A Review on Recent Progress in Preparation of Medium-Temperature Solar-Thermal Nanofluids with Stable Dispersion
Abstract
1. Introduction
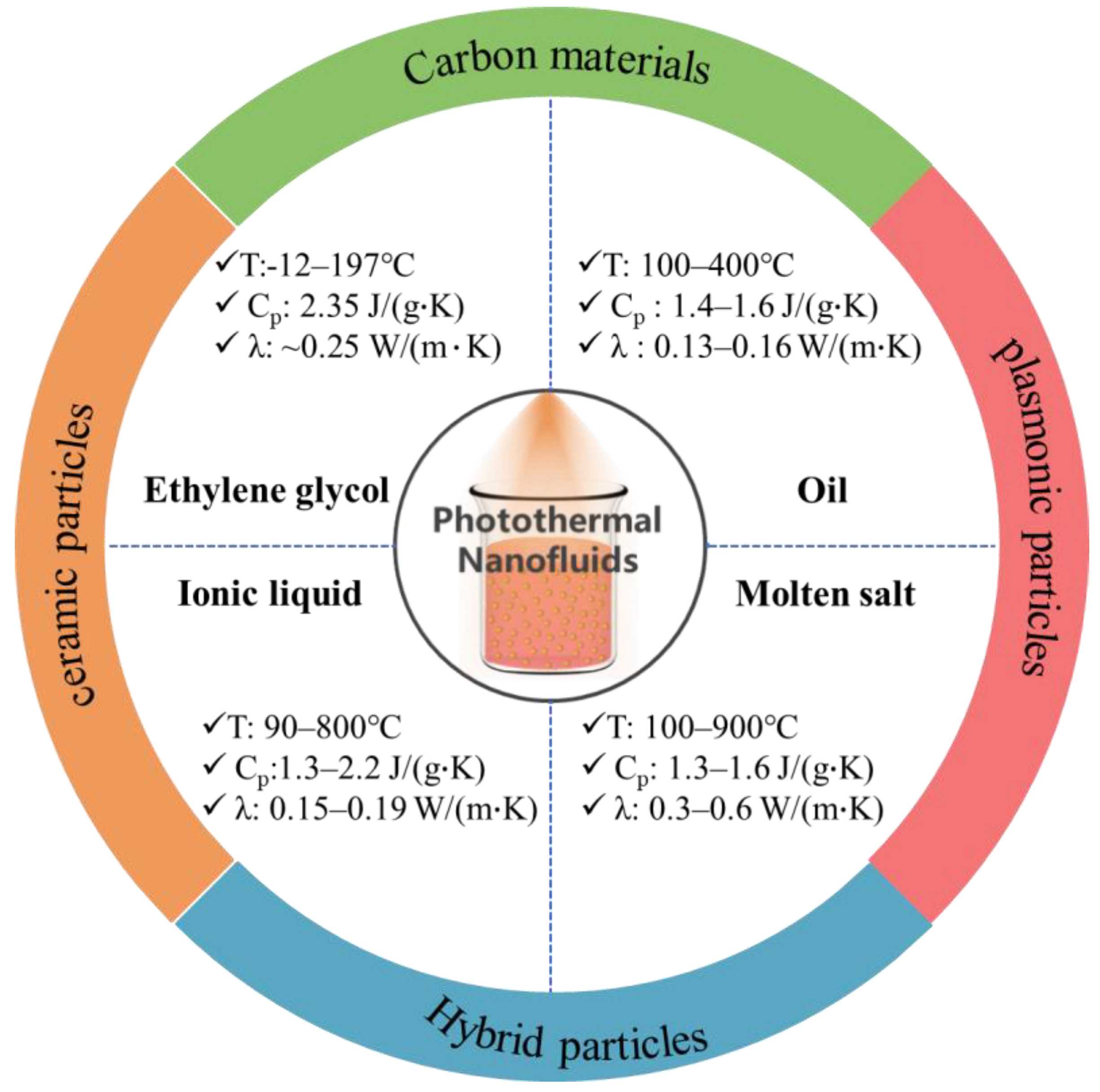
2. Medium-Temperature Solar-Thermal Nanofluids
3. Dispersion Challenge and Stabilization Strategy
3.1. Dispersion Challenge at Elevated Temperatures
3.2. Stabilization Strategy
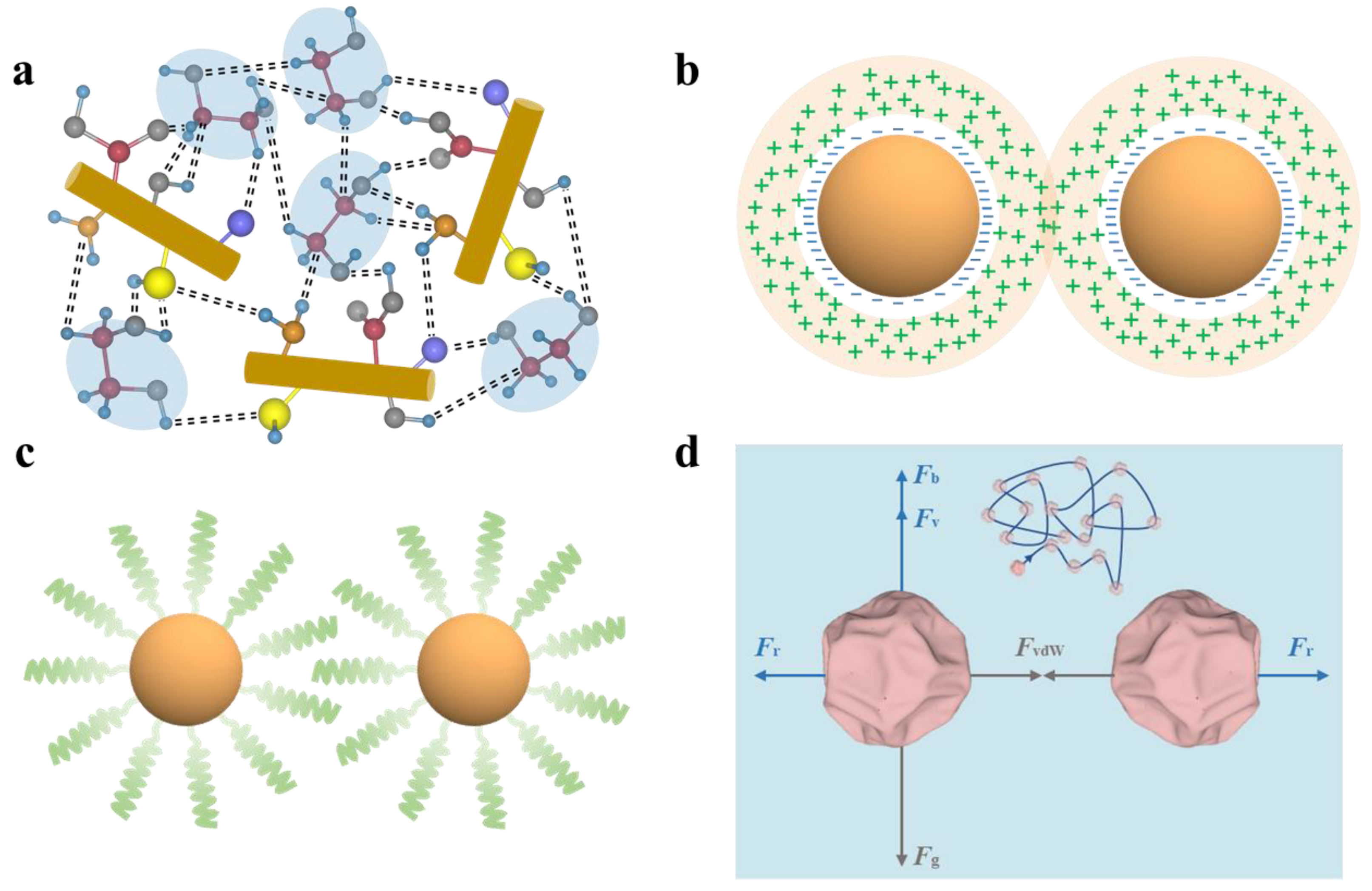
3.2.1. Hydrogen Bonding Stabilization
3.2.2. Electrostatic Stabilization
3.2.3. Steric Stabilization
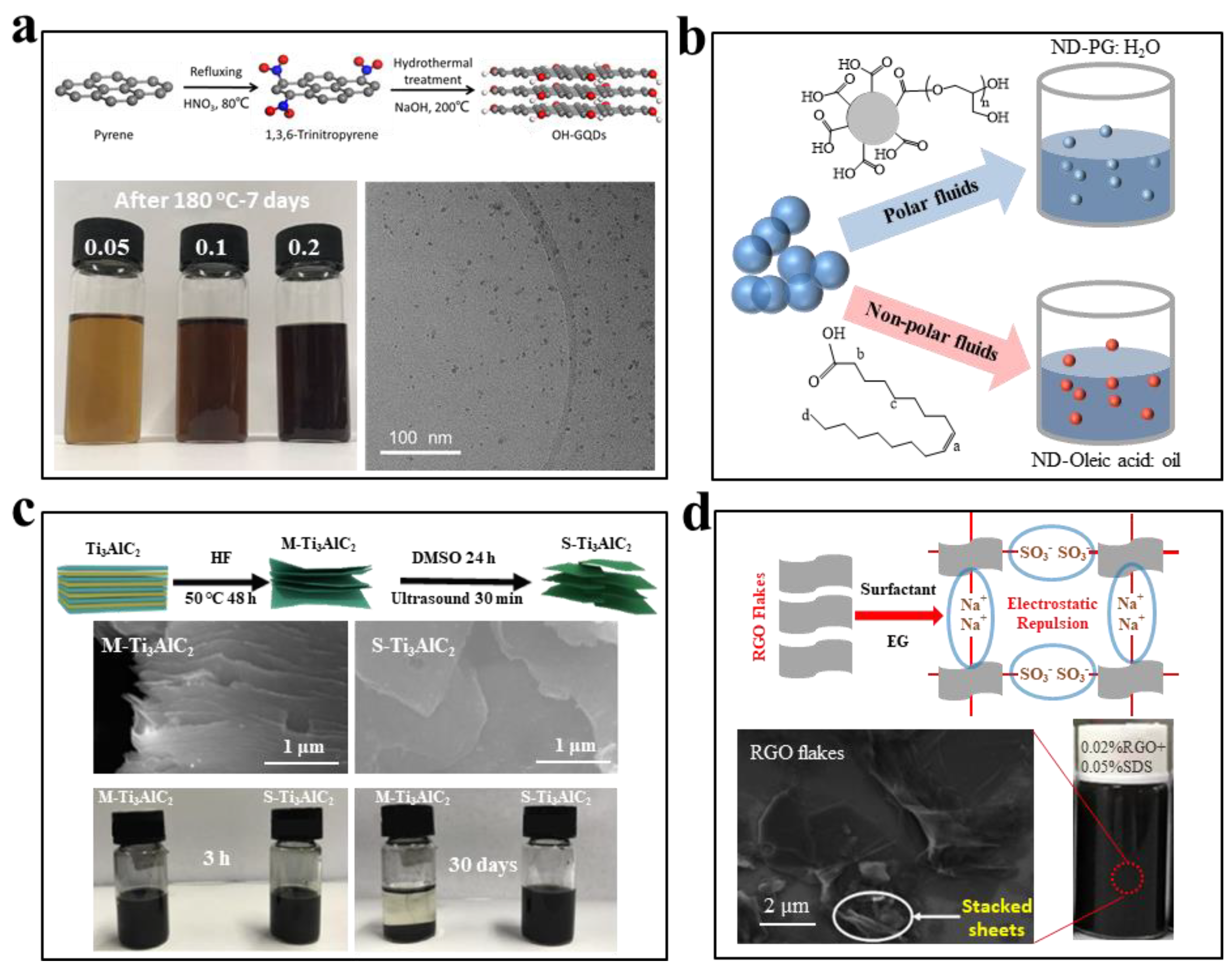
3.2.4. Self-Dispersion Stabilization
4. Stably Dispersed Medium-Temperature Solar-Thermal Nanofluids
4.1. Ethylene Glycol-Based Nanofluids
4.2. Oil-Based Nanofluids

4.3. Ionic Liquid-Based Nanofluids
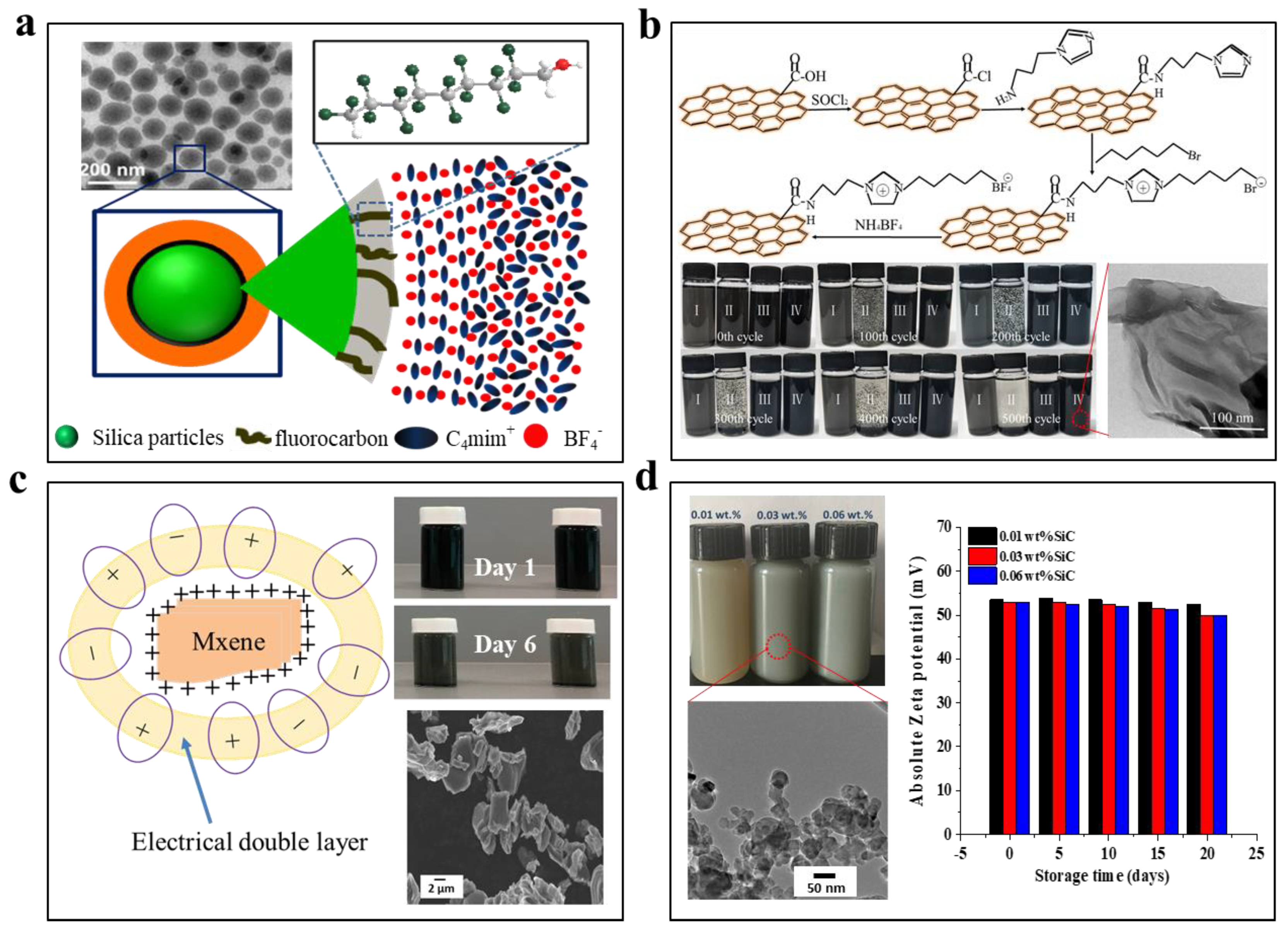
4.4. Molten Salt-Based Nanofluids

5. Summary and Outlook
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Evans, A.; Strezov, V.; Evans, T.J. Assessment of sustainability indicators for renewable energy technologies. Renew. Sustain. Energy Rev. 2009, 13, 1082–1088. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S. Research opportunities to advance solar energy utilization. Science 2016, 351, add1920. [Google Scholar] [CrossRef]
- Thirugnanasambandam, M.; Iniyan, S.; Goic, R. A review of solar thermal technologies. Renew. Sustain. Energy Rev. 2010, 14, 312–322. [Google Scholar] [CrossRef]
- Wang, R.Z.; Zhai, X.Q. Development of solar thermal technologies in China. Energy 2010, 35, 4407–4416. [Google Scholar] [CrossRef]
- Kalkan, N.; Young, E.A.; Celiktas, A. Solar thermal air conditioning technology reducing the footprint of solar thermal air conditioning. Renew. Sustain. Energy Rev. 2012, 16, 6352–6383. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Wang, Z.; Horseman, T.; Straub, A.P.; Yip, N.Y.; Li, D.; Elimelech, M.; Lin, S. Pathways and challenges for efficient solar-thermal desalination. Sci. Adv. 2019, 5, eaax0763. [Google Scholar] [CrossRef]
- Weinstein, L.A.; Loomis, J.; Bhatia, B.; Bierman, D.M.; Wang, E.N.; Chen, G. Concentrating solar power. Chem. Rev. 2015, 115, 12797–12838. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. A review on solar thermal syngas production via redox pair-based water/carbon dioxide splitting thermochemical cycles. Renew. Sustain. Energy Rev. 2015, 42, 254–285. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, B.; Li, L.; Yu, K.; Zhang, Q.; Lv, Z.; Tang, D. A high temperature tubular reactor with hybrid concentrated solar and electric heat supply for steam methane reforming. Chem. Eng. J. 2022, 428, 132073. [Google Scholar] [CrossRef]
- Schäppi, R.; Rutz, D.; Dähler, F.; Muroyama, A.; Haueter, P.; Lilliestam, J.; Patt, A.; Furler, P.; Steinfeld, A. Drop-in fuels from sunlight and air. Nature 2022, 601, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Gur, I.; Sawyer, K.; Prasher, R. Searching for a better thermal battery. Science 2012, 335, 1454–1455. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, C.Y. A review of solar collectors and thermal energy storage in solar thermal applications. Appl. Energy 2013, 104, 538–553. [Google Scholar] [CrossRef]
- Suresh, C.; Saini, R.P. Review on solar thermal energy storage technologies and their geometrical configurations. Int. J. Energy Res. 2020, 44, 4163–4195. [Google Scholar] [CrossRef]
- Tao, P.; Chang, C.; Tong, Z.; Bao, H.; Song, C.; Wu, J.; Shang, W.; Deng, T. Magnetically-accelerated large-capacity solar-thermal energy storage within high-temperature phase-change materials. Energy Environ. Sci. 2019, 12, 1613–1621. [Google Scholar] [CrossRef]
- Kalogirou, S.A. Solar thermal collectors and applications. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Minardi, J.E.; Chuang, H.N. Performance of a “black” liquid flat-plate solar collector. Sol. Energy 1975, 17, 179–183. [Google Scholar] [CrossRef]
- Farahat, S.; Sarhaddi, F.; Ajam, H. Exergetic optimization of flat plate solar collectors. Renew. Energy 2009, 34, 1169–1174. [Google Scholar] [CrossRef]
- Otanicar, T.P.; Phelan, P.E.; Prasher, R.S.; Rosengarten, G.; Taylor, R.A. Nanofluid-based direct absorption solar collector. J. Renew. Sustain. Energy 2010, 2, 033102. [Google Scholar] [CrossRef]
- Taylor, R.A.; Phelan, P.E.; Otanicar, T.P.; Walker, C.A.; Nguyen, M.; Trimble, S.; Prasher, R. Applicability of nanofluids in high flux solar collectors. J. Renew. Sustain. Energy 2011, 3, 023104. [Google Scholar] [CrossRef]
- Lenert, A.; Wang, E.N. Optimization of nanofluid volumetric receivers for solar thermal energy conversion. Sol. Energy 2012, 86, 253–265. [Google Scholar] [CrossRef]
- Joseph, A.; Thomas, S. Energy, exergy and corrosion analysis of direct absorption solar collector employed with ultra-high stable carbon quantum dot nanofluid. Renew. Energy 2022, 181, 725–737. [Google Scholar] [CrossRef]
- Hussain, M.; Shah, S.K.H.; Sajjad, U.; Abbas, N.; Ali, A. Recent developments in optical and thermal performance of direct absorption solar collectors. Energies 2022, 15, 7101. [Google Scholar] [CrossRef]
- Bretado-de los Rios, M.S.; Rivera-Solorio, C.I.; Nigam, K.D.P. An overview of sustainability of heat exchangers and solar thermal applications with nanofluids: A review. Renew. Sustain. Energy Rev. 2021, 142, 110855. [Google Scholar] [CrossRef]
- Goel, N.; Taylor, R.A.; Otanicar, T. A review of nanofluid-based direct absorption solar collectors: Design considerations and experiments with hybrid PV/thermal and direct steam generation collectors. Renew. Energy 2020, 145, 903–913. [Google Scholar] [CrossRef]
- Gorji, T.B.; Ranjbar, A.A. A review on optical properties and application of nanofluids in direct absorption solar collectors (DASCs). Renew. Sustain. Energy Rev. 2017, 72, 10–32. [Google Scholar] [CrossRef]
- Kumar, S.; Chander, N.; Gupta, V.K.; Kukreja, R. Progress, challenges and future prospects of plasmonic nanofluid based direct absorption solar collectors–a state-of-the-art review. Sol. Energy 2021, 227, 365–425. [Google Scholar] [CrossRef]
- Sun, C.; Zou, Y.; Qin, C.; Chen, M.; Li, X.; Zhang, B.; Wu, X. Solar absorption characteristics of SiO2@ Au core-shell composite nanorods for the direct absorption solar collector. Renew. Energ. 2022, 189, 402–411. [Google Scholar] [CrossRef]
- Li, X.; Zeng, G.; Lei, X. The stability, optical properties and solar-thermal conversion performance of SiC-MWCNTs hybrid nanofluids for the direct absorption solar collector (DASC) application. Sol. Energy Mater. Sol. Cells 2020, 206, 110323. [Google Scholar] [CrossRef]
- Sreekumar, S.; Shah, N.; Mondol, J.D.; Hewitt, N.; Chakrabarti, S. Broadband absorbing mono, blended and hybrid nanofluids for direct absorption solar collector: A comprehensive review. Nano Futures 2022, 6, 022002. [Google Scholar] [CrossRef]
- Tyagi, H.; Phelan, P.; Prasher, R. Predicted efficiency of a low-temperature nanofluid-based direct absorption solar collector. J. Sol. Energy Eng. 2009, 131, 041004. [Google Scholar] [CrossRef]
- Wole-osho, I.; Okonkwo, E.C.; Abbasoglu, S.; Kavaz, D. Nanofluids in solar thermal collectors: Review and limitations. Int. J. Thermophys. 2020, 41, 157. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, J.; Chen, H.; Guo, H.; Song, C.; Shang, W.; Tao, P.; Deng, T. Optical nanofluids for direct absorption-based solar-thermal energy harvesting at medium-to-high temperatures. Curr. Opin. Chem. Eng. 2019, 25, 51–56. [Google Scholar] [CrossRef]
- Said, Z.; Hachicha, A.A.; Aberoumand, S.; Yousef, B.A.A.; Sayed, E.T.; Bellos, E. Recent advances on nanofluids for low to medium temperature solar collectors: Energy, exergy, economic analysis and environmental impact. Prog. Energy Combust. Sci. 2021, 84, 100898. [Google Scholar] [CrossRef]
- Taylor, R.A.; Phelan, P.E.; Otanicar, T.P.; Adrian, R.; Prasher, R. Nanofluid optical property characterization: Towards efficient direct absorption solar collectors. Nanoscale Res. Lett. 2011, 6, 225. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, L.; Zhang, L.; Qi, F. Theoretical studies on the unimolecular decomposition of ethylene glycol. J. Phys. Chem. A 2012, 116, 55–63. [Google Scholar] [CrossRef]
- Bhalla, V.; Beejawat, S.; Doshi, J.; Khullar, V.; Singh, H.; Tyagi, H. Silicone oil envelope for enhancing the performance of nanofluid-based direct absorption solar collectors. Renew. Energy 2020, 145, 2733–2740. [Google Scholar] [CrossRef]
- Jin, H.; Andritsch, T.; Tsekmes, I.A.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Properties of mineral oil based silica nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1100–1108. [Google Scholar]
- Gulzar, O.; Qayoum, A.; Gupta, R. Photo-thermal characteristics of hybrid nanofluids based on therminol-55 oil for concentrating solar collectors. Appl. Nanosci. 2019, 9, 1133–1143. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Zhang, L.; Fang, X.; Zhang, Z. Thermodynamic properties and thermal stability of ionic liquid-based nanofluids containing graphene as advanced heat transfer fluids for medium-to-high-temperature applications. Renew. Energy 2014, 63, 519–523. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A.; Lourenço, M.J.V.; Ribeiro, A.P.C.; Langa, E.; Vieira, S.I.C.; Goodrich, P.; Hardacre, C. Thermal properties of ionic liquids and ionanofluids of imidazolium and pyrrolidinium liquids. J. Chem. Eng. Data 2010, 55, 653–661. [Google Scholar] [CrossRef]
- Shiflett, M.B. Commercial Applications of Ionic Liquids; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Bauer, T.; Pfleger, N.; Laing, D.; Steinmann, W.-D.; Eck, M.; Kaesche, S. High-Temperature Molten Salts for Solar Power Application, Molten Salts Chemistry; Elsevier: Oxford, UK, 2013; pp. 415–438. [Google Scholar]
- Guillot, S.; Faik, A.; Rakhmatullin, A.; Lambert, J.; Veron, E.; Echegut, P.; Bessada, C.; Calvet, N.; Py, X. Corrosion effects between molten salts and thermal storage material for concentrated solar power plants. Appl. Energy 2012, 94, 174–181. [Google Scholar] [CrossRef]
- Bell, S.; Steinberg, T.; Will, G. Corrosion mechanisms in molten salt thermal energy storage for concentrating solar power. Renew. Sustain. Energy Rev. 2019, 114, 109328. [Google Scholar] [CrossRef]
- Liu, B.; Wei, X.; Wang, W.; Lu, J.; Ding, J. Corrosion behavior of Ni-based alloys in molten NaCl-CaCl2-MgCl2 eutectic salt for concentrating solar power. Sol. Energy Mater. Sol. Cells 2017, 170, 77–86. [Google Scholar] [CrossRef]
- Kearney, D.; Kelly, B.; Herrmann, U.; Cable, R.; Pacheco, J.; Mahoney, R.; Price, H.; Blake, D.; Nava, P.; Potrovitza, N. Engineering aspects of a molten salt heat transfer fluid in a trough solar field. Energy 2004, 29, 861–870. [Google Scholar] [CrossRef]
- Peng, Q.; Ding, J.; Wei, X.; Yang, J.; Yang, X. The preparation and properties of multi-component molten salts. Appl. Energy 2010, 87, 2812–2817. [Google Scholar] [CrossRef]
- Trong Tam, N.; Viet Phuong, N.; Hong Khoi, P.; Ngoc Minh, P.; Afrand, M.; Van Trinh, P.; Hung Thang, B.; Żyła, G.; Estellé, P. Carbon nanomaterial-based nanofluids for direct thermal solar absorption. Nanomaterials 2020, 10, 1199. [Google Scholar] [CrossRef]
- Said, Z.; Arora, S.; Farooq, S.; Sundar, L.S.; Li, C.; Allouhi, A. Recent advances on improved optical, thermal, and radiative characteristics of plasmonic nanofluids: Academic insights and perspectives. Sol. Energy Mater. Sol. Cells 2022, 236, 111504. [Google Scholar] [CrossRef]
- Kazemian, A.; Salari, A.; Ma, T.; Lu, H. Application of hybrid nanofluids in a novel combined photovoltaic/thermal and solar collector system. Sol. Energy 2022, 239, 102–116. [Google Scholar] [CrossRef]
- Ladjevardi, S.M.; Asnaghi, A.; Izadkhast, P.S.; Kashani, A.H. Applicability of graphite nanofluids in direct solar energy absorption. Sol. Energy 2013, 94, 327–334. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Reduced graphene oxide dispersed nanofluids with improved photo-thermal conversion performance for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 163, 125–133. [Google Scholar] [CrossRef]
- Sani, E.; Vallejo, J.P.; Cabaleiro, D.; Lugo, L. Functionalized graphene nanoplatelet-nanofluids for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2018, 185, 205–209. [Google Scholar] [CrossRef]
- Hordy, N.; Rabilloud, D.; Meunier, J.L.; Coulombe, S. High temperature and long-term stability of carbon nanotube nanofluids for direct absorption solar thermal collectors. Sol. Energy 2014, 105, 82–90. [Google Scholar] [CrossRef]
- Mallah, A.R.; Mohd Zubir, M.N.; Alawi, O.A.; Salim Newaz, K.M.; Mohamad Badry, A.B. Plasmonic nanofluids for high photothermal conversion efficiency in direct absorption solar collectors: Fundamentals and applications. Sol. Energy Mater. Sol. Cells 2019, 201, 110084. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, aensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Zhang, S.; Li, D.; Zhang, X.; Zhao, Q.; Xing, B. Advances and challenges of broadband solar absorbers for efficient solar steam generation. Environ. Sci. Nano 2022, 9, 2264–2296. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, G.; Wang, M.; Yu, W.; Zeng, J.; Yu, X.; Xie, H.; Li, Q. Dual plasmonic Au/TiN nanofluids for efficient solar photothermal conversion. Sol. Energy 2019, 184, 240–248. [Google Scholar] [CrossRef]
- Shah, T.R.; Ali, H.M. Applications of hybrid nanofluids in solar energy, practical limitations and challenges: A critical review. Sol. Energy 2019, 183, 173–203. [Google Scholar] [CrossRef]
- Xiong, Q.; Altnji, S.; Tayebi, T.; Izadi, M.; Hajjar, A.; Sundén, B.; Li, L.K.B. A comprehensive review on the application of hybrid nanofluids in solar energy collectors. Sustain. Energy Technol. Assess. 2021, 47, 101341. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Hamaker, H.C. The london—van der waals attraction between spherical particles. Physica 1937, 4, 1058–1072. [Google Scholar] [CrossRef]
- Jacob, N.I.; Israelachvili, N. Intermolecular and Surface Forces; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Cates, M.E.; Evans, M.R. Soft and Fragile Matter: Nonequilibrium Dynamics, Metastability and Flow; Institute of Physics Publishing: Bristol, UK, 2000. [Google Scholar]
- Bond, W.N. LXXXII. Bubbles and drops and Stokes’ law. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1927, 4, 889–898. [Google Scholar] [CrossRef]
- Loudet, J.C.; Hanusse, P.; Poulin, P. Stokes drag on a aphere in a nematic liquid crystal. Science 2004, 306, 1525. [Google Scholar] [CrossRef]
- Rings, D.; Schachoff, R.; Selmke, M.; Cichos, F.; Kroy, K. Hot brownian motion. Phys. Rev. Lett. 2010, 105, 090604. [Google Scholar] [CrossRef]
- Pusey, P.N. Brownian motion goes ballistic. Science 2011, 332, 802–803. [Google Scholar] [CrossRef]
- Dutta, A.; Paul, A.; Chattopadhyay, A. The effect of temperature on the aggregation kinetics of partially bare gold nanoparticles. RSC Adv. 2016, 6, 82138–82149. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, P.; Liu, Y.; Xu, H.; Ye, Q.; Hu, H.; Song, C.; Chen, Z.; Shang, W.; Deng, T. Rapid charging of thermal energy storage materials through plasmonic heating. Sci. Rep. 2014, 4, 6246. [Google Scholar] [CrossRef] [PubMed]
- Meli, L.; Green, P.F. Aggregation and coarsening of ligand-stabilized gold nanoparticles in poly (methyl methacrylate) thin films. ACS Nano 2008, 2, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Copenhaver, D.; Sichmeller, C.; Peng, X. Ligand bonding and dynamics on colloidal nanocrystals at room temperature: The case of alkylamines on CdSe nanocrystals. J. Am. Chem. Soc. 2008, 130, 5726–5735. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xie, H.; Li, Y.; Chen, L.; Wang, Q. Experimental investigation on the thermal transport properties of ethylene glycol based nanofluids containing low volume concentration diamond nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 380, 1–5. [Google Scholar] [CrossRef]
- Palabiyik, I.; Musina, Z.; Witharana, S.; Ding, Y. Dispersion stability and thermal conductivity of propylene glycol-based nanofluids. J. Nanoparticle Res. 2011, 13, 5049. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Tadmor, R.; Hernández-Zapata, E.; Chen, N.; Pincus, P.; Israelachvili, J.N. Debye length and double-layer forces in polyelectrolyte solutions. Macromolecules 2002, 35, 2380–2388. [Google Scholar] [CrossRef]
- Vacic, A.; Criscione, J.M.; Rajan, N.K.; Stern, E.; Fahmy, T.M.; Reed, M.A. Determination of molecular configuration by debye length modulation. J. Am. Chem. Soc. 2011, 133, 13886–13889. [Google Scholar] [CrossRef]
- Kesler, V.; Murmann, B.; Soh, H.T. Going beyond the debye length: Overcoming charge screening limitations in next-generation bioelectronic sensors. ACS Nano 2020, 14, 16194–16201. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Cacua, K.; Ordoñez, F.; Zapata, C.; Herrera, B.; Pabón, E.; Buitrago-Sierra, R. Surfactant concentration and pH effects on the zeta potential values of alumina nanofluids to inspect stability. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123960. [Google Scholar] [CrossRef]
- Napper, D.H. Polymeric Stabilization of Colloidal Dispersions; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Somasundaran, P.; Markovic, B.; Krishnakumar, S.; Yu, X. Handbook of Surface and Colloid Chemistry; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Luo, J.; Jang, H.D.; Sun, T.; Xiao, L.; He, Z.; Katsoulidis, A.P.; Kanatzidis, M.G.; Gibson, J.M.; Huang, J. Compression and aggregation-resistant particles of crumpled soft sheets. ACS Nano 2011, 5, 8943–8949. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hao, J.; Li, H. Remarkable improvements in the stability and thermal conductivity of graphite/ethylene glycol nanofluids caused by a graphene oxide percolation structure. Dalton Trans. 2013, 42, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Zhang, J.; Fu, B.; Xu, J.; Tao, P.; Song, C.; Shang, W.; Wu, J.; Deng, T. Ethylene glycol-based solar-thermal fluids dispersed with reduced graphene oxide. RSC Adv. 2019, 9, 10282–10288. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zhang, J.; Shu, L.; Zhu, J.; Fu, B.; Song, C.; Shang, W.; Tao, P.; Deng, T. Self-dispersible graphene quantum dots in ethylene glycol for direct absorption-based medium-temperature solar-thermal harvesting. RSC Adv. 2020, 10, 45028–45036. [Google Scholar] [CrossRef]
- Branson, B.T.; Beauchamp, P.S.; Beam, J.C.; Lukehart, C.M.; Davidson, J.L. Nanodiamond nanofluids for enhanced thermal conductivity. ACS Nano 2013, 7, 3183–3189. [Google Scholar] [CrossRef]
- Bao, Z.; Bing, N.; Zhu, X.; Xie, H.; Yu, W. Ti3C2Tx MXene contained nanofluids with high thermal conductivity, super colloidal stability and low viscosity. Chem. Eng. J. 2021, 406, 126390. [Google Scholar] [CrossRef]
- Shah, S.N.A.; Shahabuddin, S.; Sabri, M.F.M.; Salleh, M.F.M.; Ali, M.A.; Hayat, N.; Sidik, N.A.C.; Samykano, M.; Saidur, R. Experimental investigation on stability, thermal conductivity and rheological properties of rGO/ethylene glycol based nanofluids. Int. J. Heat Mass Transf. 2020, 150, 118981. [Google Scholar] [CrossRef]
- Chen, Y.; Quan, X.; Wang, Z.; Lee, C.; Wang, Z.; Tao, P.; Song, C.; Wu, J.; Shang, W.; Deng, T. Stably dispersed high-temperature Fe3O4/silicone-oil nanofluids for direct solar thermal energy harvesting. J. Mater. Chem. A 2016, 4, 17503–17511. [Google Scholar] [CrossRef]
- Tao, P.; Shu, L.; Zhang, J.; Lee, C.; Ye, Q.; Guo, H.; Deng, T. Silicone oil-based solar-thermal fluids dispersed with PDMS-modified Fe3O4@graphene hybrid nanoparticles. Prog. Nat. Sci. Mater. Int. 2018, 28, 554–562. [Google Scholar] [CrossRef]
- Gómez-Villarejo, R.; Navas, J.; Martín, E.I.; Sánchez-Coronilla, A.; Aguilar, T.; Gallardo, J.J.; De los Santos, D.; Alcántara, R.; Fernández-Lorenzo, C.; Martín-Calleja, J. Preparation of Au nanoparticles in a non-polar medium: Obtaining high-efficiency nanofluids for concentrating solar power. an experimental and theoretical perspective. J. Mater. Chem. A 2017, 5, 12483–12497. [Google Scholar] [CrossRef]
- Li, X.; Gan, C.; Han, Z.; Yan, H.; Chen, D.; Li, W.; Li, H.; Fan, X.; Li, D.; Zhu, M. High dispersivity and excellent tribological performance of titanate coupling agent modified graphene oxide in hydraulic oil. Carbon 2020, 165, 238–250. [Google Scholar] [CrossRef]
- Luo, J.; Jang, H.D.; Huang, J. Effect of sheet morphology on the scalability of graphene-based ultracapacitors. ACS Nano 2013, 7, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zachariah, M.R.; Zangmeister, C.D. Crumpled nanopaper from graphene oxide. Nano Lett. 2012, 12, 486–489. [Google Scholar] [CrossRef]
- Dou, X.; Koltonow, A.R.; He, X.; Jang, H.D.; Wang, Q.; Chung, Y.-W.; Huang, J. Self-dispersed crumpled graphene balls in oil for friction and wear reduction. Proc. Natl. Acad. Sci. USA 2016, 113, 1528–1533. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, L.; Chang, C.; Li, X.; Lin, R.; Fu, B.; Song, C.; Shang, W.; Tao, P.; Deng, T. Crumpled particles of ethanol-wetted graphene oxide for medium-temperature nanofluidic solar-thermal energy harvesting. Carbon 2022, 186, 492–500. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, J.; Whyte, J.; Fu, B.; Song, C.; Shang, W.; Tao, P.; Deng, T. Silicone oil nanofluids dispersed with mesoporous crumpled graphene for medium-temperature direct absorption solar-thermal energy harvesting. Sol. Energy Mater. Sol. Cells 2022, 243, 111794. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, B.; Song, C.; Shang, W.; Tao, P.; Deng, T. Kirigami-inspired synthesis of functional mesoporous nanospheres through spray drying of holey nanosheets. Carbon 2021, 178, 382–390. [Google Scholar] [CrossRef]
- Austen Angell, C.; Ansari, Y.; Zhao, Z. Ionic liquids: Past, present and future. Faraday Discuss. 2012, 154, 9–27. [Google Scholar] [CrossRef]
- Minea, A.A.; Murshed, S.M.S. A review on development of ionic liquid based nanofluids and their heat transfer behavior. Renew. Sustain. Energy Rev. 2018, 91, 584–599. [Google Scholar] [CrossRef]
- Dash, P.; Scott, R.W.J. 1-Methylimidazole stabilization of gold nanoparticles in imidazolium ionic liquids. Chem. Commun. 2009, 7, 812–814. [Google Scholar] [CrossRef]
- Gao, J.; Ndong, R.S.; Shiflett, M.B.; Wagner, N.J. Creating nanoparticle stability in ionic liquid [C4mim][BF4] by inducing solvation layering. ACS Nano 2015, 9, 3243–3253. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, C.; Chen, L.; Fang, X.; Zhang, Z. Preparation and photo-thermal conversion performance of modified graphene/ionic liquid nanofluids with excellent dispersion stability. Sol. Energy Mater. Sol. Cells 2017, 170, 219–232. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Effect of morphology of carbon nanomaterials on thermo-physical characteristics, optical properties and photo-thermal conversion performance of nanofluids. Renew. Energy 2016, 99, 888–897. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Aslfattahi, N.; Rashedi, A. Investigation of electrical conductivity, optical oroperty, and stability of 2D MXene nanofluid containing ionic liquids. Appl. Sci. 2020, 10, 8943. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Li, X. An investigation into the thermophysical and optical properties of SiC/ionic liquid nanofluid for direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2017, 163, 157–163. [Google Scholar] [CrossRef]
- Gebbie, M.A.; Dobbs, H.A.; Valtiner, M.; Israelachvili, J.N. Long-range electrostatic screening in ionic liquids. Proc. Natl. Acad. Sci. USA 2015, 112, 7432–7437. [Google Scholar] [CrossRef]
- Gebbie, M.A.; Smith, A.M.; Dobbs, H.A.; Lee, A.A.; Warr, G.G.; Banquy, X.; Valtiner, M.; Rutland, M.W.; Israelachvili, J.N.; Perkin, S.; et al. Long range electrostatic forces in ionic liquids. Chem. Commun. 2017, 53, 1214–1224. [Google Scholar] [CrossRef]
- Zhang, H.; Dasbiswas, K.; Ludwig, N.B.; Han, G.; Lee, B.; Vaikuntanathan, S.; Talapin, D.V. Stable colloids in molten inorganic salts. Nature 2017, 542, 328–331. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Srivastava, V.; Ludwig, N.B.; Borkiewicz, O.J.; Zhang, H.; Ilavsky, J.; Lee, B.; Chapman, K.W.; Vaikuntanathan, S.; Talapin, D.V. Nanocrystals in molten salts and ionic liquids: Experimental observation of ionic correlations extending beyond the debye length. ACS Nano 2019, 13, 5760–5770. [Google Scholar] [CrossRef]
- Ma, B.; Shin, D.; Banerjee, D. Synthesis and characterization of molten salt nanofluids for thermal energy storage application in concentrated solar power plants-mechanistic understanding of specific heat capacity enhancement. Nanomaterials 2020, 10, 2266. [Google Scholar] [CrossRef]
- Cui, L.; Yu, Q.; Wei, G.; Du, X. Mechanisms for thermal conduction in molten salt-based nanofluid. Int. J. Heat Mass Transf. 2022, 188, 122648. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Zaki, A.; Grosu, Y.; González-Fernández, L.; Igartua, J.M.; Faik, A. SiO2@Al2O3 core-shell nanoparticles based molten salts nanofluids for thermal energy storage applications. J. Energy Storage 2019, 26, 101033. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Zaki, A.; Grosu, Y.; González-Fernández, L.; Anagnostopoulos, A.; Navarro, M.E.; Ding, Y.; Igartua, J.M.; Faik, A. Effect of silica nanoparticle size on the stability and thermophysical properties of molten salts based nanofluids for thermal energy storage applications at concentrated solar power plants. J. Energy Storage 2022, 51, 104276. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Apmann, K.; Fulmer, R.; Soto, A.; Vafaei, S. Thermal conductivity and viscosity: Review and optimization of effects of nanoparticles. Materials 2021, 14, 1291. [Google Scholar] [CrossRef] [PubMed]
- Bashirnezhad, K.; Bazri, S.; Safaei, M.R.; Goodarzi, M.; Dahari, M.; Mahian, O.; Dalkılıça, A.S.; Wongwises, S. Viscosity of nanofluids: A review of recent experimental studies. Int. Commun. Heat Mass Transf. 2016, 73, 114–123. [Google Scholar] [CrossRef]
- Younes, H.; Mao, M.; Murshed, S.S.; Lou, D.; Hong, H.; Peterson, G.P. Nanofluids: Key parameters to enhance thermal conductivity and its applications. Appl. Therm. Eng. 2022, 207, 118202. [Google Scholar] [CrossRef]
- Yu Hua, L.; Wei, Q.; Jian Chao, F. Temperature dependence of thermal conductivity of nanofluids. Chinese Phys. Lett. 2008, 25, 3319. [Google Scholar] [CrossRef]
- Navarrete, N.; Gimeno-Furió, A.; Forner-Escrig, J.; Juliá, J.E.; Mondragón, R. Colloidal stability of molten salt-based nanofluids: Dynamic light scattering tests at high temperature conditions. Powder Technol. 2019, 352, 1–10. [Google Scholar] [CrossRef]
- Uemura, N.; Egoshi, T.; Murakami, K.; Kizuka, T. High-power laser irradiation for high-temperature in situ transmission electron microscopy. Micron 2022, 157, 103244. [Google Scholar] [CrossRef]
- Kim, J.S.; LaGrange, T.; Reed, B.W.; Taheri, M.L.; Armstrong, M.R.; King, W.E.; Browning, N.D.; Campbell, G.H. Imaging of transient structures using nanosecond in situ TEM. Science 2008, 321, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, J.; Lin, R.; Fu, B.; Song, C.; Shang, W.; Tao, P.; Deng, T. Rapid one-step scalable microwave synthesis of Ti3C2Tx MXene. Chem. Commun. 2021, 57, 12611–12614. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Zhang, J.; Xia, J.; Li, X.; Tao, P.; Deng, T. A Review on Recent Progress in Preparation of Medium-Temperature Solar-Thermal Nanofluids with Stable Dispersion. Nanomaterials 2023, 13, 1399. https://doi.org/10.3390/nano13081399
Hu T, Zhang J, Xia J, Li X, Tao P, Deng T. A Review on Recent Progress in Preparation of Medium-Temperature Solar-Thermal Nanofluids with Stable Dispersion. Nanomaterials. 2023; 13(8):1399. https://doi.org/10.3390/nano13081399
Chicago/Turabian StyleHu, Ting, Jingyi Zhang, Ji Xia, Xiaoxiang Li, Peng Tao, and Tao Deng. 2023. "A Review on Recent Progress in Preparation of Medium-Temperature Solar-Thermal Nanofluids with Stable Dispersion" Nanomaterials 13, no. 8: 1399. https://doi.org/10.3390/nano13081399
APA StyleHu, T., Zhang, J., Xia, J., Li, X., Tao, P., & Deng, T. (2023). A Review on Recent Progress in Preparation of Medium-Temperature Solar-Thermal Nanofluids with Stable Dispersion. Nanomaterials, 13(8), 1399. https://doi.org/10.3390/nano13081399






