Anodic Aluminum Oxide-Based Chemi-Capacitive Sensor for Ethanol Gas
Abstract
:1. Introduction
2. Materials and Methods
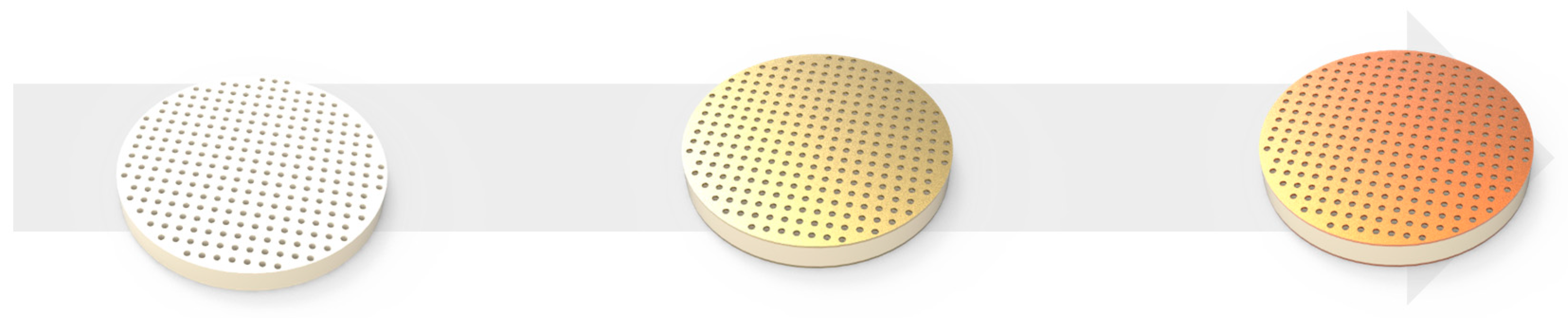
2.1. Capacitive Gas Sensor Preparation
2.2. Surface Modification
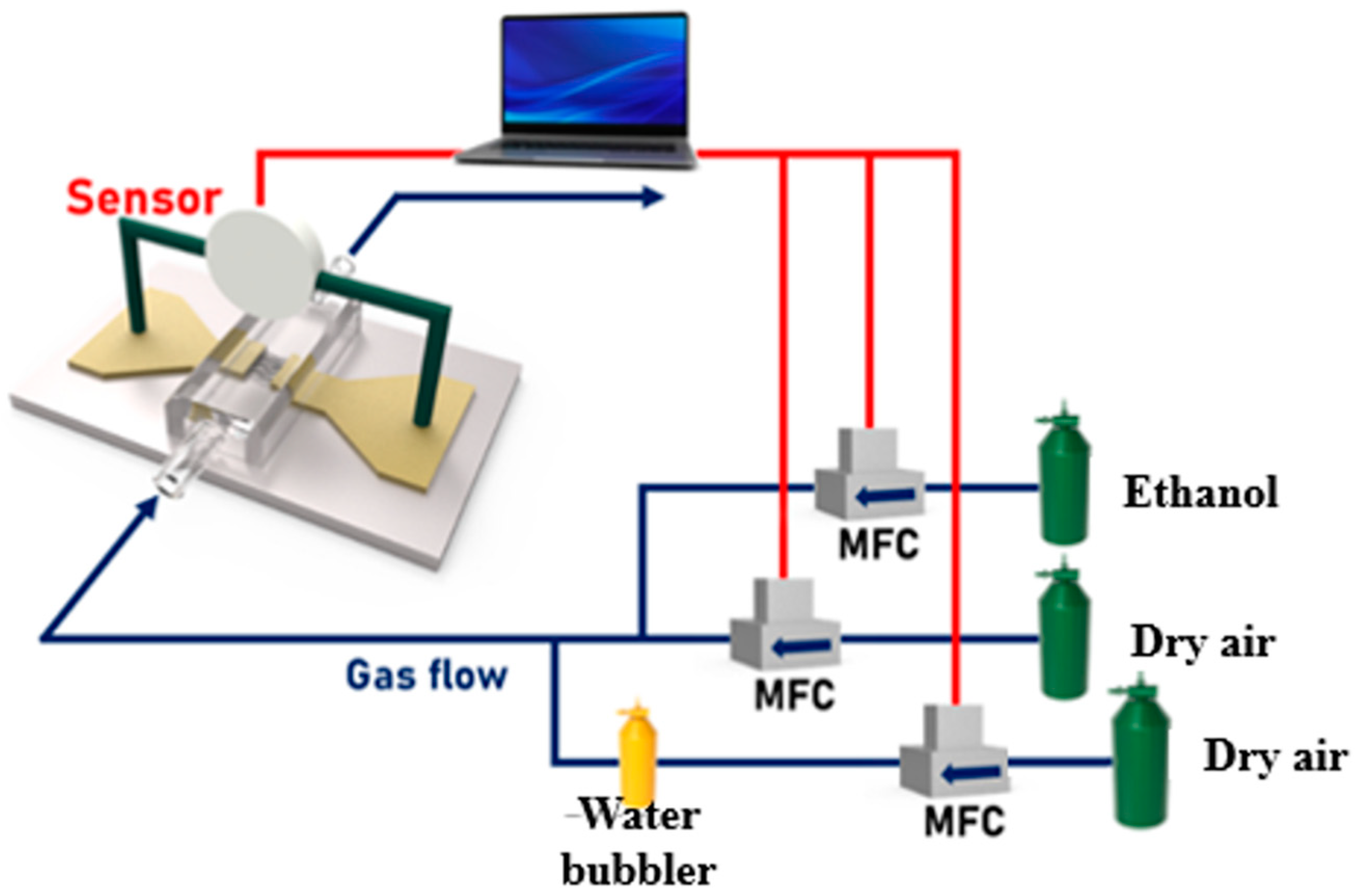
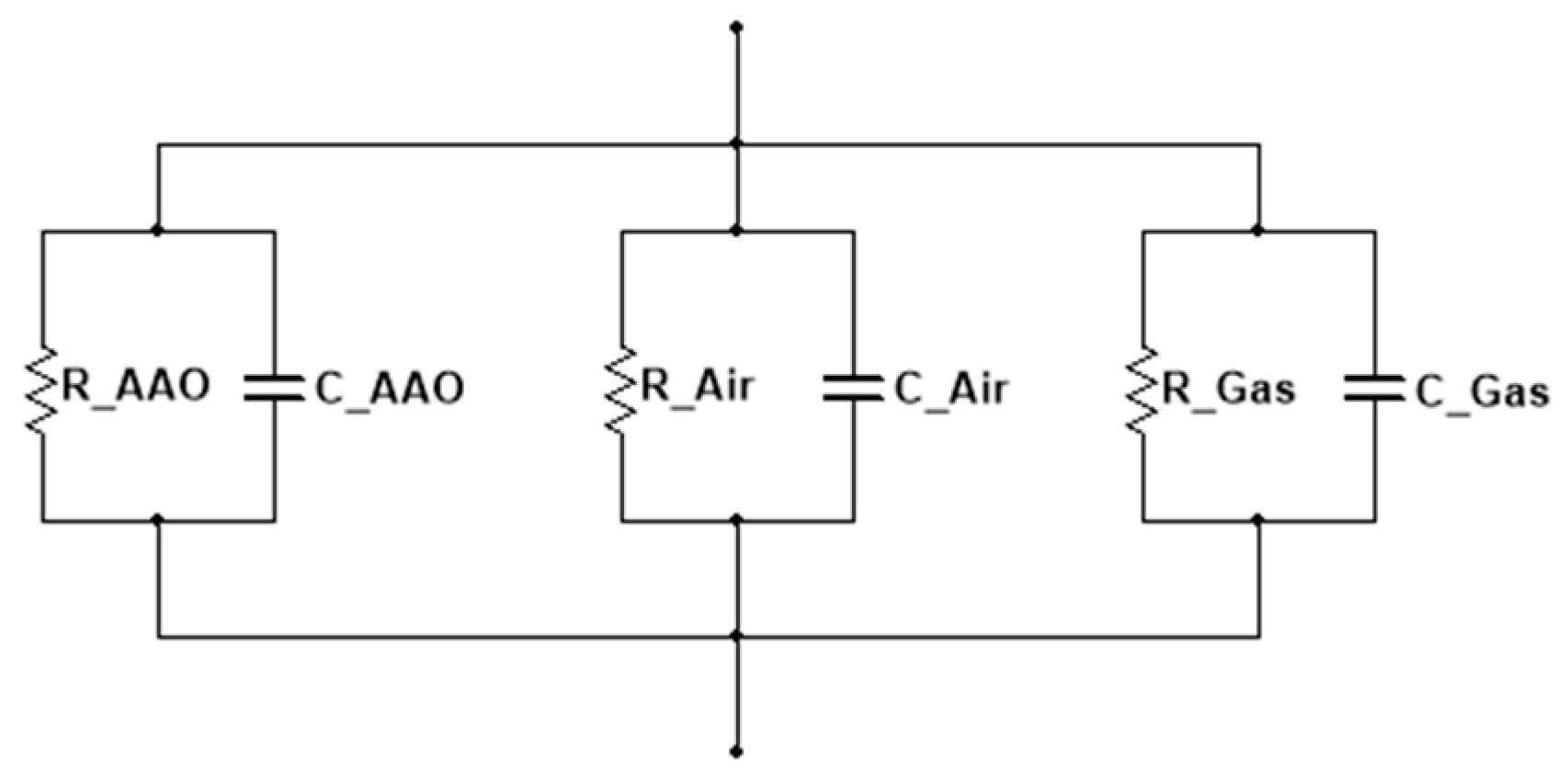
2.3. Characteristics
3. Results and Discussion
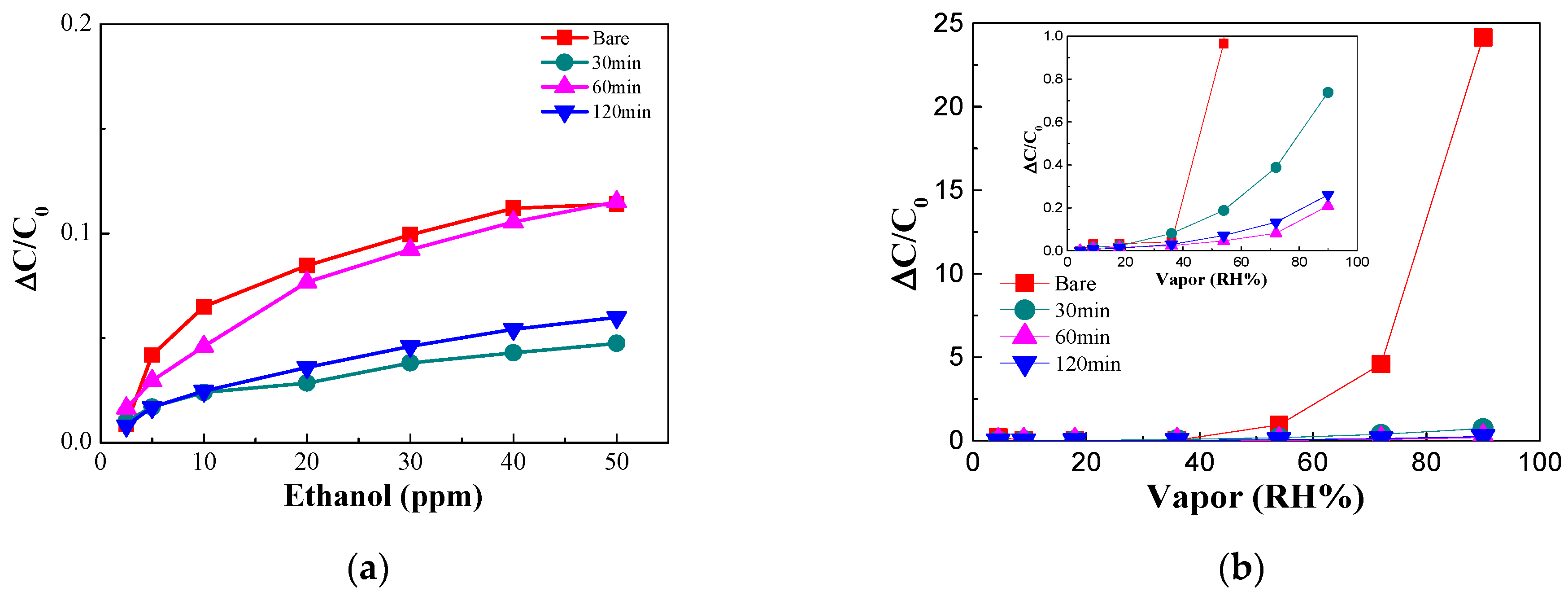
3.1. Improving the Sensitivity of the Sensor
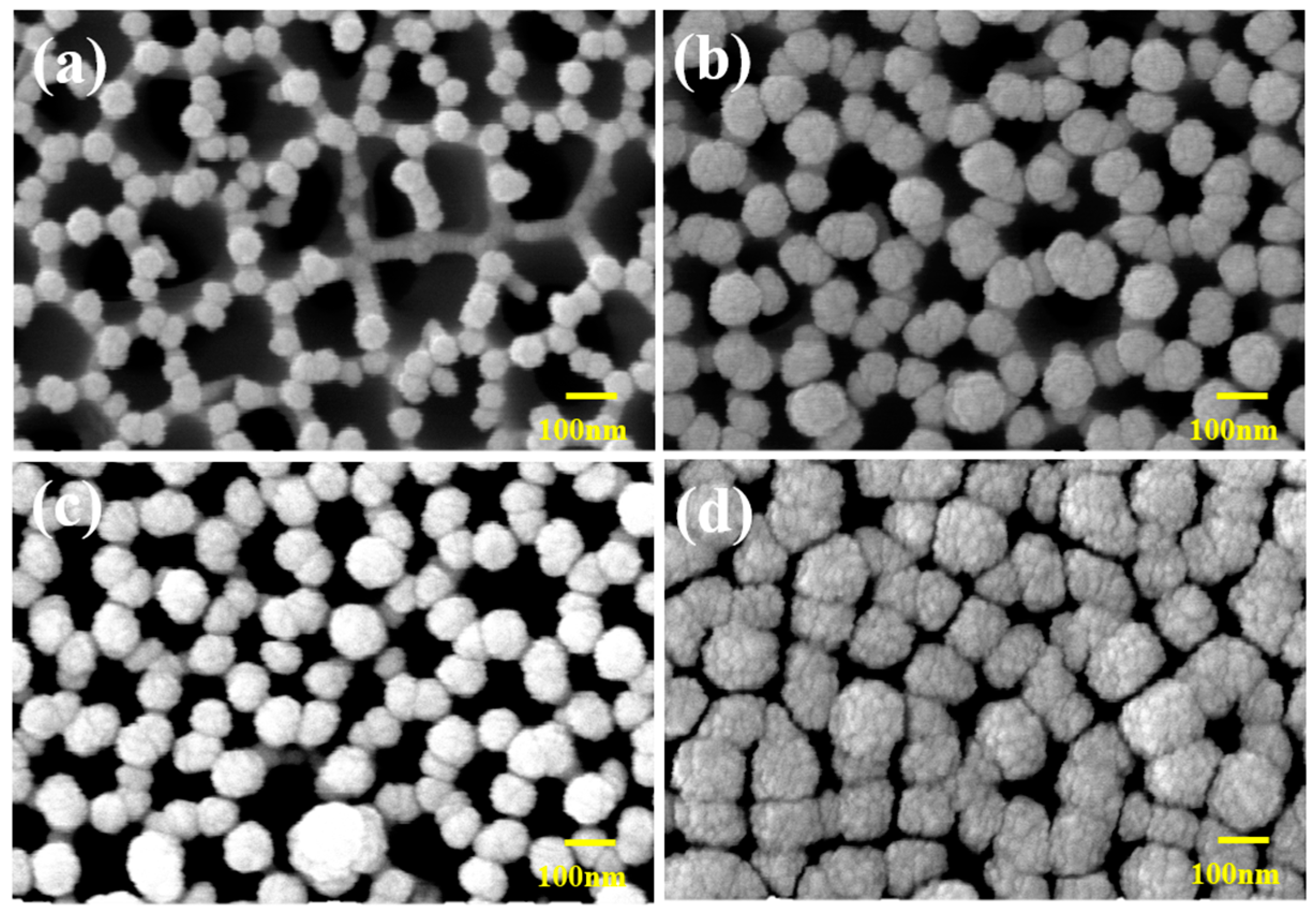
3.1.1. Change in Thickness of Sensor Electrode
3.1.2. Change in the Measuring Frequency of the Sensor
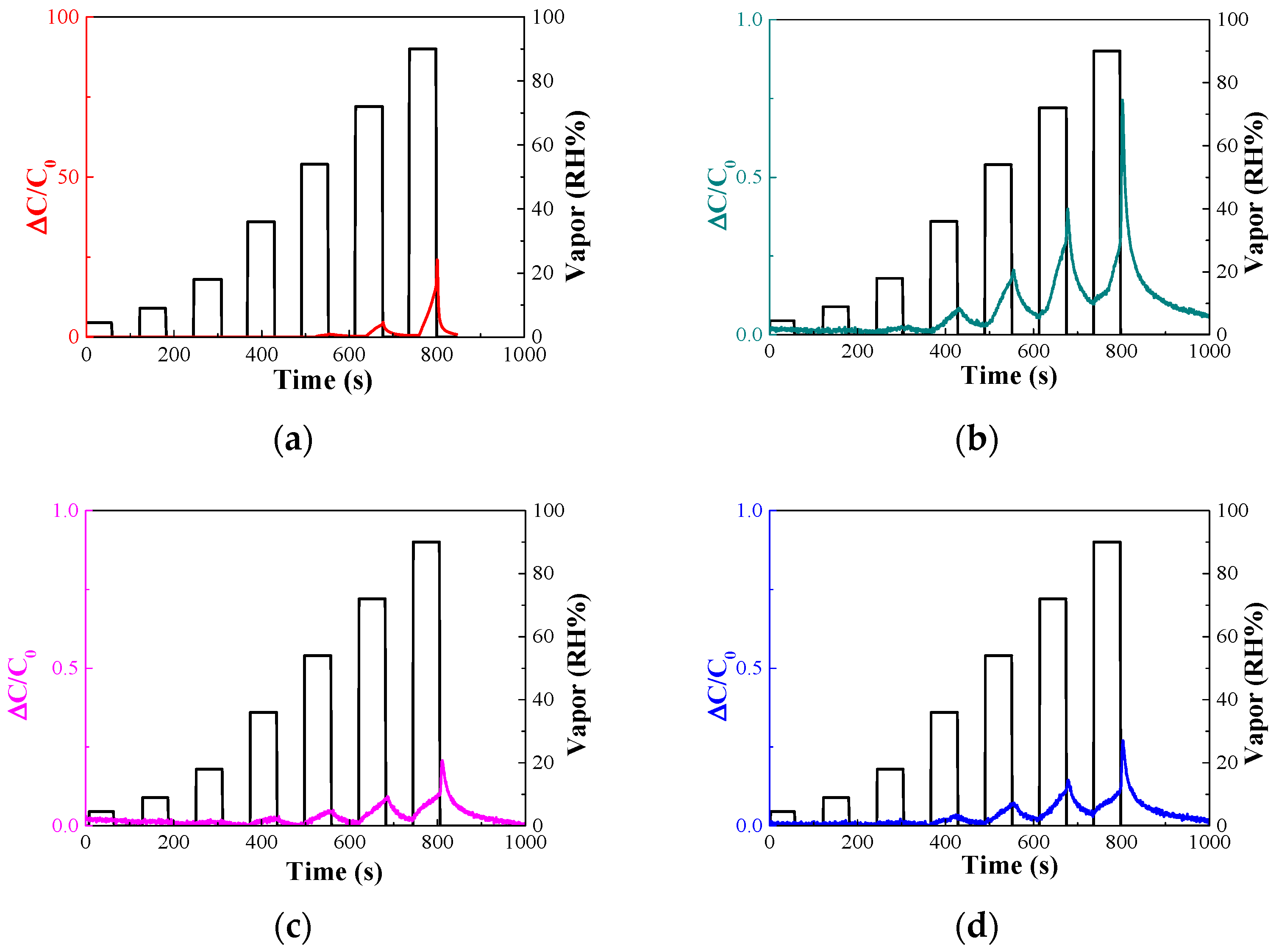
3.2. Decreased Moisture Sensitivity of the Sensor
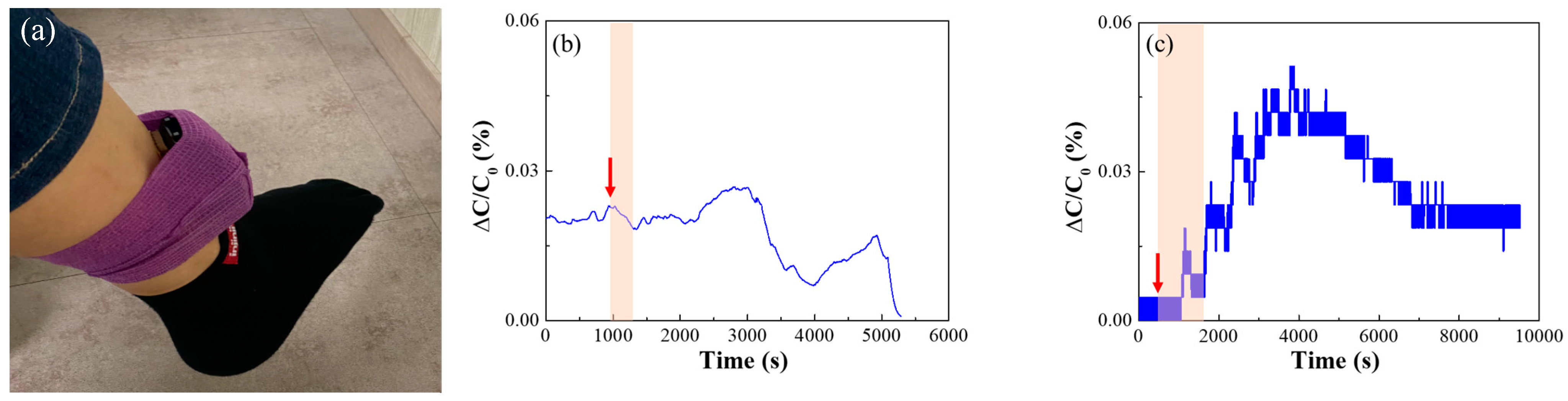
3.3. Sensing Performance of Capacitive Type Sensor Device
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lane, S.D.; Cherek, D.R.; Pietras, C.J.; Tcheremissine, O.V. Alcohol Effects on Human Risk Taking. Psychopharmacology 2004, 172, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.M.; Prazeres, D.M.F.; Cabral, J.M.S.; Fonseca, L.P. Ethanol Biosensors Based on Alcohol Oxidase. Biosens. Bioelectron. 2005, 21, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, A.; Amirfazli, A.; Farhad, S. Low Catalyst Loaded Ethanol Gas Fuel Cell Sensor. Sens. Actuators B 2016, 234, 70–79. [Google Scholar] [CrossRef]
- Benhaddouch, T.E.; Pinzon, S.K.; Landi, D.M.C.; Marcial, J.; Mehta, P.; Romero, K.; Rockward, T.; Bhansali, S.; Dong, D. Review—Micro-fuel Cell Principal Biosensors for Monitoring Transdermal Volatile Organic Compounds in Humans. ECS Sens. Plus 2022, 1, 041602. [Google Scholar] [CrossRef]
- Ozoemena, K.I.; Musa, S.; Modise, R.; Ipadeola, A.K.; Gaolatlhe, L.; Peteni, S.; Kabongo, G. Fuel Cell-Based Breath-Alcohol Sensors: Innovation-Hungry Old Electrochemistry. Curr. Opin. Electrochem. 2018, 10, 82–87. [Google Scholar] [CrossRef]
- Sorbello, J.G.; Devilly, G.J.; Allen, C.; Hughes, L.R.J.; Brown, K. Fuel-Cell breathalyser Use for Field Research on Alcohol Intoxication: An Independent Psychometric Evaluation. PeerJ 2018, 6, e4418. [Google Scholar] [CrossRef]
- Jones, A.W.; Cowan, J.M. Reflections on Variability in the Blood-Breath Ratio of Ethanol and Its Importance When Evidential Breath-Alcohol Instruments Are Used in Law Enforcement. Forensic Sci. Res. 2020, 5, 300–308. [Google Scholar] [CrossRef]
- Swift, R. Direct Measurement of Alcohol and Its Metabolites. Addiction 2003, 98 (Suppl. S2), 73–80. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-Assisted Synthesis of Metal Oxide Nanostructures for Gas Sensing Application: A Review. Sens. Actuators B 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Alcantara, G.P.; Andrade, C.G.M. A Short Review of Gas Sensors Based on Interdigital Electrode. In Proceedings of the 2015 12th IEEE International Conference on Electronic Measurement & Instruments (ICEMI), Qingdao, China, 16–18 July 2015; pp. 1616–1621. [Google Scholar]
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.L.; Hasan, T.; Huang, X.; Huang, W. Printed Gas Sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, J.; Zhang, J.; Wang, H.; Wilkinson, D.P.; Gu, C.E. Liquid Methanol Concentration Sensors for Direct Methanol Fuel Cells. J. Power Sources 2006, 159, 626–636. [Google Scholar] [CrossRef]
- Pourteimoor, S.; Haratizadeh, H. Performance of a Fabricated Nanocomposite-Based Capacitive Gas Sensor at Room Temperature. J. Mater. Sci. Mater. Electron. 2017, 28, 18529–18534. [Google Scholar] [CrossRef]
- Chung, C.K.; Khor, O.K.; Kuo, E.H.; Ku, C.A. Total Effective Surface Area Principle for Enhancement of Capacitive Humidity Sensor of Thick-Film Nanoporous Alumina. Mater. Lett. 2020, 260, 126921. [Google Scholar] [CrossRef]
- Vello, T.P.; de Oliveira, R.F.; Silva, G.O.; de Camargo, D.H.; Bufon, C.C. A Simple Capacitive Method to Evaluate Ethanol Fuel Samples. Sci. Rep. 2017, 7, 43432. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Haffer, S.; Weinberger, C.; Klaus, D.; Tiemann, M. Mesoporous Materials as Gas Sensors. Chem. Soc. Rev. 2013, 42, 4036–4053. [Google Scholar] [CrossRef] [PubMed]
- Rumiche, F.; Wang, H.H.; Hu, W.S.; Indacochea, J.E.; Wang, M.L. Anodized Aluminum Oxide (AAO) Nanowell Sensors for Hydrogen Detection. Sens. Actuators B 2008, 134, 869–877. [Google Scholar] [CrossRef]
- Norek, M.; Krasiński, A. Controlling of Water Wettability by Structural and Chemical Modification of Porous Anodic Alumina (PAA): Towards Super-Hydrophobic Surfaces. Surf. Coat. Technol. 2015, 276, 464–470. [Google Scholar] [CrossRef]
- Md Jani, A.M.; Losic, D.; Voelcker, N.H. Nanoporous Anodic Aluminium Oxide: Advances in Surface Engineering and Emerging Applications. Prog. Mater. Sci. 2013, 58, 636–704. [Google Scholar] [CrossRef]
- Lee, L.-R.; Karapala, V.K.; Lin, Y.-L.; He, H.-C.; Chen, J.-T. Intelligent Environmental Sensing: Fabrication of Switchable, Reusable, and Highly Sensitive Gas Sensors with Spiropyran-Grafted Anodic Aluminum Oxide Templates. J. Phys. Chem. C 2020, 124, 11870–11876. [Google Scholar] [CrossRef]
- Jani, A.M.M.; Anglin, E.J.; McInnes, S.J.P.; Losic, D.; Shapter, J.G.; Voelcker, N.H. Nanoporous anodic aluminium oxide membranes with layered surface chemistry. Chem. Commun. 2009, 21, 3062–3064. [Google Scholar] [CrossRef]
- Tasaltin, N.; Sanli, D.; Jonáš, A.; Kiraz, A.; Erkey, C. Preparation and Characterization of Superhydrophobic Surfaces Based on Hexamethyldisilazane-Modified Nanoporous Alumina. Nanoscale Res. Lett. 2011, 6, 487. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lee, S.H.; Jung, J.-Y.; Yoo, J.Y. Evaporating Characteristics of Sessile Droplet on Hydrophobic and Hydrophilic Surfaces. Microelectron. Eng. 2009, 86, 1350–1353. [Google Scholar] [CrossRef]
- Bindra, P.; Hazra, A. Capacitive Gas and Vapor Sensors Using Nanomaterials. J. Mater. Sci. Mater. Electron. 2018, 29, 6129–6148. [Google Scholar] [CrossRef]
- Chen, S.W.; Khor, O.K.; Liao, M.W.; Chung, C.K. Sensitivity Evolution and Enhancement Mechanism of Porous Anodic Aluminum Oxide Humidity Sensor Using Magnetic Field. Sens. Actuators B 2014, 199, 384–388. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, B.; Lee, H.; Kim, H.; Lee, K.; Park, H. Capacitive Humidity Sensor Design Based on Anodic Aluminum Oxide. Sens. Actuators B 2009, 141, 441–446. [Google Scholar] [CrossRef]
- Kim, S.-J.; Park, J.-Y.; Lee, S.-H.; Yi, S.-H. Humidity Sensors Using Porous Silicon Layer with Mesa Structure. J. Phys. D Appl. Phys. 2000, 33, 1781–1784. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.-H.; Lee, J.-Y.; Kim, K.-K.; Choa, Y.-H.; Lim, J.-H. Single-Walled Carbon Nanotube-Based Chemi-Capacitive Sensor for Hexane and Ammonia. Electron. Mater. Lett. 2019, 15, 712–719. [Google Scholar] [CrossRef]
- Chung, C.K.; Ku, C.A.; Wu, Z.E. A High-and-Rapid-Response Capacitive Humidity Sensor of Nanoporous Anodic Alumina by One-Step Anodizing Commercial 1050 Aluminum Alloy and Its Enhancement Mechanism. Sens. Actuators B 2021, 343, 130156. [Google Scholar] [CrossRef]
- Podgolin, S.K.; Petukhov, D.I.; Dorofeev, S.G.; Eliseev, A.A. Anodic Alumina Membrane Capacitive Sensors for Detection of Vapors. Talanta 2020, 219, 121248. [Google Scholar] [CrossRef]
- Zeinali, S.; Homayoonnia, S.; Homayoonnia, G. Comparative Investigation of Interdigitated and Parallel-Plate Capacitive Gas Sensors Based on Cu-BTC Nanoparticles for Selective Detection of Polar and Apolar VOCs Indoors. Sens. Actuators B 2019, 278, 153–164. [Google Scholar] [CrossRef]
- Peng, Q.; Gong, B.; Parsons, G.N. Making Inert Polypropylene Fibers Chemically Responsive by Combining Atomic Layer Deposition and Vapor Phase Chemical Grafting. Nanotechnology 2011, 22, 155601. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zeng, X.; Guo, K.; Sun, R.; Xu, J.-B. The Effect of Interfacial State on the Thermal Conductivity of Functionalized Al2O3 Filled Glass Fibers Reinforced Polymer Composites. Compos. A 2015, 69, 49–55. [Google Scholar] [CrossRef]
- Sundararajan, M.; Subramani, S.; Devarajan, M.; Jaafar, M. Synthesis and Analysis of Anodic Aluminum Oxide-Nanopore Structure on Al Substrates for Efficient Thermal Management in Electronic Packaging. J. Mater. Sci. Mater. Electron. 2020, 31, 9641–9649. [Google Scholar] [CrossRef]
- Anithambigai, P.; Dheepan Chakravarthii, M.K.; Mutharasu, D.; Huong, L.H.; Zahner, T.; Lacey, D.; Kamarulazizi, I. Potential Thermally Conductive Alumina Filled Epoxy Composite for Thermal Management of High Power LEDs. J. Mater. Sci. Mater. Electron. 2017, 28, 856–867. [Google Scholar] [CrossRef]
- Zhang, D.; Hegab, H.E.; Lvov, Y.; Dale Snow, L.; Palmer, J. Immobilization of Cellulase on a Silica Gel Substrate Modified Using a 3-APTES Self-Assembled Monolayer. SpringerPlus 2016, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.; Yim, C.; Jeon, S. Surface Wetting of Superhydrophobic Aluminum Oxide Nanostructures Investigated Using the Fiber-Optic Spectrometer and Quartz Crystal Microbalance. Sens. Actuators B 2015, 220, 799–804. [Google Scholar] [CrossRef]
- Szczepanski, V.; Vlassiouk, I.; Smirnov, S. Stability of Silane Modifiers on Alumina Nanoporous Membranes. J. Membr. Sci. 2006, 281, 587–591. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Dhiraj, K.; Addai-Mensah, J.; Losic, D. The Loading and Release Property of Nanoporous Anodic Alumina for Delivery of Drugs and Drug Carriers. In Proceedings of the 2010 International Conference on Nanoscience and Nanotechnology, Sydney, NSW, Australia, 22–26 February 2010; pp. 143–145. [Google Scholar]
- Paul, J.; Philip, J. Inter-digital Capacitive Ethanol Sensor Coated with Cobalt Ferrite Nano Composite as Gas Sensing Material. Mater. Today Proc. 2020, 25, 148–150. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Zeinali, S.; Sheikhi, M.H. Fabrication of Capacitive Sensor Based on Cu-BTC (MOF-199) Nanoporous Film for Detection of Ethanol and Methanol Vapors. Sens. Actuators B 2016, 230, 9–16. [Google Scholar] [CrossRef]
- Homayoonnia, S.; Zeinali, S. Design and Fabrication of Capacitive Nanosensor Based on MOF Nanoparticles as Sensing Layer for VOCs Detection. Sens. Actuators B 2016, 237, 776–786. [Google Scholar] [CrossRef]
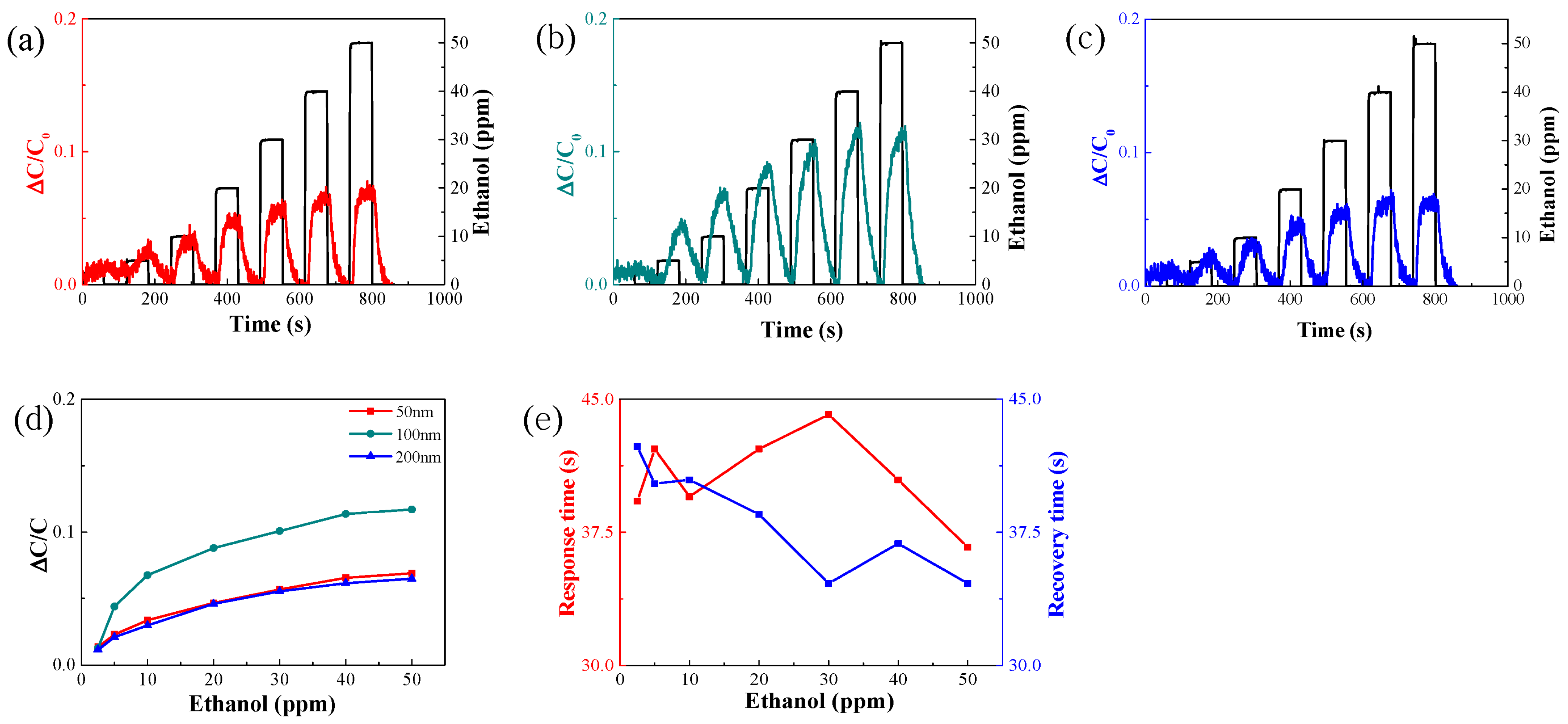
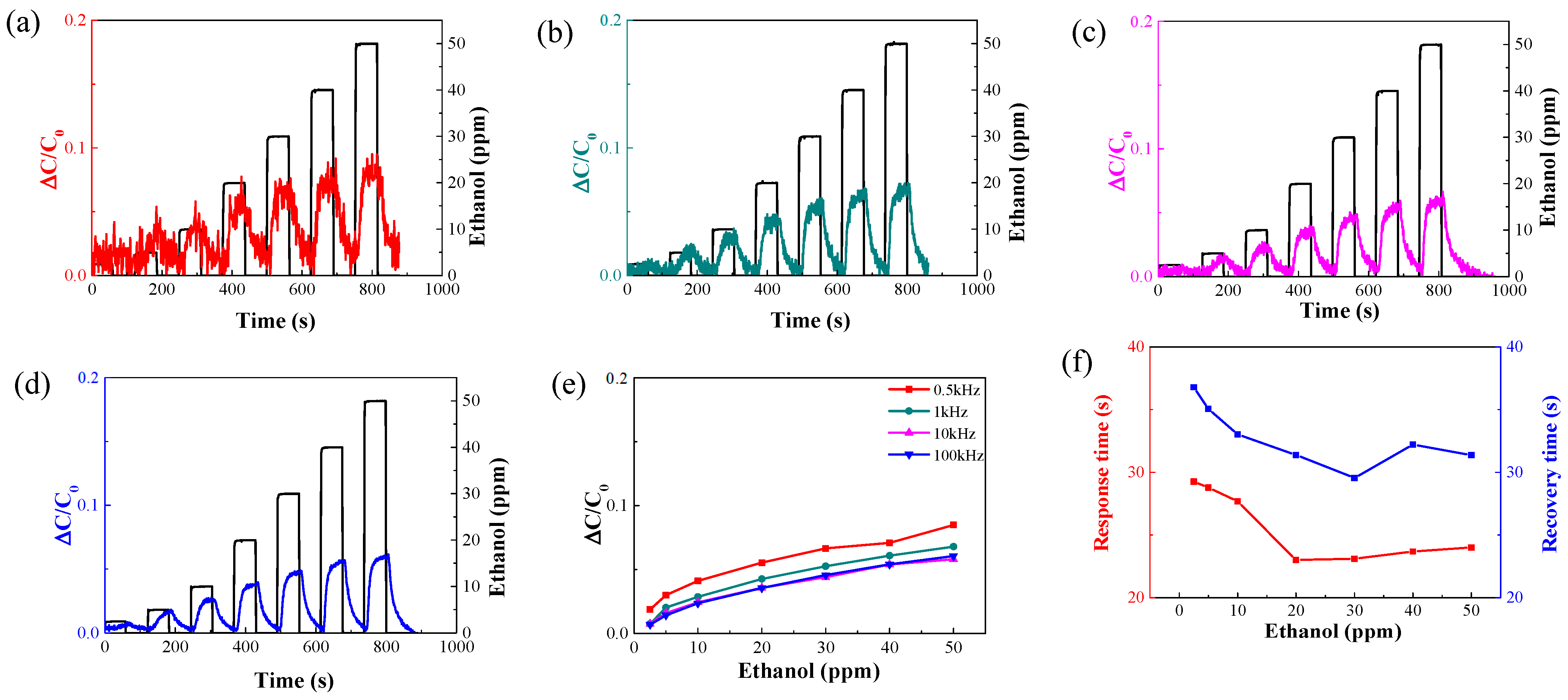
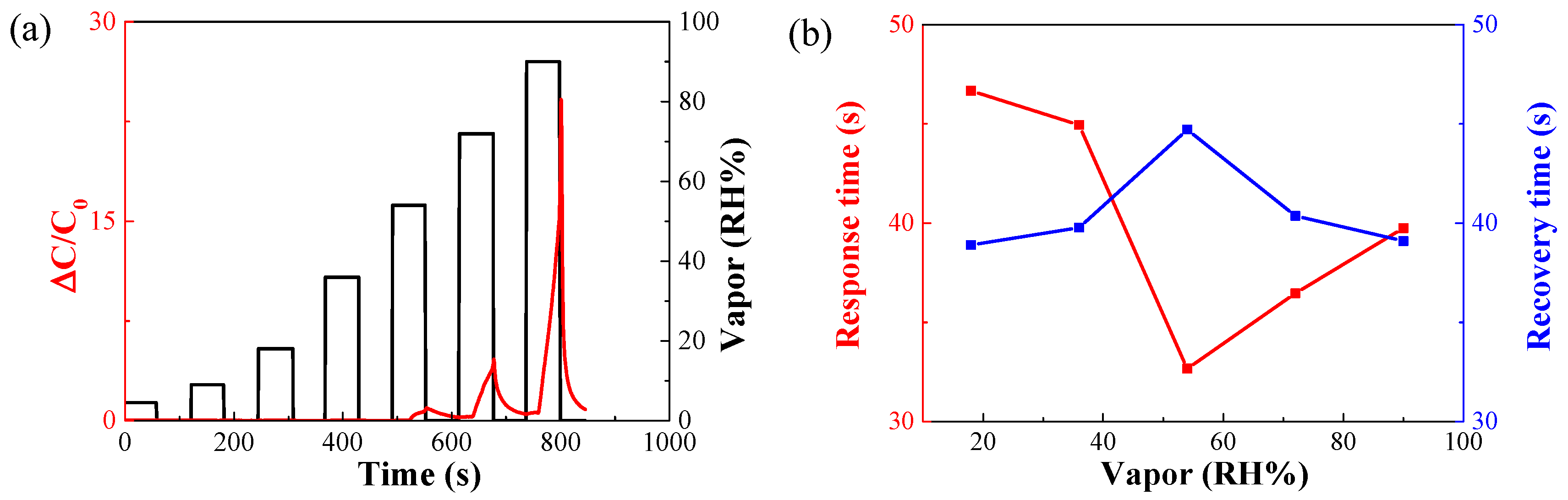
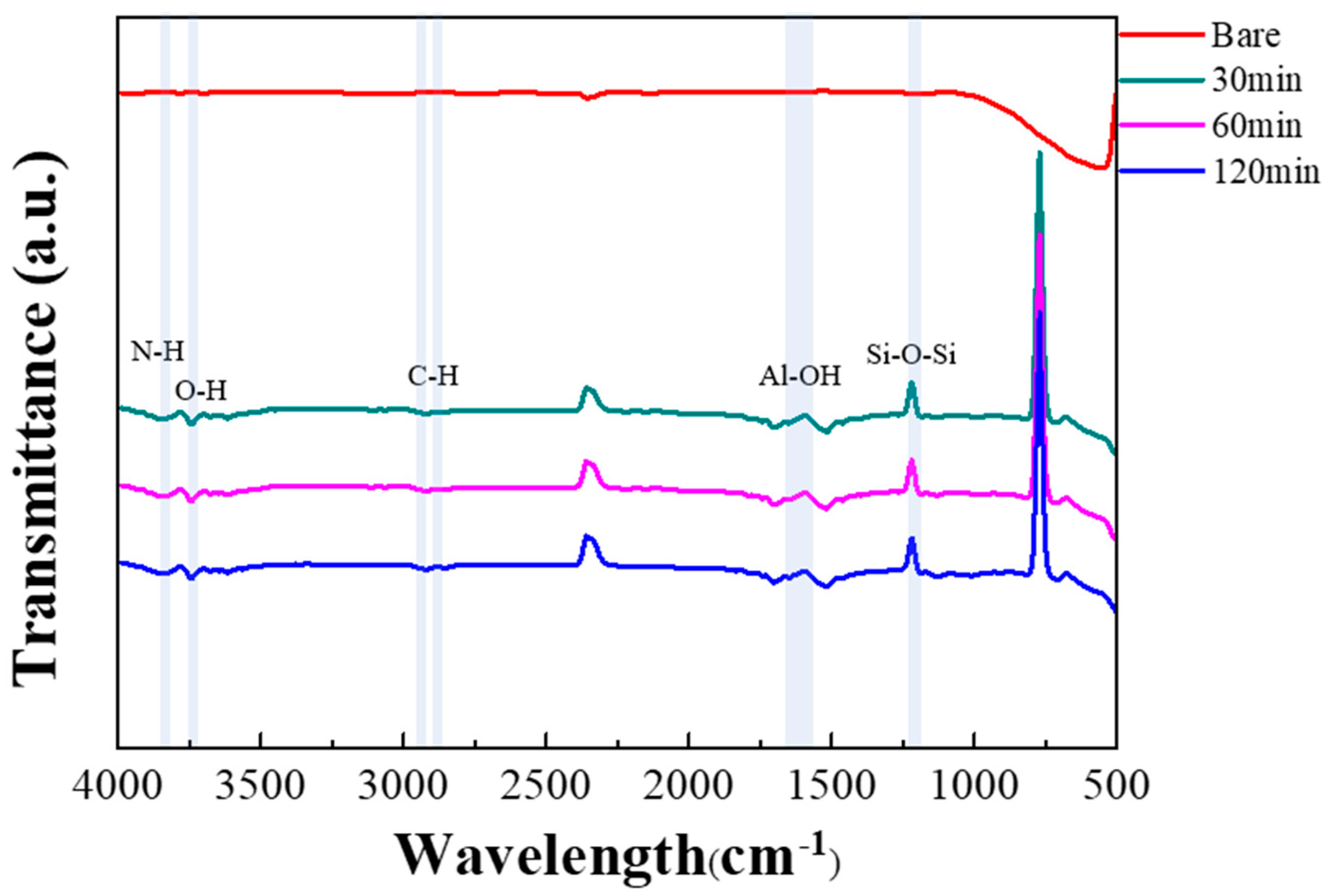


| Concentration [ppm] | Ethanol [sccm] | Dry Air [sccm] |
|---|---|---|
| 2.5 | 2.5 | 497.5 |
| 5 | 5 | 495 |
| 10 | 10 | 490 |
| 20 | 20 | 480 |
| 30 | 30 | 470 |
| 40 | 40 | 460 |
| 50 | 50 | 450 |
| Concentration [RH] | Wet Air [sccm] | Dry Air [sccm] |
|---|---|---|
| 4.5% | 25 | 475 |
| 9% | 50 | 450 |
| 18% | 100 | 400 |
| 36% | 200 | 300 |
| 54% | 300 | 200 |
| 72% | 400 | 100 |
| 90% | 500 | 0 |
| Sensing Material | C (ppm) | Response | Res/Rec Time (s) | T (℃) | Ref. |
|---|---|---|---|---|---|
| Cobalt ferrite | 7 | 1.4 | 180/180 | 31 | [40] |
| Cu-BTC nanoporous | 500 | 7.5 | about 600/180 | 25 | [41] |
| Cu-BTC thin film | 250 | 48.6 | about 140/140 | 25 | [42] |
| AAO | 10 | 0.05 | 27.54/26.26 | 25 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, G.-H.; Kim, I.-Y.; Park, J.-Y.; Choa, Y.-H.; Lim, J.-H. Anodic Aluminum Oxide-Based Chemi-Capacitive Sensor for Ethanol Gas. Nanomaterials 2024, 14, 70. https://doi.org/10.3390/nano14010070
Lim G-H, Kim I-Y, Park J-Y, Choa Y-H, Lim J-H. Anodic Aluminum Oxide-Based Chemi-Capacitive Sensor for Ethanol Gas. Nanomaterials. 2024; 14(1):70. https://doi.org/10.3390/nano14010070
Chicago/Turabian StyleLim, Gi-Hwan, In-Yea Kim, Ji-Young Park, Yong-Ho Choa, and Jae-Hong Lim. 2024. "Anodic Aluminum Oxide-Based Chemi-Capacitive Sensor for Ethanol Gas" Nanomaterials 14, no. 1: 70. https://doi.org/10.3390/nano14010070
APA StyleLim, G.-H., Kim, I.-Y., Park, J.-Y., Choa, Y.-H., & Lim, J.-H. (2024). Anodic Aluminum Oxide-Based Chemi-Capacitive Sensor for Ethanol Gas. Nanomaterials, 14(1), 70. https://doi.org/10.3390/nano14010070






