Introduction of Surface Modifiers on the Pt-Based Electrocatalysts to Promote the Oxygen Reduction Reaction Process
Abstract
:1. Introduction
2. Surface Modification with Silicon and Carbon
3. Surface Modification of Amine Compounds
3.1. Research Progress of Amine Compounds for Surface Modification
3.2. The Action Mechanism of Amine Compounds
4. Ionic Liquid Surface Modification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, E.; Wu, M.; Xu, H.; Peng, B.; Liu, Z.; Huang, Y.; Li, Y. Stability of platinum-group-metal-based electrocatalysts in proton exchange membrane fuel cells. Adv. Funct. Mater. 2022, 32, 2203883. [Google Scholar] [CrossRef]
- Yang, C.-L.; Wang, L.-N.; Yin, P.; Liu, J.; Chen, M.-X.; Yan, Q.-Q.; Wang, Z.-S.; Xu, S.-L.; Chu, S.-Q.; Cui, C.; et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chutia, A.; Brett, D.J.L.; Shearing, P.R.; He, G.; Chai, G.; Parkin, I.P. Palladium alloys used as electrocatalysts for the oxygen reduction reaction. Energy Environ. Sci. 2021, 14, 2639–2669. [Google Scholar] [CrossRef]
- Yuan, Z.; Cao, Y.; Zhang, Z.; Fang, Y.; Liu, Q.; Dang, D.; Zheng, Y. Dandelion-like titanium nitride supported platinum as an efficient oxygen reduction catalyst in acidic media. Int. J. Hydrogen Energy 2022, 47, 15035–15043. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Song, Y.; Wang, Y. A modeling study of PEM fuel cells with novel catalyst monolayers under low platinum loading. J. Mater. Chem. A 2022, 10, 4076–4086. [Google Scholar] [CrossRef]
- Xu, F.; Cai, S.; Lin, B.; Yang, L.; Le, H.; Mu, S. Geometric engineering of porous PtCu nanotubes with ultrahigh methanol oxidation and oxygen reduction capability. Small 2022, 18, 2107387. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.; Ma, W.; Zhou, Z. Oxygen reduction reaction on Pt-based electrocatalysts: Four-electron vs. two-electron pathway. Chin. J. Catal. 2022, 43, 1433–1443. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Wang, Y.; Yan, B. Synthesis of Pd nanonetworks with abundant defects for oxygen reduction electrocatalysis. New J. Chem. 2021, 45, 2814–2819. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Gao, L.; Zeng, G.; Li, M.; Huang, H. Stabilizing Pt-based electrocatalysts for oxygen reduction reaction: Fundamental understanding and design strategies. Adv. Mater. 2021, 33, 2006494. [Google Scholar] [CrossRef]
- Hsieh, B.-J.; Tsai, M.-C.; Pan, C.-J.; Su, W.-N.; Rick, J.; Lee, J.-F.; Yang, Y.-W.; Hwang, B.-J. Platinum loaded on dual-doped TiO2 as an active and durable oxygen reduction reaction catalyst. Npg Asia Mater. 2017, 9, e403. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, S.; Omasta, T.J.; Roller, J.M.; Mustain, W.E. Activity and durability of Pt-Ni nanocage electocatalysts in proton exchange membrane fuel cells. Appl. Catal. B-Environ. 2017, 203, 927–935. [Google Scholar] [CrossRef]
- Sievers, G.W.; Jensen, A.W.; Quinson, J.; Zana, A.; Bizzotto, F.; Oezaslan, M.; Dworzak, A.; Kirkensgaard, J.J.K.; Smitshuysen, T.E.L.; Kadkhodazadeh, S. Self-supported Pt-CoO networks combining high specific activity with high surface area for oxygen reduction. Nat. Mater. 2021, 20, 208–213. [Google Scholar] [CrossRef]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Ma, Z.; Cano, Z.P.; Yu, A.; Chen, Z.; Jiang, G.; Fu, X.; Yang, L.; Wu, T.; Bai, Z.; Lu, J. Enhancing oxygen reduction activity of Pt-based electrocatalysts: From theoretical mechanisms to practical methods. Angew. Chem. Int. Ed. 2020, 59, 18334–18348. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.; Adimi, S.; Shen, H.; Thomas, T.; Ma, R.; Attfield, J.P.; Yang, M. Zirconium nitride catalysts surpass platinum for oxygen reduction. Nat. Mater. 2020, 19, 282–286. [Google Scholar] [CrossRef]
- Petersen, A.S.; Jensen, K.D.; Wan, H.; Bagger, A.; Chorkendorff, I.; Stephens, I.E.L.; Rossmeisl, J.; Escudero-Escribano, M. Modeling anion poisoning during oxygen reduction on Pt near-surface alloys. ACS Catal. 2023, 13, 2735–2743. [Google Scholar] [CrossRef]
- Pham, T.M.; Im, K.; Kim, J. A highly stable tungsten-doped TiO2-supported platinum electrocatalyst for oxygen reduction reaction in acidic media. Appl. Surf. Sci. 2023, 611, 155740. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Z.; Chen, K.; Chen, C.; Zhu, Y.; Li, C. Platinum based high entropy alloy oxygen reduction electrocatalysts for proton exchange membrane fuel cells. Mater. Today Nano 2023, 21, 100282. [Google Scholar] [CrossRef]
- Takimoto, D.; Toma, S.; Suda, Y.; Shirokura, T.; Tokura, Y.; Fukuda, K.; Matsumoto, M.; Imai, H.; Sugimoto, W. Platinum nanosheets synthesized via topotactic reduction of single-layer platinum oxide nanosheets for electrocatalysis. Nat. Commun. 2023, 14, 19. [Google Scholar] [CrossRef]
- Oi, T.; Kikawada, Y.; Yanase, S. A density functional theory (DFT) study on reduced partition function ratios of oxygen species adsorbed on a Pt-19 cluster and oxygen isotope effects. Isot. Environ. Health Stud. 2021, 57, 641–663. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, S.; Jafari, M. Density functional investigation of oxygen reduction reaction on Pt3Pd alloy electrocatalyst. Mater. Res. Express 2020, 7, 015505. [Google Scholar] [CrossRef]
- Wella, S.A.; Hamamoto, Y.; Iskandar, F.; Suprijadi; Morikawa, Y.; Hamada, I. Atomic and molecular adsorption on single platinum atom at the graphene edge: A density functional theory study. J. Chem. Phys. 2020, 152, 104707. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Elangovan, A.; Liu, C.; Li, J.; Liu, B. Theoretical investigation of the oxygen reduction reaction over platinum catalysts supported by multi-edged vertically aligned carbon nanofiber for electrocatalyst preparation. ChemElectroChem 2022, 9, e202200811. [Google Scholar] [CrossRef]
- Nie, Y.; Wei, Z. Surface-confined Pt-based catalysts for strengthening oxygen reduction performance. Prog. Nat. Sci.-Mater. 2020, 30, 796–806. [Google Scholar] [CrossRef]
- Wang, H.; Hua, S.; Lin, R.; Liu, S.; Cai, X.; Ji, W.; Tian, J. Ionomer distribution control by self-assembled monolayers for high-power and low Pt-loaded proton exchange membrane fuel cells. J. Power Sources 2022, 542, 231793. [Google Scholar] [CrossRef]
- Zhang, G.-R.; Munoz, M.; Etzold, B.J.M. Accelerating oxygen-reduction catalysts through preventing poisoning with non-reactive species by using hydrophobic ionic liquids. Angew. Chem. Int. Ed. 2016, 55, 2257–2261. [Google Scholar] [CrossRef]
- Borbath, I.; Bakos, I.; Paszti, Z.; Szijjarto, G.P.; Tompos, A. Design of SnPt/C cathode electrocatalysts with optimized Sn/Pt surface composition for potential use in polymer electrolyte membrane fuel cells. Catal. Today 2021, 366, 20–30. [Google Scholar] [CrossRef]
- Mo, Y.; Feng, S.; Yu, T.; Chen, J.; Qian, G.; Luo, L.; Yin, S. Surface unsaturated WOx activating PtNi alloy nanowires for oxygen reduction reaction. J. Colloid Interface Sci. 2022, 607, 1928–1935. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Ishihara, K.; Joshi, P.; Shen, Z.; Miyake, M. Aromatic ring size effect of a surface modification agent on platinum nanoparticle electrocatalysts for oxygen reduction reaction. Electrocatalysis 2018, 9, 486–494. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, T.; Zhao, X.; Zhang, J.; Lu, Y.; Shen, J.; Lu, S.; Tu, Z.; Xin, H.L.; Wang, D. Combining structurally ordered intermetallics with N-doped carbon confinement for efficient and anti-poisoning electrocatalysis. Appl. Catal. B-Environ. 2020, 279, 119370. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Meng, D.; Guo, X.; Zhu, Y.; Peng, L.; Hu, J.; Ding, W. Nest-type NCM subset of Pt/C with oxygen capture character as advanced electrocatalyst for oxygen reduction reaction. J. Energy Chem. 2022, 71, 304–312. [Google Scholar] [CrossRef]
- Nie, Y.; Deng, J.; Chen, S.; Wei, Z. Promoting stability and activity of PtNi/C for oxygen reduction reaction via polyaniline-confined space annealing strategy. Int. J. Hydrogen Energy 2019, 44, 5921–5928. [Google Scholar] [CrossRef]
- Yamazaki, S.-I.; Asahi, M.; Taguchi, N.; Ioroi, T.; Kishimoto, Y.; Daimon, H.; Inaba, M.; Koga, K.; Kurose, Y.; Inoue, H. Creation of a highly active Pt/Pd/C core-shell-structured catalyst by synergistic combination of intrinsically high activity and surface decoration with melamine or tetra-(tert-butyl)-tetraazaporphyrin. ACS Catal. 2020, 10, 14567–14580. [Google Scholar] [CrossRef]
- Daimon, H.; Yamazaki, S.-I.; Asahi, M.; Ioroi, T.; Inaba, M. A strategy for drastic improvement in the durability of Pt/C and PtCo/C alloy catalysts for the oxygen reduction reaction by melamine surface modification. ACS Catal. 2022, 12, 8976–8985. [Google Scholar] [CrossRef]
- Yamazaki, S.-I.; Asahi, M.; Ioroi, T. Promotion of oxygen reduction on a porphyrazine-modified Pt catalyst surface. Electrochim. Acta 2019, 297, 725–734. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Miyake, M. Metal-support interactions of platinum nanoparticles modified with pyrene-functionalized alkylamine and improved electrocatalytic activity of oxygen reduction reaction. Chem. Lett. 2017, 46, 707–710. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Miyake, M. Platinum nanoparticles modified with perfluorinated alkylamines as a model cathode catalyst for fuel cells. Electroanalysis 2017, 29, 898–906. [Google Scholar] [CrossRef]
- Liu, W.; Di, S.; Wang, F.; Zhu, H. Ionic liquid modified fct-PtCo/C@ILs as high activity and durability electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 6312–6322. [Google Scholar] [CrossRef]
- George, M.; Zhang, G.-R.; Schmitt, N.; Brunnengraeber, K.; Sandbeck, D.J.S.; Mayrhofer, K.J.J.; Cherevko, S.; Etzold, B.J.M. Effect of ionic liquid modification on the ORR performance and degradation mechanism of trimetallic PtNiMo/C catalysts. ACS Catal. 2019, 9, 8682–8692. [Google Scholar] [CrossRef]
- Avid, A.; Ochoa, J.L.; Huang, Y.; Liu, Y.; Atanassov, P.; Zenyuk, I.V. Revealing the role of ionic liquids in promoting fuel cell catalysts reactivity and durability. Nat. Commun. 2022, 13, 6349. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Iguchi, Y.; Li, T.; Kato, Y.; Zhuang, Y.; Higashi, K.; Uruga, T.; Saida, T.; Miyabayashi, K.; Yagi, I. Structural transformation of Pt-Ni nanowires as oxygen reduction electrocatalysts to branched nanostructures during potential cycles. ACS Catal. 2022, 12, 259–264. [Google Scholar] [CrossRef]
- Labata, M.F.; Li, G.; Ocon, J.; Chuang, P.-Y.A. Insights on platinum-carbon catalyst degradation mechanism for oxygen reduction reaction in acidic and alkaline media. J. Power Sources 2021, 487, 229356. [Google Scholar] [CrossRef]
- Park, K.; Ohnishi, T.; Goto, M.; So, M.; Takenaka, S.; Tsuge, Y.; Inoue, G. Improvement of cell performance in catalyst layers with silica-coated Pt/carbon catalysts for polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2020, 45, 1867–1877. [Google Scholar] [CrossRef]
- Aoki, N.; Inoue, H.; Kawasaki, H.; Daimon, H.; Doi, T.; Inaba, M. Durability improvement of Pd core-Pt shell structured catalyst by porous SiO2 coating. J. Electrochem. Soc. 2018, 165, F737–F747. [Google Scholar] [CrossRef]
- Takenaka, S.; Goto, M.; Masuda, Y.; Emura, S.; Kishida, M. Improvement in the durability of carbon black-supported Pt cathode catalysts by silica-coating for use in PEFCs. Int. J. Hydrogen Energy 2018, 43, 7473–7482. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kwon, S.G.; Shin, D.Y.; Seo, P.; Yoo, J.M.; Shin, H.; Chung, Y.-H.; Kim, H.; et al. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485. [Google Scholar] [CrossRef]
- Yan, Z.; Cui, J.; Zhang, Z.; Jiang, Z.; Wei, W.; Zhu, G.; Zhao, X. Electrochemically corroded thick microporous N-C layer encapsulated Pt/C as highly active and ultra-highly stable electrocatalyst for methanol oxidation and oxygen reduction reaction in acidic media. Appl. Surf. Sci. 2023, 609, 155148. [Google Scholar] [CrossRef]
- Gawas, R.; Snyder, J.; Tang, M. Modifying interface solvation and oxygen reduction electrocatalysis with hydrophobic species. J. Phys. Chem. C 2022, 126, 14509–14517. [Google Scholar] [CrossRef]
- Casalongue, H.S.; Kaya, S.; Viswanathan, V.; Miller, D.J.; Friebel, D.; Hansen, H.A.; Norskov, J.K.; Nilsson, A.; Ogasawara, H. Direct observation of the oxygenated species during oxygen reduction on a platinum fuel cell cathode. Nat. Commun. 2013, 4, 2817. [Google Scholar] [CrossRef]
- Harn, Y.-W.; Liang, S.; Liu, S.; Yan, Y.; Wang, Z.; Jiang, J.; Zhang, J.; Li, Q.; He, Y.; Li, Z.; et al. Tailoring electrocatalytic activity of in situ crafted perovskite oxide nanocrystals via size and dopant control. Proc. Natl. Acad. Sci. USA 2021, 118, e2014086118. [Google Scholar] [CrossRef]
- Kumeda, T.; Tajiri, H.; Sakata, O.; Hoshi, N.; Nakamura, M. Effect of hydrophobic cations on the oxygen reduction reaction on single-crystal platinum electrodes. Nat. Commun. 2018, 9, 4378. [Google Scholar] [CrossRef]
- Qiao, M.; Ferrero, G.A.; Velasco, L.F.; Hor, W.V.; Yang, Y.; Luo, H.; Lodewyckx, P.; Fuertes, A.B.; Sevilla, M.; Titirici, M.-M. Boosting the oxygen reduction electrocatalytic performance of nonprecious metal nanocarbons via triple boundary engineering using protic ionic liquids. ACS Appl. Mater. Interfaces 2019, 11, 11298–11305. [Google Scholar] [CrossRef]
- Xing, Q.; Yan, W.; Wei, C.; Xiao, Z.; Jin, Z.; Liu, X.; Ren, J.; Chen, Y.; Li, X. Controlled preparation and anti-sulfate electrocatalysis of self-assembled multidimensional PtZn quasi-cubic nanodendrites. Adv. Mater. Interfaces 2022, 9, 2101944. [Google Scholar] [CrossRef]
- Safo, I.A.; Oezaslan, M. Electrochemical cleaning of polyvinylpyrrolidone-capped Pt nanocubes for the oxygen reduction reaction. Electrochim. Acta 2017, 241, 544–552. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, L.; Zhang, J.; Xiang, D.; Li, P.; Yuan, X.; Zhu, M. Co-doped Pt nanowire networks with clean surfaces for enhanced oxygen reduction reactions. Chem.-Asian J. 2020, 15, 1736–1742. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Nishihara, H.; Miyake, M. Platinum nanoparticles modified with alkylamine derivatives as an active and stable catalyst for oxygen reduction reaction. Langmuir 2014, 30, 2936–2942. [Google Scholar] [CrossRef]
- Zorko, M.; Martins, P.F.B.D.; Connell, J.G.; Lopes, P.P.; Markovic, N.M.; Stamenkovic, V.R.; Strmcnik, D. Improved rate for the oxygen reduction reaction in a sulfuric acid electrolyte using a Pt(111) surface modified with melamine. ACS Appl. Mater. Interface 2021, 13, 3369–3376. [Google Scholar] [CrossRef]
- Chung, Y.-H.; Chung, D.Y.; Jung, N.; Sung, Y.-E. Tailoring the electronic structure of nanoelectrocatalysts induced by a surface-capping organic molecule for the oxygen reduction reaction. J. Phys. Chem. Lett. 2013, 4, 1304–1309. [Google Scholar] [CrossRef]
- Asahi, M.; Yamazaki, S.-I.; Taguchi, N.; Ioroi, T. Facile approach to enhance oxygen reduction activity by modification of platinum nanoparticles by melamine-formaldehyde polymer. J. Electrochem. Soc. 2019, 166, F498–F505. [Google Scholar] [CrossRef]
- Yamazaki, S.-I.; Asahi, M.; Taguchi, N.; Ioroi, T. Electrochemical analysis of the porphyrazine-induced enhancement of ORR activity of Pt catalysts for the development of porphyrazine-adsorbed Pt catalysts. J. Electroanal. Chem. 2019, 848, 113321. [Google Scholar] [CrossRef]
- Wada, N.; Nakamura, M.; Hoshi, N. Structural effects on the oxygen reduction reaction on Pt single-crystal electrodes modified with melamine. Electrocatalysis 2020, 11, 275–281. [Google Scholar] [CrossRef]
- Suzuki, A.; Nakamura, M.; Hoshi, N. Effects of surface structures and hydrophobic species on the oxygen reduction reaction activity of Pt3Fe single-crystal electrodes. Electrocatalysis 2022, 13, 175–181. [Google Scholar] [CrossRef]
- Yamazaki, S.-I.; Asahi, M.; Taguchi, N.; Ioroi, T. Sulphonated melamine polymer for enhancing the oxygen reduction reaction activity and stability of a Pt catalyst. J. Electroanal. Chem. 2022, 908, 116103. [Google Scholar] [CrossRef]
- Hoshi, N.; Saikawa, K.; Nakamura, M. Structural effects on water molecules on the low index planes of Pt modified with alkyl amines and the correlation with the activity of the oxygen reduction reaction. Electrochem. Commun. 2019, 106, 5–8. [Google Scholar] [CrossRef]
- Shokhen, V.; Strandberg, L.; Wickman, B.; Skoglundh, M. Impact of accelerated stress tests on the cathodic catalytic layer in a proton exchange membrane (PEM) fuel cell studied by identical location scanning electron microscopy. ACS Appl. Energy Mater. 2022, 5, 11200–11212. [Google Scholar] [CrossRef]
- Nagai, T.; Yamazaki, S.-I.; Asahi, M.; Siroma, Z.; Fujiwara, N.; Ioroi, T. Metalloporphyrin-modified perovskite-type oxide for the electroreduction of oxygen. J. Power Sources 2015, 293, 760–766. [Google Scholar] [CrossRef]
- Ueno, T.; Tanaka, H.; Sugawara, S.; Shinohara, K.; Ohma, A.; Hoshi, N.; Nakamura, M. Infrared spectroscopy of adsorbed OH on n(111)-(100) and n(111)-(111) series of Pt electrode. J. Electroanal. Chem. 2017, 800, 162–166. [Google Scholar] [CrossRef]
- Chen, J.; Qian, G.; Chu, B.; Jiang, Z.; Tan, K.; Luo, L.; Li, B.; Yin, S. Tuning d-band center of Pt by PtCo-PtSn heterostructure for enhanced oxygen reduction reaction performance. Small 2022, 18, 2106773. [Google Scholar] [CrossRef]
- Sun, C.; Qiu, L.; Ke, S.; Zhang, J.; Xu, G.; Dou, M. Improved chloride tolerance of PtCo/C with a Pt-skin structure toward the oxygen reduction reaction due to a weakened Pt-Cl interaction. ACS Appl. Energy Mater. 2022, 5, 7503–7514. [Google Scholar] [CrossRef]
- Saikawa, K.; Nakamura, M.; Hoshi, N. Structural effects on the enhancement of ORR activity on Pt single-crystal electrodes modified with alkylamines. Electrochem. Commun. 2018, 87, 5–8. [Google Scholar] [CrossRef]
- Okuda, K.; Alaydrus, M.; Hoshi, N.; Hamada, I.; Nakamura, M. Electrical double layer on the Pt(111) electrode modeled under ultrahigh vacuum conditions. J. Phys. Chem. C 2022, 126, 4726–4732. [Google Scholar] [CrossRef]
- Zenyuk, I.V.; Litster, S. Spatially resolved modeling of electric double layers and surface chemistry for the hydrogen oxidation reaction in water-filled platinum-carbon electrodes. J. Phys. Chem. C 2012, 116, 9862–9875. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kudo, K.; Kodama, K.; Kitano, N.; Suzuki, T.; Minami, S.; Shinozaki, K.; Hasegawa, N.; Shinohara, A. The role of oxygen-permeable ionomer for polymer electrolyte fuel cells. Nat. Commun. 2021, 12, 4956. [Google Scholar] [CrossRef]
- Wippermann, K.; Korte, C. Effects of protic ionic liquids on the oxygen reduction reaction-a key issue in the development of intermediate-temperature polymer-electrolyte fuel cells. Curr. Opin. Electrochem. 2022, 32, 100894. [Google Scholar] [CrossRef]
- Kumeda, T.; Hoshi, N.; Nakamura, M. Effect of hydrophobic cations on the inhibitors for the oxygen reduction reaction on anions and ionomers adsorbed on single-crystal Pt electrodes. ACS Appl. Mater. Interface 2021, 13, 15866–15871. [Google Scholar] [CrossRef]
- Mcneary, W.W.; Linico, A.E.; Weimer, A.W. Water management implications for ALD-modified polymer electrolyte membrane fuel cell catalysts. J. Nanoparticle Res. 2020, 22, 185. [Google Scholar] [CrossRef]
- Li, Y.; Malkani, A.; Gawas, R.; Intikhab, S.; Xu, B.; Tang, M.; Snyder, J. Interfacial water manipulation with ionic liquids for the oxygen reduction reaction. ACS Catal. 2023, 13, 382–391. [Google Scholar] [CrossRef]
- Woitassek, D.; Lerch, S.; Jiang, W.; Shviro, M.; Roitsch, S.; Strassner, T.; Janiak, C. The facile deposition of Pt nanoparticles on reduced graphite oxide in tunable aryl alkyl ionic liquids for ORR catalysts. Molecules 2022, 27, 1018. [Google Scholar] [CrossRef]
- Zhang, G.-R.; Wolker, T.; Sandbeck, D.J.S.; Munoz, M.; Mayrhofer, K.J.J.; Cherevko, S.; Etzold, B.J.M. Tuning the electrocatalytic performance of ionic liquid modified Pt catalysts for the oxygen reduction reaction via cationic chain engineering. ACS Catal. 2018, 8, 8244–8254. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Anderson, A.B. Electronic structure calculations of liquid-solid interfaces: Combination of density functional theory and modified Poisson-Boltzmann theory. Phys. Rev. B 2008, 77, 245417. [Google Scholar] [CrossRef]
- Li, Y.; Intikhab, S.; Malkani, A.; Xu, B.; Snyder, J. Ionic liquid additives for the mitigation of nafion specific adsorption on platinum. ACS Catal. 2020, 10, 7691–7698. [Google Scholar] [CrossRef]
- Li, Y.; Hart, J.; Profit, L.; Intikhab, S.; Chatterjee, S.; Taheri, M.; Snyder, J. Sequential capacitive deposition of ionic liquids for conformal thin film coatings on oxygen reduction reaction electrocatalysts. ACS Catal. 2019, 9, 9311–9316. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active sites and mechanism of oxygen reduction reaction electrocatalysis on nitrogen-doped carbon materials. Adv. Mater. Interfaces 2019, 31, 1804297. [Google Scholar] [CrossRef] [PubMed]
- Noffke, B.W.; Li, Q.; Raghavachari, K.; Li, L.-S. A model for the pH-dependent selectivity of the oxygen reduction reaction electrocatalyzed by N-doped graphitic carbon. J. Am. Chem. Soc. 2016, 138, 13923–13929. [Google Scholar] [CrossRef]
- Sasaki, T.; Izumi, R.; Tsuda, T.; Kuwabata, S. Innovative approach for preparing a CNT-supported Pt nanoparticle functional electrocatalyst using protic ionic liquids. ACS Appl. Energy Mater. 2021, 4, 7298–7308. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, J.; Xia, B.; Li, Y.; Du, S. Ionic liquid modified Pt/C electrocatalysts for cathode application in proton exchange membrane fuel cells. Front. Chem. Sci. Eng. 2019, 13, 695–701. [Google Scholar] [CrossRef]
- Huang, K.; Song, T.; Morales-Collazo, O.; Jia, H.; Brennecke, J.F. Enhancing Pt/C catalysts for the oxygen reduction reaction with protic ionic liquids: The effect of anion structure. J. Electrochem. Soc. 2017, 164, F1448–F1459. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, F.; Zhang, H.; Tang, H.; Pan, M.; Fang, P. Improving oxygen reduction performance by using protic poly(Ionic liquid) as proton conductors. ACS Appl. Mater. Interface 2019, 11, 6111–6117. [Google Scholar] [CrossRef]
- Luo, F.; Zhang, Q.; Yang, Z.; Guo, L.; Yu, X.; Qu, K.; Ling, Y.; Yang, J.; Cai, W. Fabrication of stable and well-connected proton path in catalyst layer for high temperature polymer electrolyte fuel cells. ChemCatChem 2018, 10, 5314–5322. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Gu, W.; Ziegelbauer, J.M.; Kumaraguru, S. Carbon support microstructure impact on high current density transport resistances in PEMFC cathode. J. Electrochem. Soc. 2020, 167, 64515. [Google Scholar] [CrossRef]
- Soboleva, T.; Zhao, X.; Mallek, K.; Xie, Z.; Navessin, T.; Holdcroft, S. On the micro-, meso- and macroporous structures of polymer electrolyte membrane fuel cell catalyst layers. ACS Appl. Mater. Interface 2010, 2, 375–384. [Google Scholar] [CrossRef]
- Thompson, E.L.; Baker, D.R. Proton conduction on ionomer-free Pt surfaces. ECS Trans. 2011, 41, 709–720. [Google Scholar] [CrossRef]
- Avid, A.; Zenyuk, I.V. Confinement effects for nano-electrocatalysts for oxygen reduction reaction. Curr. Opin. Electrochem. 2021, 25, 100634. [Google Scholar] [CrossRef]
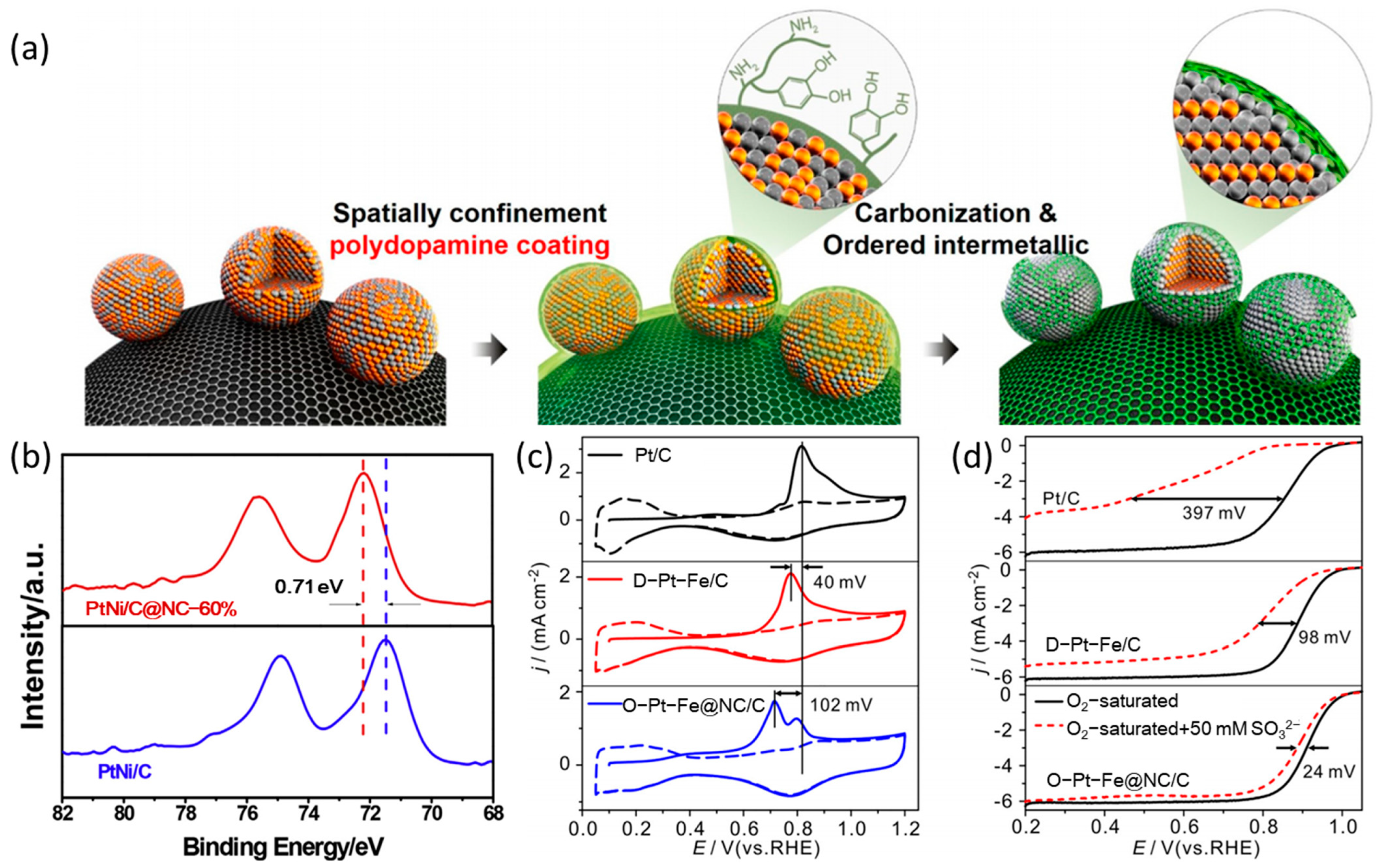

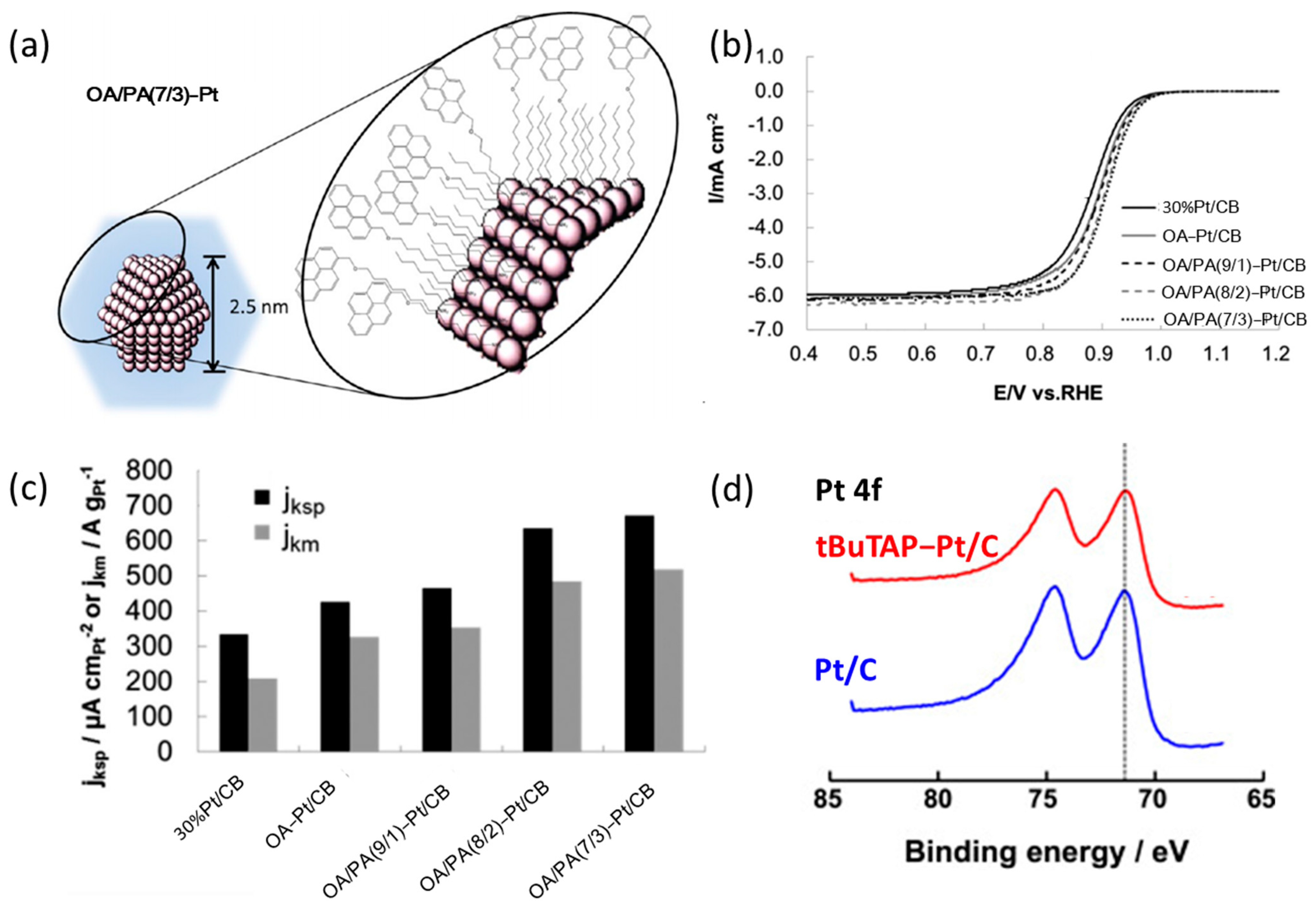
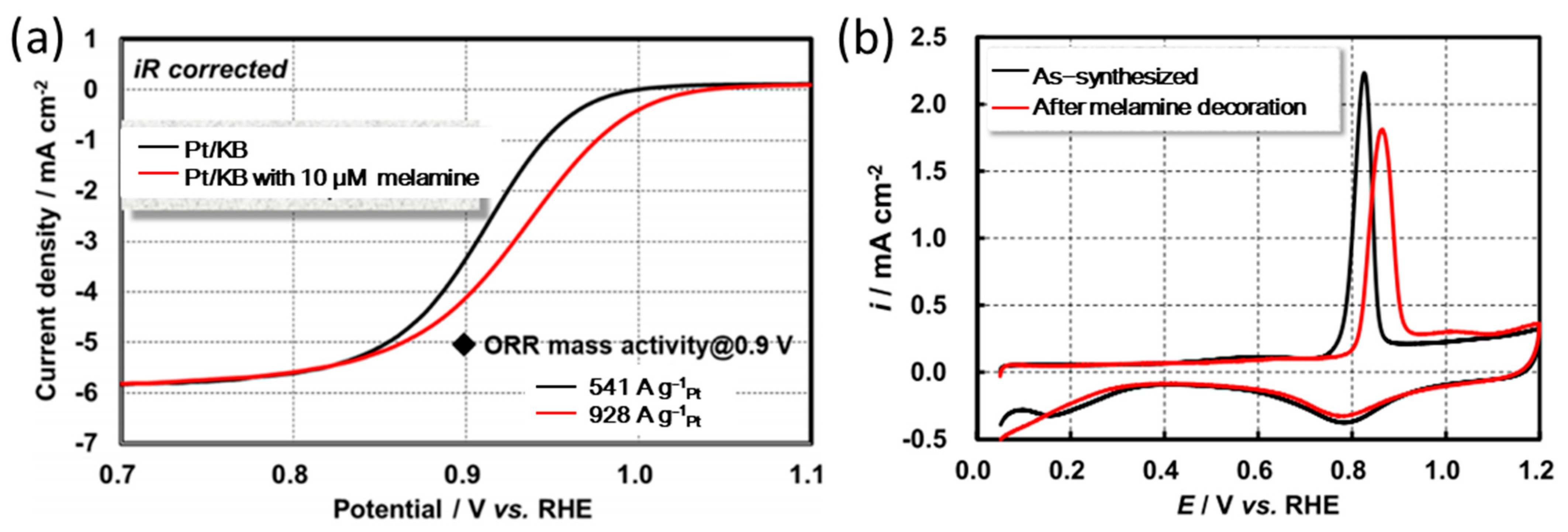
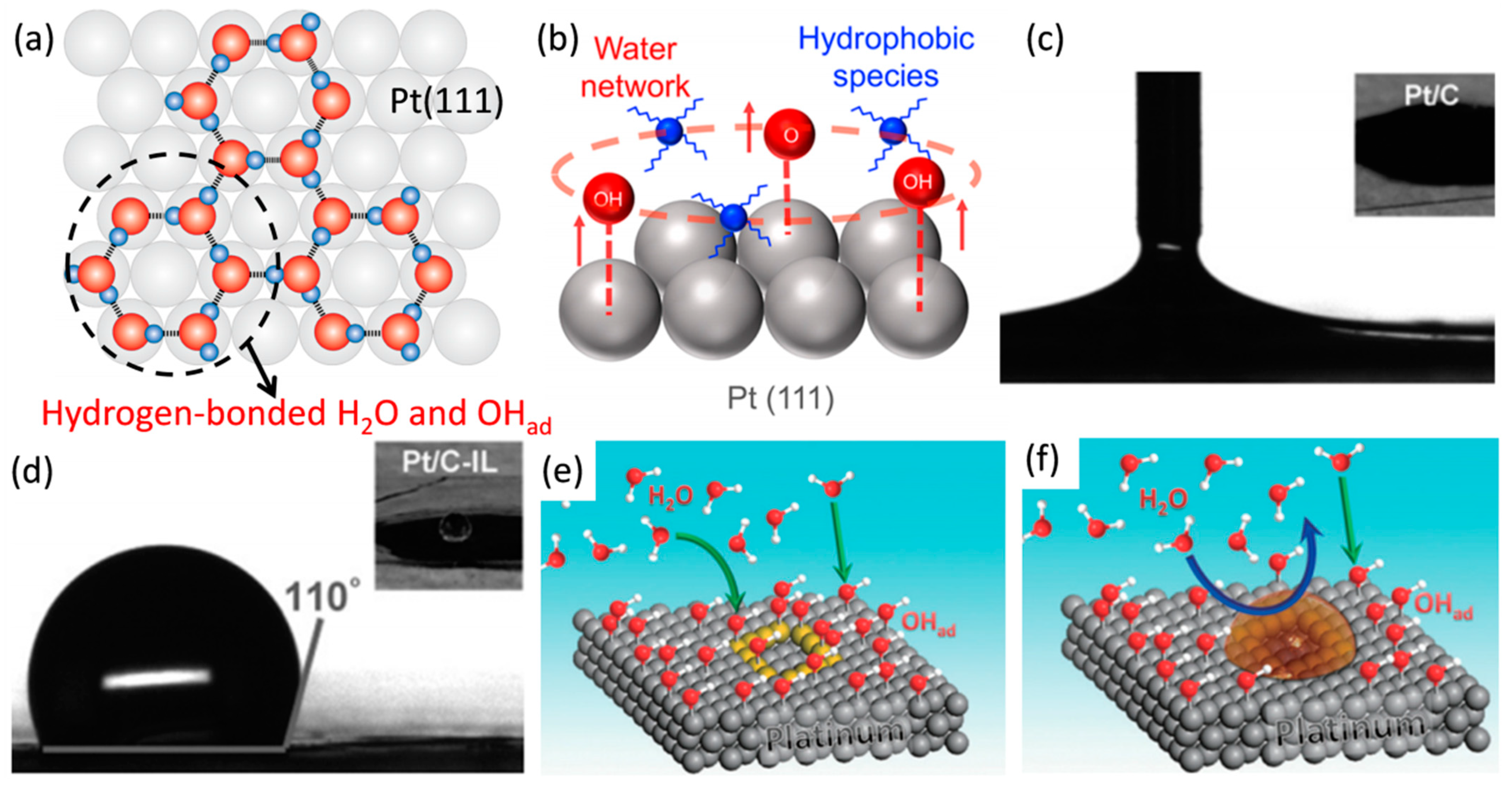
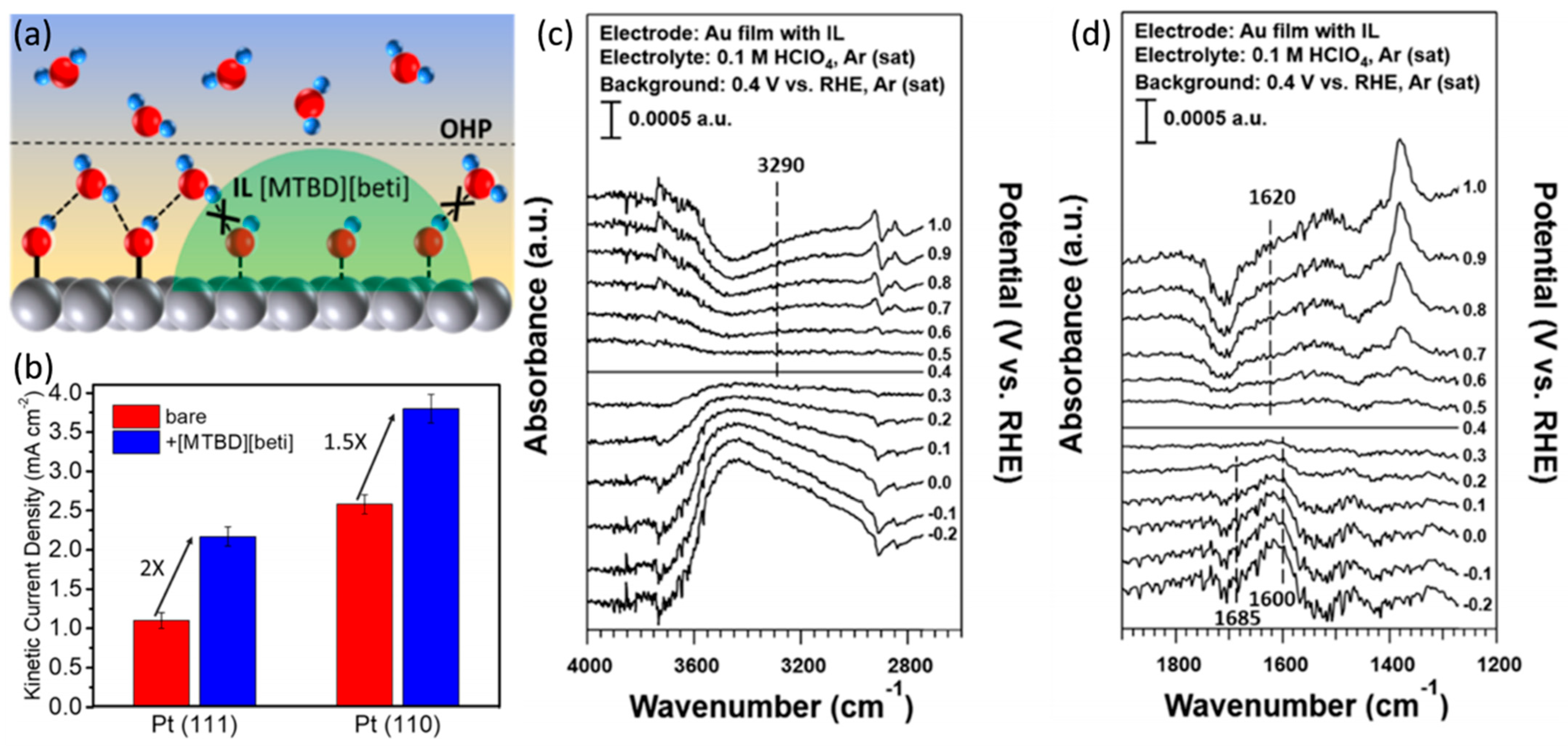
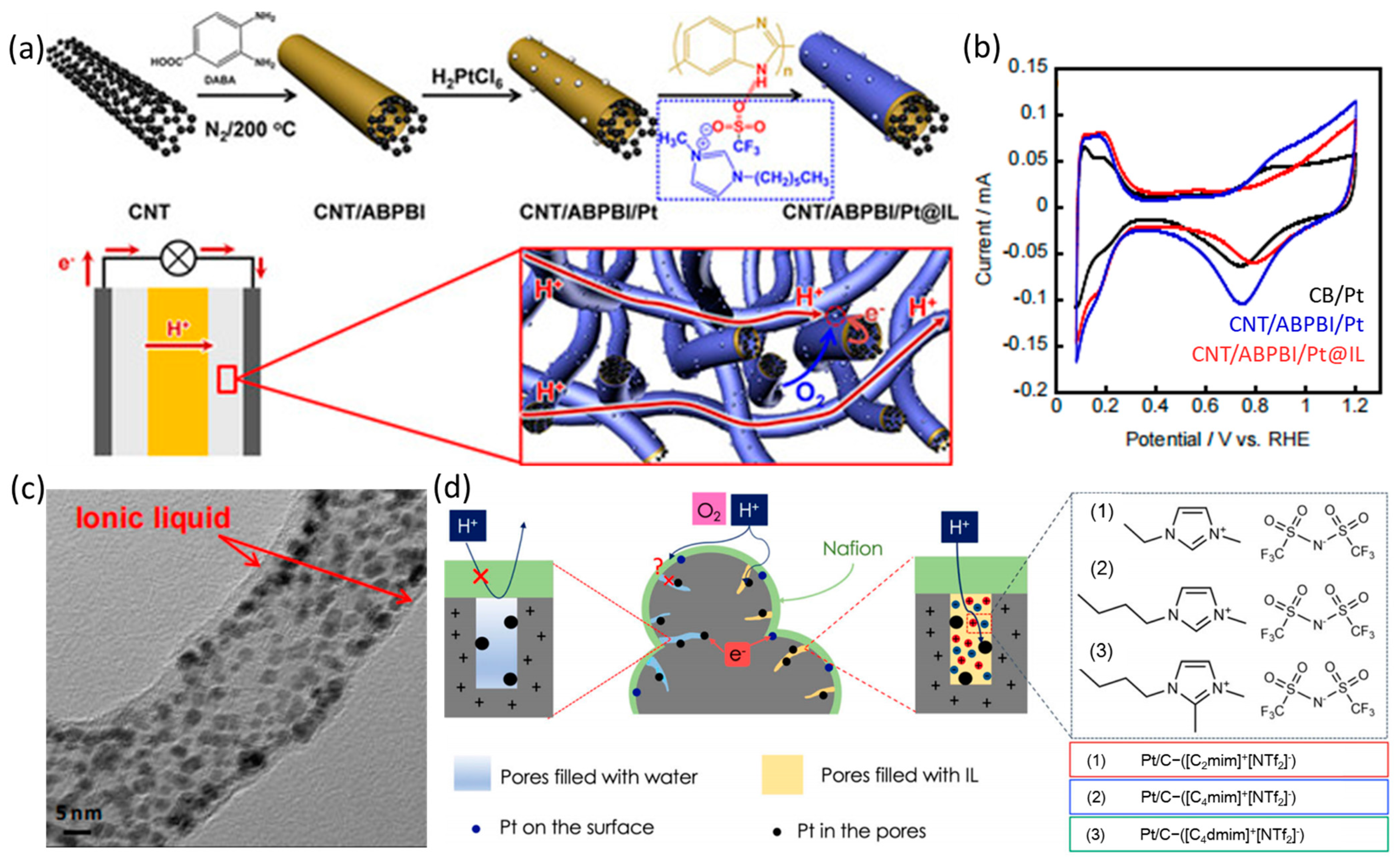
| Catalysts | Surface Modifiers | Electrolyte | MA (mA mgPt−1 @ 0.9 V vs. RHE) before Surface Modification | MA (mA mgPt−1 @ 0.9 V vs. RHE) after Surface Modification | Reference |
|---|---|---|---|---|---|
| O-Pt-Fe@NC/C | N-doped carbon layer | 0.1 M HClO4 | 260 | 530 | [31] |
| NCM-Pt/C | N-doped carbon matrix | 0.1 M HClO4 | 200 | 360 | [32] |
| PtNi/C@NC | N-doped carbon layer | 0.1 M HClO4 | 522 | 912 | [33] |
| Pt/Pd/C | Melamine | 0.1 M HClO4 | 1460 (IR corrected) | 3625 (IR corrected) | [34] |
| Pt/KB | Melamine | 0.1 M HClO4 | 541 (IR corrected) | 928 (IR corrected) | [35] |
| PtCo/KB | Melamine | 0.1 M HClO4 | 1176 (IR corrected) | 1671 (IR corrected) | |
| Pt/C | tBuTAP | 0.1 M HClO4 | 380 (IR corrected) | 764 (IR corrected) | [36] |
| OA/PA-Pt/CB | OA/PA | 0.1 M HClO4 | 285 | 518 | [37] |
| OA-Pt/CB | OA | 0.1 M HClO4 | 285 | 299 | |
| OA/UFHA-Pt/CB | OA/UFHA | 0.1 M HClO4 | 204 | 322 | [38] |
| Pt/C-[C4C1im][NTf2] | [C4C1im][NTf2] | 0.1 M HClO4 | 330 | 1010 | [27] |
| fct-PtCo/C@ ILs | [BMIM][TFSI] | 0.1 M HClO4 | 470 | 1040 | [39] |
| PtNiMo/C | [MTBD][BETI] | 0.1 M HClO4 | 706 | 1200 | [40] |
| [BMIM][NTF2] | 0.1 M HClO4 | 706 | 1059 | ||
| Pt/C | [C4mim][NTf2] | 0.1 M HClO4 | 288 | 347 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, Y.; Li, C.; Zhao, Q.; Cong, Y. Introduction of Surface Modifiers on the Pt-Based Electrocatalysts to Promote the Oxygen Reduction Reaction Process. Nanomaterials 2023, 13, 1544. https://doi.org/10.3390/nano13091544
Wang H, Wang Y, Li C, Zhao Q, Cong Y. Introduction of Surface Modifiers on the Pt-Based Electrocatalysts to Promote the Oxygen Reduction Reaction Process. Nanomaterials. 2023; 13(9):1544. https://doi.org/10.3390/nano13091544
Chicago/Turabian StyleWang, Haibin, Yi Wang, Chunlei Li, Qiuping Zhao, and Yuanyuan Cong. 2023. "Introduction of Surface Modifiers on the Pt-Based Electrocatalysts to Promote the Oxygen Reduction Reaction Process" Nanomaterials 13, no. 9: 1544. https://doi.org/10.3390/nano13091544





