Radial Nano-Heterojunctions Consisting of CdS Nanorods Wrapped by 2D CN:PDI Polymer with Deep HOMO for Photo-Oxidative Water Splitting, Dye Degradation and Alcohol Oxidation
Abstract
:1. Introduction
2. Materials and Methods
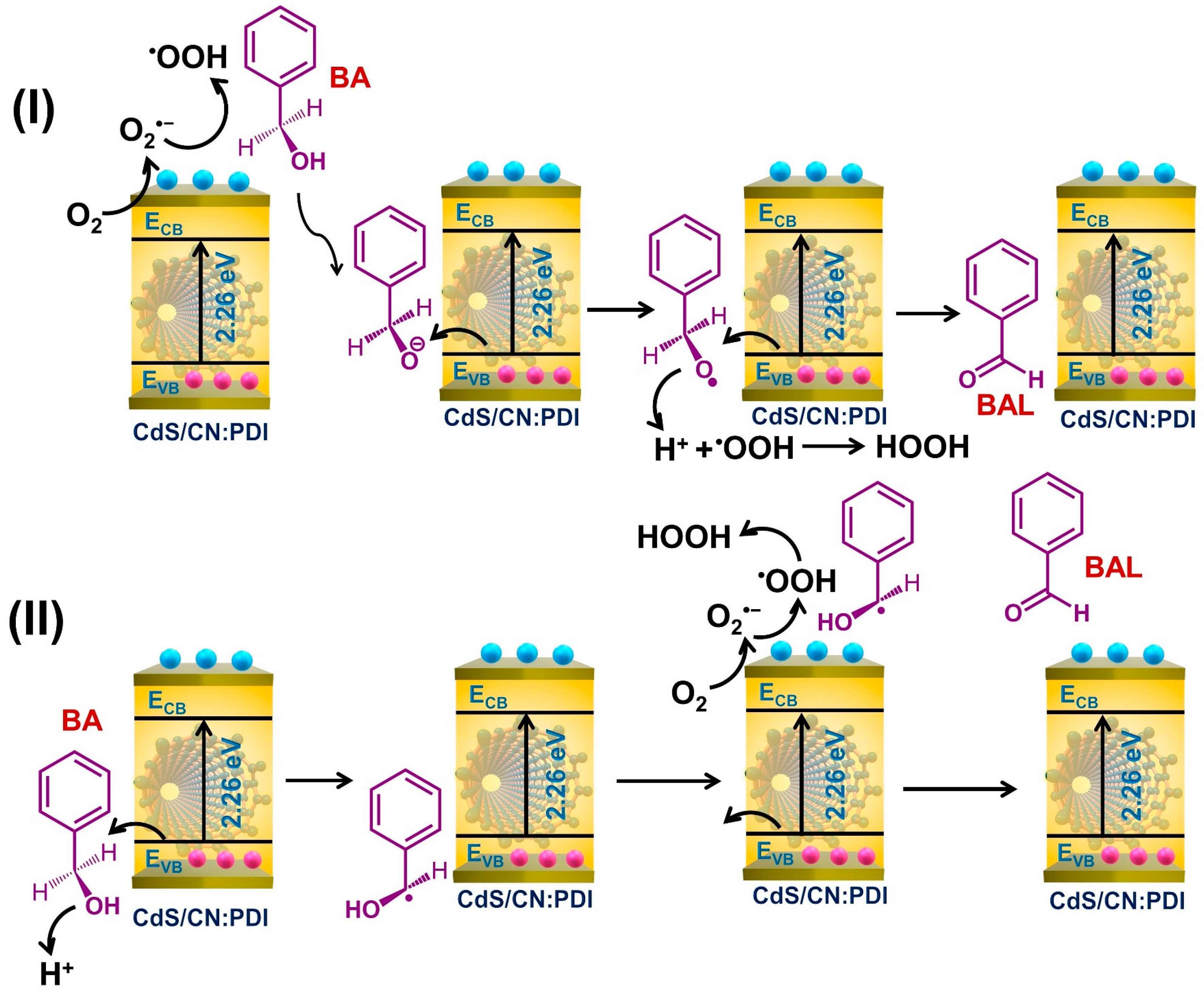
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Tang, C.; Cheng, M.; Lai, C.; Li, L.; Yang, X.; Du, L.; Zhang, G.; Wang, G.; Yang, L. Recent progress in the applications of non-metal modified graphitic carbon nitride in photocatalysis. Coord. Chem. Rev. 2023, 474, 214846. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 2019, 4, 430. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Kumar, P.; Laishram, D.; Sharma, R.K.; Vinu, A.; Hu, J.; Kibria, M.G. Boosting photocatalytic activity using carbon nitride based 2D/2D van der Waals heterojunctions. Chem. Mater. 2021, 33, 9012–9092. [Google Scholar] [CrossRef]
- Kar, P.; Zhang, Y.; Farsinezhad, S.; Mohammadpour, A.; Wiltshire, B.D.; Sharma, H.; Shankar, K. Rutile phase n- and p-type anodic titania nanotube arrays with square-shaped pore morphologies. Chem. Commun. 2015, 51, 7816–7819. [Google Scholar] [CrossRef]
- Wang, H.; Xia, Y.; Li, H.; Wang, X.; Yu, Y.; Jiao, X.; Chen, D. Highly active deficient ternary sulfide photoanode for photoelectrochemical water splitting. Nat. Commun. 2020, 11, 3078. [Google Scholar] [CrossRef]
- Farsinezhad, S.; Shanavas, T.; Mahdi, N.; Askar, A.M.; Kar, P.; Sharma, H.; Shankar, K. Core–shell titanium dioxide–titanium nitride nanotube arrays with near-infrared plasmon resonances. Nanotechnology 2018, 29, 154006. [Google Scholar] [CrossRef]
- Ott, C.; Reiter, F.; Baumgartner, M.; Pielmeier, M.; Vogel, A.; Walke, P.; Burger, S.; Ehrenreich, M.; Kieslich, G.; Daisenberger, D.; et al. Flexible and Ultrasoft Inorganic 1D Semiconductor and Heterostructure Systems Based on SnIP. Adv. Funct. Mater. 2019, 29, 1900233. [Google Scholar] [CrossRef]
- Bagal, I.V.; Arunachalam, M.; Waseem, A.; Abdullah, A.; Kang, S.H.; Ryu, S.-W. Gallium phosphide photoanodes coated with nickel oxyhydroxide cocatalyst for stable photoelectrochemical water splitting reactions. Appl. Surf. Sci. 2021, 558, 149873. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Alam, K.M.; Jensen, C.E.; Kumar, P.; Hooper, R.W.; Bernard, G.M.; Patidar, A.; Manuel, A.P.; Amer, N.; Palmgren, A.; Purschke, D.N.; et al. Photocatalytic mechanism control and study of carrier dynamics in CdS@C3N5 core–shell nanowires. ACS Appl. Mater. Interfaces 2021, 13, 47418–47439. [Google Scholar] [CrossRef]
- Sakizadeh, J.; Cline, J.P.; Snyder, M.A.; Kiely, C.J.; McIntosh, S. Biomineralization of Nanocrystalline CdS/ZnS Photocatalysts via Controlled Surface Passivation for Enhanced Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 2293–2304. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.-J.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, T.; Yu, W.; Si, R.; Liu, Y.; Zhao, Z. Modulating location of single copper atoms in polymeric carbon nitride for enhanced photoredox catalysis. ACS Catal. 2020, 10, 5715–5722. [Google Scholar] [CrossRef]
- Kumar, P.; Mulmi, S.; Laishram, D.; Alam, K.M.; Thakur, U.K.; Thangadurai, V.; Shankar, K. Water-splitting photoelectrodes consisting of heterojunctions of carbon nitride with a p-type low bandgap double perovskite oxide. Nanotechnology 2021, 32, 485407. [Google Scholar] [CrossRef]
- Laishram, D.; Zeng, S.; Alam, K.M.; Kalra, A.P.; Cui, K.; Kumar, P.; Sharma, R.K.; Shankar, K. Air-and water-stable halide perovskite nanocrystals protected with nearly-monolayer carbon nitride for CO2 photoreduction and water splitting. Appl. Surf. Sci. 2022, 592, 153276. [Google Scholar] [CrossRef]
- Alam, K.M.; Kumar, P.; Kar, P.; Goswami, A.; Thakur, U.K.; Zeng, S.; Vahidzadeh, E.; Cui, K.; Shankar, K. Heterojunctions of halogen-doped carbon nitride nanosheets and BiOI for sunlight-driven water-splitting. Nanotechnology 2019, 31, 084001. [Google Scholar] [CrossRef]
- Ran, Y.; Cui, Y.; Zhang, Y.; Fang, Y.; Zhang, W.; Yu, X.; Lan, H.; An, X. Assembly-synthesis of puff pastry-like g-C3N4/CdS heterostructure as S-junctions for efficient photocatalytic water splitting. Chem. Eng. J. 2022, 431, 133348. [Google Scholar] [CrossRef]
- Wang, X.; Wu, K.; Cao, W.; Rui, K.; Wang, W.; Zhu, R.; Zhu, J.; Yan, Z. Z-Scheme Strategy in Polymeric Graphitic C3N5/CdS Core–Shell Heterojunction Drives Long-Lived Carriers Separation for Robust Visible-Light Hydrogen Production. Adv. Mater. Interfaces 2022, 10, 2201627. [Google Scholar] [CrossRef]
- Gao, S.; Wan, S.; Yu, J.; Cao, S. Donor–acceptor modification of carbon nitride for enhanced photocatalytic hydrogen evolution. Adv. Sustain. Syst. 2023, 7, 2200130. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.; Wang, C.; Gan, L.; Chen, X.; Zhang, P.; Wang, Y.; Li, H.; Wang, L.; Zhou, X. Unraveling the dual defect sites in graphite carbon nitride for ultra-high photocatalytic H2O2 evolution. Energy Environ. Sci. 2022, 15, 830–842. [Google Scholar] [CrossRef]
- Kumar, P.; Vahidzadeh, E.; Thakur, U.K.; Kar, P.; Alam, K.M.; Goswami, A.; Mahdi, N.; Cui, K.; Bernard, G.M.; Michaelis, V.K.; et al. C3N5: A low bandgap semiconductor containing an azo-linked carbon nitride framework for photocatalytic, photovoltaic and adsorbent applications. J. Am. Chem. Soc. 2019, 141, 5415–5436. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kanazawa, S.; Kofuji, Y.; Sakamoto, H.; Ichikawa, S.; Tanaka, S.; Hirai, T. Sunlight-driven hydrogen peroxide production from water and molecular oxygen by metal-free photocatalysts. Angew. Chem. Int. Ed. 2014, 53, 13454–13459. [Google Scholar] [CrossRef]
- Long, B.; Yan, G.; He, H.; Meng, S. Porous and Few-Layer Carbon Nitride Nanosheets via Surface Steam Etching for Enhanced Photodegradation Activity. ACS Appl. Nano Mater. 2022, 5, 7798–7810. [Google Scholar] [CrossRef]
- Dang, H.; Mao, S.; Li, Q.; Li, M.; Shao, M.; Wang, W.; Liu, Q. Synergy of nitrogen vacancies and partially broken hydrogen bonds in graphitic carbon nitride for superior photocatalytic hydrogen evolution under visible light. Catal. Sci. Technol. 2022, 12, 5032–5044. [Google Scholar] [CrossRef]
- Li, Y.; Ouyang, S.; Xu, H.; Hou, W.; Zhao, M.; Chen, H.; Ye, J. Targeted exfoliation and reassembly of polymeric carbon nitride for efficient photocatalysis. Adv. Funct. Mater. 2019, 29, 1901024. [Google Scholar] [CrossRef]
- Shahini, E.; Shankar, K.; Tang, T. Liquid-phase exfoliation of graphitic carbon nitrides studied by molecular dynamics simulation. J. Colloid Interface Sci. 2023, 630, 900–910. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Lin, L.; Wang, X. Sol processing of conjugated carbon nitride powders for thin-film fabrication. Angew. Chem. Int. Ed. 2015, 54, 6297–6301. [Google Scholar] [CrossRef]
- Kumar, P.; Kar, P.; Manuel, A.P.; Zeng, S.; Thakur, U.K.; Alam, K.M.; Zhang, Y.; Kisslinger, R.; Cui, K.; Bernard, G.M.; et al. Noble Metal Free, Visible Light Driven Photocatalysis Using TiO2 Nanotube Arrays Sensitized by P-Doped C3N4 Quantum Dots. Adv. Opt. Mater. 2020, 8, 1901275. [Google Scholar] [CrossRef]
- Chaulagain, N.; Alam, K.M.; Kadian, S.; Kumar, N.; Garcia, J.; Manik, G.; Shankar, K. Synergistic Enhancement of the Photoelectrochemical Performance of TiO2 Nanorod Arrays through Embedded Plasmon and Surface Carbon Nitride Co-sensitization. ACS Appl. Mater. Interfaces 2022, 14, 24309–24320. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Zhang, J.; Zhang, H.; Tian, W.; Li, X.; Tade, M.O.; Sun, H.; Wang, S. Flower-like MoS2 on graphitic carbon nitride for enhanced photocatalytic and electrochemical hydrogen evolutions. Appl. Catal. B Environ. 2018, 239, 334–344. [Google Scholar] [CrossRef]
- Yi, J.; She, X.; Song, Y.; Mao, M.; Xia, K.; Xu, Y.; Mo, Z.; Wu, J.; Xu, H.; Li, H. Solvothermal synthesis of metallic 1T-WS2: A supporting co-catalyst on carbon nitride nanosheets toward photocatalytic hydrogen evolution. Chem. Eng. J. 2018, 335, 282–289. [Google Scholar] [CrossRef]
- Zhu, M.; Kim, S.; Mao, L.; Fujitsuka, M.; Zhang, J.; Wang, X.; Majima, T. Metal-Free Photocatalyst for H2 Evolution in Visible to Near-Infrared Region: Black Phosphorus/Graphitic Carbon Nitride. J. Am. Chem. Soc. 2017, 139, 13234–13242. [Google Scholar] [CrossRef]
- Prakash, J.; Prasad, U.; Alexander, R.; Bahadur, J.; Dasgupta, K.; Kannan, A.N.M. Photoelectrochemical Solar Water Splitting: The Role of the Carbon Nanomaterials in Bismuth Vanadate Composite Photoanodes toward Efficient Charge Separation and Transport. Langmuir 2019, 35, 14492–14504. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, H.; Peng, T.; Li, S.; Xu, L.; Zhang, J.; Zhang, L. Ag@CeO2–Au Nanorod Plasmonic Nanohybrids for Enhanced Photocatalytic Conversion of Benzyl Alcohol to Benzaldehyde. ACS Appl. Nano Mater. 2022, 5, 4972–4982. [Google Scholar] [CrossRef]
- Li, K.; Pei, Y.; Xiao, P.; He, Z.; Carabineiro, S.A.; Jiang, H.; Zhu, J. Templated Synthesis of Mesoporous Co3O4 Nanostructures for the Liquid-Phase Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde. ACS Appl. Nano Mater. 2022, 5, 3722–3732. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Pan, C.; Wang, G.; Zhao, H.; Dong, Y.; Zhu, Y. Selectively Permeable FeOOH Amorphous Layer Coating CdS for Enhanced Oxidation of Benzyl Alcohol to Benzaldehyde. ChemSusChem 2023, e202202355. [Google Scholar] [CrossRef]
- Lee, S.G.; Kang, M.J.; Park, M.; Kim, K.-j.; Lee, H.; Kim, H.S. Selective photocatalytic conversion of benzyl alcohol to benzaldehyde or deoxybenzoin over ion-exchanged CdS. Appl. Catal. B 2022, 304, 120967. [Google Scholar] [CrossRef]
- Zhong, J.-J.; To, W.-P.; Liu, Y.; Lu, W.; Che, C.-M. Efficient acceptorless photo-dehydrogenation of alcohols and N-heterocycles with binuclear platinum (II) diphosphite complexes. Chem. Sci. 2019, 10, 4883–4889. [Google Scholar] [CrossRef]
- Xiang, X.; Zhu, B.; Zhang, J.; Jiang, C.; Chen, T.; Yu, H.; Yu, J.; Wang, L. Photocatalytic H2-production and benzyl-alcohol-oxidation mechanism over CdS using Co2+ as hole cocatalyst. Appl. Catal. B 2023, 324, 122301. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, T.; Han, F.; Yang, Q.; Li, B. Synthesis of Ni modified Au@CdS core–shell nanostructures for enhancing photocatalytic coproduction of hydrogen and benzaldehyde under visible light. J. Colloid Interface Sci. 2022, 606, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Sun, Z.; Liu, X.; Jia, H.; Du, P. Cadmium sulfide/graphitic carbon nitride heterostructure nanowire loading with a nickel hydroxide cocatalyst for highly efficient photocatalytic hydrogen production in water under visible light. Nanoscale 2016, 8, 4748–4756. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Joshi, U.A.; Lee, J.S. Solvothermal synthesis of CdS nanowires for photocatalytic hydrogen and electricity production. J. Phys. Chem. C 2007, 111, 13280–13287. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Zou, Z. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Xu, J.; Chen, T.; Jiang, Q.; Li, Y.X. Utilization of Environmentally Benign Dicyandiamide as a Precursor for the Synthesis of Ordered Mesoporous Carbon Nitride and its Application in Base-Catalyzed Reactions. Chem. Asian J. 2014, 9, 3269–3277. [Google Scholar] [CrossRef] [PubMed]
- Sattler, A.; Schönberger, S.; Schnick, W. Melemium Methylsulfonates HC6N7(NH2)3H2C6N7 (NH2)3(SO3Me)3·H2O and H2C6N7(NH2)3 (SO3Me)2·H2O. Z. Anorg. Allg. Chem. 2010, 636, 476–482. [Google Scholar] [CrossRef]
- Chu, S.; Wang, Y.; Guo, Y.; Feng, J.; Wang, C.; Luo, W.; Fan, X.; Zou, Z. Band structure engineering of carbon nitride: In search of a polymer photocatalyst with high photooxidation property. ACS Catal. 2013, 3, 912–919. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.C.; Kang, X.; Wang, L.; Liu, G.; Cheng, H.M. Selective breaking of hydrogen bonds of layered carbon nitride for visible light photocatalysis. Adv. Mater. 2016, 28, 6471–6477. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, X.; Zhang, Z.; Zhang, L.; Wang, Y.; Sun, Z.; Irfan, R.M.; Du, P. Highly efficient simultaneous hydrogen evolution and benzaldehyde production using cadmium sulfide nanorods decorated with small cobalt nanoparticles under visible light. J. Catal. 2018, 357, 147–153. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Thongtem, T.; Thongtem, S. Effects of ethylenediamine to water ratios on cadmium sulfide nanorods and nanoparticles produced by a solvothermal method. Mater. Lett. 2009, 63, 1538–1541. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Wang, W. Direct functionalization of methane into ethanol over copper modified polymeric carbon nitride via photocatalysis. Nat. Commun. 2019, 10, 506. [Google Scholar]
- Li, Y.; He, Z.; Liu, L.; Jiang, Y.; Ong, W.-J.; Duan, Y.; Ho, W.; Dong, F. Inside-and-out modification of graphitic carbon nitride (g-C3N4) photocatalysts via defect engineering for energy and environmental science. Nano Energy 2022, 105, 108032. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Wu, S.; Liu, Y.; Tan, Y.Z.; Luo, H.; Yuan, X.; Chew, J.W. Petal-like CdS nanostructures coated with exfoliated sulfur-doped carbon nitride via chemically activated chain termination for enhanced visible-light–driven photocatalytic water purification and H2 generation. Appl. Catal. B 2018, 229, 181–191. [Google Scholar] [CrossRef]
- Wang, Z.; Almatrafi, E.; Wang, H.; Qin, H.; Wang, W.; Du, L.; Chen, S.; Zeng, G.; Xu, P. Cobalt Single Atoms Anchored on Oxygen-Doped Tubular Carbon Nitride for Efficient Peroxymonosulfate Activation: Simultaneous Coordination Structure and Morphology Modulation. Angew. Chem. 2022, 134, e202202338. [Google Scholar]
- Garza-Hernández, R.; Carrillo-Castillo, A.; Martínez-Landeros, V.; Martínez-Puente, M.; Martínez-Guerra, E.; Aguirre-Tostado, F. In-situ X-ray photoelectron spectroscopy analysis of the initial growth of CdS thin films by chemical bath deposition. Thin Solid Films 2019, 682, 142–146. [Google Scholar] [CrossRef]
- Turnbull, M.J.; Yiu, Y.M.; Goldman, M.; Sham, T.-K.; Ding, Z. Favorable bonding and band structures of Cu2ZnSnS4 and CdS films and their photovoltaic interfaces. ACS Appl. Mater. Interfaces 2022, 14, 32683–32695. [Google Scholar] [CrossRef]
- DiMeglio, J.L.; Bartlett, B.M. Interplay of corrosion and photocatalysis during nonaqueous benzylamine oxidation on cadmium sulfide. Chem. Mater. 2017, 29, 7579–7586. [Google Scholar] [CrossRef]
- Gerken, V.C.; Carreira, E.M. Carbon Nitride Photoredox Catalysis Enables the Generation of the Dioxolanyl Radical for Conjugate Addition Reactions. ACS Catal. 2022, 12, 10787–10792. [Google Scholar] [CrossRef]
- Chen, C.-C.; Wu, J.-J. Simultaneous Growth of the SnO2 Nanostructure and Phase Transformation of the Carbon Nitride Matrix by a Hydrothermal Process for Efficient Solar Hydrogen Evolution. ACS Appl. Energy Mater. 2022, 5, 9733–9741. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, X.; Yu, L.; Wang, Y.; Ning, J.; Xu, S.; Lou, X.W. Carbon-Coated CdS Petalous Nanostructures with Enhanced Photostability and Photocatalytic Activity. Angew. Chem. Int. Ed. 2013, 52, 5636–5639. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.M.; Kumar, P.; Kar, P.; Thakur, U.K.; Zeng, S.; Cui, K.; Shankar, K. Enhanced charge separation in g-C3N4–BiOI heterostructures for visible light driven photoelectrochemical water splitting. Nanoscale Adv. 2019, 1, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, J.; Guo, W.; Xu, Q.; Min, Y. A high efficiency water hydrogen production method based on CdS/WN composite photocatalytic. J. Colloid Interface Sci. 2022, 613, 652–660. [Google Scholar] [CrossRef]
- Wang, R.; Cao, X.; Huang, H.; Ji, X.; Chen, X.; Liu, J.; Yan, P.; Wei, S.; Chen, L.; Wang, Y. Facile Chemical Vapor Modification Strategy to Construct Surface Cyano-Rich Polymer Carbon Nitrides for Highly Efficient Photocatalytic H2 Evolution. ChemSusChem 2022, 15, e202201575. [Google Scholar] [CrossRef] [PubMed]
- Ciria-Ramos, I.; Navascués, N.; Diaw, F.; Furgeaud, C.; Arenal, R.; Ansón-Casaos, A.; Haro, M.; Juarez-Perez, E.J. Formamidinium halide salts as precursors of carbon nitrides. Carbon 2022, 196, 1035–1046. [Google Scholar] [CrossRef]
- Meng, P.; Heng, H.; Sun, Y.; Huang, J.; Yang, J.; Liu, X. Positive effects of phosphotungstic acid on the in-situ solid-state polymerization and visible light photocatalytic activity of polyimide-based photocatalyst. Appl. Catal. B 2018, 226, 487–498. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Borkar, R.; Bansiwal, A.; Labhsetwar, N.; Jain, S.L. Metal-organic hybrid: Photoreduction of CO2 using graphitic carbon nitride supported heteroleptic iridium complex under visible light irradiation. Carbon 2017, 123, 371–379. [Google Scholar] [CrossRef]
- Zhou, T.; Li, T.; Hou, J.; Wang, Y.; Hu, B.; Sun, D.; Wu, Y.; Jiang, W.; Che, G.; Liu, C. Tailoring boron doped intramolecular donor–acceptor integrated carbon nitride skeleton with propelling photocatalytic activity and mechanism insight. Chem. Eng. J. 2022, 445, 136643. [Google Scholar] [CrossRef]
- Wen, J.; Li, R.; Lu, R.; Yu, A. Photophysics and Photocatalysis of Melem: A Spectroscopic Reinvestigation. Chem. Asian J. 2018, 13, 1060–1066. [Google Scholar] [CrossRef]
- Li, K.; Jiang, Y.; Rao, W.; Li, Y.; Liu, X.; Zhang, J.; Xu, X.; Lin, K. Cooperative coupling strategy for constructing 0D/2D carbon nitride composites with strengthened chemical interaction for enhanced photocatalytic applications. Chem. Eng. J. 2022, 431, 134075. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, S.; Wang, J.; Zhang, R.; Wang, X. Cadmium sulfide 3D photonic crystal with hierarchically ordered macropores for highly efficient photocatalytic hydrogen generation. Inorg. Chem. 2022, 61, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, J.; Chu, S.; Kong, F.; Luo, L.; Wang, Y.; Zou, Z. Theoretical and experimental study on narrowing the band gap of carbon nitride photocatalyst by coupling a wide gap molecule. Chem. Phys. Lett. 2012, 550, 175–180. [Google Scholar] [CrossRef]
- Kofuji, Y.; Isobe, Y.; Shiraishi, Y.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Carbon nitride–aromatic diimide–graphene nanohybrids: Metal-free photocatalysts for solar-to-hydrogen peroxide energy conversion with 0.2% efficiency. J. Am. Chem. Soc. 2016, 138, 10019–10025. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, Y.; Isobe, Y.; Shiraishi, Y.; Sakamoto, H.; Ichikawa, S.; Tanaka, S.; Hirai, T. Hydrogen Peroxide Production on a Carbon Nitride–Boron Nitride-Reduced Graphene Oxide Hybrid Photocatalyst under Visible Light. ChemCatChem 2018, 10, 2070–2077. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Ramacharyulu, P.; Abbas, S.J.; Sahoo, S.R.; Ke, S.-C. Mechanistic insights into 4-nitrophenol degradation and benzyl alcohol oxidation pathways over MgO/g-C3N4 model catalyst systems. Catal. Sci. Technol. 2018, 8, 2825–2834. [Google Scholar] [CrossRef]
- Zheng, C.; He, G.; Xiao, X.; Lu, M.; Zhong, H.; Zuo, X.; Nan, J. Selective photocatalytic oxidation of benzyl alcohol into benzaldehyde with high selectivity and conversion ratio over Bi4O5Br2 nanoflakes under blue LED irradiation. Appl. Catal. B 2017, 205, 201–210. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, L.; Wang, W.; Zeng, S. Modification of heterogeneous photocatalysts for selective organic synthesis. Catal. Sci. Technol. 2018, 8, 1229–1250. [Google Scholar] [CrossRef]
- Murphy, A.; Barnes, P.; Randeniya, L.; Plumb, I.; Grey, I.; Horne, M.; Glasscock, J. Efficiency of solar water splitting using semiconductor electrodes. Int. J. Hydrogen Energy 2006, 31, 1999–2017. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Hu, S.; Xiang, C.; Haussener, S.; Berger, A.D.; Lewis, N.S. An analysis of the optimal band gaps of light absorbers in integrated tandem photoelectrochemical water-splitting systems. Energy Environ. Sci. 2013, 6, 2984–2993. [Google Scholar] [CrossRef]
- Nellist, M.R.; Laskowski, F.A.; Lin, F.; Mills, T.J.; Boettcher, S.W. Semiconductor–electrocatalyst interfaces: Theory, experiment, and applications in photoelectrochemical water splitting. Acc. Chem. Res. 2016, 49, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Üzer, E.; Kumar, P.; Kisslinger, R.; Kar, P.; Thakur, U.K.; Zeng, S.; Shankar, K.; Nilges, T. Vapor deposition of semiconducting P allotropes into TiO2 nanotube arrays for photo-electrocatalytic water splitting. ACS Appl. Nano Mater. 2019, 2, 3358–3367. [Google Scholar] [CrossRef]
- Ameen, F.; Aygun, A.; Seyrankaya, A.; Tiri, R.N.E.; Gulbagca, F.; Kaynak, İ.; Majrashi, N.; Orfali, R.; Dragoi, E.N.; Sen, F. Photocatalytic investigation of textile dyes and E. coli bacteria from wastewater using Fe3O4@MnO2 heterojunction and investigation for hydrogen generation on NaBH4 hydrolysis. Environ. Res. 2023, 220, 115231. [Google Scholar] [CrossRef] [PubMed]
- Mali, G.; Walekar, L.; Kolhe, N.; Kadam, A.N.; Kore, R.; Mhamane, D.; Parbat, H.; Lee, S.-W.; Lokhande, B.; Patil, V. Multifunctional polyoxotungstocobaltate anchored fern-leaf like BiVO4 microstructures for enhanced photocatalytic and supercapacitive performance. Colloids Surf. A 2023, 662, 130974. [Google Scholar] [CrossRef]
- Thakur, S.; Kaur, R.; Mandal, S.K. Size dependence of CdS nanoparticles on the precursor concentration and visible light driven photocatalytic degradation of methylene blue. New J. Chem. 2021, 45, 12227–12235. [Google Scholar] [CrossRef]
- Laishram, D.; Shejale, K.P.; Gupta, R.; Sharma, R.K. Heterostructured HfO2/TiO2 spherical nanoparticles for visible photocatalytic water remediation. Mater. Lett. 2018, 231, 225–228. [Google Scholar] [CrossRef]
- Shejale, K.P.; Laishram, D.; Gupta, R.; Sharma, R.K. Engineered ZnO-TiO2 nanospheres for high performing membrane assimilated photocatalytic water remediation and energy harvesting. ChemistrySelect 2018, 3, 7291–7301. [Google Scholar] [CrossRef]
- Ghorai, K.; Bhattacharjee, M.; Mandal, D.; Hossain, A.; Bhunia, T.; Das, M.; Ray, P.P.; Show, B.; Bera, P.; Mandal, T.K. Facile synthesis of CuCr2O4/BiOBr nanocomposite and its photocatalytic activity towards RhB and tetracycline hydrochloride degradation under household visible LED light irradiation. J. Alloys Compd. 2021, 867, 157947. [Google Scholar] [CrossRef]
- Vignesh, S.; Kim, H. Rational construction of efficient ZnS quantum dots-supported g-C3N4 with Co3O4 heterostructure composite for bifunctional electrocatalytic hydrogen evolution reaction and environmental pollutant degradation. J. Alloys Compd. 2023, 942, 169077. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, M.; Krishnan, V.; Mohapatra, S. Facile synthesis of Ce doped ZnO nanowires for efficient photocatalytic removal of organic pollutants from water. Mater. Today Commun. 2023, 34, 105361. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Okab, A.A.; Graimed, B.H.; Issa, M.A.; Ammar, S.H. Photocatalytic destruction of Congo red dye in wastewater using a novel Ag2WO4/Bi2S3 nanocomposite decorated g-C3N4 nanosheet as ternary S-scheme heterojunction: Improving the charge transfer efficiency. Diam. Relat. Mater. 2023, 133, 109711. [Google Scholar] [CrossRef]
- Hamzad, S.; Kumar, K.-Y.; Prashanth, M.; Radhika, D.; Parashuram, L.; Alharti, F.-A.; Jeon, B.-H.; Raghu, M. Boron doped RGO from discharged dry cells decorated Niobium pentoxide for enhanced visible light-induced hydrogen evolution and water decontamination. Surf. Interfaces 2023, 36, 102544. [Google Scholar] [CrossRef]
- Aouf, D.; Henni, A.; Selloum, D.; Khane, Y.; Fenniche, F.; Zerrouki, D.; Belkhalfa, H.; Dizge, N. Facile preparation and characterization of nanostructured ZnS/PbS heterojunction thin films for enhanced microbial inhibition and photocatalytic degradation. Mater. Chem. Phys. 2023, 295, 127059. [Google Scholar] [CrossRef]
- Song, Z.; Liu, Y.; Zhang, B.; Song, S.; Zhou, Z.; Huang, Y.; Zhao, Z. Magnetic grinding synthesis of copper sulfide-based photocatalytic composites for the degradation of organic dyes under visible light. New J. Chem. 2023, 47, 2286–2295. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, G.; Liu, A. Facet-dependent Cu2O@Zn(OH)2 composites with enhanced visible-light photocatalysis. Mater. Lett. 2023, 330, 133334. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, X.; Liu, X.; Li, H.; Gao, S. Synthesis and visible light catalytic activity of Ag3PO4/Bi2SiO5 nanocomposites. J. Solid State Chem. 2023, 317, 123708. [Google Scholar] [CrossRef]
- Tahir, M.Y.; Sillanpaa, M.; Almutairi, T.M.; Mohammed, A.A.; Ali, S. Excellent photocatalytic and antibacterial activities of bio-activated carbon decorated magnesium oxide nanoparticles. Chemosphere 2023, 312, 137327. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Q.; Liu, H. In situ growth of gold nanoparticles onto polydopamine-modified MXene to quickly and efficiently degrade dyes. J. Mater. Sci. 2023, 15, 1026–1043. [Google Scholar] [CrossRef]
- Schünemann, S.; van Gastel, M.; Tüysüz, H. A CsPbBr3/TiO2 composite for visible-light-driven photocatalytic benzyl alcohol oxidation. ChemSusChem 2018, 11, 2057–2061. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Zou, J.; Li, L.; Yu, Y.; Wu, L. The cooperation effect in the Au–Pd/LDH for promoting photocatalytic selective oxidation of benzyl alcohol. Catal. Sci. Technol. 2018, 8, 268–275. [Google Scholar] [CrossRef]
- Jing, K.; Ma, W.; Ren, Y.; Xiong, J.; Guo, B.; Song, Y.; Liang, S.; Wu, L. Hierarchical Bi2MoO6 spheres in situ assembled by monolayer nanosheets toward photocatalytic selective oxidation of benzyl alcohol. Appl. Catal. B 2019, 243, 10–18. [Google Scholar] [CrossRef]
- Shen, M.; Shi, Y.; Wang, Z.; Wu, T.; Hu, L.; Wu, L. Enhanced photocatalytic benzyl alcohol oxidation over Bi4Ti3O12 ultrathin nanosheets. J. Colloid Interf. Sci. 2022, 608, 2529–2538. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, C.; Wang, G.; Leng, Y.; Jiang, P.; Dong, Y.; Zhu, Y. Improving the photocatalytic activity of benzyl alcohol oxidation by Z-scheme SnS/gC3N4. New J. Chem. 2021, 45, 6611–6617. [Google Scholar] [CrossRef]
- Du, M.; Zeng, G.; Huang, J.; Sun, D.; Li, Q.; Wang, G.; Li, X. Green photocatalytic oxidation of benzyl alcohol over noble-metal-modified H2Ti3O7 nanowires. ACS Sustain. Chem. Eng. 2019, 7, 9717–9726. [Google Scholar] [CrossRef]
- Cheng, R.; Steele, J.A.; Roeffaers, M.B.; Hofkens, J.; Debroye, E. Dual-channel charge carrier transfer in CsPbX3 perovskite/W18O49 composites for selective photocatalytic benzyl alcohol oxidation. ACS Appl. Energy Mater. 2021, 4, 3460–3468. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, T.; Zheng, Z.; Xing, B.; Li, C.; Li, B. Constructing interfacial active sites in Ru/g-C3N4−x photocatalyst for boosting H2 evolution coupled with selective benzyl-alcohol oxidation. Appl. Catal. B 2022, 315, 121575. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, P.; Bao, S.; Wang, Z.; Tian, B.; Zhang, J. Synthesis of sandwich-structured AgBr@Ag@TiO2 composite photocatalyst and study of its photocatalytic performance for the oxidation of benzyl alcohols to benzaldehydes. Chem. Eng. J. 2016, 306, 1151–1161. [Google Scholar] [CrossRef]
- She, H.; Zhou, H.; Li, L.; Wang, L.; Huang, J.; Wang, Q. Nickel-doped excess oxygen defect titanium dioxide for efficient selective photocatalytic oxidation of benzyl alcohol. ACS Sustain. Chem. Eng. 2018, 6, 11939–11948. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, X.; Yang, W.; Liu, Y. Photothermal effect and continuous hot electrons injection synergistically induced enhanced molecular oxygen activation for efficient selective oxidation of benzyl alcohol over plasmonic W18O49/ZnIn2S4 photocatalyst. Appl. Catal. B 2021, 299, 120675. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Y.; Yang, Z.; Xie, Y.; Zhang, J.; Shang, X.; Zhao, H.; Zhang, Z.; Wang, X. The effect of halogen on BiOX (X = Cl, Br, I)/Bi2WO6 heterojunction for visible-light-driven photocatalytic benzyl alcohol selective oxidation. Appl. Catal. A 2018, 567, 65–72. [Google Scholar] [CrossRef]
- Xing, F.; Zeng, R.; Cheng, C.; Liu, Q.; Huang, C. POM-incorporated ZnIn2S4 Z-scheme dual- functional photocatalysts for cooperative benzyl alcohol oxidation and H2 evolution in aqueous solution. Appl. Catal. B 2022, 306, 121087. [Google Scholar] [CrossRef]
- Bao, X.; Lv, X.; Wang, Z.; Wang, M.; Liu, M.; Dai, D.; Zheng, L.; Zheng, Z.; Cheng, H.; Wang, P. Nitrogen vacancy enhanced photocatalytic selective oxidation of benzyl alcohol in g-C3N4. Int. J. Hydrogen Energy 2021, 46, 37782–37791. [Google Scholar] [CrossRef]

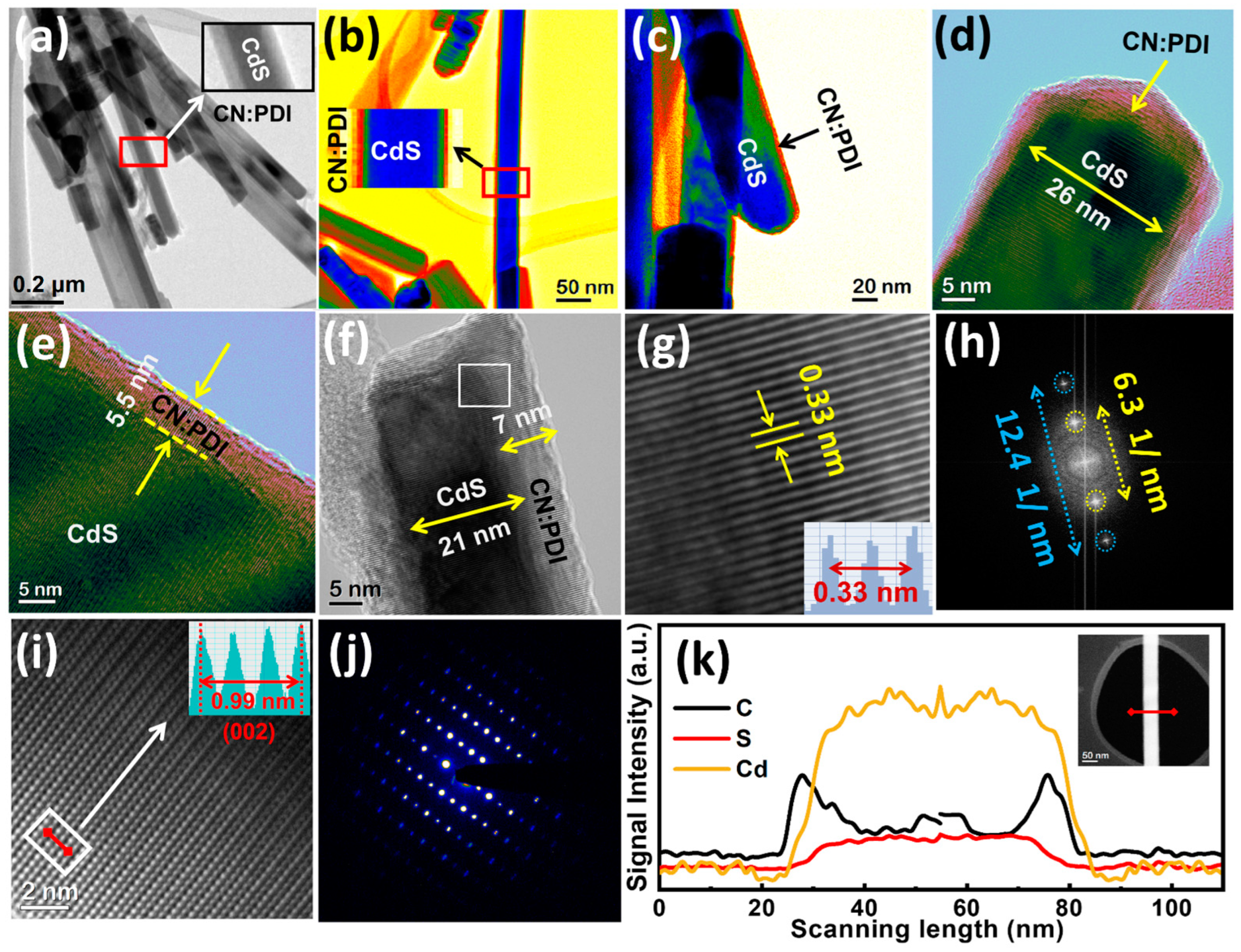
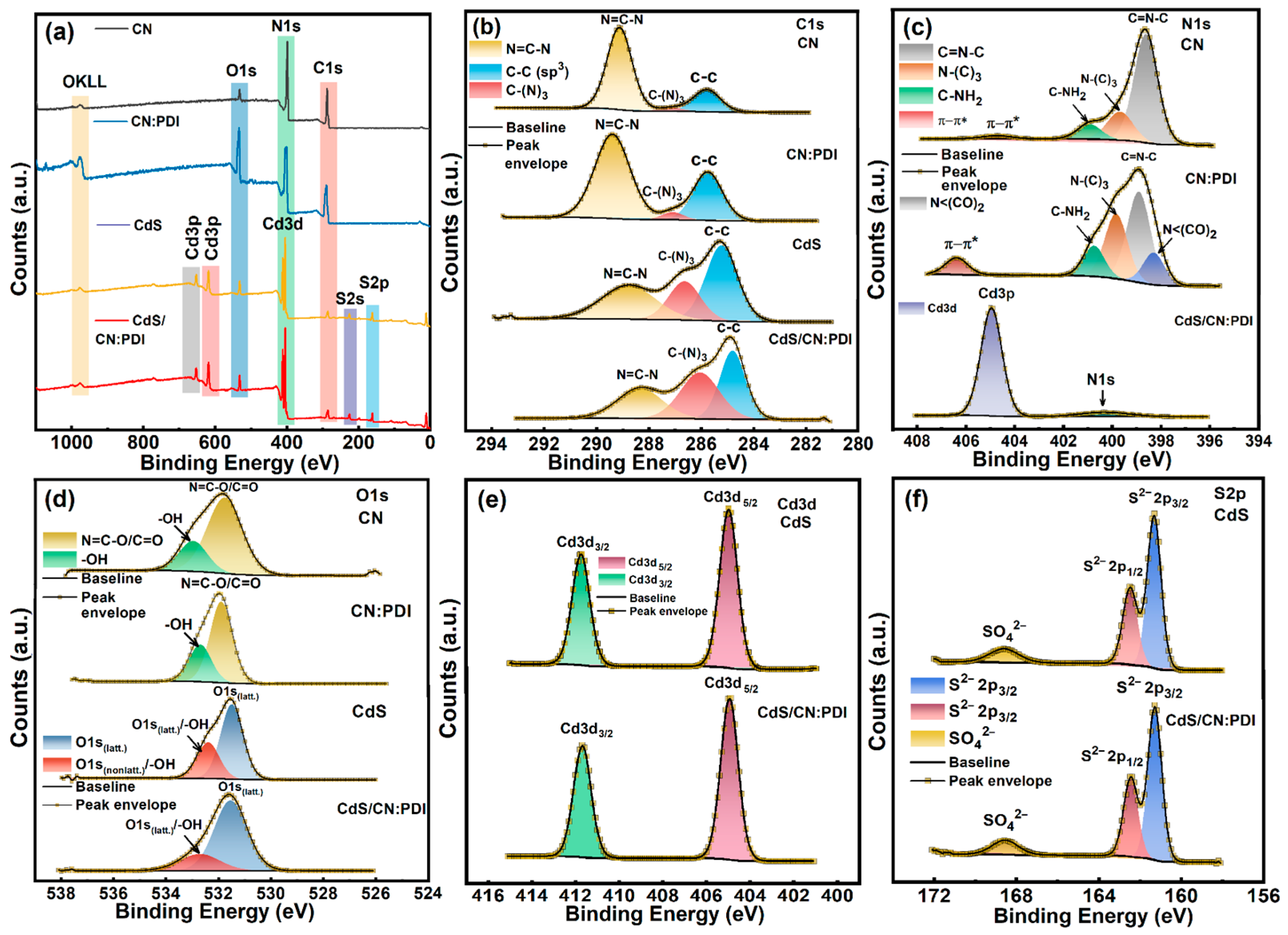
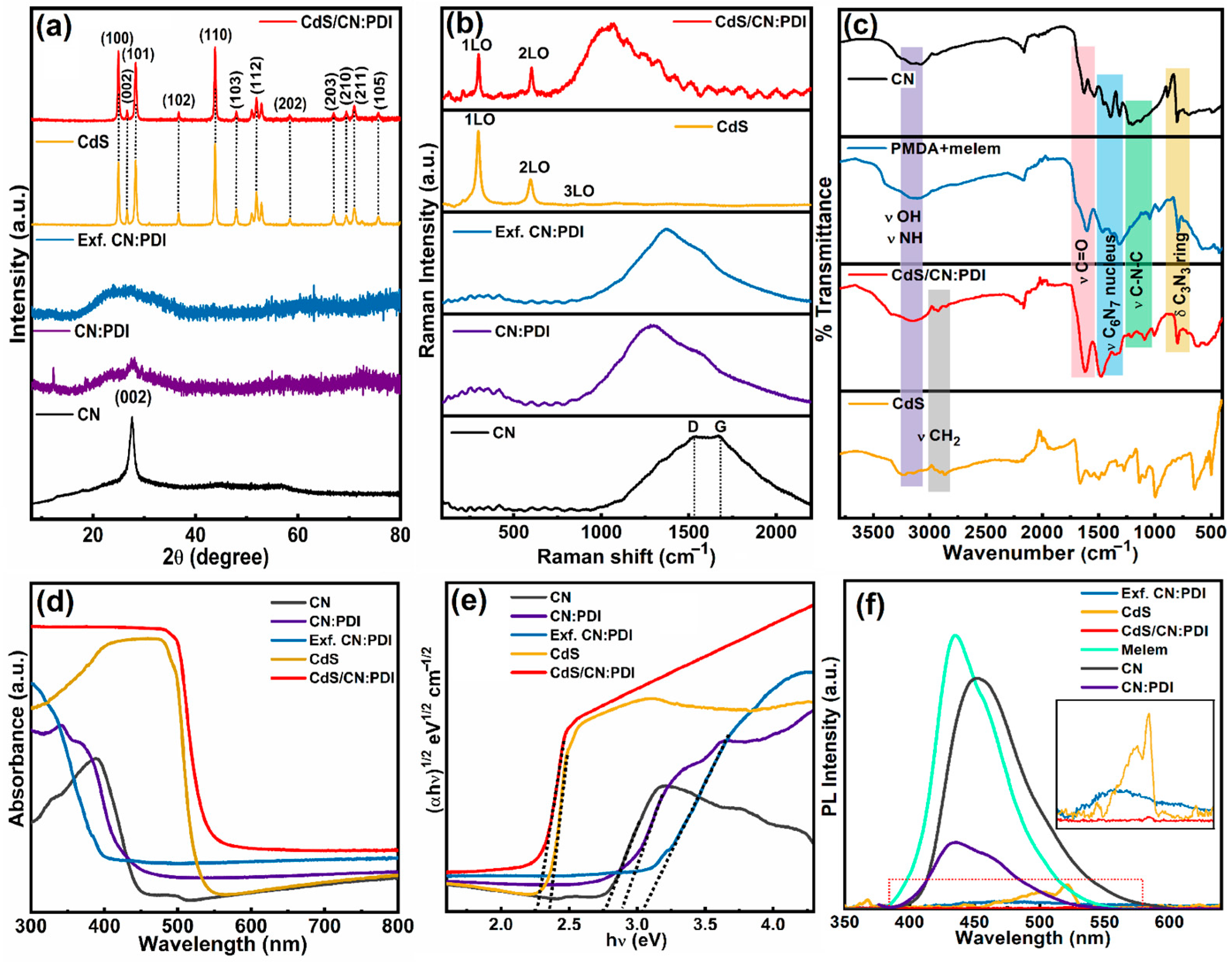
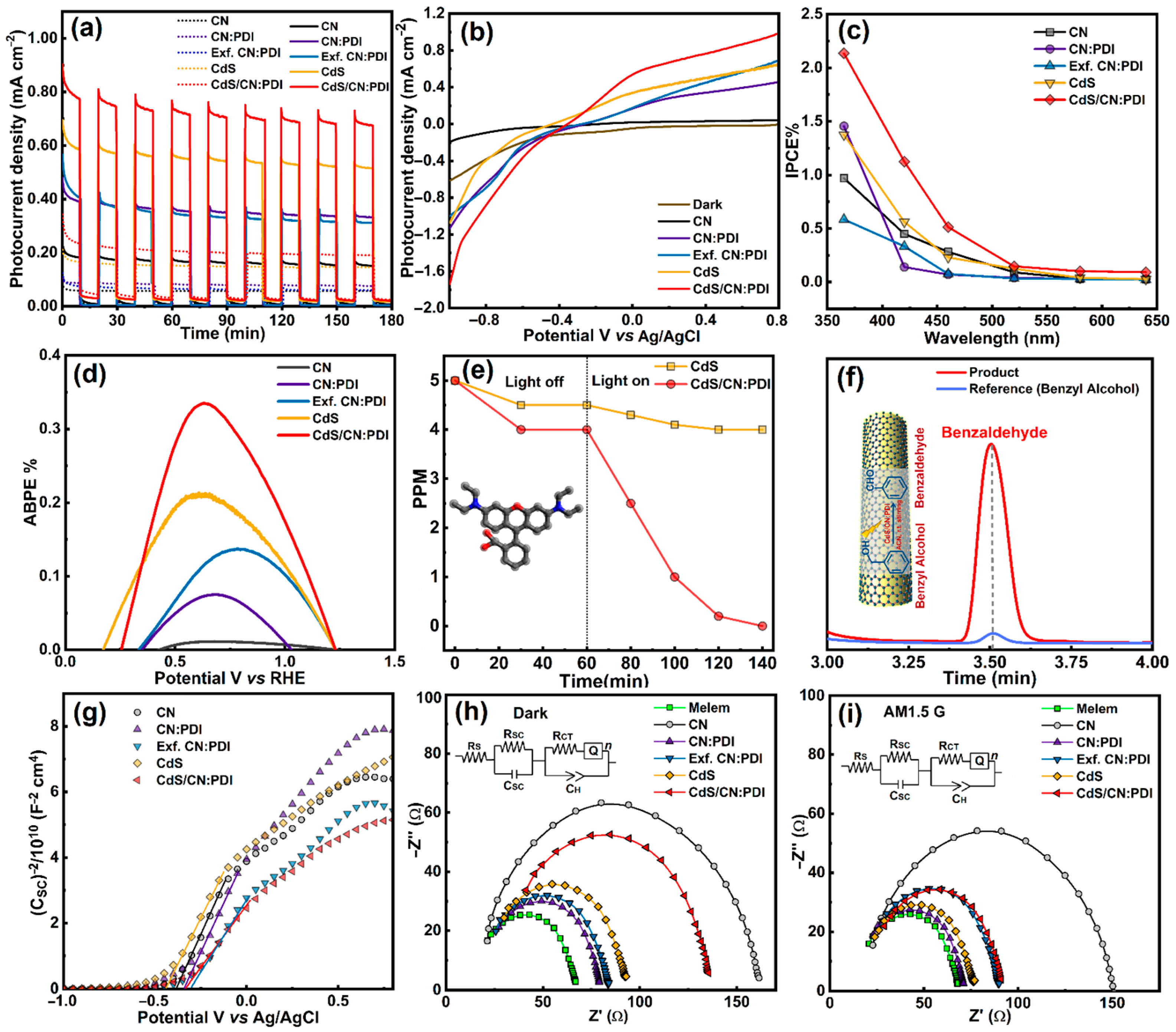

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Vahidzadeh, E.; Alam, K.M.; Laishram, D.; Cui, K.; Shankar, K. Radial Nano-Heterojunctions Consisting of CdS Nanorods Wrapped by 2D CN:PDI Polymer with Deep HOMO for Photo-Oxidative Water Splitting, Dye Degradation and Alcohol Oxidation. Nanomaterials 2023, 13, 1481. https://doi.org/10.3390/nano13091481
Kumar P, Vahidzadeh E, Alam KM, Laishram D, Cui K, Shankar K. Radial Nano-Heterojunctions Consisting of CdS Nanorods Wrapped by 2D CN:PDI Polymer with Deep HOMO for Photo-Oxidative Water Splitting, Dye Degradation and Alcohol Oxidation. Nanomaterials. 2023; 13(9):1481. https://doi.org/10.3390/nano13091481
Chicago/Turabian StyleKumar, Pawan, Ehsan Vahidzadeh, Kazi M. Alam, Devika Laishram, Kai Cui, and Karthik Shankar. 2023. "Radial Nano-Heterojunctions Consisting of CdS Nanorods Wrapped by 2D CN:PDI Polymer with Deep HOMO for Photo-Oxidative Water Splitting, Dye Degradation and Alcohol Oxidation" Nanomaterials 13, no. 9: 1481. https://doi.org/10.3390/nano13091481







