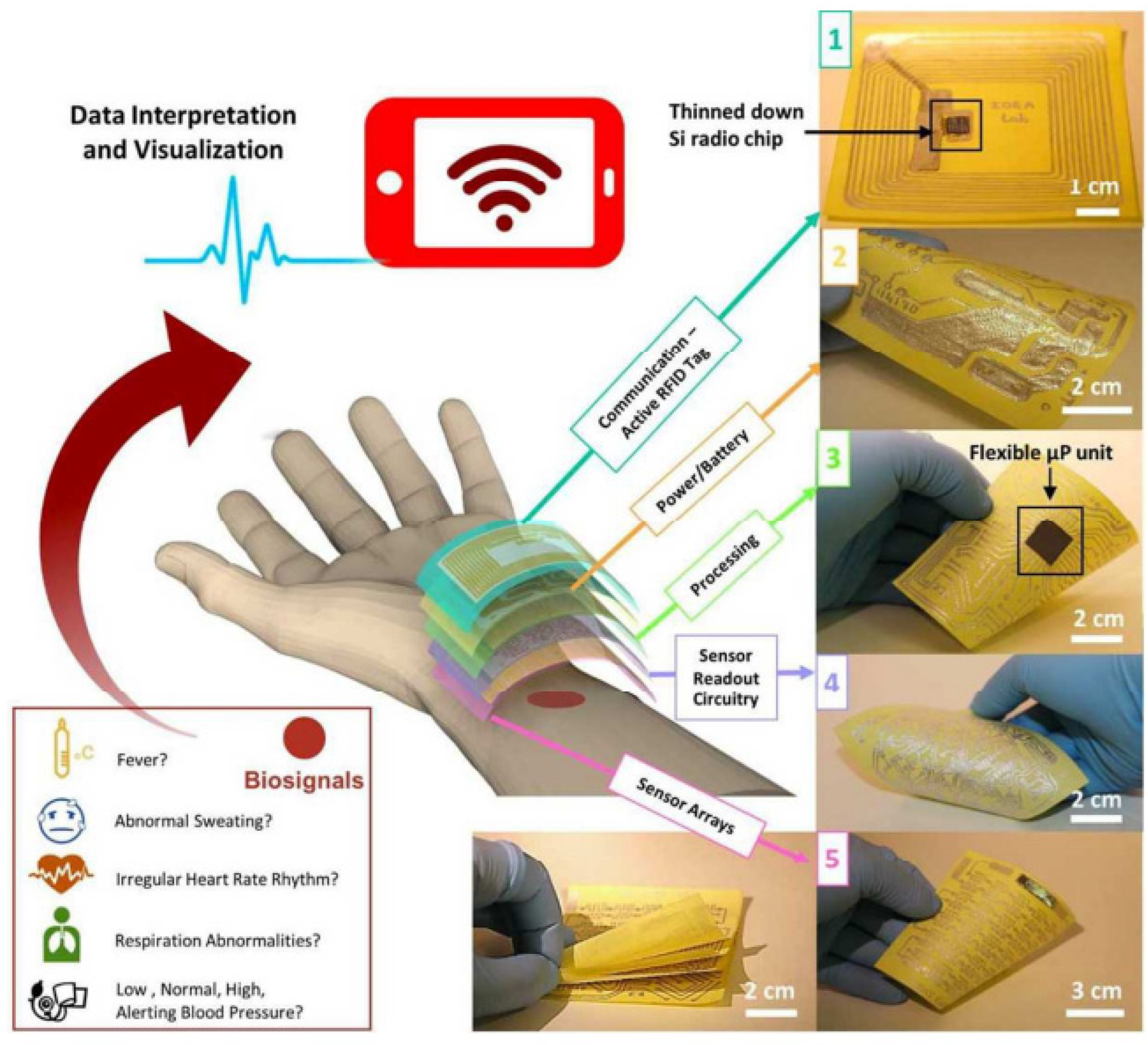


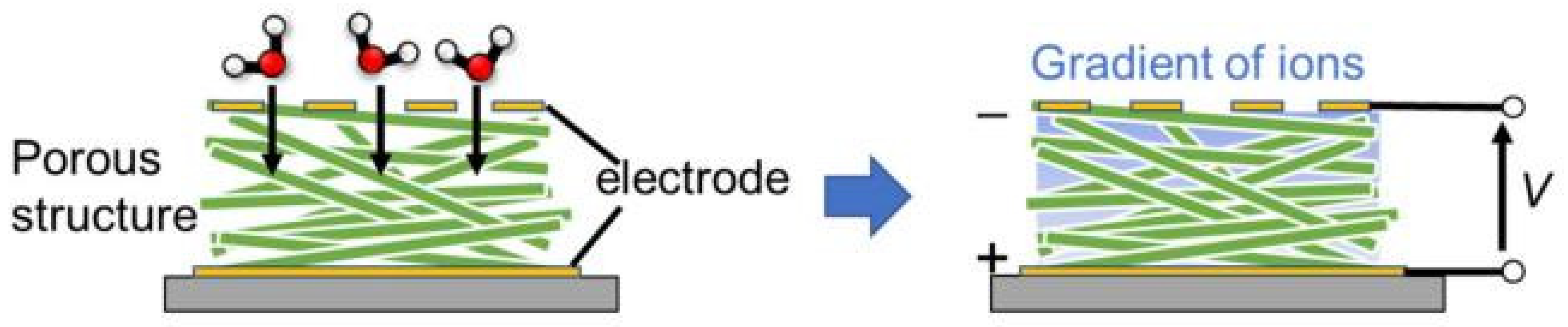
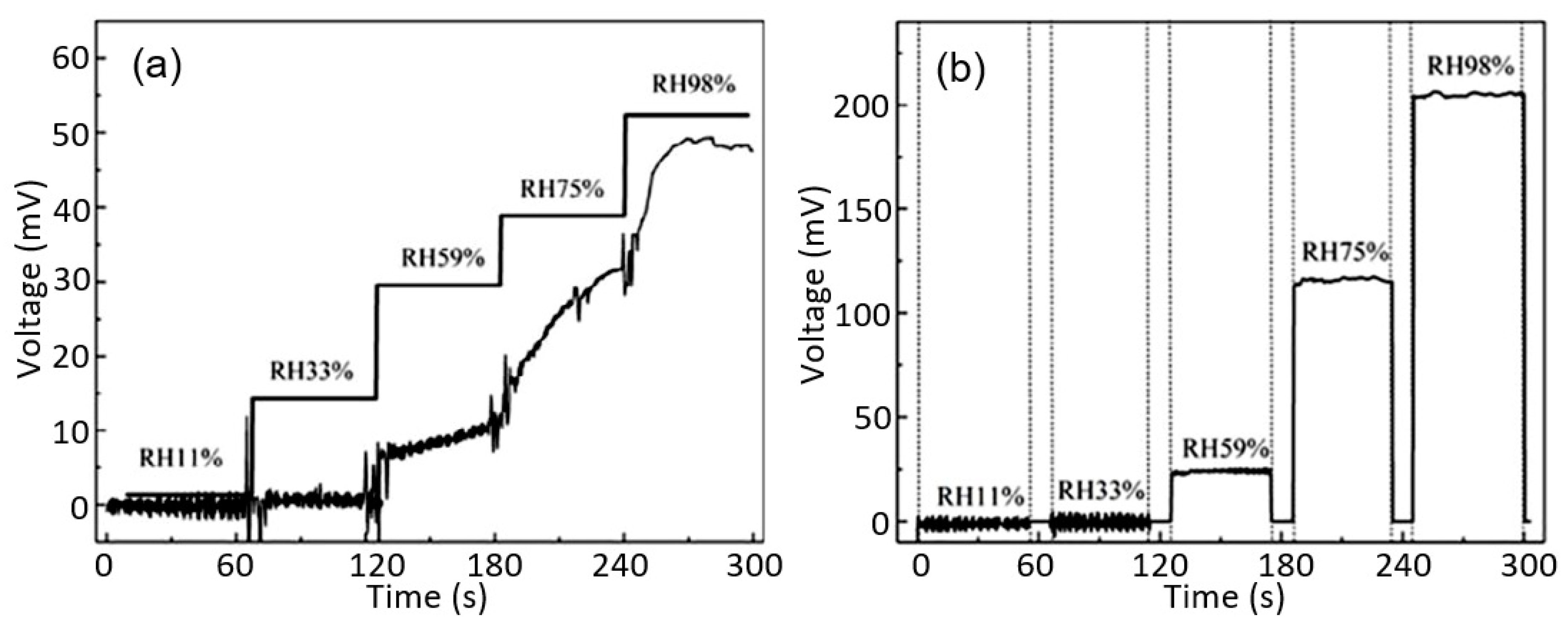
| Sensing Material | Electrode | RH Range (%) | Response | Res/Rec Time | Ref. |
|---|---|---|---|---|---|
| Recycled paper, cardboard, food-packaging paper | Ag, screen printing | 35–80 | ~3–6 (C/C0) | N/A | [17] |
| Paper (Lumi silk) | Ag, inkjet printing | 40–100 | ∼3.5 (C/C0); 2 pF/% RH (ΔC/ΔRH) | 4/3 min | [22] |
| Cellulose paper | Pencil drawing | 40–90 | ~2 (C/C0) 4.5%/% RH (ΔC/ΔRH) | 2/7 s | [25] |
| Cellulose paper | Pencil drawing | 31–90 | ∼2.2 (C/C0); 0.06 pF/% RH | 10/32 s | [26] |
| Tissue paper | Copper tape | 40–95 | ~4 (C/C0); 11 pF/% RH | 1.5/2.2 s | [21] |
| Functionalized copy paper | Ag, gravure printings | 30–90 | ~15 (C/C0); | ~1/~1 s | [27] |
| Post-it-note paper | Ag ink pen | 10–95 | 0.18%/% RH | 1.2/2.3 s | [28] |
| Functionalized p_e:smart paper type 2 | Ag, inkjet printing | 20–80 | ~10 pF/% RH ~10 (C/C0) | Long response | [16] |
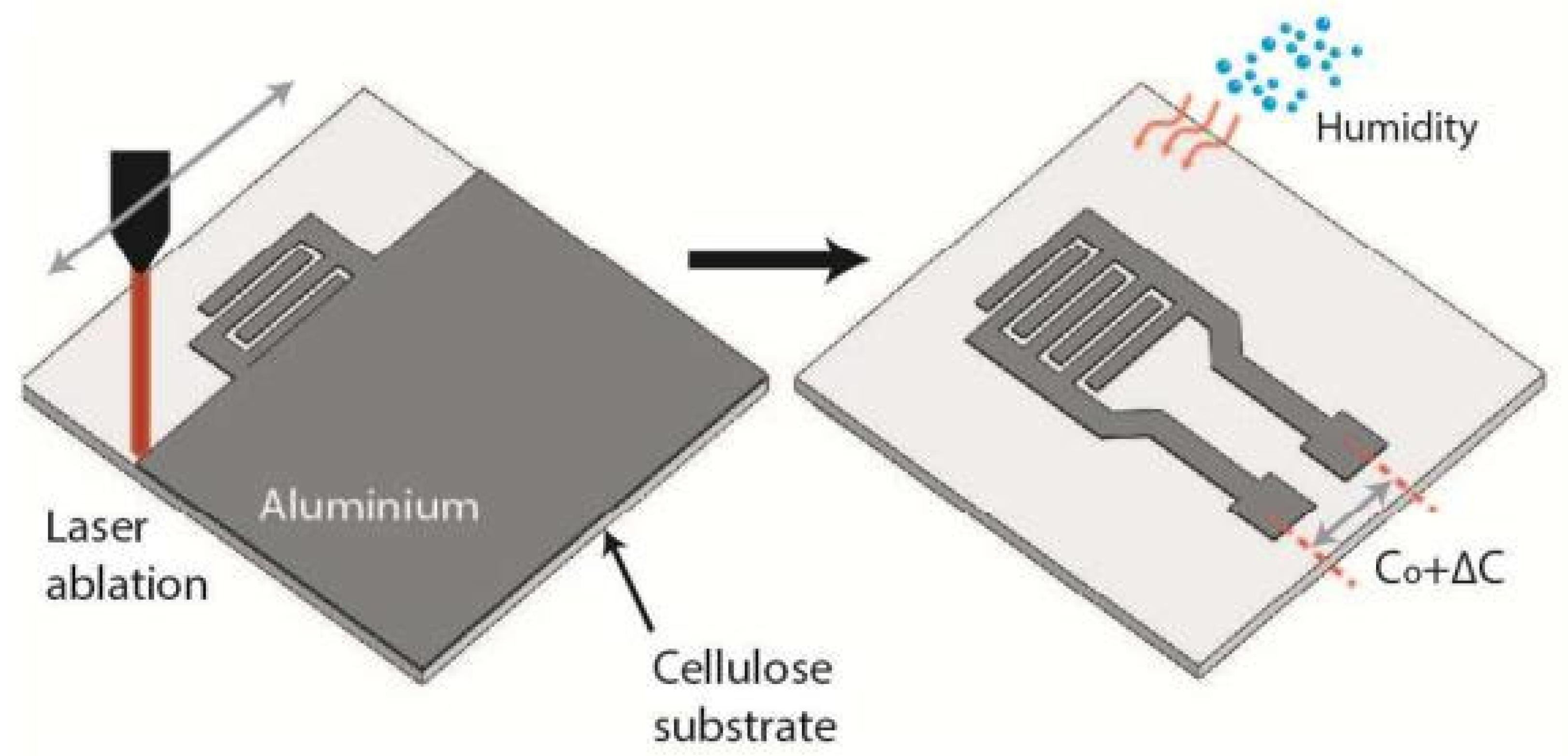
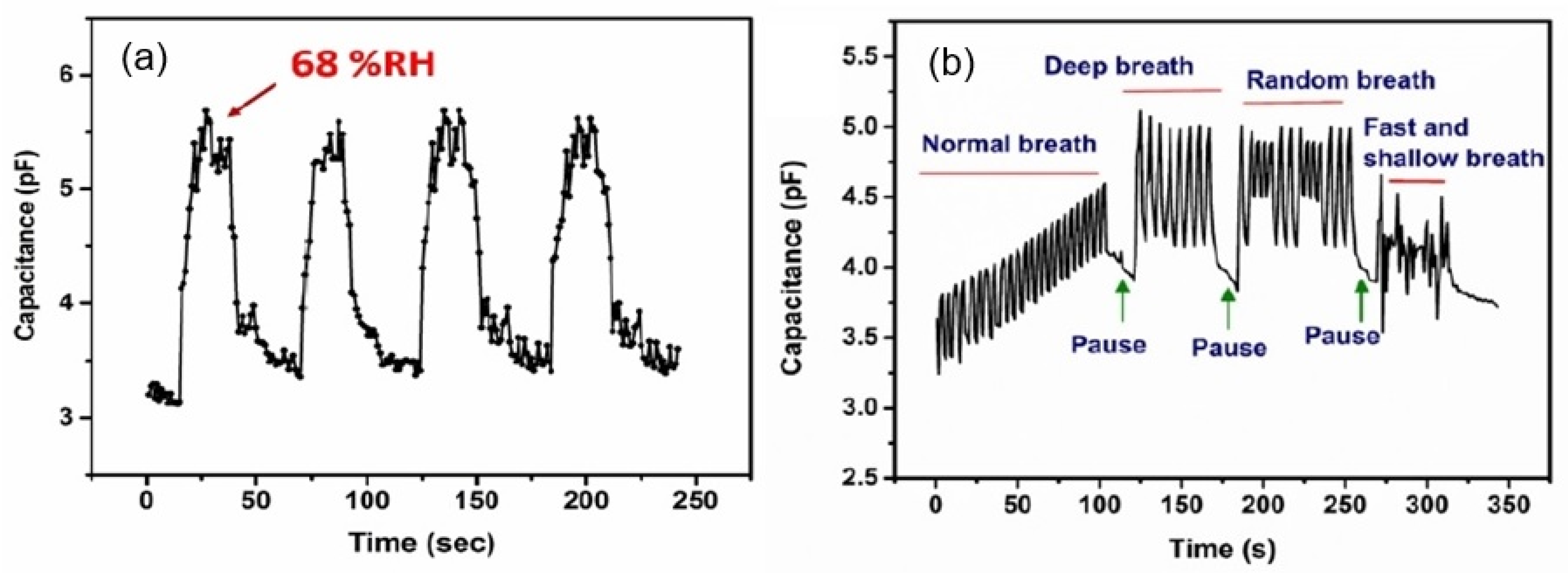
| Metallized papers | Al, laser ablation | 2–60 | 0.1 pF/% RH ~1.5 (C/C0) | 260/20 s | [18] |
| 60–85 | 6 pF/% RH ~15 (C/C0) | ||||
| Glossy paper | Graphene, inject printing | 10–70 | 1.8 (C/C0) 0.03 pF/% RH (ΔC/ΔRH) | N/A | [29] |
| Sensing Material | Method | RH Range (%) | Response | Res/Rec Time | Ref. |
|---|---|---|---|---|---|
| CNT (10%)—paper composite | CNT-cellulose suspensions were filtered, pressed, and dried to form hand sheets | 40–80 | 22% (ΔC/C0); ~9 fF/% RH (ΔC/ΔRH) | Slow | [30] |
| MWCNT/PDMS (photo paper) | Screen printing | 70 70–95 | 0.375 pF/% RH 8.24 pF/% RH | N/A | [31] |
| GO | Soaking | 30–90 | 209% (ΔC/C0); 5.65 fF/% RH (ΔC/ΔRH) | Slow recovery | [32] |
| GO | Printing | 30–90 | 85–320 (C/C0) | 170/40 s | [33] |
| GO/CNC (on PET) | Drop coating | 25–90 | ~2·103 (C/C0) | 17/22 s | [34] |
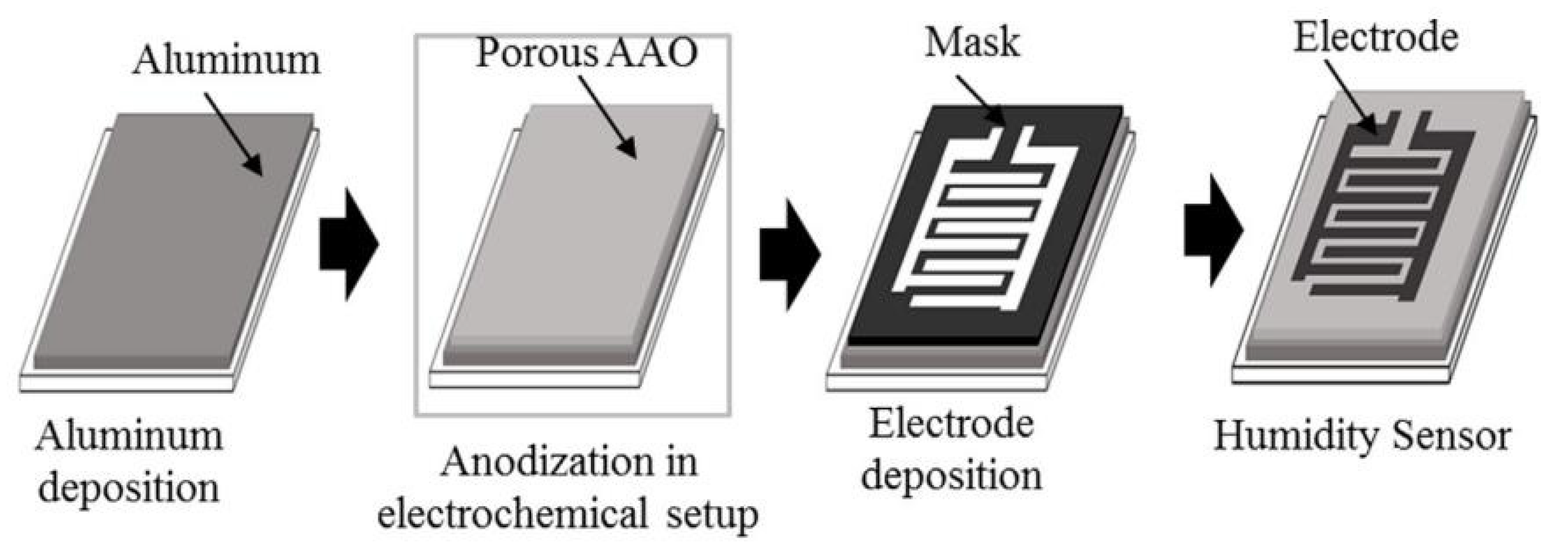
| Aluminum oxide | Electrochemical oxidation | 50–80 | 4.5 pF/% RH (ΔC/ΔRH) | Slow | [19] |
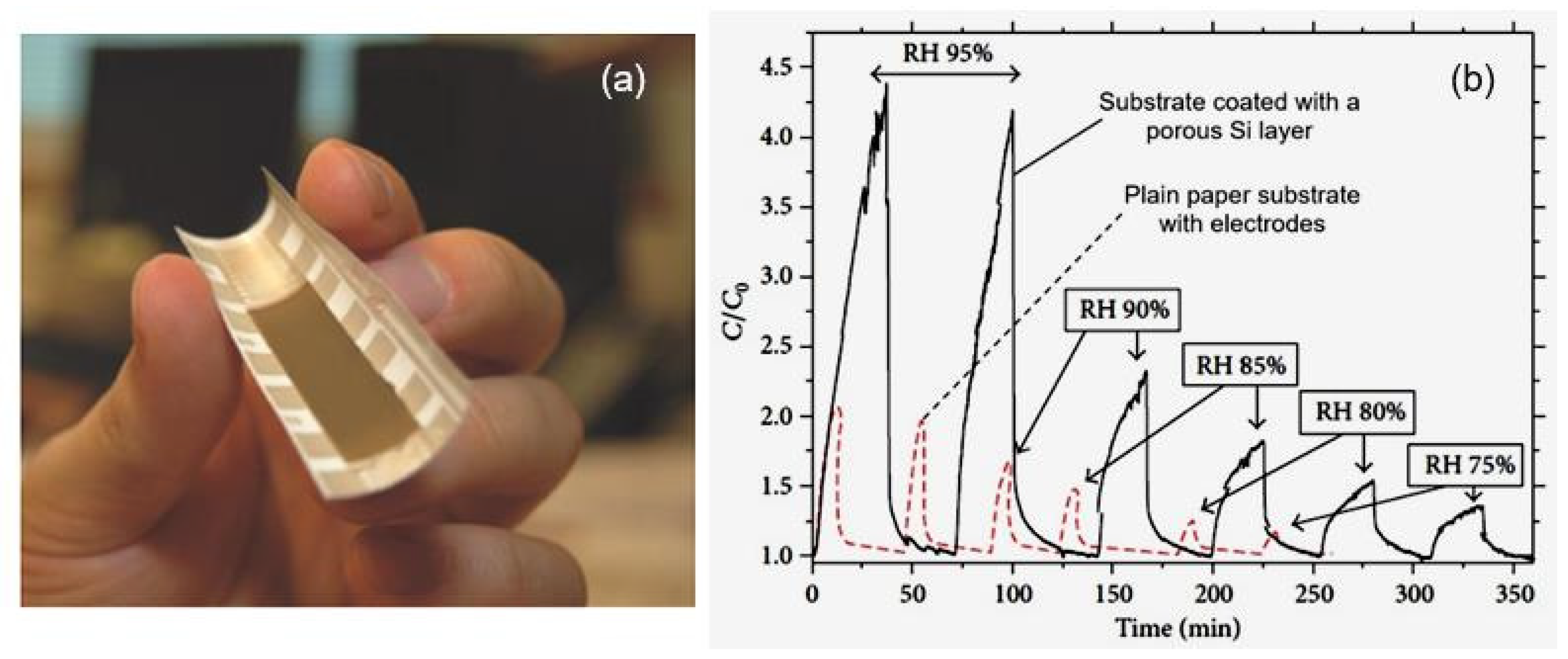
| Porous silicon | Spray-coating | 60–95 | >4.5 (C/C0) | >50/20 min | [35] |
| Type of Paper | Electrode/Method | RH Range (%) | Response | Res./Rec. Time | Ref |
|---|---|---|---|---|---|
| Printing paper | Tape pasting | 41–72 72–91.5 | ~8 (I/I0) ~200 (I/I0) | 472/19 s | [37] |
| Printing paper | 58–75 75–99 | 5.45 kΩ/% RH (ΔR/ΔRH) 411 Ω/% RH. | 382/22 s 991/48 s | [38] | |
| Tracing paper | Graphite, line-patterning | 20–70 | 215% (ΔV/V) | N/A | [39] |
| Whatman 3MM Chr | Graphite, ball-point pen | 30–90 | N/A | 1500/- s | [40] |
| Printing paper | Au, sputtering | 20–70 | 14 (R0/R) | N/A | [41] |
| Glossy photo paper | 12.5 (R0/R) | ||||
| Glossy photo paper | Ag, inkjet printing | 20–90 | ~104 (R0/R) | N/A | [42] |
| Glossy photo paper | Ag, PEDOT:PSS inkjet printing; | 5–85 | ~3 × 104 (R0/R) | ~10/~5 s | [43] |
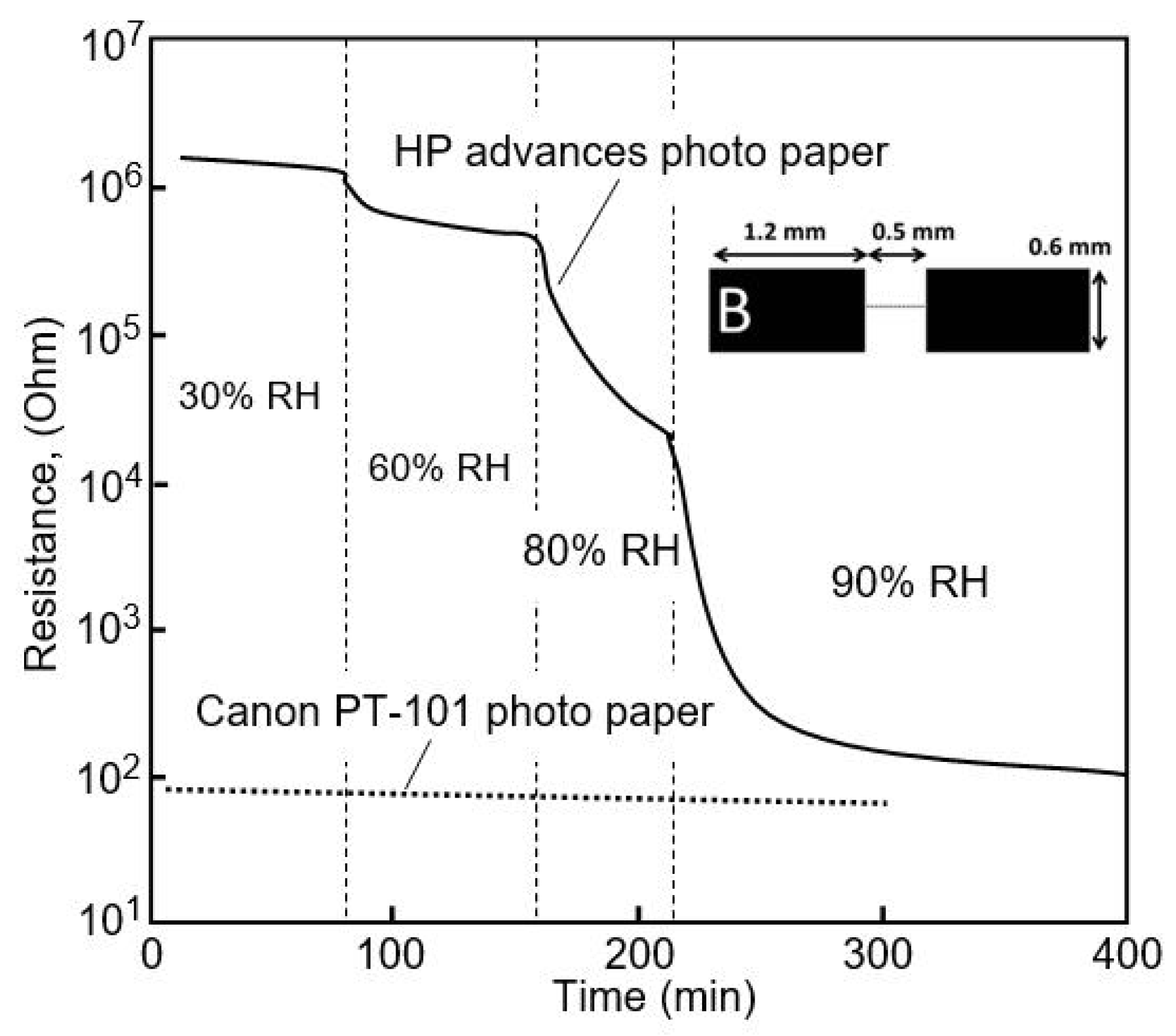
| HP advanced photo paper | Ag, inkjet printing | 30–60 60–90 | ~3 (R0/R) 7 × 103 (R0/R) | N/A | [18] |
| Printing paper | Carbon, spray | 20–90 | ~800 (I/I0) | 237/29 s | [44] |
| TEMPO-oxidized paper | Laser irradiation | 11–98 | 1.2 × 103 (R0/R) | 60/495 s | [45] |
| Conventional cellulose A4 paper | Ag, inject printing | 15–92 | ~1.25 (R0/R) 0.57 kΩ/% RH | 294/306 s | [46] |
| Cellulose/KOH composite | N/A | 11–98 | ~2 × 102 (R0/R) | 6/11 s | [47] |
| Material | Deposition | Electrode/Method | Range % RH | Response | Res./Rec. Time | Ref. |
|---|---|---|---|---|---|---|
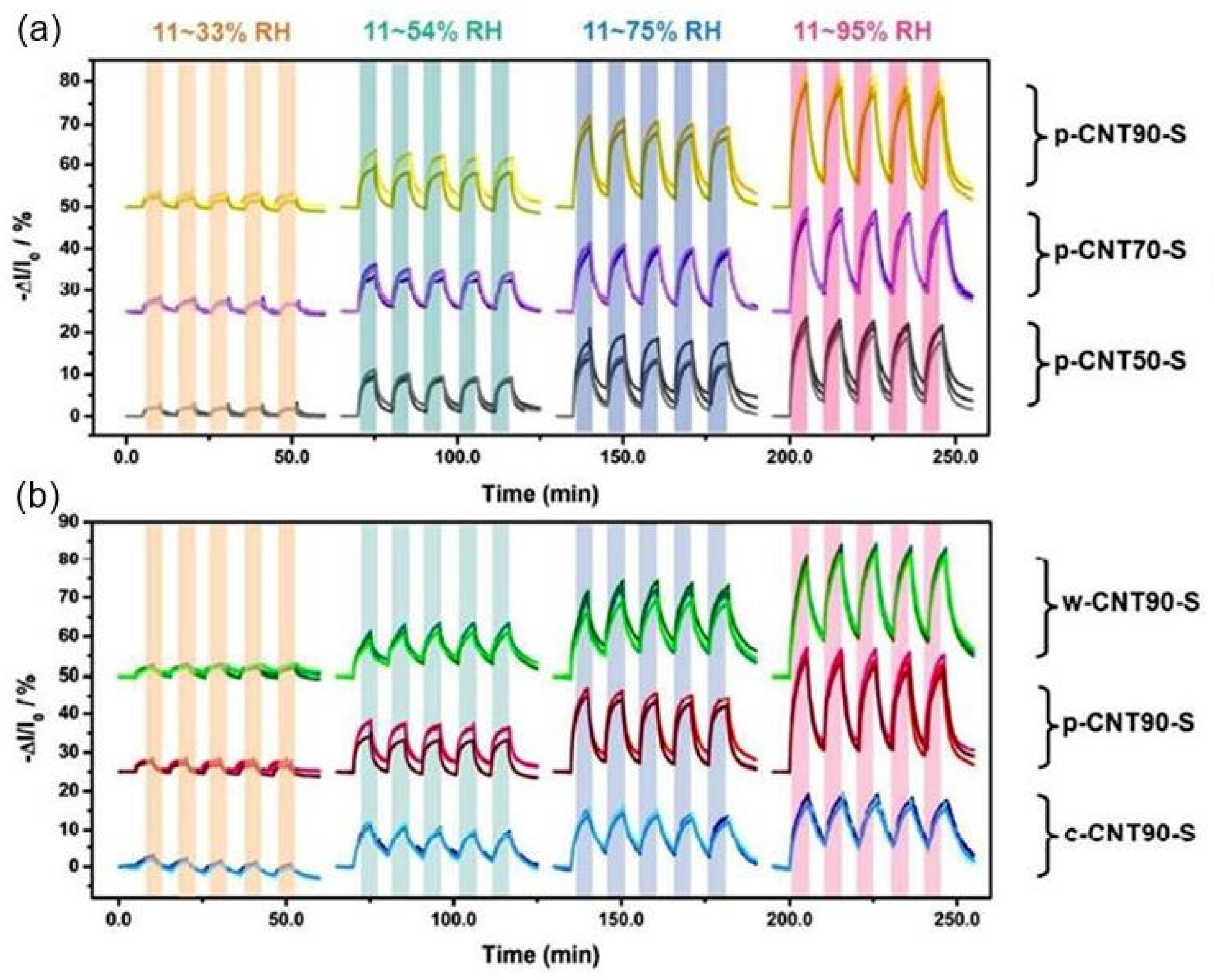
| CNT/cellulose | Rod-wire coater | Silver | 10–95 | ∼70% (−ΔI/I0) | 321/435 s | [54] |
| TEMPO-oxidized cellulose fibers/functionalized CNTs paper | Vacuum filtering | Silver | 11–95 | ∼87% (−ΔI/I0) | 333/523 s | [55] |
| Nanofibrillated cellulose/MWCNT composite film | Vacuum filtration | Copper-foil tape | 11–95 | ∼70% (−ΔI/I0) | 330/377 s | [56] |
| Regenerated cellulose/CNT composite fiber | Wet spinning | N/A | 35–86 | 44% (ΔR/R0) | N/A | [57] |
| Cellulose/CNT composite film | N/A | 35–85 | ~60% (ΔR/R0) | N/A | [58] | |
| Functionalized MWCNT-coated paper | Spray coating | Nano-metal inkjet printing | 20–90 | ∼60% (−ΔG/G0) | N/A | [59] |
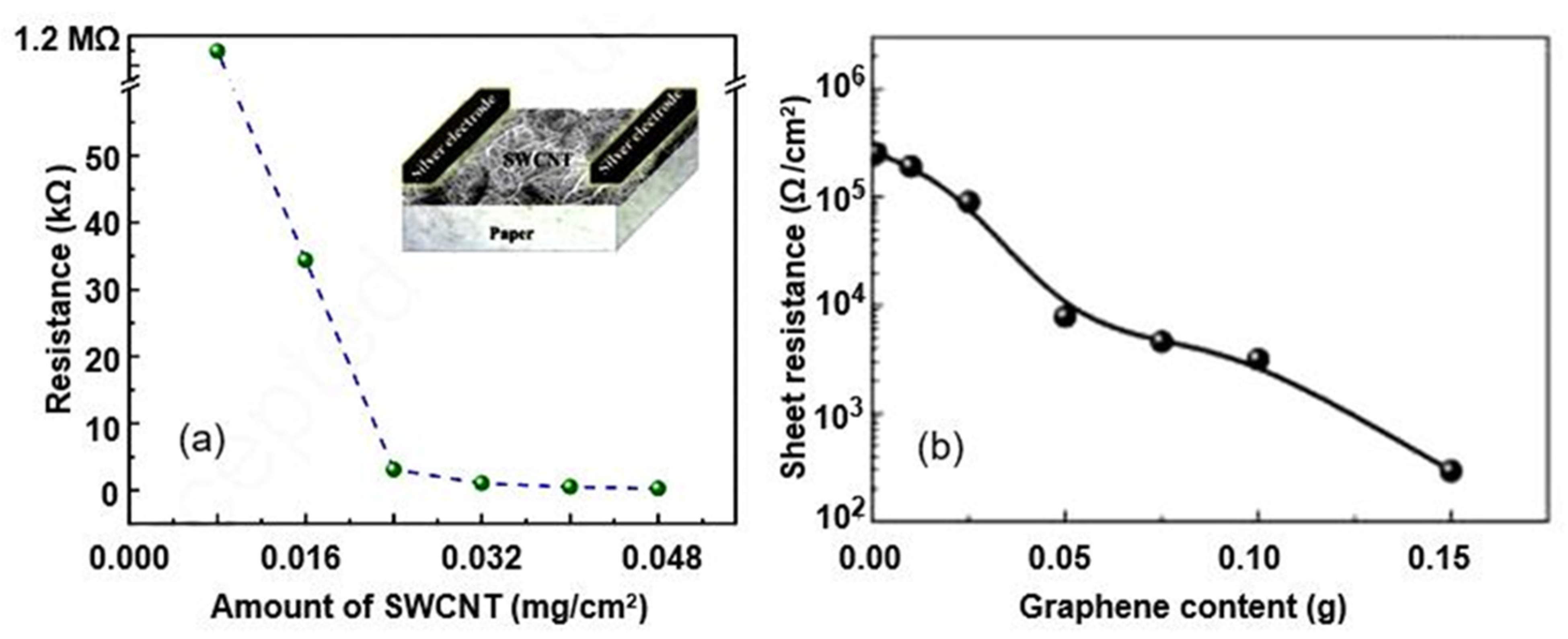
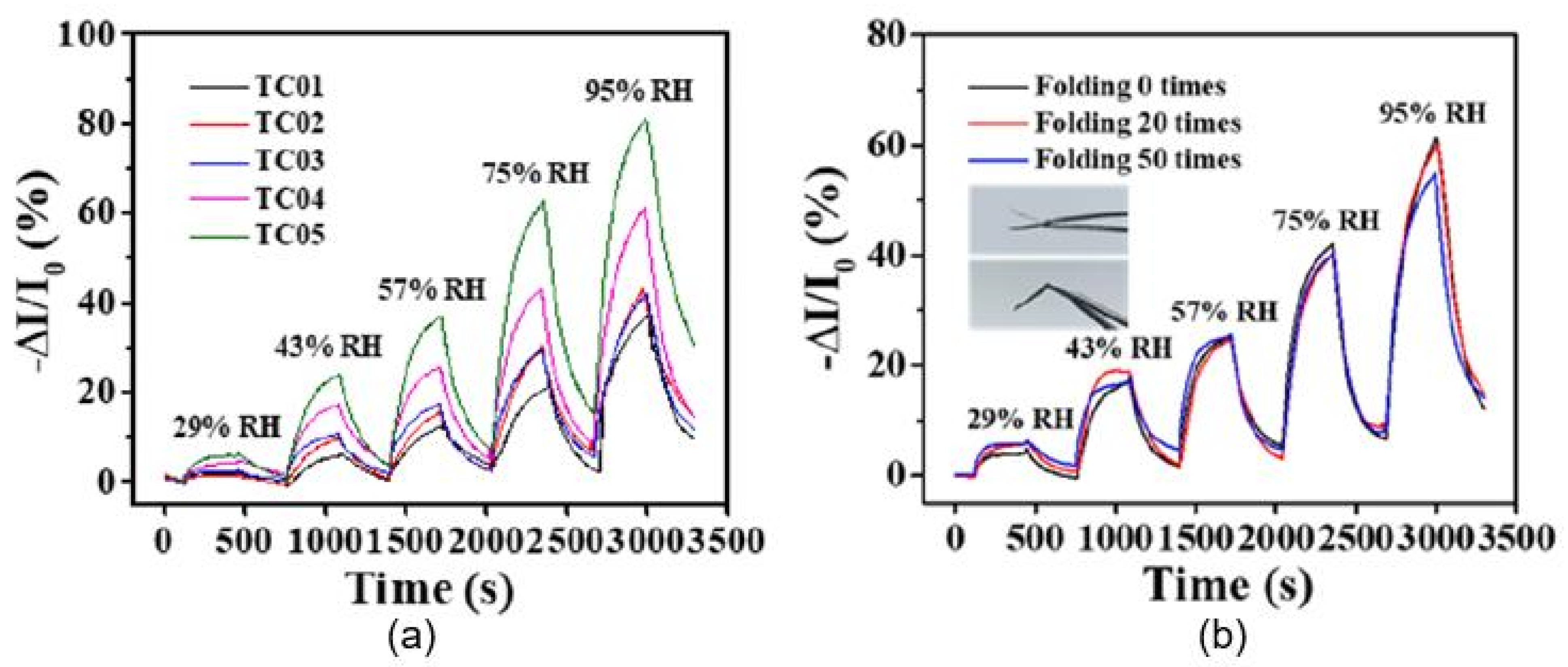
| H2SO4 and HNO3 (3:1)-treated o-MWCNT-coated paper | Inkjet printing | Graphite, pencil, hand-drawing | 33–95 | ∼30% (−ΔI/I0) | 470/500 s | [51] |
| COOH-functionalized SWCNT-coated paper | Filtration | Copper-foil tape | 10–75 | 37.5% (ΔS/S0) | 6/200 s | [60] |
| Graphene nanoplatelet | Vacuum filtration | Copper | 5–90 | 290% (ΔR/R0) | 12/20 s | [61] |
| Dip coating | 75% (ΔR/R0) | 9/15 s | ||||
| CNF/GNP composite on PEN | Screen printing | Ag, screen printing; GNP/CNF | 30–90 | ∼140–240% (ΔR/R0) | 17/22 s | [62] |
| Graphene–carbon (glossy photo paper) | Screen printing | Graphene–carbon | 30–92 | 13 Ω/% RH | 4/6 s | [63] |
| Graphite | Screen printing | Ag, inject printing, laser ablation | N/A | 0.0564% | N/A | [64] |
| Graphite (hard cellulose paper (business card)) | Pencil drawing | Graphite | 43–83 | 1.4–8.6 kΩ/% RH 0.5–2.5%/RH | 270/420 s | [65] |
| Sensing Material | Method | Range RH (%) | Response | Res./Rec. Time | Ref. |
|---|---|---|---|---|---|
| SiO2/acetate film | Hand drawing | 50–80 | ~104 (R0/R) | 31/7 s | [72] |
| CdS NPs | Drop casting | 5–99 | 55% (ΔR/R) ~2 (R0/R) | ~75/~50 s | [73] |
| ZnO NPs | Spin coating | 20–70 | 14 (R0/R) | 600/- s | [41,74] |
| Sensing Material/Paper | Method/Electrode | Range RH (%) | Response/Sensitivity | Res./Rec. Time | Ref. |
|---|---|---|---|---|---|
| PANI (photographic paper) | Inkjet printing/ | 13–95 | ~2.5 (R0/R); 220 Ω/% RH (ΔR/% RH) | N/A | [77] |
| PANI-CMC copolymer (glass) | Spin coating/- | 5–95 | ~2.5 (R0/R); 2.5 MΩ/% RH (ΔR/% RH) | 45/60 s | [78] |
| PANI/CNF sheet (cellulose sheet) | -/- | 30–50 | 1.3 µA/% RH (ΔI/% RH) | 370/1500 s | [79] |
| PANI/SLS (highly porous cellulose paper) | Polymerization/ Ag, printing | 5–95 | ~120 (R0/R); 35 kΩ/% RH 99.2% (ΔR/R) | 18/35 s | [80] |
| PANI/RGO (polypropylene filter paper) | Filtration/Ag | 11–98 | 580% (ΔI/I0) | 50/100 s | [81] |
| Ng PANI/paper composite (filter paper) | Polymerization/ Copper tape | 20–95 | ~10 (R0/R); 10 kΩ/% RH | 1300/2800 s | [82] |
| Nf PANI/paper composite | 75–95 | ~5 (R/R0); 50 Ω/% RH | N/A | ||
| PEDOT: PVMA (photographic paper) | Inkjet printing/- | 11–98 | 71–98% (ΔR/R); 3–50 (R0/R) | <5/<5 s | [83] |
| Polypyrrole (chromatography paper 1CHR) | Polymerization/ graphite, printing | 33–90 | ~1.3 (R/R0); ~17 Ω/% RH (ΔR/% RH) | N/A | [84] |
| Sensing Material | Method/Electrode | Range (% RH) | Response, (Z0/Z) | Res./Rec. Time | Ref |
|---|---|---|---|---|---|
| PEDOT:PSS (bond paper) | Inkjet printing | 16–90 | ~3 (Z0/Z) | N/A | [89] |
| PANI (bond paper) | Inkjet printing | 16–45 45–90 | ~104 (Z0/Z) ~10 (Z0/Z) | 9 min 12 min | |
| PANI (bond and photographic paper) | Inkjet printing | 13–90 | ~2.5 (Z0/Z) −0.23 kΩ/% RH | N/A | [77] |
| PANI (chromatography paper) | Drop coating | 0–97 | 48% | 220/150 s | [94] |
| PANI-CMC composite (glass plate) | Spin coating | 25–75 75–95 | ~3 × 102 (Z0/Z) ~2 (Z0/Z) | 10/90 s | [95] |
| Paper (print paper) | Pencil-trace electrodes | 11–50 50–95 | ~3 (Z0/Z) 400 (Z0/Z) | N/A 56/86 s | [96] |
| PILs (print paper) | Drop casting | 11–95 | ~103 (Z0/Z) | 13/145 s | |
| Paper (Whatman paper) | /PEDOT:PSS electrode, screen printing | 25–60 60–85 | <2 (Z0/Z) ~300 (Z0/Z) | N/A | [97] |
| Paper functionalized with NaCl | Spray coating, dip coating | 25–60 60–85 | <2 (Z0/Z) 2×103 (Z0/Z) | N/A | |
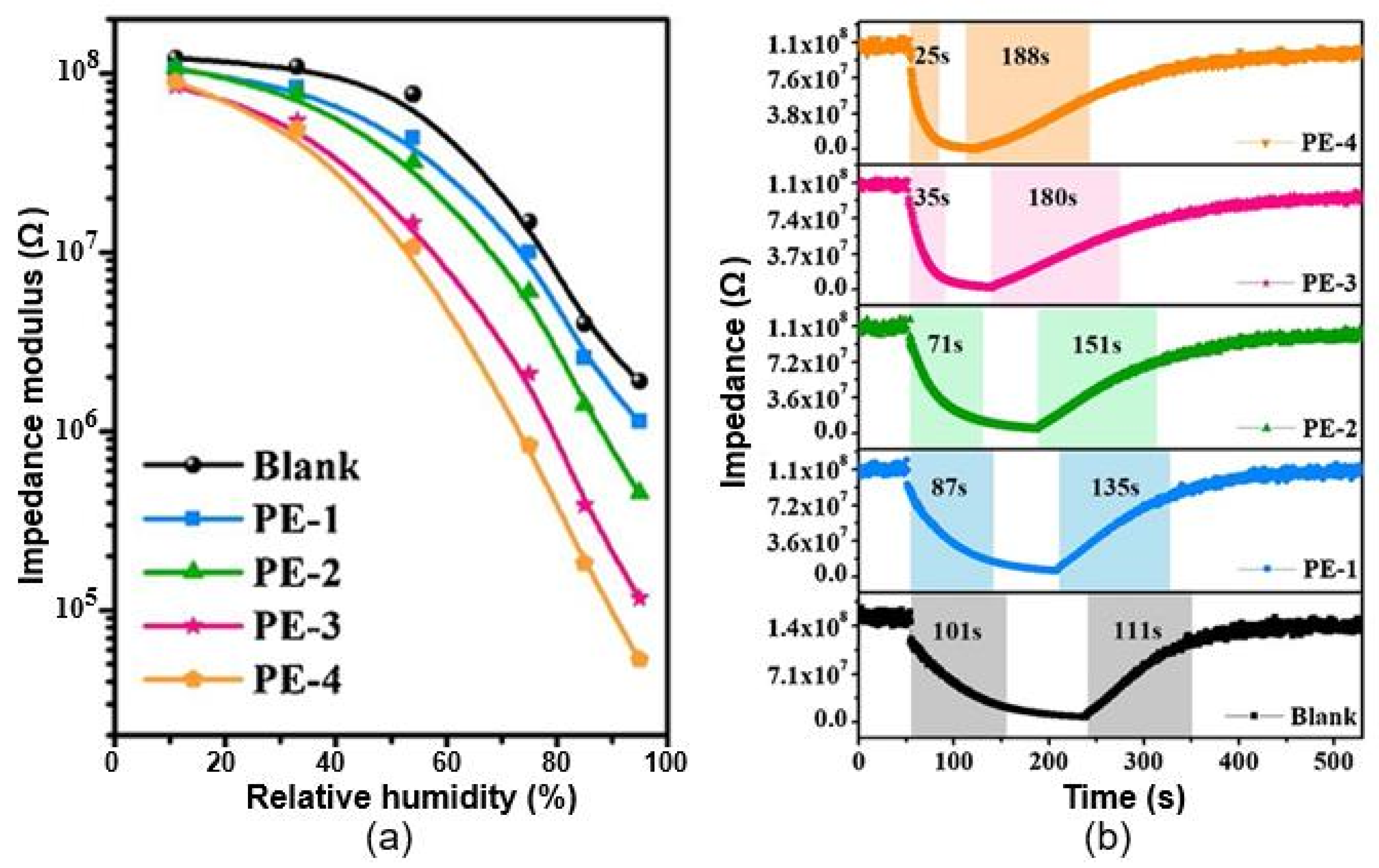
| Paper (printing paper) | /Ag, screen printing | 11–60 60–95 | ~2 (Z0/Z) ~65 (Z0/Z) | N/A 101/111 s | [98] |
| Paper functionalized with EPTAC | Immersion | 11–95 | ~2×103 (Z0/Z) | 25/188 s | |
| TEMPO-oxidized cellulose nanofibers (CNF) | Free-standing film/PEDOT:PSS, Ag, screen printing | 20–85 | −0.1 Ω/% RH ~103 (Z0/Z) | 6–7/7–8 s | [99] |
| ZnO/CNF | Free-standing pellet/graphite pencil drawing | 40–80 | ~5 (Z0/Z) −0.45 MΩ/% RH | N/A | [100] |
| 80–90 | 160 (Z0/Z) 4 MΩ/% RH | ||||
| CNF | Free-standing film/carbon, screen printing | 20–90 | 47 (Z0/Z) | 200/1020 s | [101] |
| CNF/PEG | 1036 (Z0/Z) | 265/490 s |
| Sensor | PILs Content | Sensitivity | Hysteresis (% RH) | Response/Recovery Times |
|---|---|---|---|---|
| Blank Paper | 0% | 408.2 | 19 | 56 s/86 s |
| PILs@Paper1 | 6.24% | 408.4 | 11 | 45 s/99 s |
| PILs@Paper2 | 9.05% | 616.5 | 9 | 38 s/105 s |
| PILs@Paper3 | 15.07% | 961.3 | 7 | 25 s/113 s |
| PILs@Paper4 | 24.08% | 1426.6 | 22 | 13 s/145 s |
| PILs@Paper5 | 34.02% | 2662.4 | 25 | 7 s/202 s |
| Type | Sensing Material | Method | RH Range, % | Resonance Frequency | Frequency Shift | Res./Rec. Times | Ref. |
|---|---|---|---|---|---|---|---|
| QCM | Bacterial cellulose (BC) | Drop coating | 5–55 55–97 | 5 MHz | 7 Hz/% RH 43 Hz/% RH | N/A | [112] |
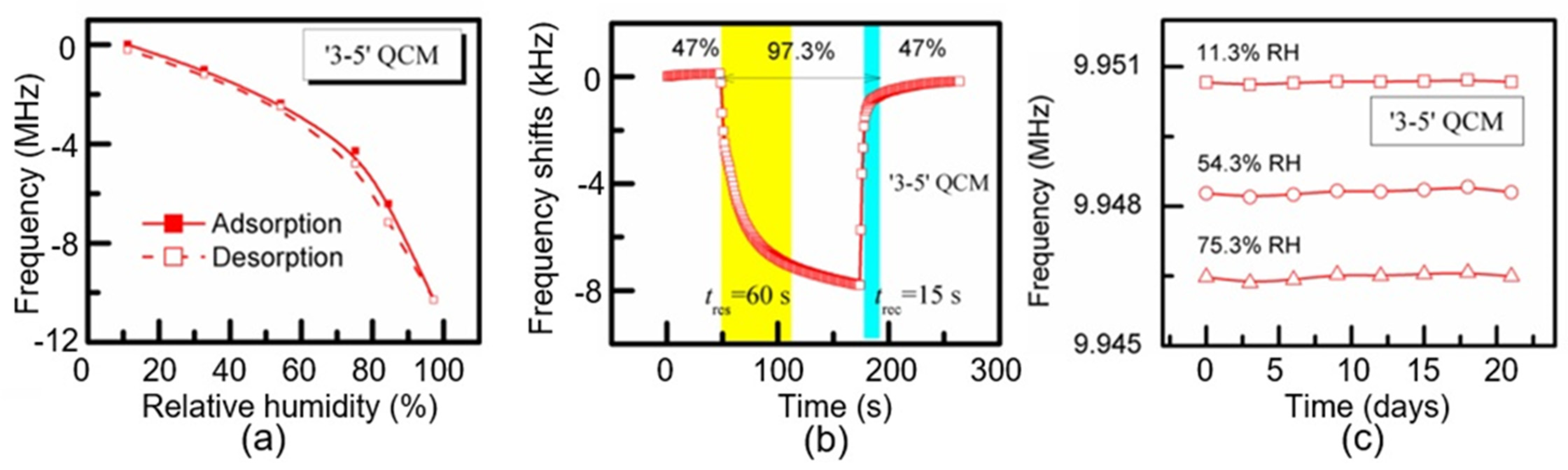
| CNC | Air-brush method | 11–60 60–97 | 10 MHz | 60 Hz/% RH ~200 Hz/% RH | 60/15 s | [106] | |
| Polydopamine/CNC/GO | Drop coating | 10–60 60–97 | 10 MHz | 26 Hz/% RH 90 Hz/% RH | 11/4 s 37/5 s | [109] | |
| CNC | Drop coating | 11–50 50–95 | ~5 MHz | ~6 Hz/% RH ~40 Hz/% RH | 90–120/50–60 s | [107] | |
| Nitro-modified CNC | Drop coating | 11–84 | 20 MHz | 28 Hz/% RH | 18/10 s | [108] | |
| SAW | BC | Spin coating | 5–25 30–85 85–95 | 200 MHz | 790 Hz/% RH ~1300 Hz/% RH ~3000 Hz/% RH | 3/3 s 7/4 s 12/5 s | [110] |
| CMUT | CNC | Spin coating | 11–53 53–94 | 10 MHz | 900 Hz/% RH 2000 Hz/% RH | 7/2 s | [111] |
| Sensing Material | Film Deposition Technique | RH Range, % | Method | Response | Res./Rec. Times | Ref. |
|---|---|---|---|---|---|---|
| HEC/PVDF | Drop coating | 50–80 | Light intensity | 0.0228 dB/% RH | N/A | [115] |
| HEC/PVDF | Drop coating | 50–80 | Light intensity | 0.03 dB/% RH | N/A | [114] |
| Carboxymethyl cellulose (CMC) | Deep coating | 20–70 70–85 | Light intensity | 0.07 dB/% RH 0.86 dB/% RH | N/A | [116] |
| CMC | Deep coating | 35–85 | Wavelength change | 0.17 nm/% RH | 3/3 s | [118] |
| CMC/CNTs | 0.23 nm/% RH | |||||
| CLC | Program-controlled coating system | 38–98 | Wavelength change | 0.28 nm/% RH | 30 min/ | [117] |
| Cellulose-acetate butyrate (CAB) | N/A | 10–95 | Wavelength change | ~0.5 nm/% RH | 125/ s | [119] |
| CMC fiber | Dry-jet wet spinning | 50–90 | Light intensity | N/A | N/A | [120] |
| Sensing Material | Method | RH Range, % | Resonance Frequency | Response | Res./Rec. Times | Ref. |
|---|---|---|---|---|---|---|
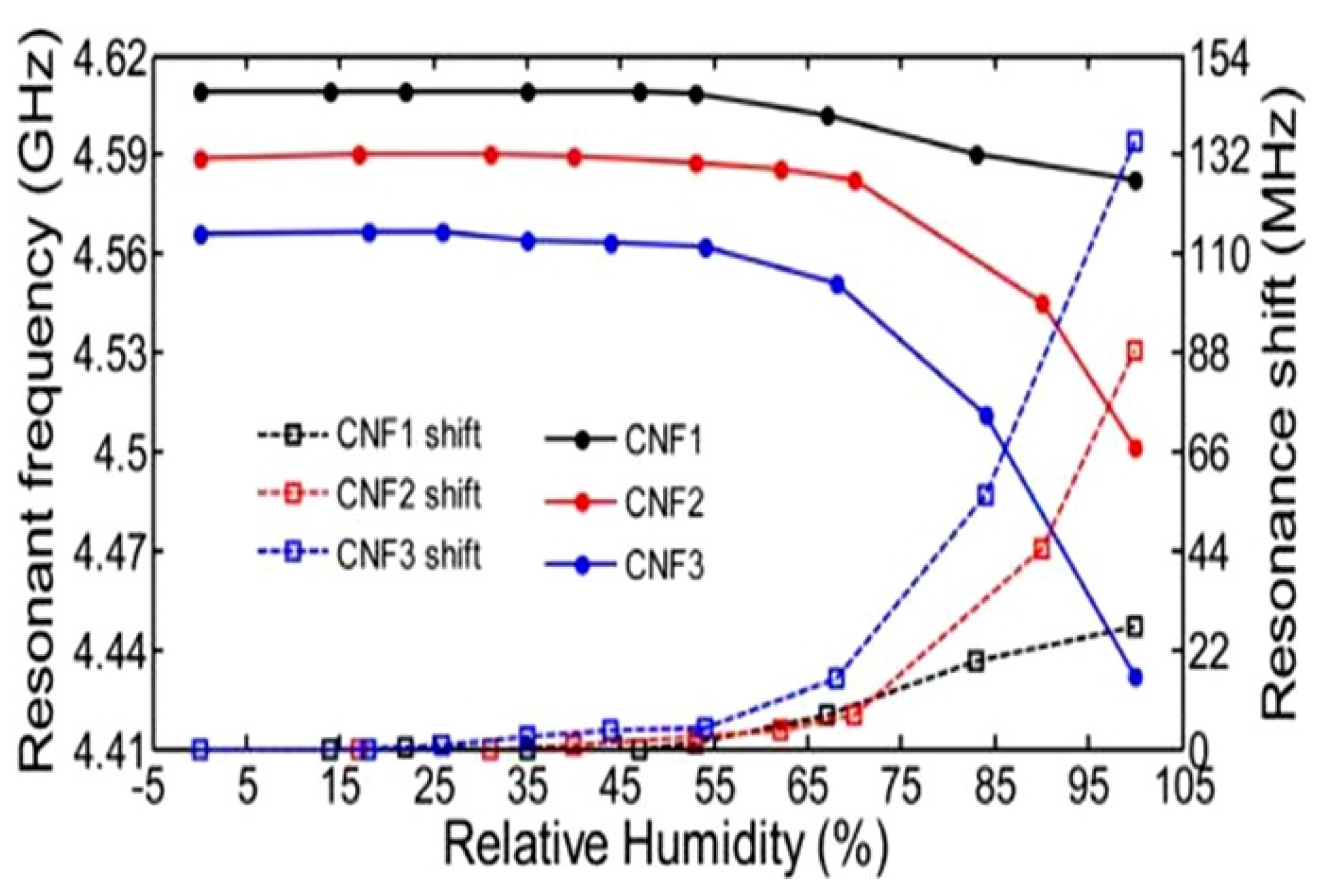
| TEMPO-oxidized CNF | Films and sheet | 5–65 65–97 | f0 = 4.7 GHz | ~0.3 MHz/% RH ~3.6 MHz/% RH | N/A | [121] |
| 22–89 | f0~8.1 GHz | 2.5 MHz/% RH | N/A | [122] | ||
| TEMPO-oxidized CNF/polyvinyl alcohol (PVOH) | 22–89 | f0~6.9 GHz | ~4 MHz/% RH | N/A | ||
| PEDOT:PSS/CNF | 0–90 | f0 = 2.78 GHz | N/A | N/A | [123] |
| Ref. | f0 | Dimension | Sensitivity | Quality Factor |
|---|---|---|---|---|
| [137] | 24 MHz | - | 13 kHz/% RH | - |
| [133] | 157 MHz | 402 mm2 | 370 kHz/% RH | 3.7 |
| [136] | 182 MHz | 202 mm2 | 140 kHz/% RH | 6.5 |
| Sensing Material | RH Range. % | λmax (Reflection) Change, nm | Response/Recovery Times | Ref. |
|---|---|---|---|---|
| CNC | 70–95 | 500–550 | 7 min/>5 min | [167] |
| CNC | 43–98 | 360–525 | N/A | [168] |
| CNC/poly(ethylene glycol) (PEG) | 50–90 | 520–670 | N/A | [169] |
| CNC/PEG | 30–100 | 500–910 | N/A | [170] |
| CNC/PEG/PBD-PEGE | 30–100 | 558–805 | N/A | [171] |
| CNC/glycerol | 16–98 | 620–710 | N/A | [172] |
| CNC/glycerol | 33–98 | 525–820 | /300 s | [173] |
| CNC/polyol | 30–95 | 425–652 455–695 501–750 | N/A | [174] |
| CNC/polyacrylamide (PAM) | 11–97 | 590–708 | 2–3 min/ | [175] |
| CNC/PNIPAM | 70–90 | 424–518 524–654 600–745 | 30 s/180 s | [176] |
| CNC/PNIPAM | 9–98 | 490–680 | N/A | [177] |
| CNC/NaCl | 50–95 | 520–680 | 240 min/30 min | [178] |
| CNC/NMMO | 13–97 | 590–680 | <2 min/~10 min | [179] |
| Au/CNC | 35–70 | N/A | /2 min | [180] |
| Au/(CHI/CMC-N3)25/Au | 10–98 | N/A | N/A | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korotcenkov, G.; Simonenko, N.P.; Simonenko, E.P.; Sysoev, V.V.; Brinzari, V. Paper-Based Humidity Sensors as Promising Flexible Devices, State of the Art, Part 2: Humidity-Sensor Performances. Nanomaterials 2023, 13, 1381. https://doi.org/10.3390/nano13081381
Korotcenkov G, Simonenko NP, Simonenko EP, Sysoev VV, Brinzari V. Paper-Based Humidity Sensors as Promising Flexible Devices, State of the Art, Part 2: Humidity-Sensor Performances. Nanomaterials. 2023; 13(8):1381. https://doi.org/10.3390/nano13081381
Chicago/Turabian StyleKorotcenkov, Ghenadii, Nikolay P. Simonenko, Elizaveta P. Simonenko, Victor V. Sysoev, and Vladimir Brinzari. 2023. "Paper-Based Humidity Sensors as Promising Flexible Devices, State of the Art, Part 2: Humidity-Sensor Performances" Nanomaterials 13, no. 8: 1381. https://doi.org/10.3390/nano13081381





