Toward Long-Term-Dispersible, Metal-Free Single-Chain Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
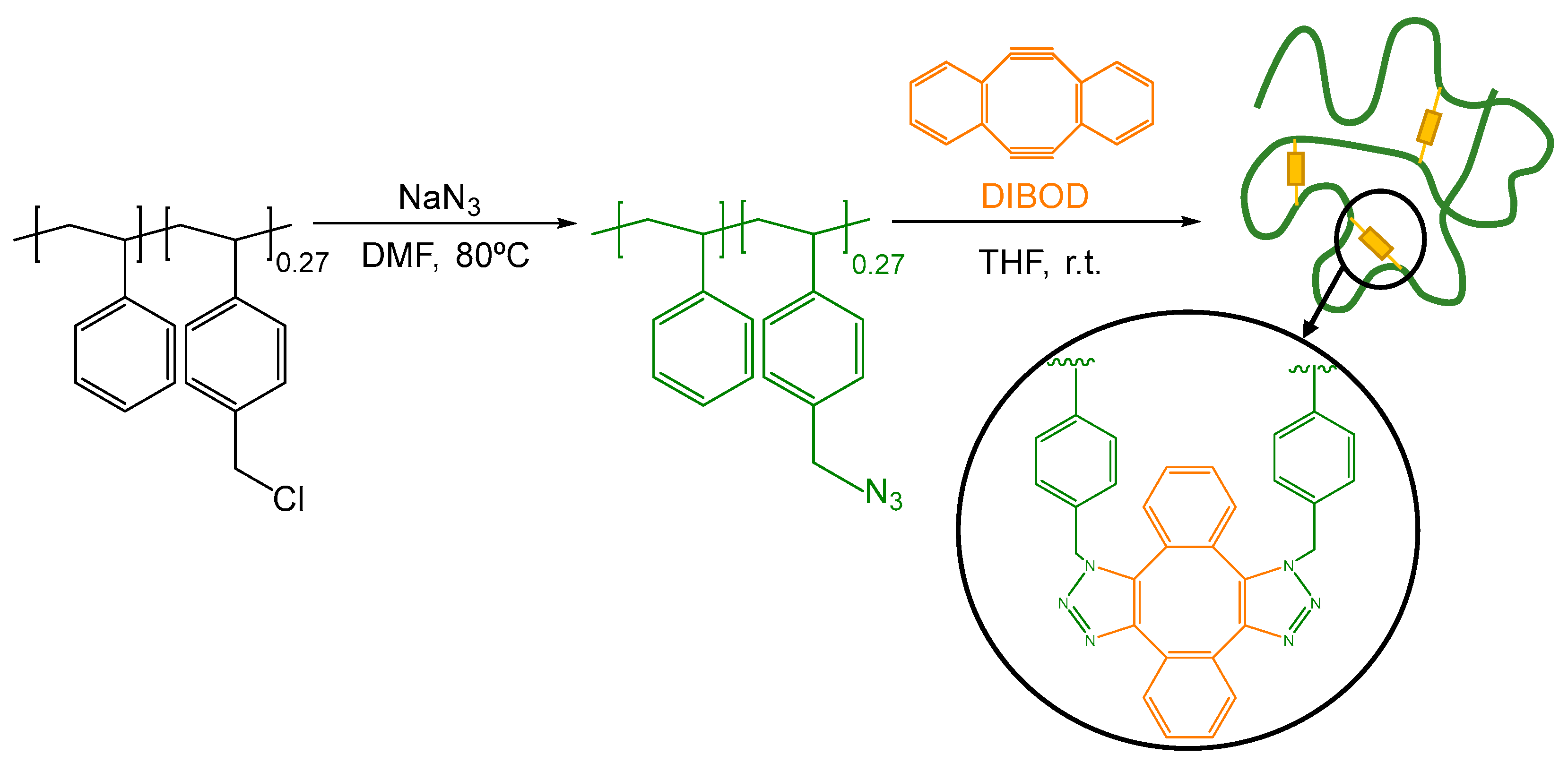
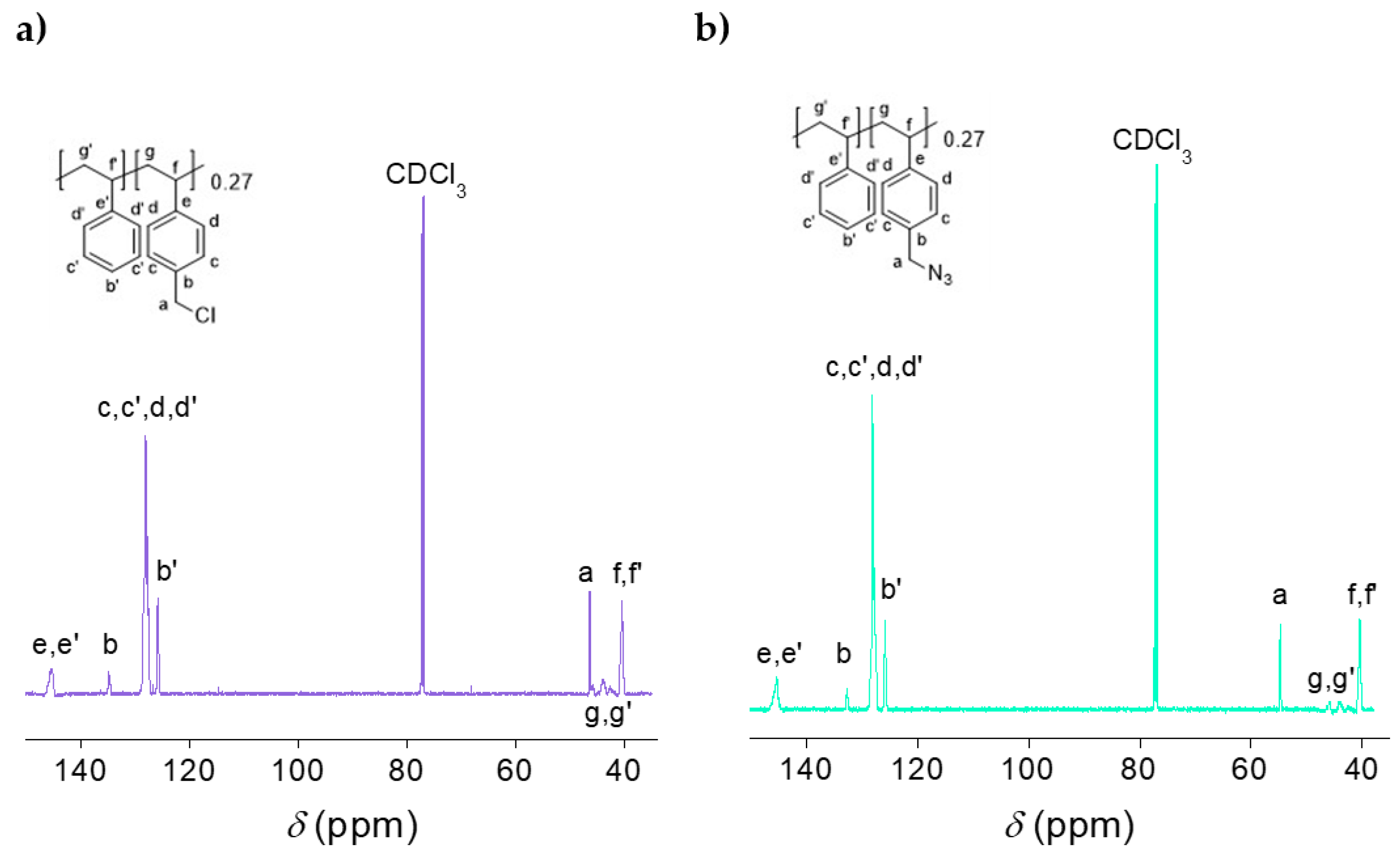
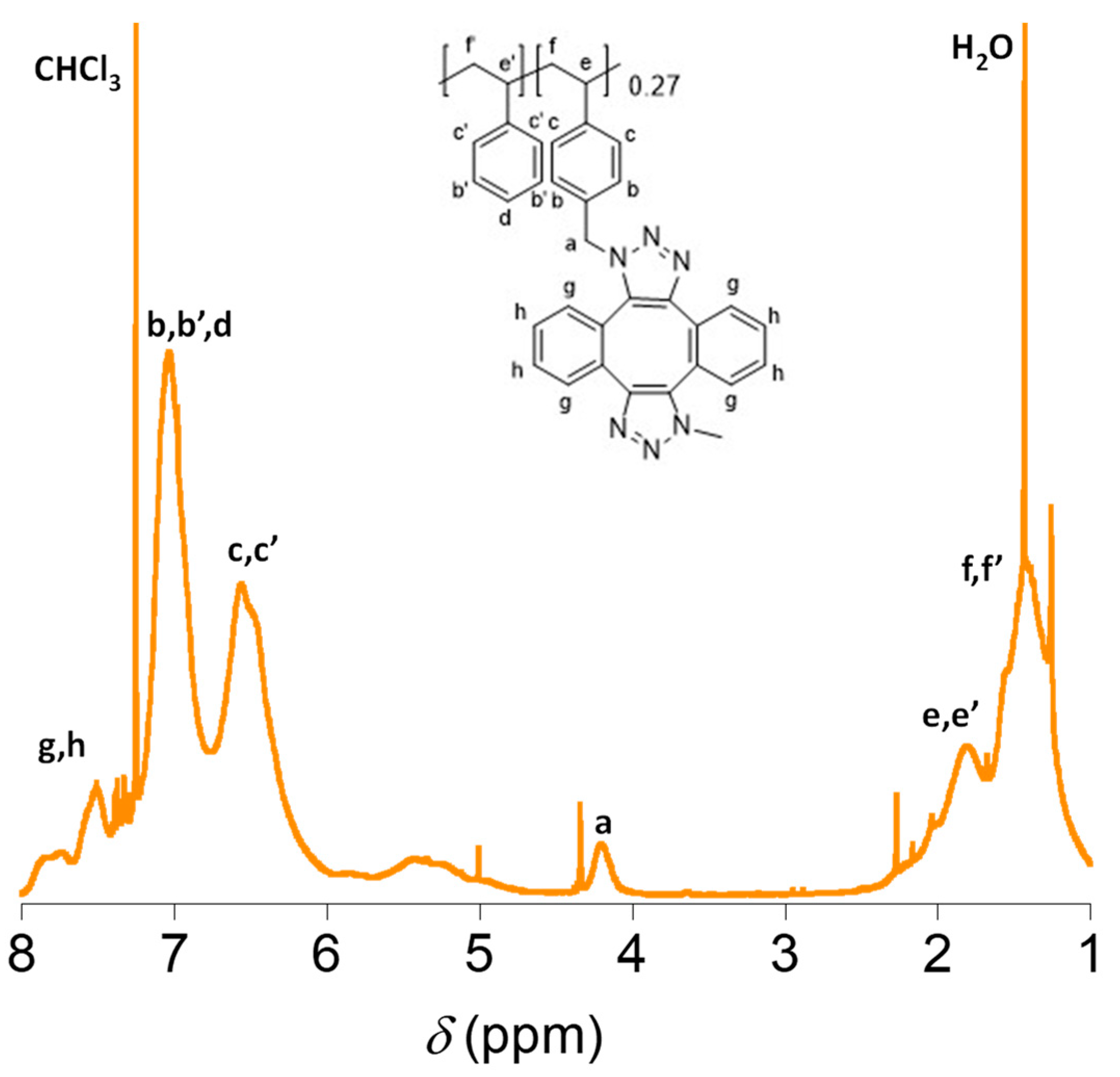
2.1. Synthesis and Characterization of the Polystyrene-Based Precursor Decorated with Azide Functional Groups (2)
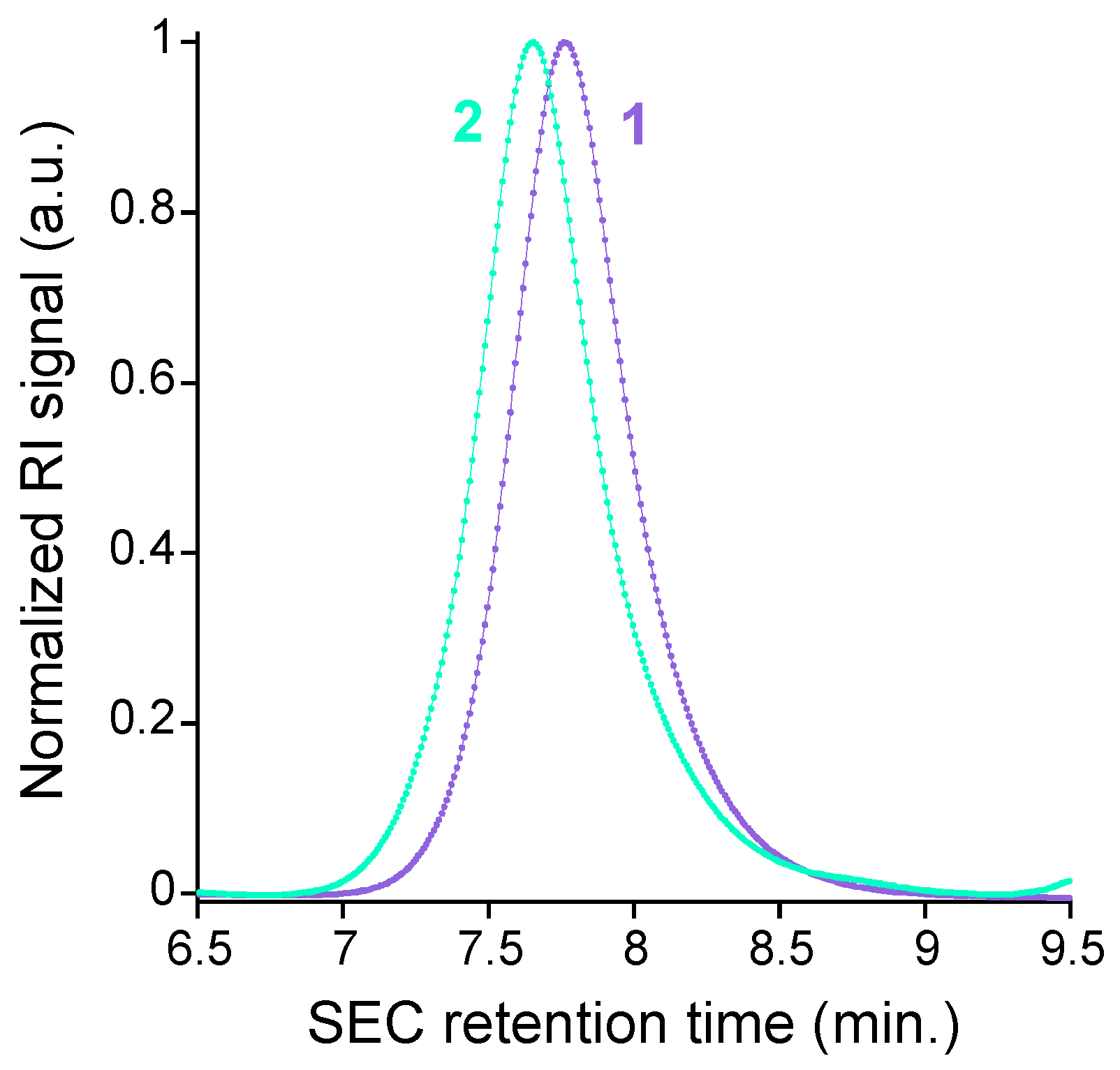
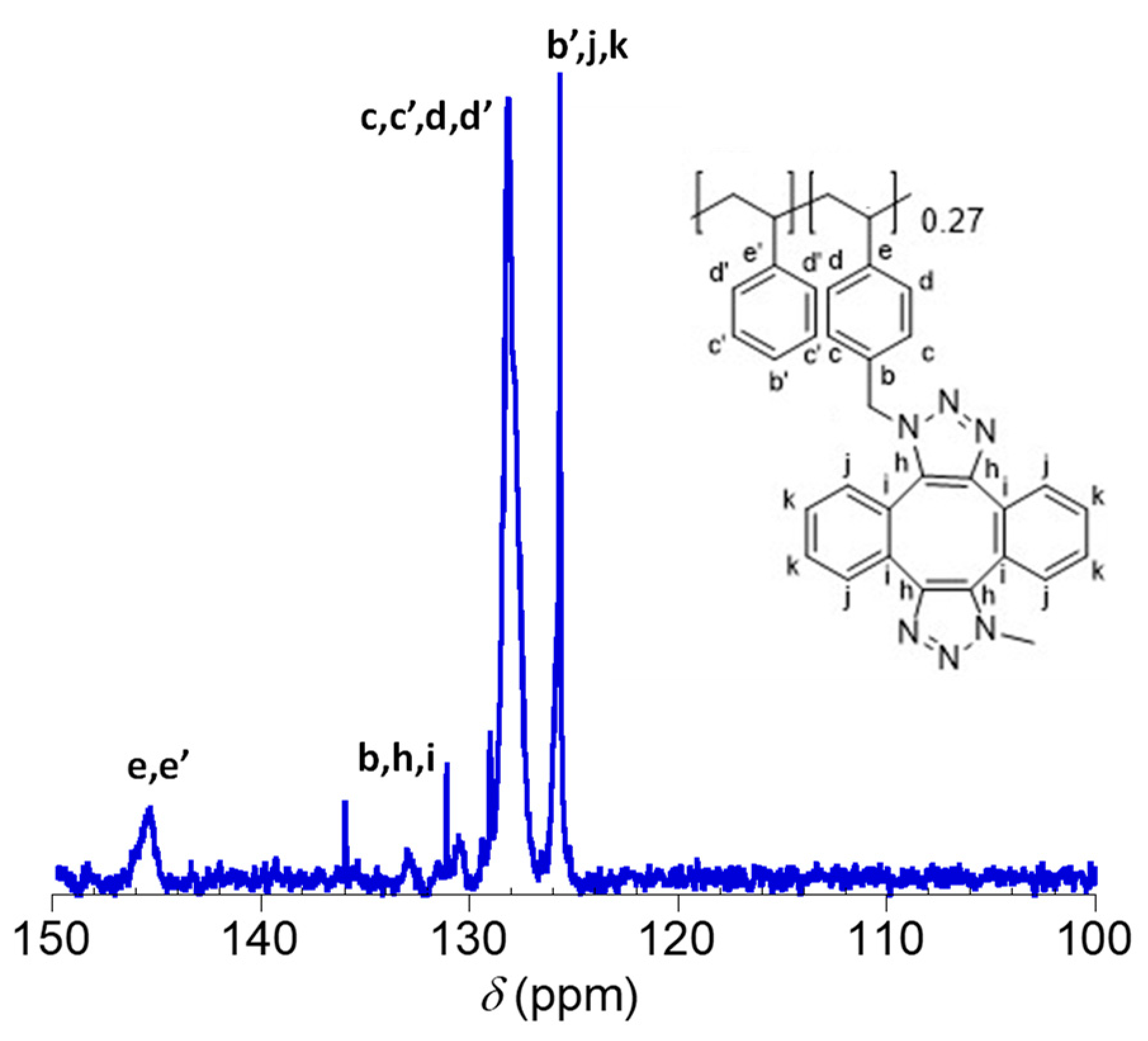
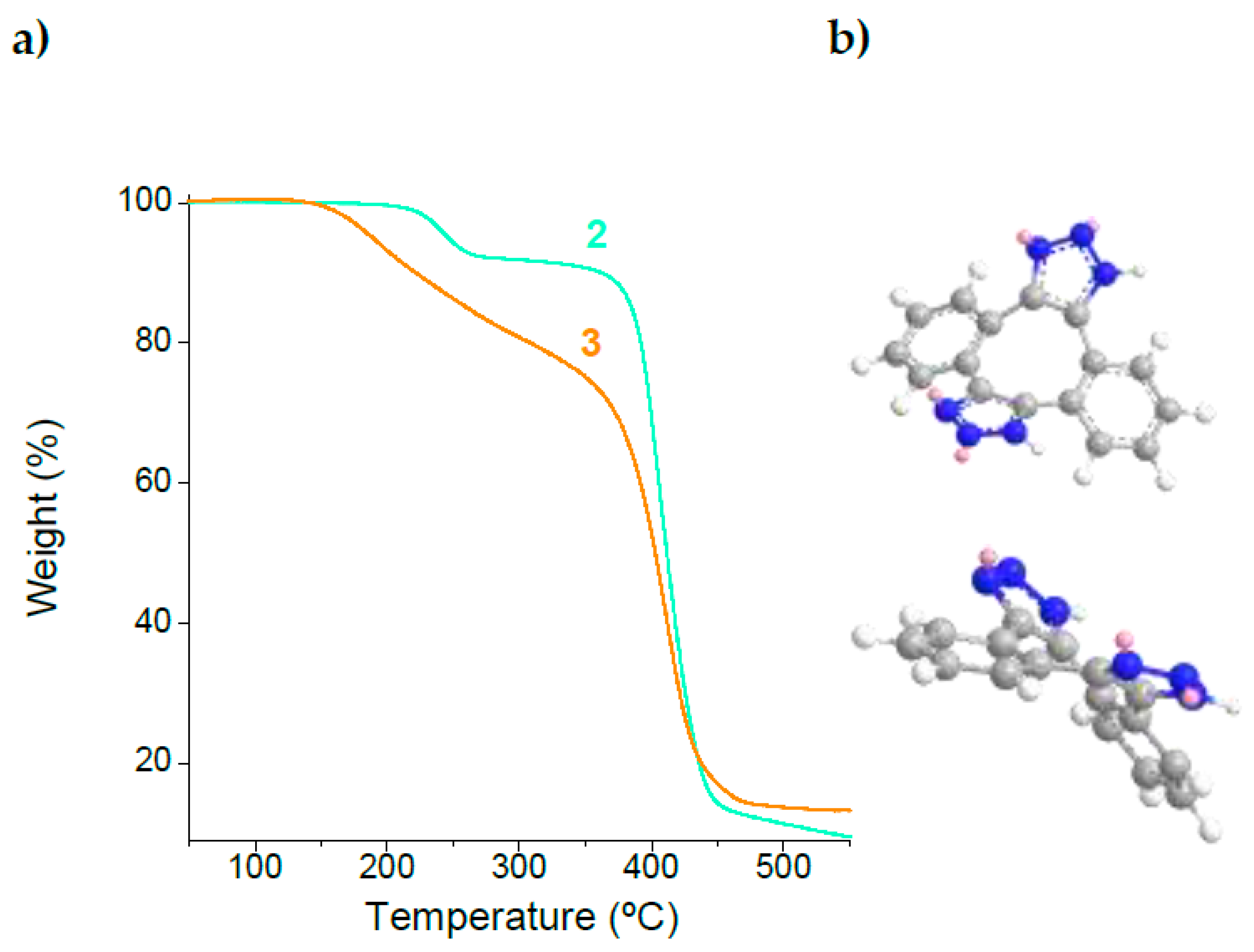
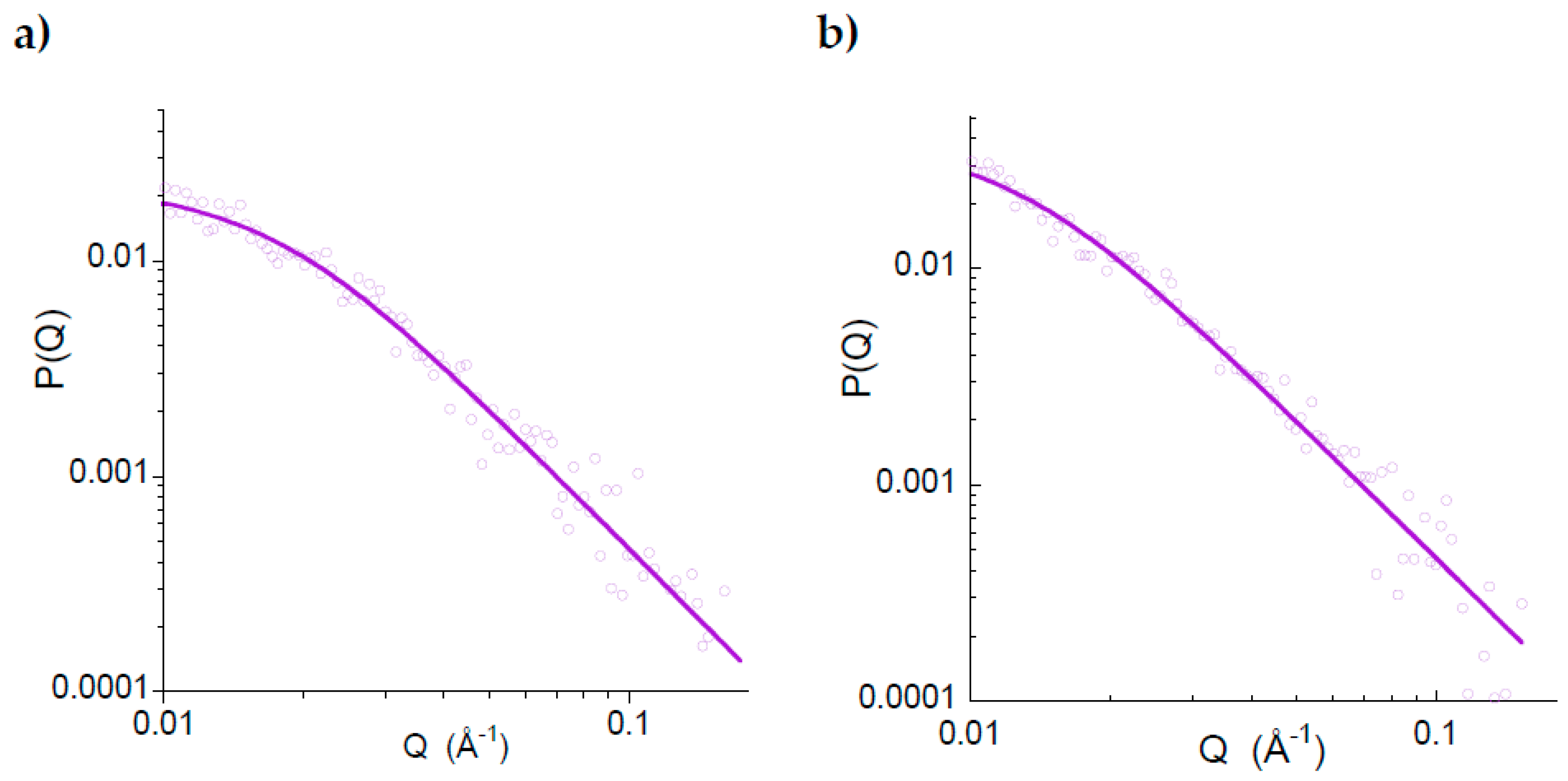
2.2. Synthesis and Characterization of Metal-Free Single-Chain Nanoparticles (3) from the Polystyrene-Based Precursor Decorated with Azide Functional Groups (2)
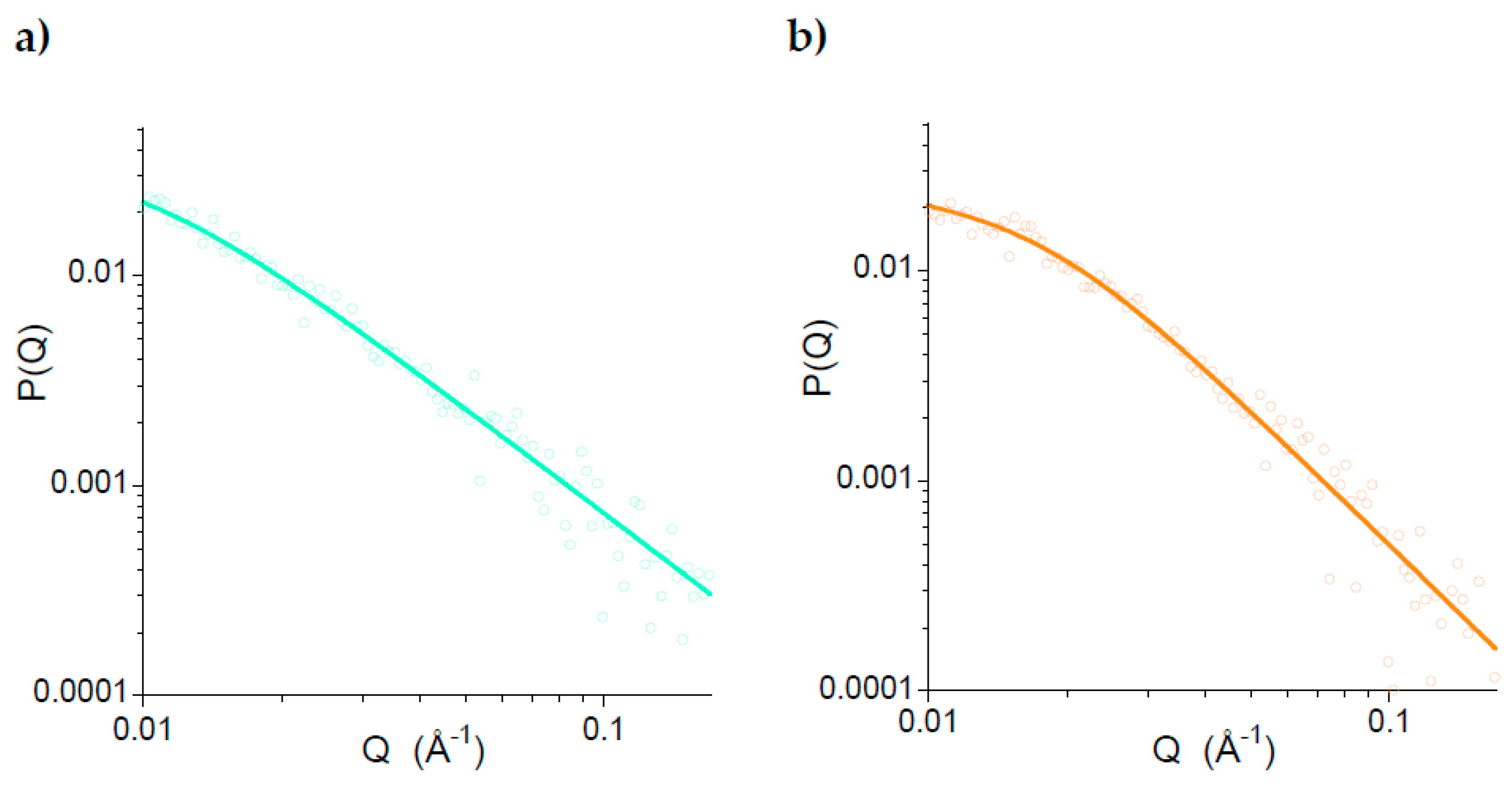
2.3. Long-Term Stability against Aggregation of Metal-Free Polystyrene-Single-Chain Nanoparticles (3)
3. Materials and Methods
3.1. Materials
3.2. Techniques
3.2.1. Size-Exclusion Chromatography (SEC)
3.2.2. Nuclear Magnetic Resonance (NMR)
3.2.3. Dynamic Light Scattering (DLS)
3.2.4. Fourier Transform Infra-Red (FTIR) Spectroscopy
3.2.5. Thermogravimetric Analysis (TGA)
3.2.6. Small-Angle X-ray Scattering (SAXS)
3.3. Procedures
3.3.1. Synthesis of Poly(S-co-CMS), 1
3.3.2. Synthesis of Poly(S-co-AMS), 2
3.3.3. Synthesis of Metal-Free PS-SCNPs, 3
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pomposo, J.A. (Ed.) Single-Chain Polymer Nanoparticles: Synthesis, Characterization, Simulations, and Applications; John Wiley & Sons: Weinheim, Germany, 2017. [Google Scholar]
- Alqarni, M.A.M.; Waldron, C.; Yilmaz, G.; Becer, C.R. Synthetic Routes to Single Chain Polymer Nanoparticles (SCNPs): Current Status and Perspectives. Macromol. Rapid Commun. 2021, 42, 2100035. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, Z. Progress in polymer single-chain based hybrid nanoparticles. Prog. Polym. Sci. 2022, 133, 101593. [Google Scholar] [CrossRef]
- Nitti, A.; Carfora, R.; Assanelli, G.; Notari, M.; Pasini, D. Single-Chain Polymer Nanoparticles for Addressing Morphologies and Functions at the Nanoscale: A Review. ACS Appl. Nano Mater. 2022, 5, 13985–13997. [Google Scholar] [CrossRef]
- Chen, R.; Berda, E.B. 100th Anniversary of Macromolecular Science Viewpoint: Reexamining Single-Chain Nanoparticles. ACS Macro Lett. 2020, 9, 1836–1843. [Google Scholar] [CrossRef]
- Terashima, T. Controlled Self-Assembly of Amphiphilic Random Copolymers into Folded Micelles and Nanostructure Materials. J. Oleo Sci. 2020, 69, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Palmans, A.R.A. Chap. 25—Single-Chain Polymeric Nanoparticles: Toward In Vivo Imaging and Catalysis in Complex Media, in Self-Assembling Biomaterials; Azevedo, H.S., da Silva, R.M.P., Eds.; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Gonzalez-Burgos, M.; Latorre-Sanchez, A.; Pomposo, J.A. Advances in Single Chain Technology. Chem. Soc. Rev. 2015, 44, 6122–6142. [Google Scholar] [CrossRef]
- Lyon, C.K.; Prasher, A.; Hanlon, A.M.; Tuten, B.T.; Tooley, C.A.; Frank, P.G.; Berda, E.B. A Brief User’s Guide to Single-Chain Nanoparticles. Polym. Chem. 2015, 6, 181–197. [Google Scholar] [CrossRef]
- Mavila, S.; Eivgi, O.; Berkovich, I.; Lemcoff, N.G. Intramolecular Cross-Linking Methodologies for the Synthesis of Polymer Nanoparticles. Chem. Rev. 2016, 116, 878–961. [Google Scholar] [CrossRef] [PubMed]
- Huurne, G.M.T.; Palmans, A.R.A.; Meijer, E.W. Supramolecular Single-Chain Polymeric Nanoparticles. CCS Chem. 2019, 1, 64–82. [Google Scholar] [CrossRef]
- Altintas, O.; Barner-Kowollik, C. Single-Chain Folding of Synthetic Polymers: A Critical Update. Macromol. Rapid Commun. 2016, 37, 29–46. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Pomposo, J.A. Single-Chain Polymer Nanoparticles via Non-Covalent and Dynamic Covalent Bonds. Part. Part. Syst. Char. 2014, 31, 11–23. [Google Scholar] [CrossRef]
- Altintas, O.; Barner-Kowollik, C. Single Chain Folding of Synthetic Polymers by Covalent and Non-Covalent Interactions: Current Status and Future Perspectives. Macromol. Rapid Commun. 2012, 33, 958–971. [Google Scholar] [CrossRef]
- Aiertza, M.K.; Odriozola, I.; Cabañero, G.; Grande, H.-J.; Loinaz, I. Single-Chain Polymer Nanoparticles. Cell. Mol. Life Sci. 2012, 69, 337–346. [Google Scholar] [CrossRef]
- Hamelmann, N.M.; Paulusse, J.M.J. Single-chain polymer nanoparticles in biomedical applications. J. Control. Release 2023, 356, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Barbee, M.H.; Wright, Z.M.; Allen, B.P.; Taylor, H.F.; Patteson, E.F.; Knight, A.S. Protein-Mimetic Self-Assembly with Synthetic Macromolecules. Macromolecules 2021, 54, 3585–3612. [Google Scholar] [CrossRef]
- Chen, J.; Garcia, E.S.; Zimmerman, S.C. Intramolecularly Cross-Linked Polymers: From Structure to Function with Applications as Artificial Antibodies and Artificial Enzymes. Acc. Chem. Res. 2020, 53, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Kroger, A.P.P.; Paulusse, J.M.J. Single-Chain Polymer Nanoparticles in Controlled Drug Delivery and Targeted Imaging. J. Control. Release 2018, 286, 326–347. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Sánchez, A.; Pomposo, J.A. Recent bioinspired applications of single-chain nanoparticles. Polym. Int. 2016, 65, 855–860. [Google Scholar] [CrossRef]
- Verde-Sesto, E.; Arbe, A.; Moreno, A.J.; Cangialosi, D.; Alegria, A.; Colmenero, J.; Pomposo, J.A. Single-chain nanoparticles: Opportunities provided by internal and external confinement. Mater. Horiz. 2020, 7, 2292–2313. [Google Scholar] [CrossRef]
- Rothfuss, H.; Knofel, N.D.; Roesky, P.W.; Barner-Kowollik, C. Single-Chain Nanoparticles as Catalytic Nanoreactors. J. Am. Chem. Soc. 2018, 140, 5875–5881. [Google Scholar] [CrossRef]
- Rubio-Cervilla, J.; González, E.; Pomposo, J.A. Advances in Single-Chain Nanoparticles for Catalysis Applications. Nanomaterials 2017, 7, 341. [Google Scholar] [CrossRef]
- Blasco, E.; Tuten, B.T.; Frisch, H.; Lederer, A.; Barner-Kowollik, C. Characterizing Single Chain Nanoparticles (SCNPs): A Critical Survey. Polym. Chem. 2017, 8, 5845–5851. [Google Scholar] [CrossRef]
- Perez-Baena, I.; Barroso-Bujans, F.; Gasser, U.; Arbe, A.; Moreno, A.J.; Colmenero, J.; Pomposo, J.A. Endowing Single-Chain Polymer Nanoparticles with Enzyme-Mimetic Activity. ACS Macro Lett. 2013, 2, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanchez, A.; Pérez-Baena, I.; Pomposo, J.A. Advances in Click Chemistry for Single-Chain Nanoparticle Construction. Molecules 2013, 18, 3339–3355. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Ormategui, N.; Grande, H.J.; Odriozola, I.; Pomposo, J.A.; Loinaz, I. Intramolecular click cycloaddition: An efficient room-temperature route towards bioconjugable polymeric nanoparticles. Macromol. Rapid Commun. 2008, 29, 1156–1160. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Perez-Baena, I.; Montes, S.; Loinaz, I.; Odriozola, I.; García, I.; Pomposo, J.A. New route to polymeric nanoparticles by click chemistry using bifunctional cross-linkers. Macromol. Symp. 2010, 296, 303–310. [Google Scholar] [CrossRef]
- Oria, L.; Aguado, R.; Pomposo, J.A.; Colmenero, J. A versatile “click” chemistry precursor of functional polystyrene nanoparticles. Adv. Mater. 2010, 22, 3038–3041. [Google Scholar] [CrossRef]
- Jasinski, N.; Lauer, A.; Stals, P.J.M.; Behrens, S.; Essig, S.; Walther, A.; Goldmann, A.S.; Barner-Kowollik, C. Cleaning the Click: A Simple Electrochemical Avenue for Copper Removal from Strongly Coordinating Macromolecules. ACS Macro Lett. 2015, 4, 298–301. [Google Scholar] [CrossRef]
- Macdonald, J.E.; Kelly, J.A.; Veinot, J.G. Iron/iron oxide nanoparticle sequestration of catalytic metal impurities from aqueous media and organic reaction products. Langmuir 2007, 23, 9543–9545. [Google Scholar] [CrossRef] [PubMed]
- Duguid, J.; Bloomfield, V.A.; Benevides, J.; Thomas, G.J., Jr. Raman spectroscopy of DNA-metal complexes. I. Interactions and conformational effects of the divalent cations: Mg, Ca, Sr, Ba, Mn, Co, Ni, Cu, Pd, and Cd. Biophys. J. 1993, 65, 1916–1928. [Google Scholar] [CrossRef]
- Usluer, Ö.; Abbas, M.; Wantz, G.; Vignau, L.; Hirsch, L.; Grana, E.; Brochon, C.; Cloutet, E.; Hadziioannou, G. Metal Residues in Semiconducting Polymers: Impact on the Performance of Organic Electronic Devices. ACS Macro Lett. 2014, 3, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Middleton, L.R.; Winey, K.I. Nanoscale Aggregation in Acid- and Ion-Containing Polymers. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 499–523. [Google Scholar] [CrossRef]
- De-La-Cuesta, J.; Verde-Sesto, E.; Arbe, A.; Pomposo, J.A. Self-Reporting of Folding and Aggregation by Orthogonal Hantzsch Luminophores within A Single Polymer Chain. Angew. Chem. Int. Ed. 2021, 60, 3534–3539. [Google Scholar] [CrossRef] [PubMed]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A Strain-Promoted [3+2] Azide−Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Kim, E.; Koo, H. Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. [Google Scholar] [CrossRef]
- Biyogo, A.M.; Hespel, L.; Humblot, V.; Lebrun, L.; Estour, F. Cellulose fibers modification through metal-free click chemistry for the elaboration of versatile functional surfaces. Eur. Polym. J. 2020, 135, 109866. [Google Scholar] [CrossRef]
- Escorihuela, J.; Marcelis, A.T.M.; Zuilhof, H. Metal-Free Click Chemistry Reactions on Surfaces. Adv. Mater. Interfaces 2015, 2, 1500135. [Google Scholar] [CrossRef]
- Wong, H.N.C.; Garratt, P.J.; Sondheimer, F. Unsaturated eight-membered ring compounds. XI. Synthesis of sym-dibenzo-1,5-cyclooctadiene-3,7-diyne and sym-dibenzo-1,3,5-cyclooctatrien-7-yne, presumably planar conjugated eight-membered ring compounds. J. Am. Chem. Soc. 1974, 96, 5604–5605. [Google Scholar] [CrossRef]
- Kii, I.; Shiraishi, A.; Hiramatsu, T.; Matsushita, T.; Uekusa, H.; Yoshida, S.; Yamamoto, M.; Kudo, A.; Hagiwara, M.; Hosoya, T. Strain-promoted double-click reaction for chemical modification of azido-biomolecules. Org. Biomol. Chem. 2010, 8, 4051–4055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Wu, Y.; Li, S.M.; Zhang, Y.X.; Zhang, K. Scalable Bimolecular Ring-Closure Method for Cyclic Polymers. Macromolecules 2020, 53, 8621–8630. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Zhao, Y.; Zhang, L.; Zhang, K. Versatile Bimolecular Ring-Closure Method for Cage-Shaped Polymers. Macromolecules 2021, 54, 6901–6910. [Google Scholar] [CrossRef]
- González-Burgos, M.; Alegría, A.; Arbe, A.; Colmenero, J.; Pomposo, J.A. An unexpected route to aldehyde-decorated single-chain nanoparticles from azides. Polym. Chem. 2016, 7, 6570–6574. [Google Scholar] [CrossRef]
- Harth, E.; Horn, B.V.; Lee, V.Y.; Germack, D.S.; Gonzales, C.P.; Miller, R.D.; Hawker, C.J. A Facile Approach to Architecturally Defined Nanoparticles via Intramolecular Chain Collapse. J. Am. Chem. Soc. 2002, 124, 8653–8660. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, B. Small-Angle Scattering From Branched Polymers. Macromol. Theory Simul. 2012, 21, 372–381. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blázquez-Martín, A.; Ruiz-Bardillo, A.; Verde-Sesto, E.; Iturrospe, A.; Arbe, A.; Pomposo, J.A. Toward Long-Term-Dispersible, Metal-Free Single-Chain Nanoparticles. Nanomaterials 2023, 13, 1394. https://doi.org/10.3390/nano13081394
Blázquez-Martín A, Ruiz-Bardillo A, Verde-Sesto E, Iturrospe A, Arbe A, Pomposo JA. Toward Long-Term-Dispersible, Metal-Free Single-Chain Nanoparticles. Nanomaterials. 2023; 13(8):1394. https://doi.org/10.3390/nano13081394
Chicago/Turabian StyleBlázquez-Martín, Agustín, Ainara Ruiz-Bardillo, Ester Verde-Sesto, Amaia Iturrospe, Arantxa Arbe, and José A. Pomposo. 2023. "Toward Long-Term-Dispersible, Metal-Free Single-Chain Nanoparticles" Nanomaterials 13, no. 8: 1394. https://doi.org/10.3390/nano13081394





