Epigallocatechin Gallate Potentiates the Anticancer Effect of AFP-siRNA-Loaded Polymeric Nanoparticles on Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Biological Reagents
2.2. Synthesis of siRNA-Loaded PLGA Nanoparticles
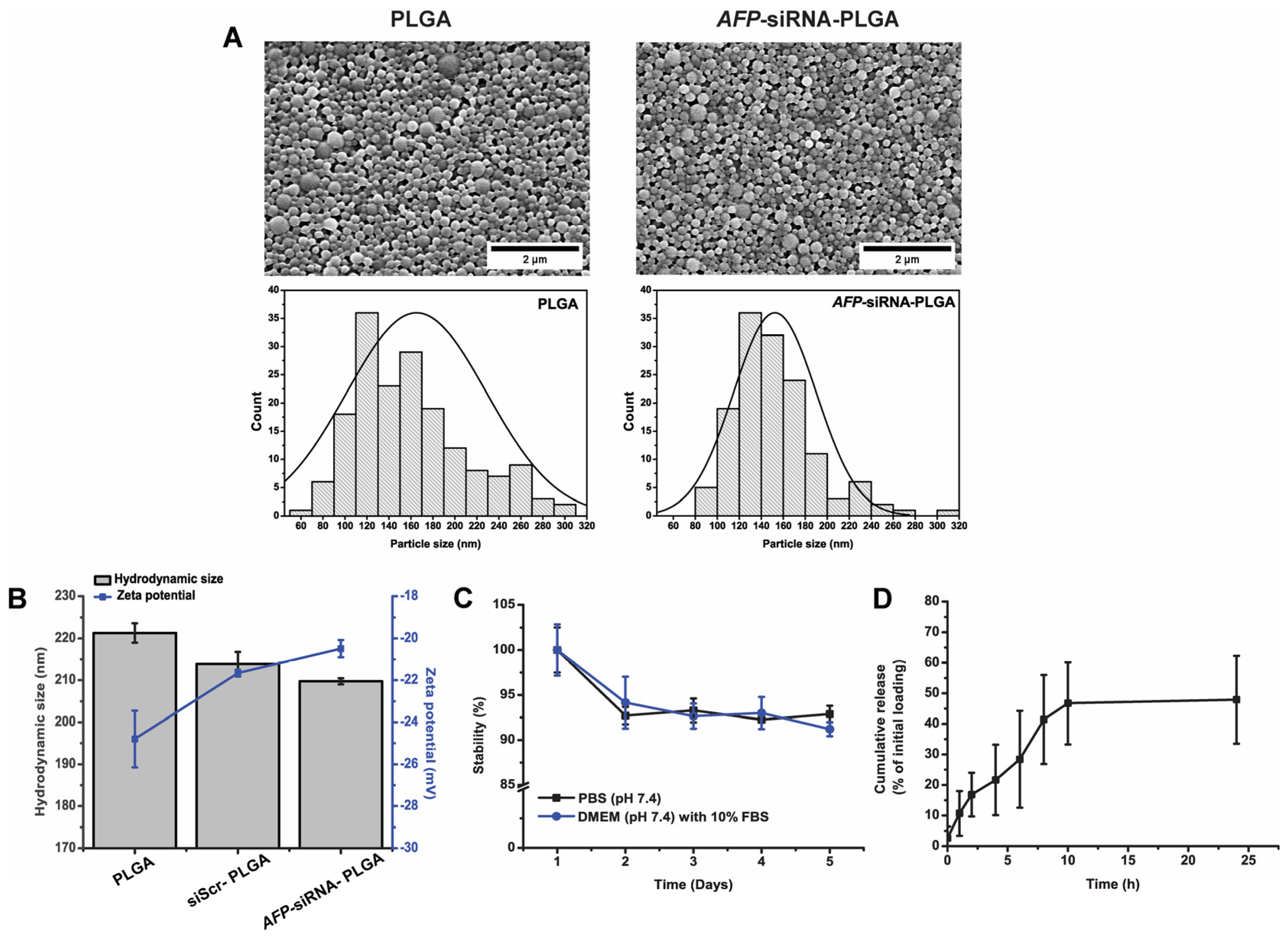
2.3. Characterization of the Synthesized Nanoparticles
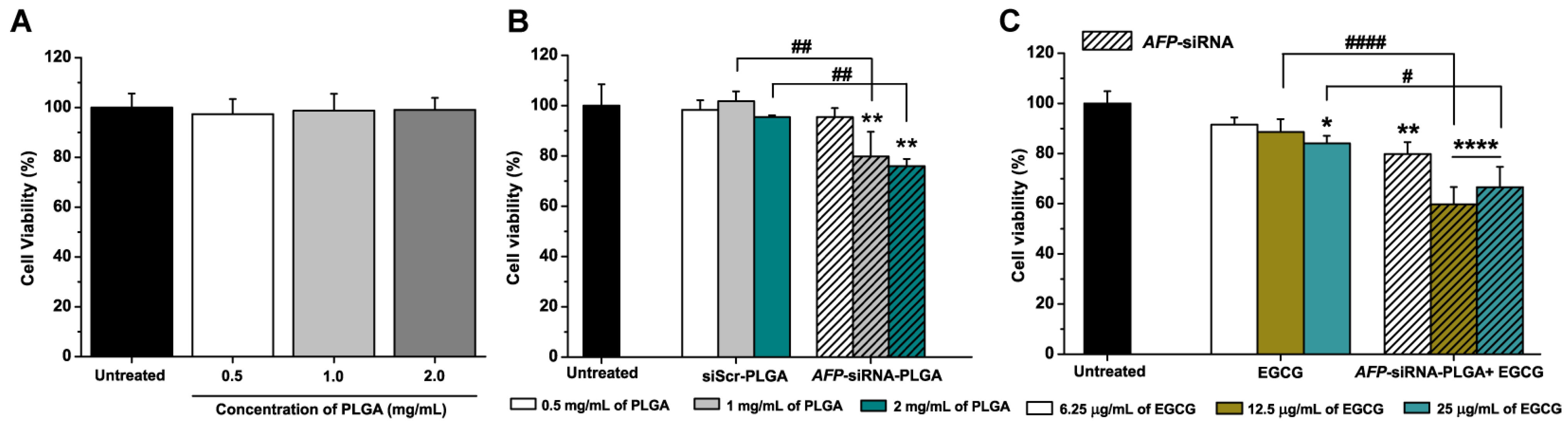
2.4. Cell Viability and Caspase-3/7 Activity
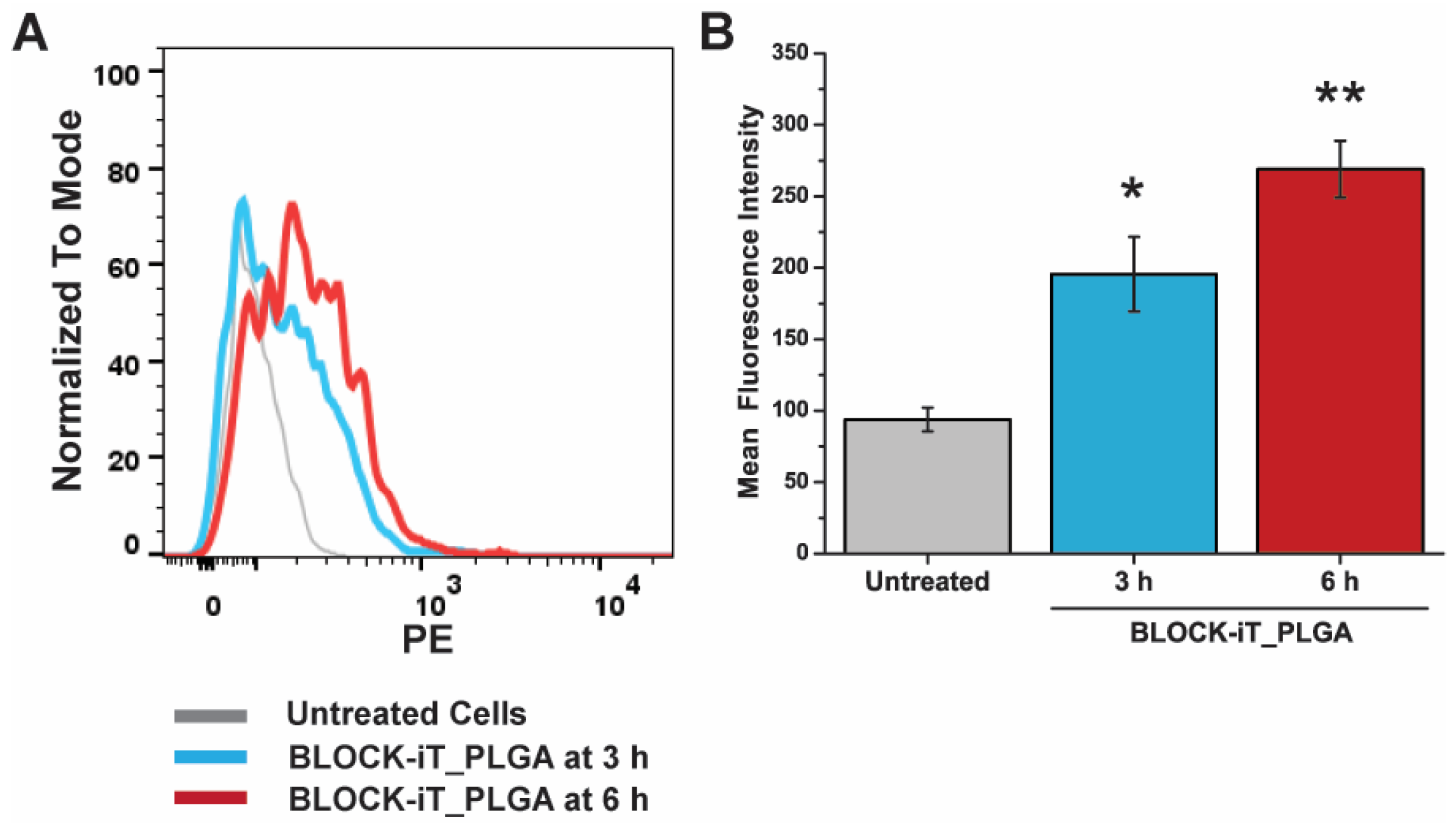
2.5. Cellular Internalization
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physical Characterization of the Synthesized Nanoparticles
3.2. Anticancer Effects of AFP-siRNA and EGCG
3.3. Anticancer Effects of AFP-siRNA-Loaded PLGA
3.4. Anticancer Effects of AFP-siRNA-Loaded PLGA Combined with EGCG
3.5. Cellular Internalization of PLGA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 2 June 2023).
- International Agency for Research on Cancer World Health Organization. Number of New Cases and Deaths from Liver Cancer Predicted to Rise by More Than 55% by 2040. Available online: https://www.iarc.who.int/news-events/number-of-new-cases-and-deaths-from-liver-cancer-predicted-to-rise-by-more-than-55-by-2040 (accessed on 16 June 2023).
- Kidshealth. Side Effects of Chemotherapy and Radiation. Available online: https://kidshealth.org/en/parents/side-effects.html (accessed on 2 June 2023).
- American Cancer Society. Chemotherapy Side Effects. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/chemotherapy-side-effects.html (accessed on 16 June 2023).
- National Cancer Institute. Radiation Therapy Side Effects. Available online: https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy/side-effects (accessed on 16 June 2023).
- Lohitesh, K.; Chowdhury, R.; Mukherjee, S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: An insight. Cancer Cell Int. 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Nakajima, T.; Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Takashima, H.; Katagishi, T.; Kimura, H.; Minami, M.; et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J. Hepatol. 2006, 44, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Tatebe, H.; Nakagawa, T.; Hara, Y.; Weinstein, I.B.; Moriwaki, H. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008, 262, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Feng, Y.; Zhang, L.; Li, J.; Xie, Y.A.; Luo, X. Epigallocatechin-3-gallate inhibits cell growth, induces apoptosis and causes S phase arrest in hepatocellular carcinoma by suppressing the AKT pathway. Int. J. Oncol. 2014, 44, 791–796. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, S.; Xu, J.; Li, W.; Duan, G.; Wang, H.; Yang, H.; Yang, Z.; Zhou, R. A new molecular mechanism underlying the EGCG-mediated autophagic modulation of AFP in HepG2 cells. Cell Death Dis. 2017, 8, e3160. [Google Scholar] [CrossRef]
- Debruyne, E.N.; Delanghe, J.R. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: New aspects and applications. Clin. Chim. Acta 2008, 395, 19–26. [Google Scholar] [CrossRef]
- Fang, Z.L.; Fang, N.; Han, X.N.; Huang, G.; Fu, X.J.; Xie, G.S.; Wang, N.R.; Xiong, J.P. Effects of AFP gene silencing on Survivin mRNA expression inhibition in HepG2 cells. Genet. Mol. Res. 2015, 14, 3184–3190. [Google Scholar] [CrossRef]
- Zhang, L.; He, T.; Cui, H.; Wang, Y.; Huang, C.; Han, F. Effects of AFP gene silencing on apoptosis and proliferation of a hepatocellular carcinoma cell line. Discov. Med. 2012, 14, 115–124. [Google Scholar]
- Schmidt, B.; Wei, L.; DePeralta, D.K.; Hoshida, Y.; Tan, P.S.; Sun, X.; Sventek, J.P.; Lanuti, M.; Tanabe, K.K.; Fuchs, B.C. Molecular subclasses of hepatocellular carcinoma predict sensitivity to fibroblast growth factor receptor inhibition. Int. J. Cancer 2016, 138, 1494–1505. [Google Scholar] [CrossRef]
- Young, S.W.; Stenzel, M.; Yang, J.L. Nanoparticle-siRNA: A potential cancer therapy? Crit. Rev. Oncol. Hematol. 2016, 98, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Assadollahi, V.; Khosravian, P.; Mirzaei, S.A.; Elahian, F. Engineered mesoporous silica nanoparticles, new insight nanoplatforms into effective cancer gene therapy. Int. J. Biol. Macromol. 2023, 253, 127060. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shen, X.T.; Li, Y.Y.; Xu, S.Q. Carbon nanotubes-based drug delivery to cancer and brain. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Sadat Tabatabaei Mirakabad, F.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Jabeen, M.; Chauhan, A.; Ansari, M.A. Therapeutic potential of functionalized siRNA nanoparticles on regression of liver cancer in experimental mice. Sci. Rep. 2019, 9, 15825. [Google Scholar] [CrossRef]
- Pho-Iam, T.; Punnakitikashem, P.; Somboonyosdech, C.; Sripinitchai, S.; Masaratana, P.; Sirivatanauksorn, V.; Sirivatanauksorn, Y.; Wongwan, C.; Nguyen, K.T.; Srisawat, C. PLGA nanoparticles containing α-fetoprotein siRNA induce apoptosis and enhance the cytotoxic effects of doxorubicin in human liver cancer cell line. Biochem. Biophys. Res. Commun. 2021, 553, 191–197. [Google Scholar] [CrossRef]
- Pantazis, P.; Dimas, K.; Wyche, J.H.; Anant, S.; Houchen, C.W.; Panyam, J.; Ramanujam, R.P. Preparation of siRNA-encapsulated PLGA nanoparticles for sustained release of siRNA and evaluation of encapsulation efficiency. Methods Mol. Biol. 2012, 906, 311–319. [Google Scholar]
- Shin, N.; Shin, H.J.; Yi, Y.; Beom, J.; Lee, W.; Lee, C.H.; Kim, D.W. p66shc siRNA-encapsulated PLGA nanoparticles ameliorate neuropathic pain following spinal nerve ligation. Polymers 2020, 12, 1014. [Google Scholar] [CrossRef]
- Zhang, D.; Kong, Y.Y.; Sun, J.H.; Huo, S.J.; Zhou, M.; Gui, Y.L.; Mu, X.; Chen, H.; Yu, S.Q.; Xu, Q. Co-delivery nanoparticles with characteristics of intracellular precision release drugs for overcoming multidrug resistance. Int. J. Nanomed. 2017, 12, 2081–2108. [Google Scholar] [CrossRef] [PubMed]
- Han, H.D.; Byeon, Y.; Kang, T.H.; Jung, I.D.; Lee, J.W.; Shin, B.C.; Lee, Y.J.; Sood, A.K.; Park, Y.M. Toll-like receptor 3-induced immune response by poly(D,L-lactide-co-glycolide) nanoparticles for dendritic cell-based cancer immunotherapy. Int. J. Nanomed. 2016, 11, 5729–5742. [Google Scholar] [CrossRef] [PubMed]
- Risnayanti, C.; Jang, Y.S.; Lee, J.; Ahn, H.J. PLGA nanoparticles co-delivering MDR1 and BCL2 siRNA for overcoming resistance of paclitaxel and cisplatin in recurrent or advanced ovarian cancer. Sci. Rep. 2018, 8, 7498. [Google Scholar] [CrossRef]
- Feczkó, T.; Tóth, J.; Dósa, G.; Gyenis, J. Influence of process conditions on the mean size of PLGA nanoparticles. Chem. Eng. Process. 2011, 50, 846–853. [Google Scholar] [CrossRef]
- Tu, F.; Lee, D. Controlling the stability and size of double-emulsion-templated poly(lactic-co-glycolic) acid microcapsules. Langmuir 2012, 28, 9944–9952. [Google Scholar] [CrossRef]
- Whiteley, Z.; Ho, H.M.K.; Gan, Y.X.; Panariello, L.; Gkogkos, G.; Gavriilidis, A.; Craig, D.Q.M. Microfluidic synthesis of protein-loaded nanogels in a coaxial flow reactor using a design of experiments approach. Nanoscale Adv. 2021, 3, 2039–2055. [Google Scholar] [CrossRef]
- Li, J.; Stayshich, R.M.; Meyer, T.Y. Exploiting sequence to control the hydrolysis behavior of biodegradable PLGA copolymers. J. Chem. Soc. 2011, 133, 6910–6913. [Google Scholar] [CrossRef]
- Rawat, S.; Gupta, P.; Kumar, A.; Garg, P.; Suri, C.R.; Sahoo, D.K. Molecular mechanism of poly(vinyl alcohol) mediated prevention of aggregation and stabilization of insulin in nanoparticles. Mol. Pharm. 2015, 12, 1018–1030. [Google Scholar] [CrossRef]
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, T.; Chen, Z.; Geng, Y.; Xie, X.; Li, S.; Yang, H.; Wu, C.; Liu, Y. Luminescent/magnetic PLGA-based hybrid nanocomposites: A smart nanocarrier system for targeted codelivery and dual-modality imaging in cancer theranostics. Int. J. Nanomed. 2017, 12, 4299–4322. [Google Scholar] [CrossRef] [PubMed]
- Jonderian, A.; Maalouf, R. Formulation and in vitro interaction of rhodamine-B loaded PLGA nanoparticles with cardiac myocytes. Front. Pharmacol. 2016, 7, 458. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Park, H.; Shin, N.; Kwon, H.H.; Yin, Y.; Hwang, J.A.; Kim, S.I.; Kim, S.R.; Kim, S.; Joo, Y.; et al. p47phox siRNA-loaded PLGA nanoparticles suppress ROS/oxidative stress-induced chondrocyte damage in osteoarthritis. Polymers 2020, 12, 443. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Liang, Y.; Si, R.; Jiang, Z.; Ma, B.; Gao, P. Knockdown of alpha-fetoprotein expression inhibits HepG2 cell growth and induces apoptosis. J. Cancer Res. Ther. 2018, 14, S634–S643. [Google Scholar] [PubMed]
- Punuch, K.; Wongwan, C.; Jantana, S.; Somboonyosdech, C.; Rodponthukwaji, K.; Kunwong, N.; Nguyen, K.T.; Sirivatanauksorn, V.; Sirivatanauksorn, Y.; Srisawat, C.; et al. Study of siRNA delivery via polymeric nanoparticles in combination with angiogenesis inhibitor for the treatment of AFP-related liver cancer. Int. J. Mol. Sci. 2022, 23, 12666. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, Y.; Feng, Y.; Zhang, L.; Huang, X.; Shen, X.; Luo, X. (-)-Epigallocatechingallate induces apoptosis in B lymphoma cells via caspase-dependent pathway and Bcl-2 family protein modulation. Int. J. Oncol. 2015, 46, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020, 154–155, 64–78. [Google Scholar] [CrossRef]
- Caputo, T.M.; Cusano, A.M.; Principe, S.; Cicatiello, P.; Celetti, G.; Aliberti, A.; Micco, A.; Ruvo, M.; Tagliamonte, M.; Ragone, C.; et al. Sorafenib-loaded PLGA carriers for enhanced drug delivery and cellular uptake in liver cancer cells. Int. J. Nanomed. 2023, 18, 4121–4142. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Zhang, Y.; Li, Q.; Zhu, Y.; Fan, C. Size-dependent cellular uptake and sustained drug release of PLGA particles. Particuology 2023, 73, 1–7. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodponthukwaji, K.; Pingrajai, P.; Jantana, S.; Taya, S.; Duangchan, K.; Nguyen, K.T.; Srisawat, C.; Punnakitikashem, P. Epigallocatechin Gallate Potentiates the Anticancer Effect of AFP-siRNA-Loaded Polymeric Nanoparticles on Hepatocellular Carcinoma Cells. Nanomaterials 2024, 14, 47. https://doi.org/10.3390/nano14010047
Rodponthukwaji K, Pingrajai P, Jantana S, Taya S, Duangchan K, Nguyen KT, Srisawat C, Punnakitikashem P. Epigallocatechin Gallate Potentiates the Anticancer Effect of AFP-siRNA-Loaded Polymeric Nanoparticles on Hepatocellular Carcinoma Cells. Nanomaterials. 2024; 14(1):47. https://doi.org/10.3390/nano14010047
Chicago/Turabian StyleRodponthukwaji, Kamonlatth, Ponpawee Pingrajai, Saranrat Jantana, Seri Taya, Kongpop Duangchan, Kytai T. Nguyen, Chatchawan Srisawat, and Primana Punnakitikashem. 2024. "Epigallocatechin Gallate Potentiates the Anticancer Effect of AFP-siRNA-Loaded Polymeric Nanoparticles on Hepatocellular Carcinoma Cells" Nanomaterials 14, no. 1: 47. https://doi.org/10.3390/nano14010047
APA StyleRodponthukwaji, K., Pingrajai, P., Jantana, S., Taya, S., Duangchan, K., Nguyen, K. T., Srisawat, C., & Punnakitikashem, P. (2024). Epigallocatechin Gallate Potentiates the Anticancer Effect of AFP-siRNA-Loaded Polymeric Nanoparticles on Hepatocellular Carcinoma Cells. Nanomaterials, 14(1), 47. https://doi.org/10.3390/nano14010047






