A Visible-Light-Enhanced Heterogeneous Photo Degradation of Tetracycline by a Nano-LaFeO3 Catalyst with the Assistance of Persulfate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Experimental
2.3. Characterization and Analysis
3. Results and Discussion
3.1. Physicochemical and Structure Properties
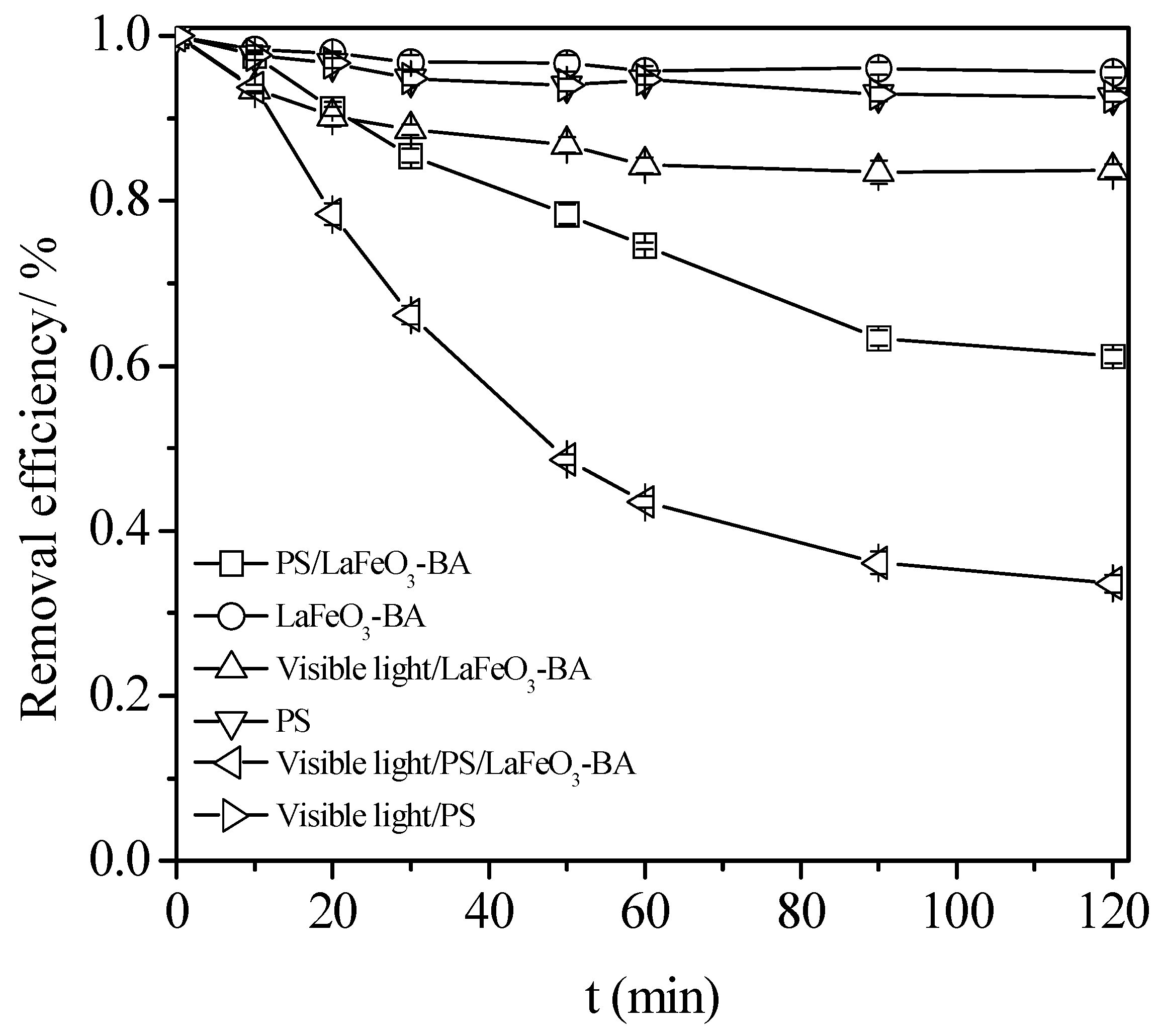
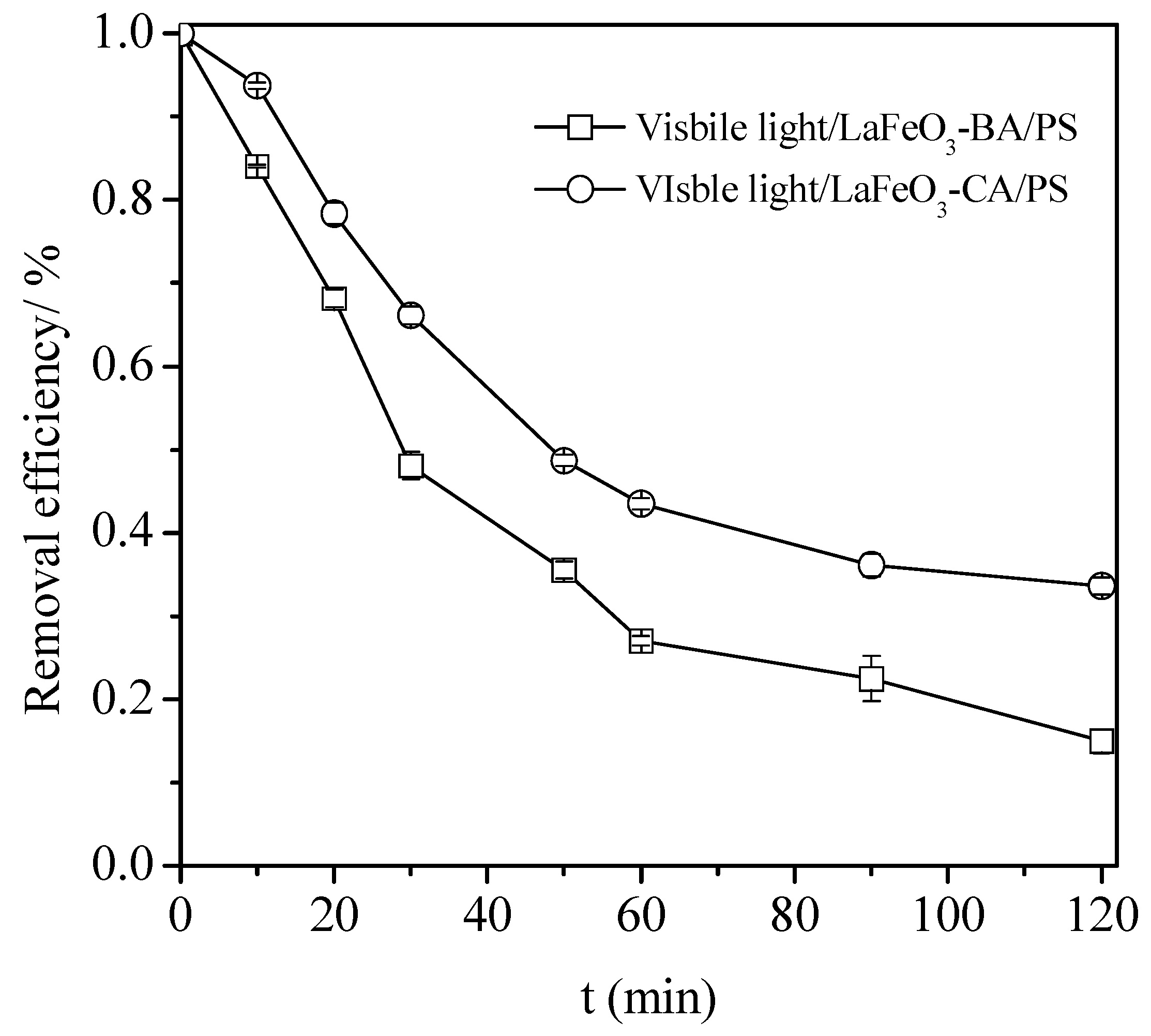
3.2. Process Configuration Effect on the Degradation of the TC Degradation
- The PS alone (single process);
- The catalyst alone (single process);
- A combination of visible light/PS (dual process);
- A combination of PS/BA (dual process);
- A combination of visible light/BA (dual process);
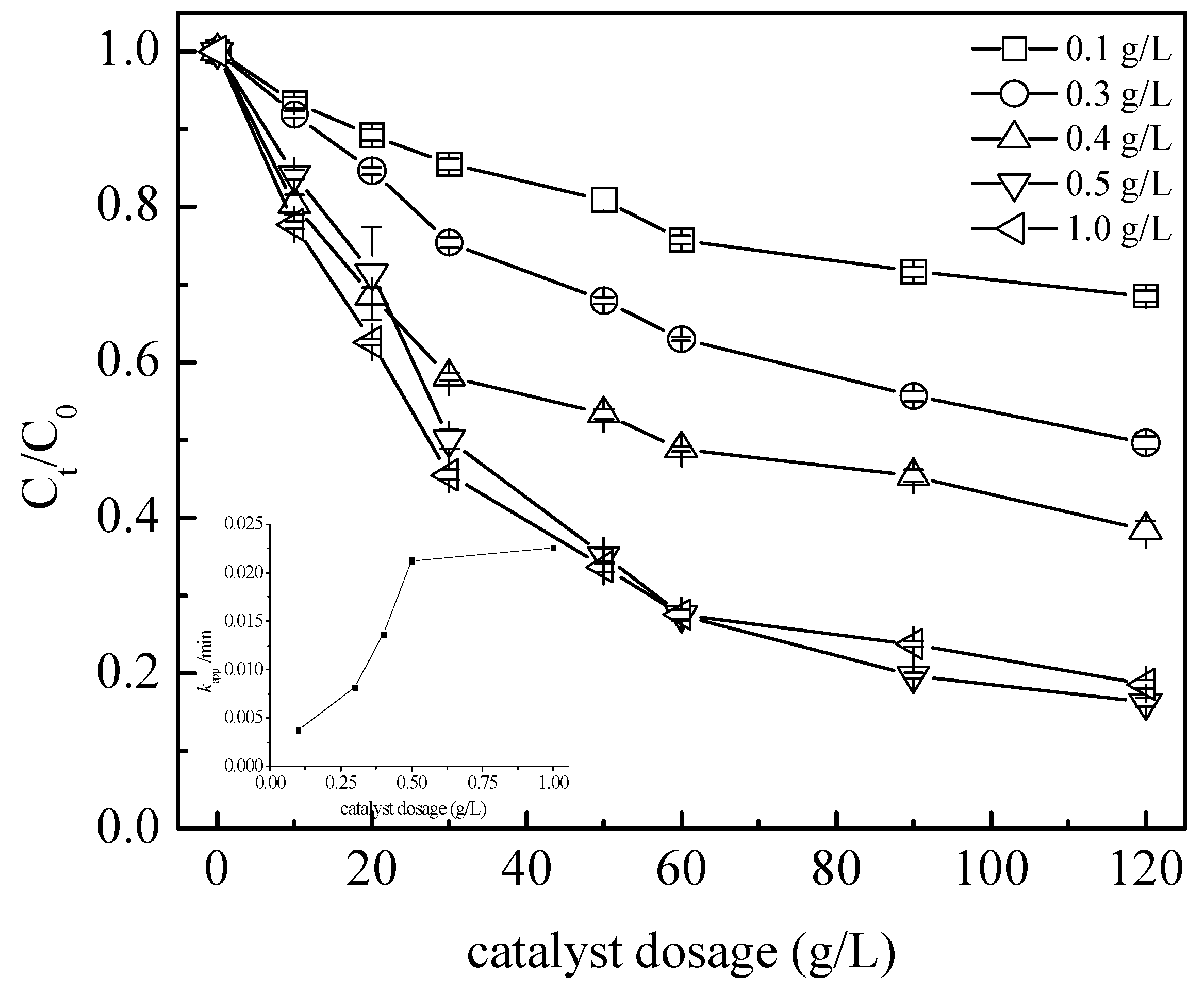
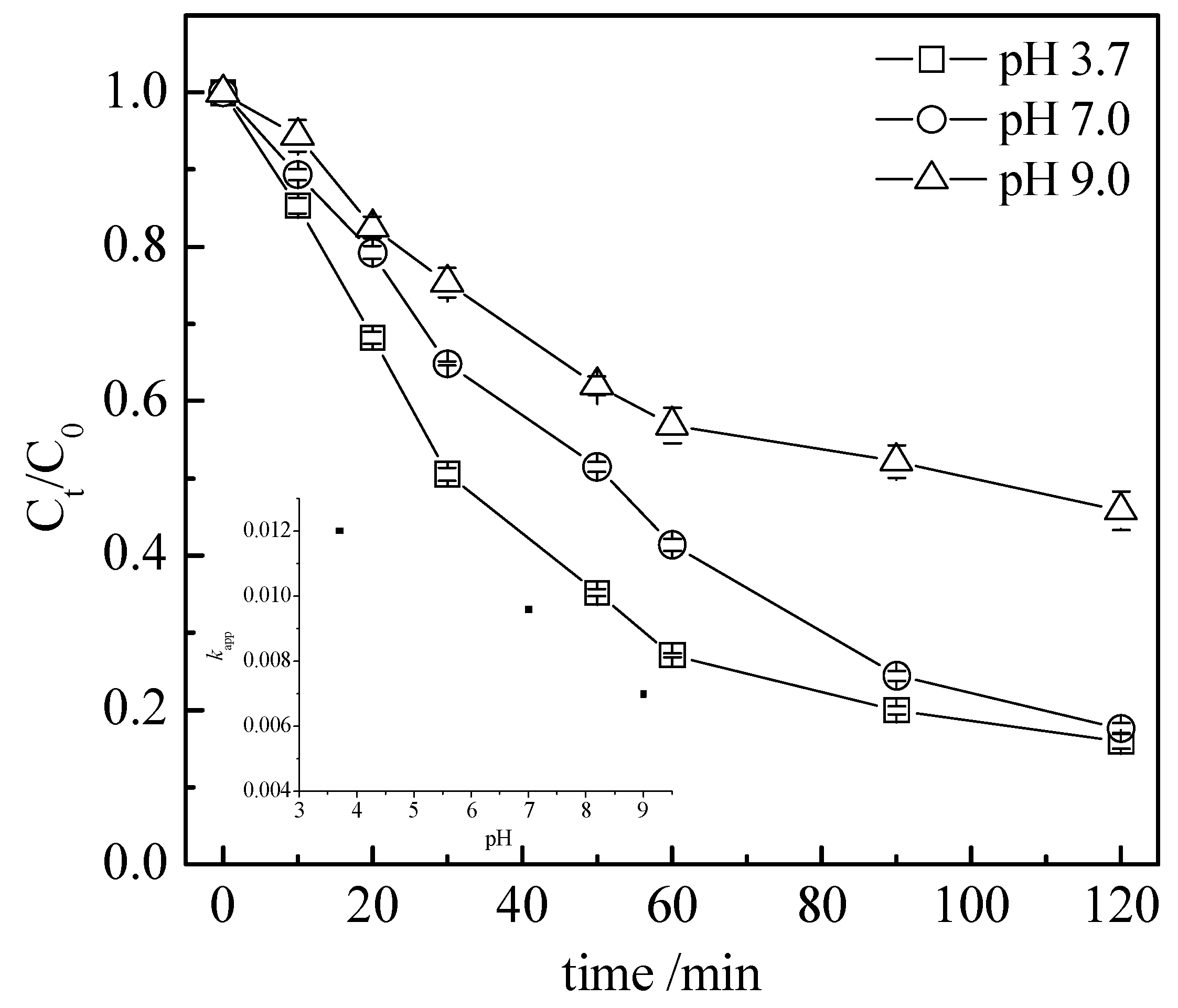
3.3. Operating Parameter Impact on the Process Global Efficiency
3.3.1. Catalyst Dosage and Oxidant Concentration Effect
3.3.2. pH Value Effect
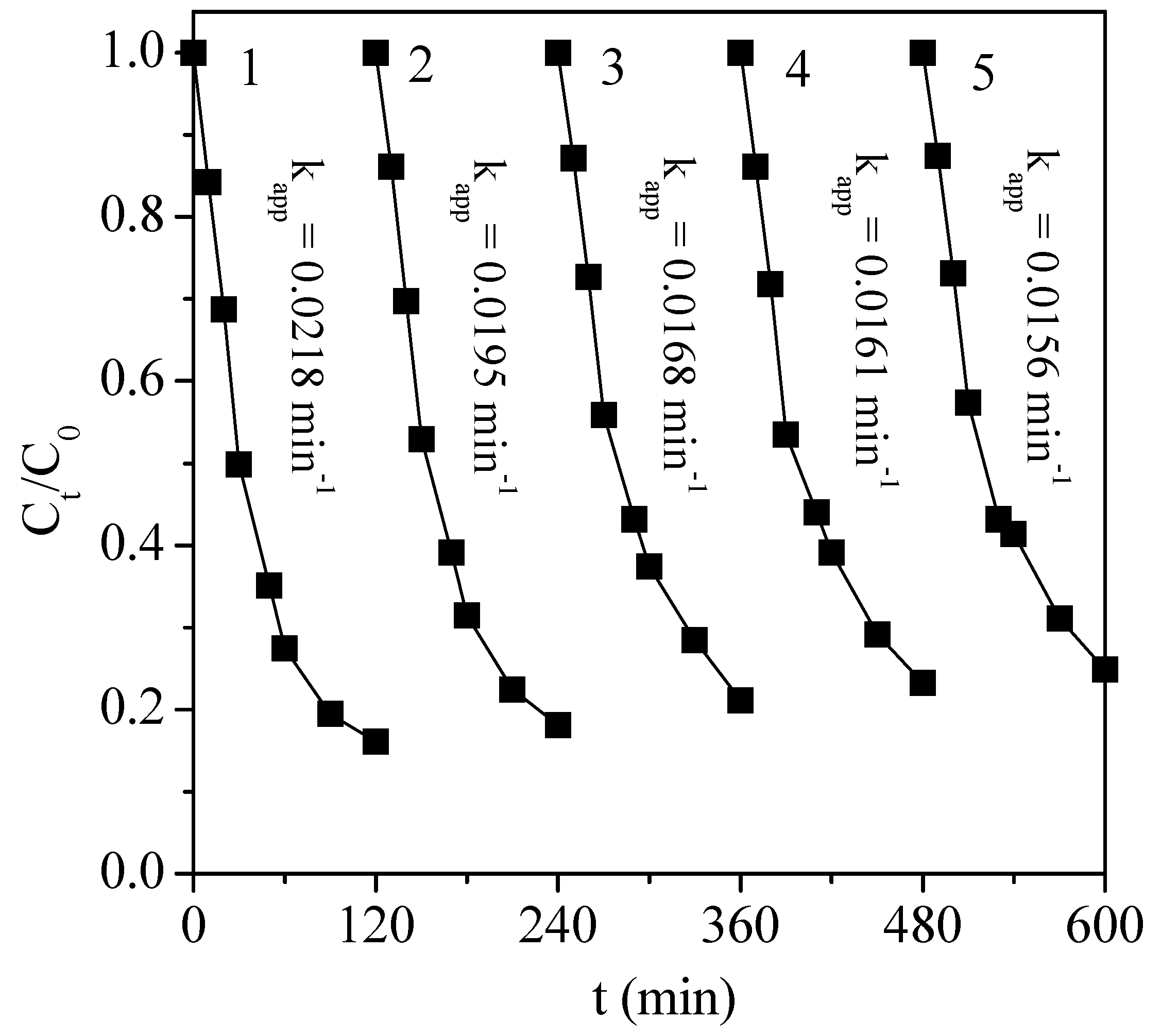
3.4. Catalyst Stability Test
3.5. Toxicity Evolution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ogunlaja, A.; Ogunlaja, O.O.; Olukannia, O.D.; Taylor, G.O.; Olorunnisola, C.G.; Dougnon, V.T.; Mousse, W.; Fatta-Kassinos, D.; Msagati TA, M.; Unuabonah, E.I. Antibiotic resistomes and their chemical residues in aquatic environments in Africa. Environ. Pollut. 2022, 312, 119783. [Google Scholar] [CrossRef]
- Do, S.H.; Jo, J.H.; Jo, Y.H.; Lee, H.K.; Kong, S.H. Application of a peroxymonosulfate Cobalt (PMS/Co(II)) system to treat diesel-contaminated soil. Chemosphere 2009, 77, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Enivron. 2009, 85, 171–175. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Miller, I.K. The chemistry of persulfate. I. The kinetics and mechanism of the decomposition of the persulfate ion in aqueous medium. J. Phys. Chem. B 1951, 73, 3055–3059. [Google Scholar]
- Anipsitakis, P.G.; Stathatos, E.; Dionysiou, D.D. Heterogeneous Activation of Oxone Using Co3O4. J. Phys. Chem. B 2005, 109, 13052–13055. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe–Co/SBA-15 catalyst for the degradation of Orange II in water. J. Hazard. Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef]
- House, D.A.; House, D.A. Kinetics and mechanism of oxidations by peroxydisulfate. Chem. Rev. 1962, 62, 185–203. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Zhang, Y.; Meng, W.; Yu, B.; Pu, S.; Yuan, D.; Qi, F.; Xu, B.; Chu, W. Sulfate radical-based photo-Fenton reaction derived by CuBi2O4 and its composites with α-Bi2O3 under visible light irradiation: Catalyst fabrication, performance and reaction mechanism. Appl. Catal. B Enivron. 2018, 235, 264–273. [Google Scholar] [CrossRef]
- Chudzińska, J.; Woźniak, B.; Sprynskyy, M.; Nowak, I.; Feliczak-Guzik, A. Photoremoval of bisphenol A using hierarchical zeolites and diatom biosilica. Int. J. Mol. Sci. 2023, 24, 2878. [Google Scholar] [CrossRef] [PubMed]
- Sprynskyy, M.; Szczyglewska, P.; Wojtczak, I.; Nowak, I.; Witkowski, A.; Buszewski, B.; Feliczak-Guzik, A. Diatom biosilica doped with palladium(II) chloride nanoparticles as new efficient photocatalysts for methyl orange degradation. Int. J. Mol. Sci. 2021, 22, 6734. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as substitutes of noble metals for heterogeneous catalysis: Dream or reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.; Bérubé, F.; Kaliaguine, S. Effect of the synthesis conditions on the redox and catalytic properties in oxidation reactions of LaCo1−xFexO3. Appl. Catal. A 2005, 282, 273–284. [Google Scholar] [CrossRef]
- Kakihana, M.; Yashima, M.; Yoshimura, M.; Ikeda, N.; Sugitani, Y. Synthesis of high surface area LaMnO3+d by a polymerizable complex method. J. Alloys Compd. 1999, 283, 102–105. [Google Scholar] [CrossRef]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.; Fufori, S.; Fino, D.; Saracco, G.; Specchia, V. Lanthanum cobaltite catalysts for diesel soot combustion. Appl. Catal. B Enivron. 2008, 83, 85–95. [Google Scholar] [CrossRef]
- Peňa, M.A.; Fierro, J.L. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Szabo, V.; Bassir, M.; Neste, A.V.; Kaliaguine, S. Perovskite-type oxides synthesized by reactive grinding: Part IV. Catalytic properties of LaCo1− xFexO3 in methane oxidation. Appl. Catal. B Enivron. 2003, 43, 81–92. [Google Scholar] [CrossRef]
- Royer, S.; Neste, A.V.; Davidson, R.; Mclntyre, S.; Kaliaguine, S. Methane Oxidation over Nanocrystalline LaCo1-xFexO3: Resistance to SO2 Poisoning. Ind. Eng. Chem. Res. 2004, 43, 5670–5680. [Google Scholar] [CrossRef]
- Meríno, N.A.; Barbero, B.P.; Grange, P.; Cadús, L.E. La1−xCaxCoO3 perovskite-type oxides: Preparation, characterisation, stability, and catalytic potentiality for the total oxidation of propane. J. Catal. 2005, 231, 232–244. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, L.; Li, Y.; Hu, C. Enhanced Fenton-like degradation of refractory organic compounds by surface complex formation of LaFeO3 and H2O2. J. Hazard. Mater. 2015, 294, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jing, L.; Fu, W.; Yang, L.; Xin, B.; Fu, H. Photoinduced charge property of nanosized perovskite-type LaFeO3 and its relationships with photocatalytic activity under visible irradiation. Mater. Res. Bull. 2007, 42, 203–212. [Google Scholar] [CrossRef]
- Parida, K.M.; Reddy, K.H.; Martha, S.; Das, D.P.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sol–gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydro. Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Tang, P.; Tong, Y.; Chen, H.; Cao, F.; Pan, G. Microwave-assisted synthesis of nanoparticulate perovskite LaFeO3 as a high active visible-light photocatalyst. Curr. Appl. Phys. 2013, 13, 340–343. [Google Scholar] [CrossRef]
- Su, H.; Jing, L.; Shi, K.; Yao, C.; Fu, H. Synthesis of large surface area LaFeO3 nanoparticles by SBA-16 template method as high active visible photocatalysts. J. Nanoparticle. Res. 2010, 12, 967–974. [Google Scholar] [CrossRef]
- Tijare, S.N.; Joshi, M.V.; Padole, P.S.; Mangrulkar, P.A.; Rayalu, S.S.; Labhsetwar, N.K. Photocatalytic hydrogen generation through water splitting on nano-crystalline LaFeO3 perovskite. Inter. J. Hydrogen Engery 2012, 37, 10451–10456. [Google Scholar] [CrossRef]
- Libby, W.F. Promising catalyst for auto exhaust. Science 1971, 171, 499–500. [Google Scholar] [CrossRef]
- Safizade, B.; Masoudpanah, S.M.; Hasheminiasari, M.; Ghasemi, A. Photocatalytic activity of BiFeO3/ZnFe2O4 nanocomposites under visible light irradiation. RSC Adv. 2018, 8, 6988–6995. [Google Scholar] [CrossRef]
- Shi, J.; Guo, L. ABO3-based photocatalysts for water splitting. Prog. Nat. Sci. Mat. Int. 2012, 22, 592–615. [Google Scholar] [CrossRef]
- Xu, K.; Feng, J. Superior photocatalytic performance of LaFeO3/gC3N4 heterojunction nanocomposites under visible light irradiation. RSC Adv. 2017, 7, 45369–45376. [Google Scholar] [CrossRef]
- Lu, S.; Wang, G.L.; Chen, S.; Yu, H.T.; Quan, X. Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for degradation of organic pollutants. J. Hazard. Mater. 2018, 353, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.P.; Tian, X.K.; Nie, Y.L.; Yang, C.; Zhou, Z.X.; Wang, Y.X. Promoted peroxymonosulfate activation into singlet oxygen over perovskite for ofloxacin degradation by controlling the oxygen defect concentration. Chem. Eng. J. 2019, 359, 828–839. [Google Scholar] [CrossRef]
- Rao, Y.F.; Zhang, Y.Y.; Fan, J.H.; Wei, G.L.; Wang, D.; Han, F.M.; Huang, Y.; Croue, J.P. Enhanced peroxymonosulfate activation by Cu-doped LaFeO3 with rich oxygen vacancies: Compound-specific mechanisms. Chem. Eng. J. 2022, 435, 134882. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Liu, Y.F.; Miller, K.A.; Zhu, H.Y.; Egap, E. Lead halide perovskite nanocrystals as photocatalysts for PET-RAFT polymerization under visible and near-infrared irradiation. ACS Macro. Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Ma, K.L.; Gao, H.F. Tunable luminescence and enhanced polar solvent resistance of perovskite nanocrystals achieved by surface-initiated photopolymerization. J. Am. Chem. Soc. 2022, 144, 20411–20420. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Liu, Y.F.; Ai, Q.; Gao, G.H.; Yuan, L.; Fang, Q.Y.; Tian, X.Y.; Zhang, X.; Egap, E.; Ajayan, P.M.; et al. In situ synthesis of lead-free halide perovskite–COF nanocomposites as photocatalysts for photoinduced polymerization in both organic and aqueous phases. ACS Materials Lett. 2022, 4, 464–471. [Google Scholar] [CrossRef]
- Koo, P.L.; Jaafar, N.F.; Yap, P.S.; Oh, W.D. A review on the application of perovskite as peroxymonosulfate activator for organic pollutants removal. J. Environ. Chem. Eng. 2022, 10, 107093. [Google Scholar] [CrossRef]
- Courty, P.; Ajot, H.; Marcilly, C.; Delmon, B. Oxydes mixtes ou en solution solide sous forme très divisée obtenus par décomposition thermique de précurseurs amorphes. Powder Technol. 1973, 7, 21–38. [Google Scholar] [CrossRef]
- Baythoun, M.S.G.; Sale, F.R. Production of strontium-substituted lanthanum manganite perovskite powder by the amorphous citrate process. J. Mater. Sci. 1982, 17, 2757–2769. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Dujardin, C.; Granger, P. Surface reconstruction of supported Pd on LaCoO3: Consequences on the catalytic properties in the decomposition of N2O. J. Catal. 2008, 253, 37–49. [Google Scholar] [CrossRef]
- Lisi, L.; Bagnasco, G.; Ciambelli, P.; Rossi, S.D.; Porta, P.; Russo, G.; Turco, M. Perovskite-type oxides: II. Redox properties of LaMn1− xCuxO3 and LaCo1−xCuxO3 and methane catalytic combustion. J. Solid State Chem. 1999, 146, 176–183. [Google Scholar] [CrossRef]
- Zhang, R.; Villanueva, A.; Alamdari, H.; Kaliaguine, S. SCR of NO by propene over nanoscale LaMn1−xCuxO3 perovskites. Appl. Catal. A 2006, 307, 85–97. [Google Scholar] [CrossRef]
- Kaliaguine, S.; Neste, A.V.; Szabo, V.; Gallot, J.E.; Bassir, M.; Muzychuk, R. Perovskite-type oxides synthesized by reactive grinding: Part I. Preparation and characterization. Appl. Catal. A 2001, 209, 345–358. [Google Scholar] [CrossRef]
- Levasseur, B.; Kaliaguine, S. Methanol oxidation on LaBO3 (B = Co, Mn, Fe) perovskite-type catalysts prepared by reactive grinding. Appl. Catal. A 2008, 343, 29–38. [Google Scholar] [CrossRef]
- Alamdari, H.; Royer, S. Handbook of Perovskites and Related Mixed Oxides, Chapter 2: Mechanochemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2016; pp. 25–46. [Google Scholar]
- Properties and Applications of Perovskite-Type Oxides; Tejuca, L.G.; Fierro, J.L.G. (Eds.) Marcel Dekker: New York, NY, USA, 1992; p. 271. [Google Scholar]
- Campagnoli, E.; Tavares, A.; Fabbrini, L.; Rossetti, I.; Dubitsky, Y.A.; Zaopo, A.L. Forni, Effect of preparation method on activity and stability of LaMnO3 and LaCoO3 catalysts for the flameless combustion of methane. Appl. Catal. B Enivron. 2005, 55, 133–139. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, R.; Chen, B.; Bion, N.; Duprez, D.; Hou, L.; Zhang, H.; Royer, S. Design of nanocrystalline mixed oxides with improved oxygen mobility: A simple non-aqueous route to nano-LaFeO3 and the consequences on the catalytic oxidation performances. Chem. Comm. 2013, 49, 4923–4925. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Rossetti, I.; Forni, L. Flame-spray pyrolysis preparation of perovskites for methane catalytic combustion. J. Catal. 2005, 236, 251–261. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia 3 d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Comm. 2003, 17, 2136–2137. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, J.; Wu, F.; Zhang, Y. Rapid photooxidation of As(III) through surface complexation with nascent colloidal ferric hydroxide. Environ. Sci. Technol. 2014, 48, 272–278. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, L.; Zhang, C.; Yu, Y.; Zhang, L.; Wu, F. A novel photochemical system of ferrous sulfite complex: Kinetics and mechanisms of rapid decolorization of Acid Orange 7 in aqueous solutions. Water Res. 2014, 57, 87–95. [Google Scholar] [CrossRef]
- Gao, P.; Tian, X.K.; Fu, W.; Wang, Y.X.; Nie, Y.L.; Yang, C.; Deng, Y. Copper in LaMnO3 to promote peroxymonosulfate activation by regulating the reactive oxygen species in sulfamethoxazole degradation. J. Hazard. Mater. 2021, 411, 125163. [Google Scholar] [CrossRef]
- Liu, D.D.; Tang, Y.B.; Hao, Z.K.; Chen, D.Q.; Li, T.Q.; Jiang, L.P.; Tian, B.; Yan, C.P.; Luo, Y.; Jia, B.Y. Comparative study of H2O2/PDS-based advanced oxidation process using nanopaticles for Rhodamine B degradation: Mechanism, stability and applicability. J. Water. Pro. Eng. 2022, 47, 102757. [Google Scholar] [CrossRef]

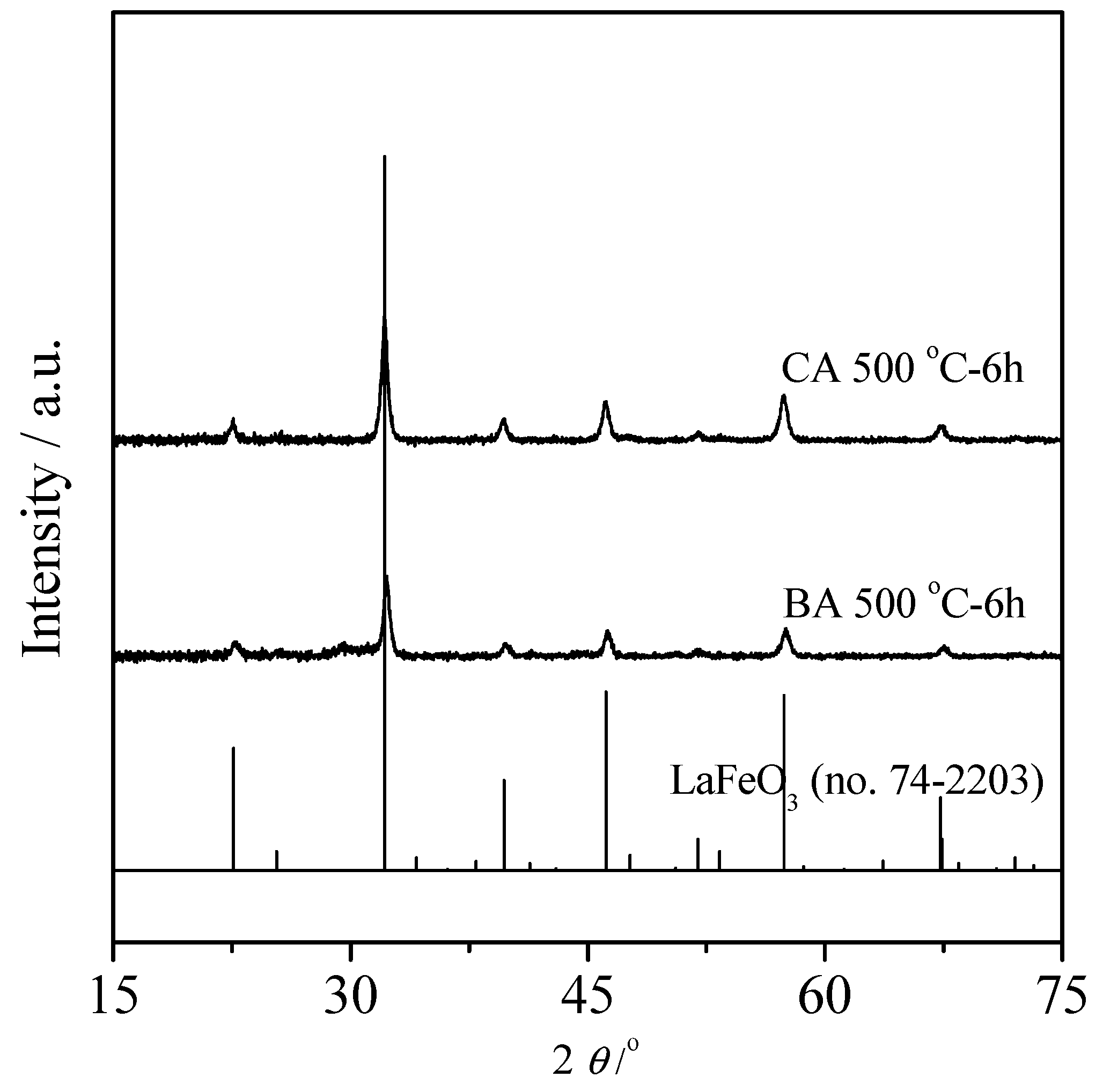
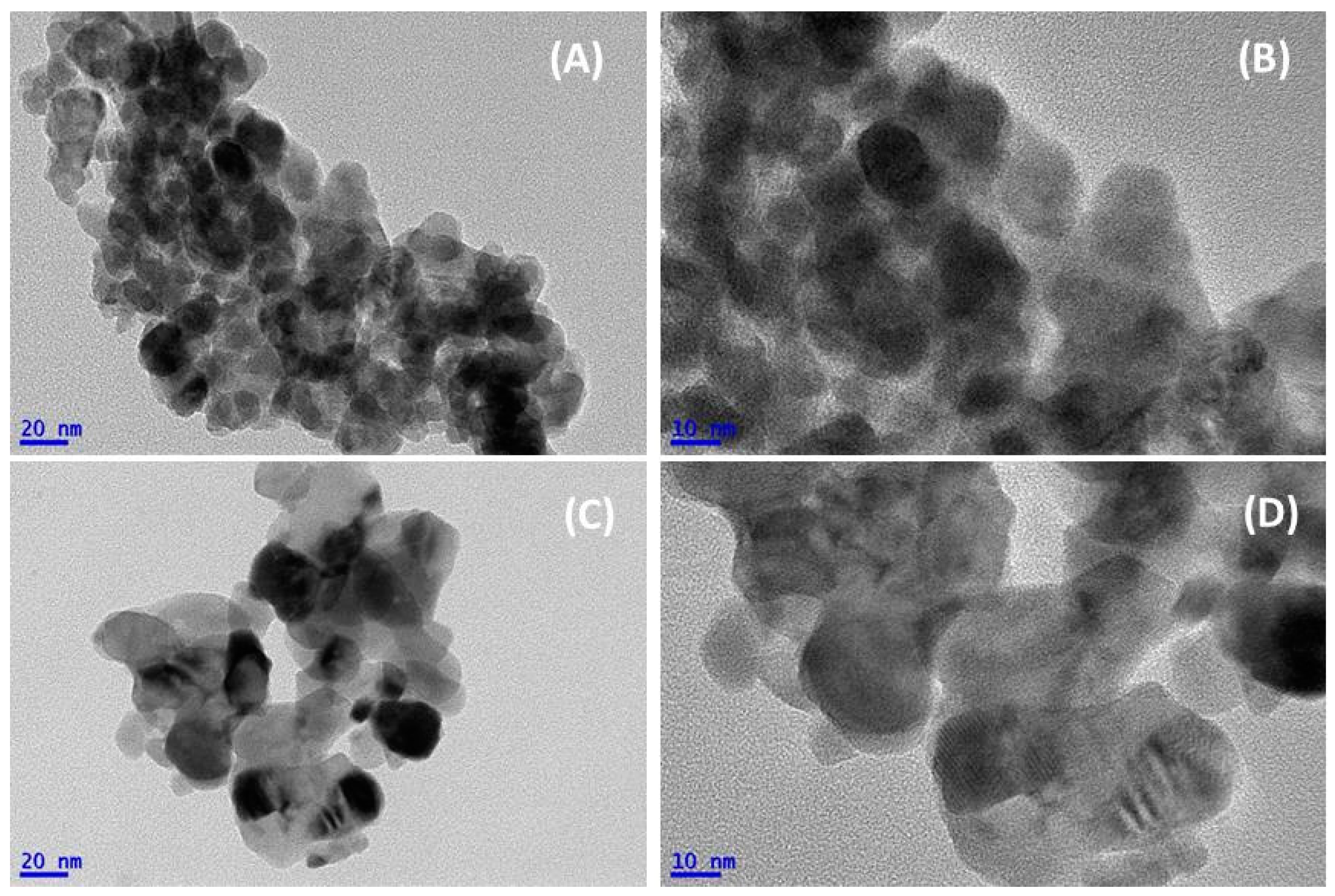

| Samples | BA Sample | CA Sample |
|---|---|---|
| Chemical composition | LaxFeyO3±δ | LaxFeyO3±δ |
| DXRD/a nm | 18.4 | 22.3 |
| Sth/b m2 g−1 | 49.2 | 36.9 |
| SBET/c m2 g−1 | 32.5 | 15.3 |
| R/d - | 0.66 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Wang, Y.; Zhou, F.; Liu, S.; Fu, L.; Wang, L.; Zhang, C.; Xue, W. A Visible-Light-Enhanced Heterogeneous Photo Degradation of Tetracycline by a Nano-LaFeO3 Catalyst with the Assistance of Persulfate. Nanomaterials 2023, 13, 1388. https://doi.org/10.3390/nano13081388
Hou L, Wang Y, Zhou F, Liu S, Fu L, Wang L, Zhang C, Xue W. A Visible-Light-Enhanced Heterogeneous Photo Degradation of Tetracycline by a Nano-LaFeO3 Catalyst with the Assistance of Persulfate. Nanomaterials. 2023; 13(8):1388. https://doi.org/10.3390/nano13081388
Chicago/Turabian StyleHou, Liwei, Yanan Wang, Fan Zhou, Shuangyue Liu, Lin Fu, Lei Wang, Changbo Zhang, and Weijie Xue. 2023. "A Visible-Light-Enhanced Heterogeneous Photo Degradation of Tetracycline by a Nano-LaFeO3 Catalyst with the Assistance of Persulfate" Nanomaterials 13, no. 8: 1388. https://doi.org/10.3390/nano13081388






