Terpenes-Modified Lipid Nanosystems for Temozolomide, Improving Cytotoxicity against Glioblastoma Human Cancer Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
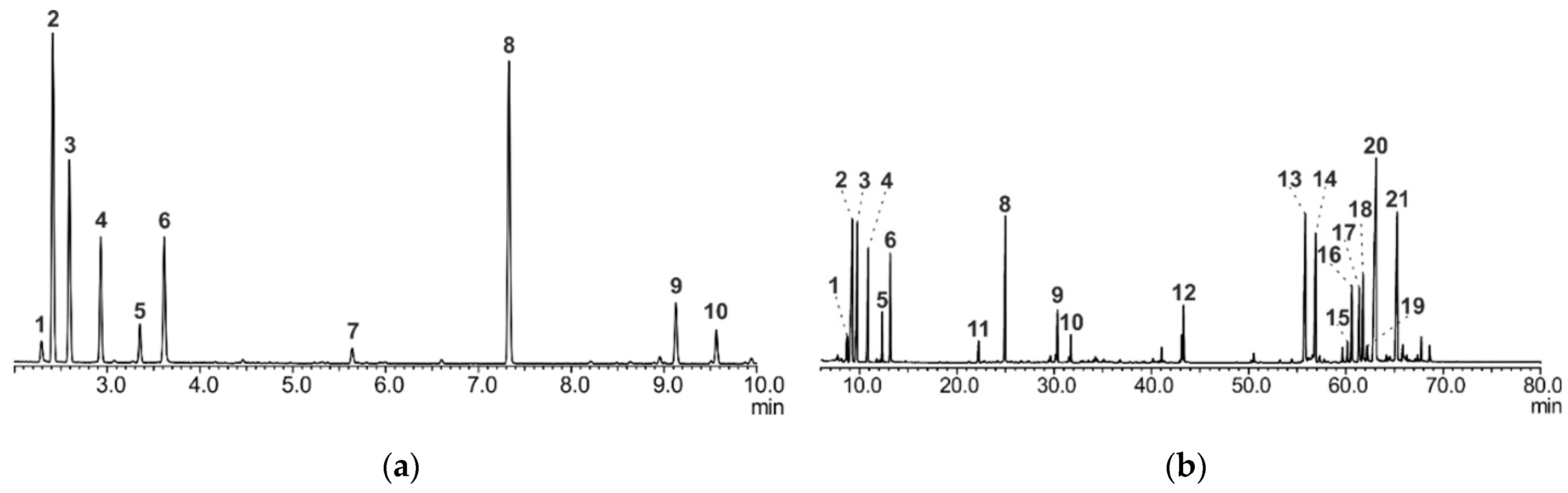
2.2. GC/MS Analysis of A. sibirica Resin
2.2.1. Method A
2.2.2. Method B
2.3. Preparation of A. sibirica Resin-, Abietic Acid- and TMZ–Loaded Lipid Nanosystems
2.4. Characterization of A. sibirica Resin-, Abietic Acid and TMZ-Loaded Lipid Nanosystems
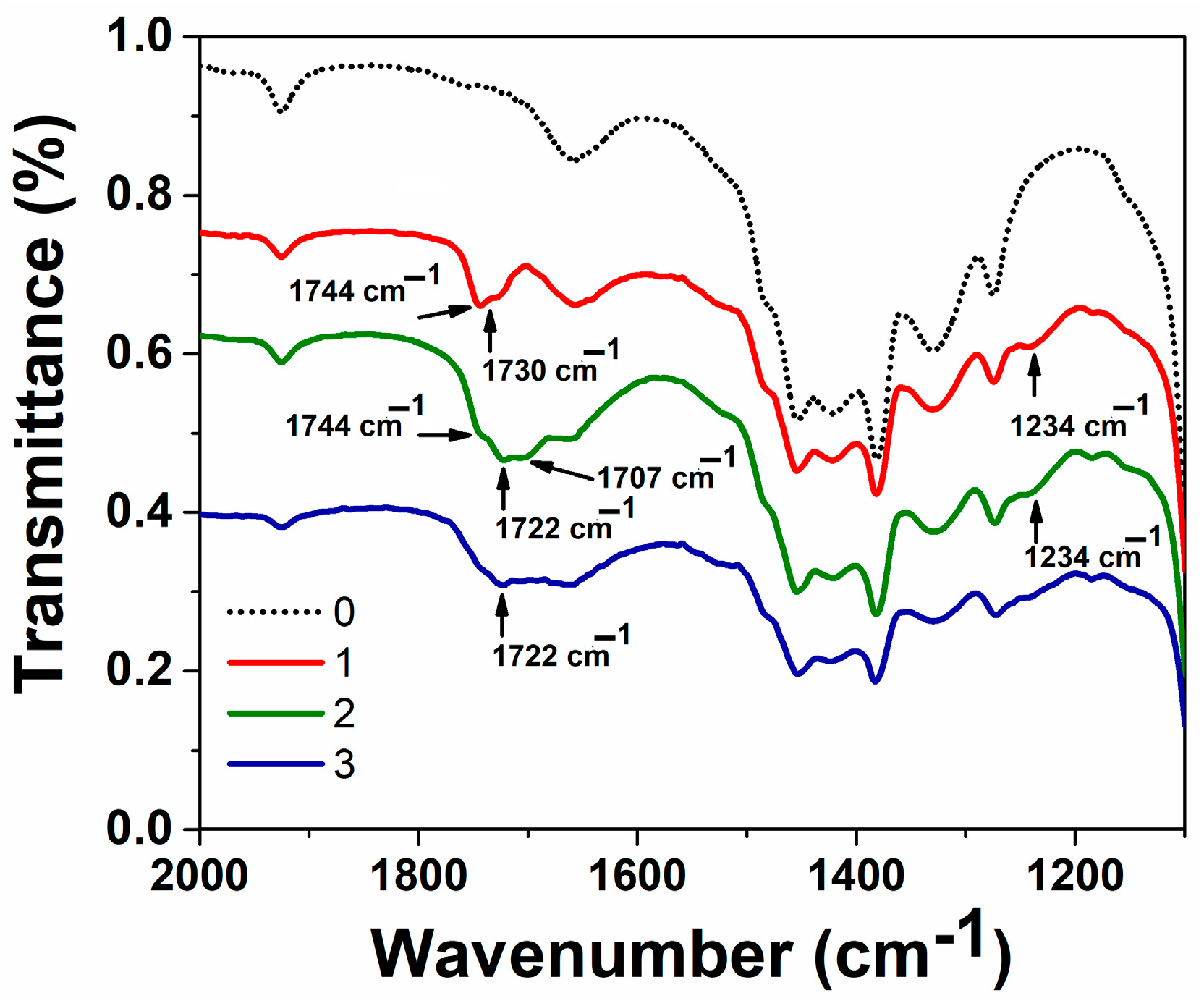
2.4.1. IR Spectroscopy
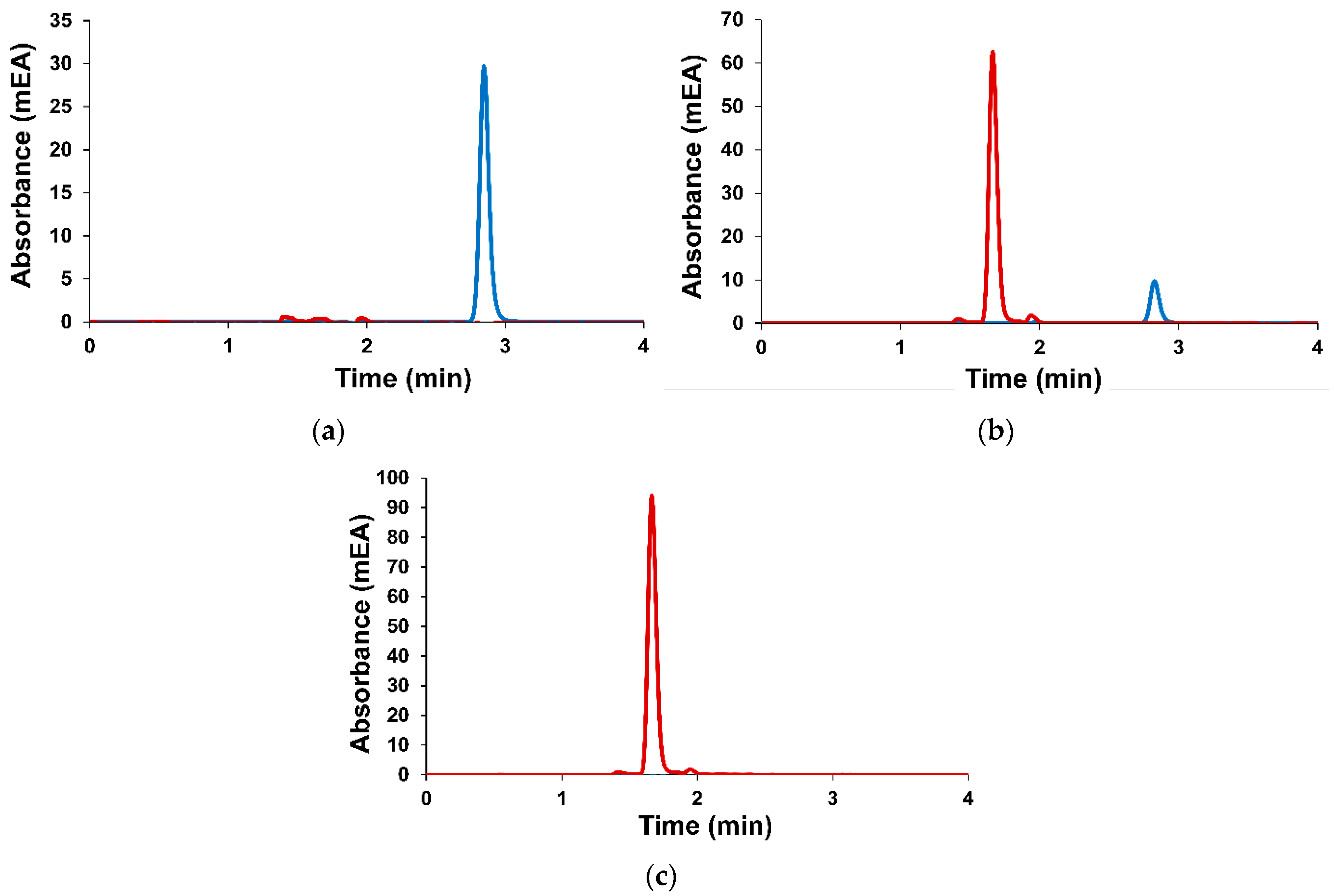
2.4.2. Encapsulation Efficiency and Loading Capacity
2.4.3. In Vitro Drug Release Profile
2.5. Cytotoxicity on Human Cell Lines
Statistical Analysis
3. Results
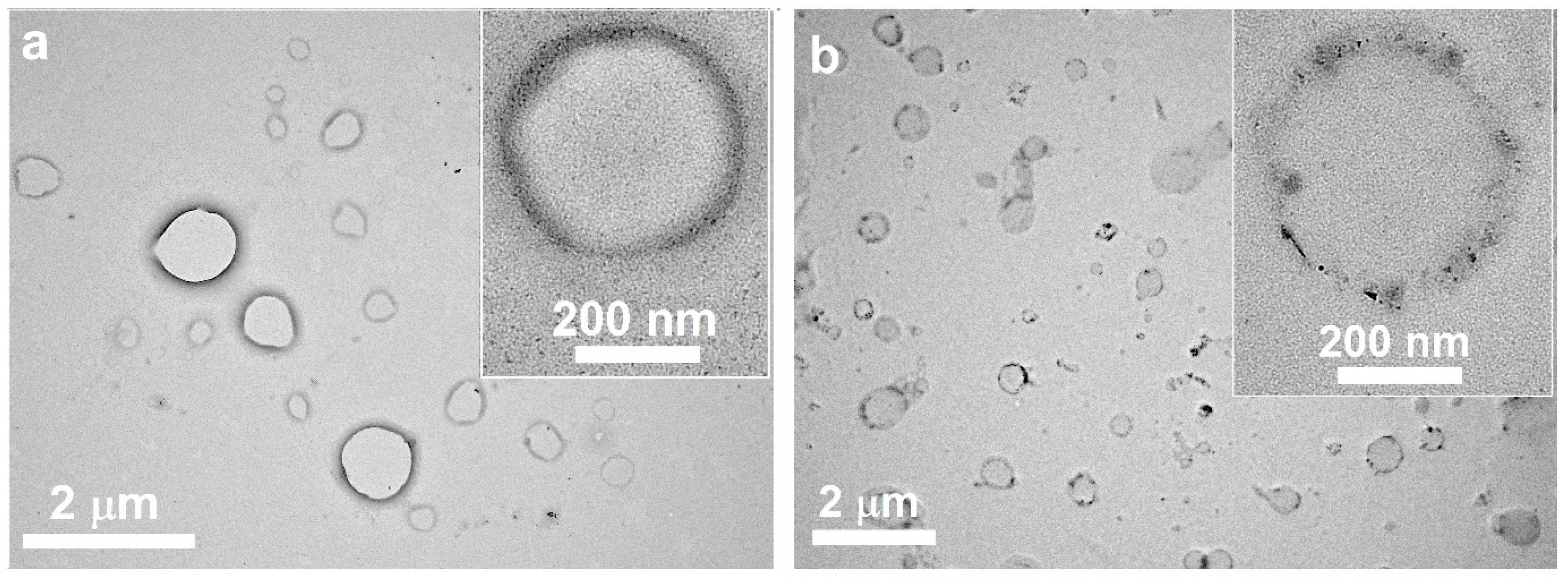
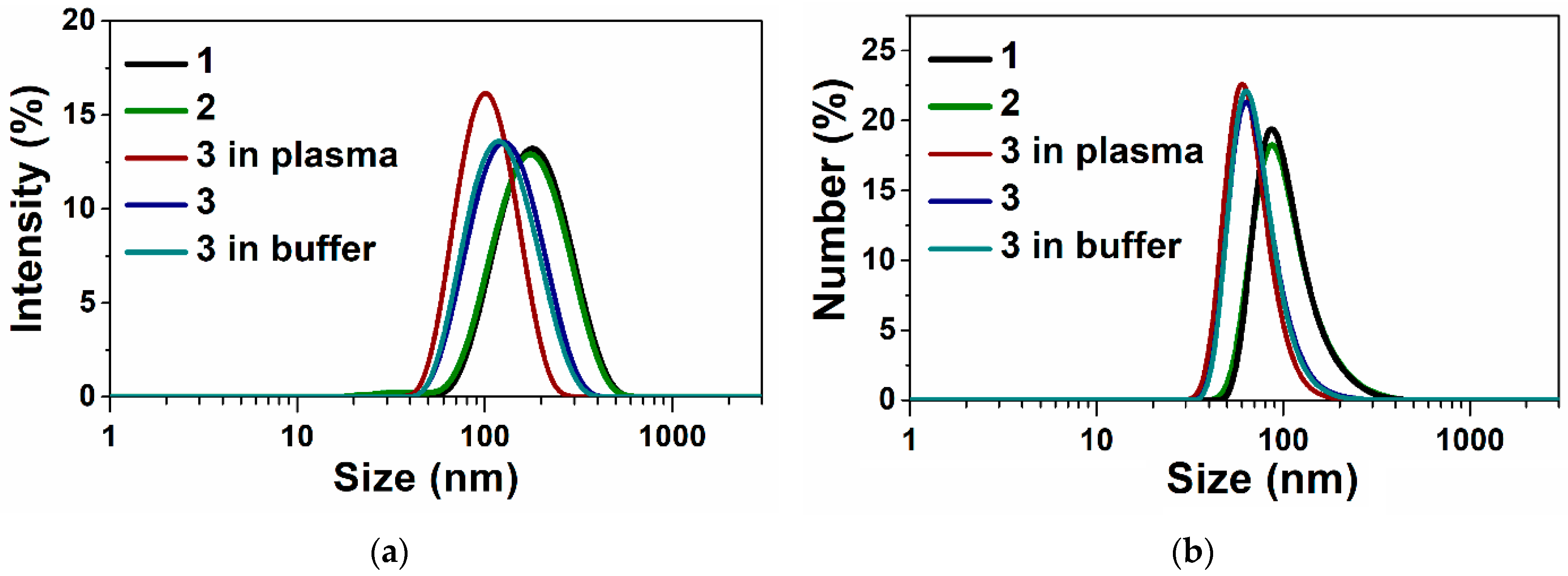
3.1. Preparation and Characteristics of Terpene-Containing and TMZ-Loaded Lipid Nanosystems
3.2. Cytotoxicity on Human Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mrugala, M.M.; Chamberlain, M.C.; Hutchinson, F. Mechanisms of disease: Temozolomide and glioblastoma—Look to the future. Nat. Clin. Pract. Oncol. 2008, 5, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, D.-Y. Present and Future of Anti-Glioblastoma Therapies: A Deep Look into Molecular Dependencies/Features. Molecules 2020, 25, 4641. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Weller, M.; Van Den Bent, M.; Sanson, M.; Weiler, M.; Von Deimling, A.; Plass, C.; Hegi, M.; Platten, M.; Reifenberger, G. MGMT testing—The challenges for biomarker-based glioma treatment. Nat. Rev. Neurol. 2014, 10, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Reardon, D.A. Neuro-oncology in 2015: Progress in glioma diagnosis, classification and treatment. Nat. Rev. Neurol. 2016, 12, 2015–2016. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Lukas, R.V.; Hegi, M.E. Improving survival in molecularly selected glioblastoma. Lancet 2019, 393, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Stevens, M.F.G.; Bradshaw, T.D. Temozolomide: Mechanisms of Action, Repair and Resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef]
- Sestito, S.; Runfola, M.; Tonelli, M.; Chiellini, G.; Rapposelli, S. New Multitarget Approaches in the War Against Glioblastoma: A Mini-Perspective. Front. Pharmacol. 2018, 9, 874. [Google Scholar] [CrossRef]
- Bahadur, S.; Sahu, A.K.; Baghel, P.; Saha, S. Current promising treatment strategy for glioblastoma multiform: A review. Oncol. Rev. 2019, 13, 114–124. [Google Scholar] [CrossRef]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide Nanoparticles for Targeted Glioblastoma Therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef]
- Afzalipour, R.; Khoei, S.; Khoee, S.; Shirvalilou, S.; Jamali Raoufi, N.; Motevalian, M.; Karimi, M.R. Dual-Targeting Temozolomide Loaded in Folate-Conjugated Magnetic Triblock Copolymer Nanoparticles to Improve the Therapeutic Efficiency of Rat Brain Gliomas. ACS Biomater. Sci. Eng. 2019, 5, 6000–6011. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Rapalli, V.K.; Singhvi, G.; Gorantla, S.; Khosa, A.; Dubey, S.K.; Saha, R.N. Design of temozolomide-loaded proliposomes and lipid crystal nanoparticles with industrial feasible approaches: Comparative assessment of drug loading, entrapment efficiency, and stability at plasma pH. J. Liposome Res. 2021, 31, 158–168. [Google Scholar] [CrossRef]
- Song, S.; Mao, G.; Du, J.; Zhu, X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016, 23, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Moses-Gardner, A.; Huang, J.; Smith, E.R.; Moses, M.A. ITGA2 as a potential nanotherapeutic target for glioblastoma. Sci. Rep. 2019, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Nano-therapies for glioblastoma treatment. Cancers 2020, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Yasaswi, P.S.; Shetty, K.; Yadav, K.S. Temozolomide nano enabled medicine: Promises made by the nanocarriers in glioblastoma therapy. J. Control. Release 2021, 336, 549–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, J.; Liu, W.; Zheng, X.; Zhang, W.; Lee, C.S.; Wang, P. Hypocrellin-Based Multifunctional Phototheranostic Agent for NIR-Triggered Targeted Chemo/Photodynamic/Photothermal Synergistic Therapy against Glioblastoma. ACS Appl. Bio Mater. 2020, 3, 3817–3826. [Google Scholar] [CrossRef]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef]
- Di Filippo, L.D.; de Carvalho, S.G.; Duarte, J.L.; Luiz, M.T.; Paes Dutra, J.A.; de Paula, G.A.; Chorilli, M.; Conde, J. A receptor-mediated landscape of druggable and targeted nanomaterials for gliomas. Mater. Today Bio 2023, 20, 100671. [Google Scholar] [CrossRef]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef]
- Kim, A.; Walz, W. Nanotherapy for Brain Tumor Drug Delivery; Agrahari, V., Kim, A., Agrahari, V., Eds.; Neuromethods; Springer: New York, NY, USA, 2021; Volume 163, ISBN 978-1-0716-1051-0. [Google Scholar]
- Krajcer, A.; Grzywna, E.; Lewandowska-Łańcucka, J. Strategies increasing the effectiveness of temozolomide at various levels of anti-GBL therapy. Biomed. Pharmacother. 2023, 165, 115174. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, Y.; Fu, Z.; Chen, J.; Fan, W.; Wu, X. Progress in research and development of temozolomide brain-targeted preparations: A review. J. Drug Target. 2023, 31, 119–133. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, B.; Lam, N.W.; Dua, K.; Haghi, M. Exploring the impact of physicochemical properties of liposomal formulations on their in vivo fate. Life Sci. 2022, 300, 120574. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Yadav, K.; Shukla, R.P.; Gautam, S.; Marwaha, D.; Sharma, M.; Mishra, P.R. Surface modification strategies in translocating nano-vesicles across different barriers and the role of bio-vesicles in improving anticancer therapy. J. Control. Release 2023, 363, 290–348. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Kommineni, N.; Chaudhari, R.; Conde, J.; Tamburaci, S.; Cecen, B.; Chandra, P.; Prasad, R. Engineered Liposomes in Interventional Theranostics of Solid Tumors. ACS Biomater. Sci. Eng. 2023, 9, 4527–4557. [Google Scholar] [CrossRef]
- Moosavian, S.A.; Bianconi, V.; Pirro, M.; Sahebkar, A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin. Cancer Biol. 2021, 69, 337–348. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Amarandi, R.-M.; Ibanescu, A.; Carasevici, E.; Marin, L.; Dragoi, B. Liposomal-Based Formulations: A Path from Basic Research to Temozolomide Delivery Inside Glioblastoma Tissue. Pharmaceutics 2022, 14, 308. [Google Scholar] [CrossRef] [PubMed]
- Vanza, J.; Jani, P.; Pandya, N.; Tandel, H. Formulation and statistical optimization of intravenous temozolomide-loaded PEGylated liposomes to treat glioblastoma multiforme by three-level factorial design. Drug Dev. Ind. Pharm. 2018, 44, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Buzyurova, D.N.; Pashirova, T.N.; Zueva, I.V.; Burilova, E.A.; Shaihutdinova, Z.M.; Rizvanov, I.K.; Babaev, V.M.; Petrov, K.A.; Souto, E.B. Surface modification of pralidoxime chloride-loaded solid lipid nanoparticles for enhanced brain reactivation of organophosphorus-inhibited AChE: Pharmacokinetics in rat. Toxicology 2020, 444, 152578. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.A.; Jiang, S.X.; Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Rich, J.N.; Gururangan, S.; Friedman, A.H.; Bigner, D.D.; Sampson, J.H.; et al. Phase II Trial of Temozolomide Plus O 6 -Benzylguanine in Adults With Recurrent, Temozolomide-Resistant Malignant Glioma. J. Clin. Oncol. 2009, 27, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Lanza, M.; Casili, G.; Paterniti, I.; Filippone, A.; Caffo, M.; Cardali, S.M.; Puliafito, I.; Colarossi, C.; Raciti, G.; et al. TAK1 Inhibitor Enhances the Therapeutic Treatment for Glioblastoma. Cancers 2020, 13, 41. [Google Scholar] [CrossRef]
- Goker Bagca, B.; Ozates, N.P.; Asik, A.; Caglar, H.O.; Gunduz, C.; Biray Avci, C. Temozolomide treatment combined with AZD3463 shows synergistic effect in glioblastoma cells. Biochem. Biophys. Res. Commun. 2020, 533, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.S.; Molenaar, P.; Teng, J.; Cornelissen, F.M.G.; Roelofs, I.; Menezes, R.; Dik, R.; Lagerweij, T.; Broersma, Y.; Petersen, N.; et al. A cancer drug atlas enables synergistic targeting of independent drug vulnerabilities. Nat. Commun. 2020, 11, 2935. [Google Scholar] [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22199–22224. [Google Scholar] [CrossRef]
- Agarwal, S.; Muniyandi, P.; Maekawa, T.; Kumar, D.S. Vesicular systems employing natural substances as promising drug candidates for MMP inhibition in glioblastoma: A nanotechnological approach. Int. J. Pharm. 2018, 551, 339–361. [Google Scholar] [CrossRef]
- Carbone, C.; Martins-Gomes, C.; Caddeo, C.; Silva, A.M.; Musumeci, T.; Pignatello, R.; Puglisi, G.; Souto, E.B. Mediterranean essential oils as precious matrix components and active ingredients of lipid nanoparticles. Int. J. Pharm. 2018, 548, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Crooker, K.; Aliani, R.; Ananth, M.; Arnold, L.; Anant, S.; Thomas, S.M. A review of promising natural chemopreventive agents for head and neck cancer. Cancer Prev. Res. 2018, 11, 441–450. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Limonta, P.; Moretti, R.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Montagnani Marelli, M. Role of Endoplasmic Reticulum Stress in the Anticancer Activity of Natural Compounds. Int. J. Mol. Sci. 2019, 20, 961. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, F.; Yan, J.; Xia, Z.; Jiang, D.; Ma, P. Hispidulin: A promising flavonoid with diverse anti-cancer properties. Life Sci. 2020, 259, 118395. [Google Scholar] [CrossRef]
- Gomez-Cadena, A.; Barreto, A.; Fioretino, S.; Jandus, C. Immune system activation by natural products and complex fractions: A network pharmacology approach in cancer treatment. Cell Stress 2020, 4, 154–166. [Google Scholar] [CrossRef]
- Domínguez-Martín, E.M.; Magalhães, M.; Efferth, T.; Díaz-Lanza, A.M.; Cabral, C.; Rijo, P. Terpenes: A hope for glioblastoma patients. In New Insights into Glioblastoma; Elsevier: Amsterdam, The Netherlands, 2023; pp. 227–269. [Google Scholar]
- Chang, K.-F.; Huang, X.-F.; Chang, J.T.; Huang, Y.-C.; Weng, J.-C.; Tsai, N.-M. Cedrol suppresses glioblastoma progression by triggering DNA damage and blocking nuclear translocation of the androgen receptor. Cancer Lett. 2020, 495, 180–190. [Google Scholar] [CrossRef]
- Chang, K.-F.; Huang, X.-F.; Chang, J.T.; Huang, Y.-C.; Lo, W.-S.; Hsiao, C.-Y.; Tsai, N.-M. Cedrol, a Sesquiterpene Alcohol, Enhances the Anticancer Efficacy of Temozolomide in Attenuating Drug Resistance via Regulation of the DNA Damage Response and MGMT Expression. J. Nat. Prod. 2020, 83, 3021–3029. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Wang, G.; Yao, Z.; Dang, X. Pharmacokinetics and antitumor efficacy of DSPE-PEG2000 polymeric liposomes loaded with quercetin and temozolomide: Analysis of their effectiveness in enhancing the chemosensitization of drug-resistant glioma cells. Int. J. Mol. Med. 2016, 37, 690–702. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Abdullin, T.I.; Grigor’Eva, L.R.; Kuznetsova, E.V.; Akhmadishina, R.A.; Ziganshina, L.E.; Cong, H.H.; Mironov, V.F. Design, Synthesis, and Cancer Cell Growth Inhibitory Activity of Triphenylphosphonium Derivatives of the Triterpenoid Betulin. J. Nat. Prod. 2017, 80, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Tsepaeva, O.V.; Salikhova, T.I.; Ishkaeva, R.A.; Kundina, A.V.; Abdullin, T.I.; Laikov, A.V.; Tikhomirova, M.V.; Idrisova, L.R.; Nemtarev, A.V.; Mironov, V.F. Bifunctionalized Betulinic Acid Conjugates with C-3-Monodesmoside and C-28-Triphenylphosphonium Moieties with Increased Cancer Cell Targetability. J. Nat. Prod. 2023, 86, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Li, S.M.; Shen, Y.H.; Zhang, W.D. Phytochemical and biological studies of Abies species. Chem. Biodivers. 2008, 5, 56–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, X.; Liu, Y.; Wang, Y.; Tian, T.; Zhao, X.; Li, J.; Ruan, H. Triterpenoids from the branch and leaf of Abies fargesii. Phytochemistry 2016, 130, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Babaie, S.; Del Bakhshayesh, A.R.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A Novel Nanocarrier for Transdermal Drug Delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.H.; Eltabeeb, M.A.; El-Nabarawi, M.A.; Abdellatif, M.M. Utilization of propranolol hydrochloride mucoadhesive invasomes as a locally acting contraceptive: In-vitro, ex-vivo, and in-vivo evaluation. Drug Deliv. 2022, 29, 2549–2560. [Google Scholar] [CrossRef]
- Song, H.; Wei, M.; Zhang, N.; Li, H.; Tan, X.C.; Zhang, Y.J.; Zheng, W.S. Enhanced permeability of blood-brain barrier and targeting function of brain via borneol-modified chemically solid lipid nanoparticle. Int. J. Nanomed. 2018, 13, 1869–1879. [Google Scholar] [CrossRef]
- Sinyashin, K.O.; Zakharova, L.Y.; Pashirova, T.N.; Petrov, K.A.; Voloshina, A.D.; Vyshtakalyuk, A.B.; RizvanovI, K.H.; Babaev, V.M.; Maganova, F.I. Liposomal Form of Abies sibirica Resin for Non-Invasive Delivery of Biologically Active Substance of Abies sibirica Oleoresin to Brain, Which Has Antioxidant, Cytotoxic Activity against Cervical Cancer Cells, and Methods for Its Preparation. Russian Patent RU 2757873 C2, 22 October 2021. [Google Scholar]
- Khan, A.; Imam, S.S.; Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Brain Targeting of Temozolomide via the Intranasal Route Using Lipid-Based Nanoparticles: Brain Pharmacokinetic and Scintigraphic Analyses. Mol. Pharm. 2016, 13, 3773–3782. [Google Scholar] [CrossRef]
- Gelsleichter, N.E.; de Souza, P.O.; Teixeira, F.C.; Debom, G.N.; Lenz, G.S.; Roliano, G.G.; de Cássia Sant’ana, R.; Visioli, F.; Fachel, F.N.S.; Michels, L.R.; et al. Metastatic Melanoma: A Preclinical Model Standardization and Development of a Chitosan-Coated Nanoemulsion Containing Temozolomide to Treat Brain Metastasis. Cell. Mol. Neurobiol. 2023, 43, 2939–2951. [Google Scholar] [CrossRef]
- Hsieh, Y.-S.; Yang, S.-F.; Hsieh, Y.-H.; Hung, C.-H.; Chu, S.-C.; Yang, S.-H.; Chen, P.-N. Erratum: “The Inhibitory Effect of Abietic Acid on Melanoma Cancer Metastasis and Invasiveness In Vitro and In Vivo”. Am. J. Chin. Med. 2017, 45, 403. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Liu, Q.; Dai, J. Abietic acid suppresses non-small-cell lung cancer cell growth via blocking IKKβ/NF-κB signaling. OncoTargets Ther. 2019, 12, 4825–4837. [Google Scholar] [CrossRef]
- Voloshina, A.D.; Semenov, V.E.; Strobykina, A.S.; Kulik, N.V.; Krylova, E.S.; Zobov, V.V.; Reznik, V.S. Synthesis and antimicrobial and toxic properties of novel 1,3-bis(alkyl)-6-methyluracil derivatives containing 1,2,3- and 1,2,4-triazolium fragments. Russ. J. Bioorganic Chem. 2017, 43, 170–176. [Google Scholar] [CrossRef]
- Kirby, C.; Gregoriadis, G. Dehydration-Rehydration Vesicles: A Simple Method for High Yield Drug Entrapment in Liposomes. Nat. Biotechnol. 1984, 2, 979–984. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Sapunova, A.S.; Lukashenko, S.S.; Burilova, E.A.; Lubina, A.P.; Shaihutdinova, Z.M.; Gerasimova, T.P.; Kovalenko, V.I.; Voloshina, A.D.; Souto, E.B.; et al. Synthesis, structure-activity relationship and biological evaluation of tetracationic gemini Dabco-surfactants for transdermal liposomal formulations. Int. J. Pharm. 2020, 575, 118953. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Yang, S.F.; Hsieh, Y.H.; Hung, C.H.; Chu, S.C.; Yang, S.H.; Chen, P.N. The Inhibitory Effect of Abietic Acid on Melanoma Cancer Metastasis and Invasiveness In Vitro and In Vivo. Am. J. Chin. Med. 2015, 43, 1697–1714. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Chandler, S.G.; Ilium, L.; Thomas, N.W. Nasal absorption in rats. II. Effect of enhancers on insulin absorption and nasal histology. Int. J. Pharm. 1991, 76, 61–70. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Lu, Y.; Du, S.; Bai, J.; Li, P.; Wen, R.; Zhao, X. Bioavailability and Brain-Targeting of Geniposide in Gardenia-Borneol Co-Compound by Different Administration Routes in Mice. Int. J. Mol. Sci. 2012, 13, 14127–14135. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Du, J.; Liu, M.; Feng, J.; Hu, K. Improved brain delivery of pueraria flavones via intranasal administration of borneol-modified solid lipid nanoparticles. Nanomedicine 2019, 14, 2105–2119. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Sawant, N.; Kolapkar, A.; Huprikar, A.; Desai, N. Borneol: A Promising Monoterpenoid in Enhancing Drug Delivery Across Various Physiological Barriers. AAPS PharmSciTech 2021, 22, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, L.; Du, J.; Zhao, X.; Liu, M.; Feng, J.; Hu, K. Nose-to-brain delivery of borneol modified tanshinone IIA nanoparticles in prevention of cerebral ischemia/reperfusion injury. Drug Deliv. 2021, 28, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Suckling, K.E.; Blair, H.A.F.; Boyd, G.S.; Craig, I.F.; Malcolm, B.R. The importance of the phospholipid bilayer and the length of the cholesterol molecule in membrane structure. Biochim. Biophys. Acta Biomembr. 1979, 551, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Owensiii, D.; Peppas, N. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Ma, Z.; Wu, B. Effects of pharmaceutical PEGylation on drug metabolism and its clinical concerns. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Aranda, F.J.; Villalaín, J. The interaction of abietic acid with phospholipid membranes. Biochim. Biophys. Acta Biomembr. 1997, 1327, 171–180. [Google Scholar] [CrossRef]
- Villalaín, J. Location of the toxic molecule abietic acid in model membranes by MAS–NMR. Biochim. Biophys. Acta Biomembr. 1997, 1328, 281–289. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chuang, H.-S. Use of abietic acid or derivative thereof for modulation permeability of plasma membrane. United States Patent US 2004/0063788 Al, 2004. [Google Scholar]
- Faustino, C.; Neto, Í.; Fonte, P.; Macedo, A. Cytotoxicity and Chemotherapeutic Potential of Natural Rosin Abietane Diterpenoids and their Synthetic Derivatives. Curr. Pharm. Des. 2019, 24, 4362–4375. [Google Scholar] [CrossRef]
- Zentar, H.; Jannus, F.; Gutierrez, P.; Medina-O’Donnell, M.; Lupiáñez, J.A.; Reyes-Zurita, F.J.; Alvarez-Manzaneda, E.; Chahboun, R. Semisynthesis and Evaluation of Anti-Inflammatory Activity of the Cassane-Type Diterpenoid Taepeenin F and of Some Synthetic Intermediates. J. Nat. Prod. 2022, 85, 2372–2384. [Google Scholar] [CrossRef] [PubMed]
- Haffez, H.; Osman, S.; Ebrahim, H.Y.; Hassan, Z.A. Growth Inhibition and Apoptotic Effect of Pine Extract and Abietic Acid on MCF-7 Breast Cancer Cells via Alteration of Multiple Gene Expressions Using In Vitro Approach. Molecules 2022, 27, 293. [Google Scholar] [CrossRef] [PubMed]
- Kawaoka, Y.; Feng, H.; Watanabe, T.; Yamashita, M. Adjuvant and vaccine containing adjuvant. United States Patent US 202110106680 A1, 2021. [Google Scholar]
- Xu, Y.; Tong, Y.; Lei, Z.; Zhu, J.; Wan, L. Abietic acid induces ferroptosis via the activation of the HO-1 pathway in bladder cancer cells. Biomed. Pharmacother. 2023, 158, 114154. [Google Scholar] [CrossRef] [PubMed]

| № | Lipid System | Terpene, % w/w | Loaded Drug | Size, (nm) | Zaver (nm) | PDI | ζ (mV) | EE (%) | LC (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PC/DSPE–PEG2000/Ch | – | - | 78 ± 2 | 145 ± 1 | 0.17 ± 0.02 | –27.5 ± 0.5 | – | – |
| 2 | PC/DSPE–PEG2000/Ch | A. sibirica, 1 | - | 68 ± 1 | 137 ± 1 | 0.2 ± 0.01 | –27 ± 1 | – | – |
| 3 | PC/DSPE–PEG2000/Ch | Ab. acid, 1 | - | 68 ± 1 | 123 ± 1 | 0.14 ± 0.01 | –15 ± 1 | – | – |
| 4 | PC/DSPE–PEG2000/Ch | – | TMZ | 91 ± 1 | 157 ± 1 | 0.19 ± 0.01 | –32 ± 1 | 83 ± 1 | 1.5 ± 0.1 |
| 4 * | PC/DSPE–PEG2000/Ch | – | TMZ | 190 ± 2 | 297 ± 5 | 0.16 ± 0.02 | –28 ± 1 | - | - |
| 5 | PC/DSPE–PEG2000/Ch | A. sibirica, 1 | TMZ | 91 ± 1 | 145 ± 1 | 0.21 ± 0.01 | –25 ± 1 | 74 ± 10 | 1.4 ± 0.1 |
| 5 * | PC/DSPE–PEG2000/Ch | A. sibirica, 1 | TMZ | 164 ± 8 | 225 ± 1 | 0.21 ± 0.01 | –17 ± 1 | - | - |
| 6 | PC/DSPE–PEG2000/Ch | Ab. acid, 1 | TMZ | 68 ± 1 | 118 ± 1 | 0.14 ± 0.01 | –18 ± 1 | 74 ± 15 | 1.4 ± 0.1 |
| 6 * | PC/DSPE–PEG2000/Ch | Ab. acid, 1 | TMZ | 68 ± 1 | 130 ± 1 | 0.16 ± 0.01 | –15 ± 1 | - | - |
| Systems | IC50 (mg/mL) | |||
|---|---|---|---|---|
| T 98G | M-Hela | Chang Liver | M-Hela | |
| Empty lipid system | 18.4 | 15.1 | 13.5 | |
| Abietic acid–lipid system | 0.7 | 0.98 | 0.6 | 45.8 ± 3.5 |
| A. sibirica–lipid system | 0.75 | 1.3 | 1.2 | 13 ± 1 |
| TMZ | 0.04 | 0.007 | 0.03 | – |
| TMZ–lipid system | 0.3 | 0.15 | 0.15 | – |
| TMZ–abietic acid–lipid system | 0.07 | 0.13 | 0.09 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashirova, T.N.; Nemtarev, A.V.; Buzyurova, D.N.; Shaihutdinova, Z.M.; Dimukhametov, M.N.; Babaev, V.M.; Voloshina, A.D.; Mironov, V.F. Terpenes-Modified Lipid Nanosystems for Temozolomide, Improving Cytotoxicity against Glioblastoma Human Cancer Cells In Vitro. Nanomaterials 2024, 14, 55. https://doi.org/10.3390/nano14010055
Pashirova TN, Nemtarev AV, Buzyurova DN, Shaihutdinova ZM, Dimukhametov MN, Babaev VM, Voloshina AD, Mironov VF. Terpenes-Modified Lipid Nanosystems for Temozolomide, Improving Cytotoxicity against Glioblastoma Human Cancer Cells In Vitro. Nanomaterials. 2024; 14(1):55. https://doi.org/10.3390/nano14010055
Chicago/Turabian StylePashirova, Tatiana N., Andrey V. Nemtarev, Daina N. Buzyurova, Zukhra M. Shaihutdinova, Mudaris N. Dimukhametov, Vasily M. Babaev, Alexandra D. Voloshina, and Vladimir F. Mironov. 2024. "Terpenes-Modified Lipid Nanosystems for Temozolomide, Improving Cytotoxicity against Glioblastoma Human Cancer Cells In Vitro" Nanomaterials 14, no. 1: 55. https://doi.org/10.3390/nano14010055







