Stable Supercapacitors Based on Activated Carbon Prepared from Italian Orange Juice
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation Process
2.3. Characterization
3. Results and Discussions
3.1. Brunauer–Emmett–Teller (BET) Analysis
3.2. Morphological Analysis
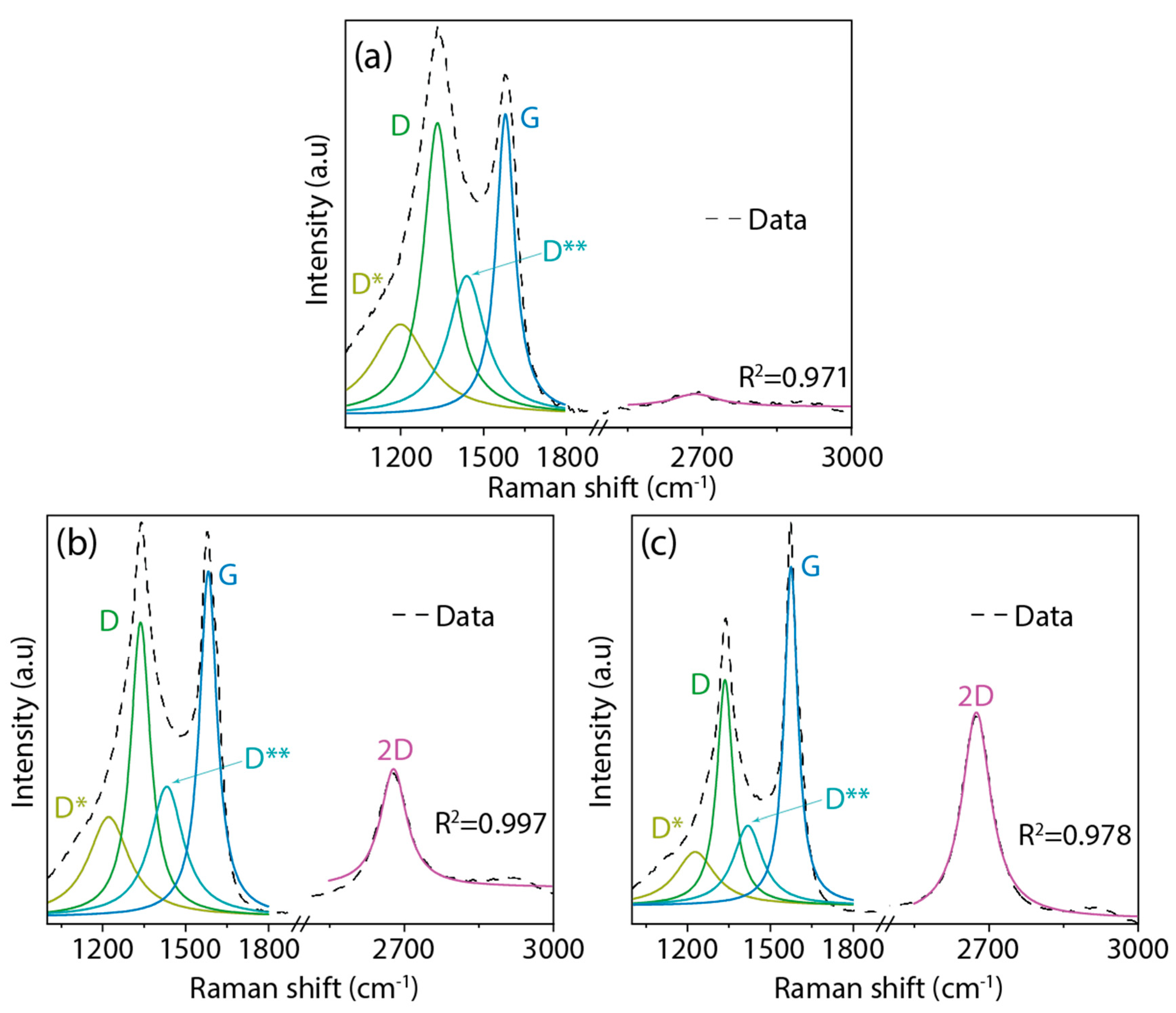
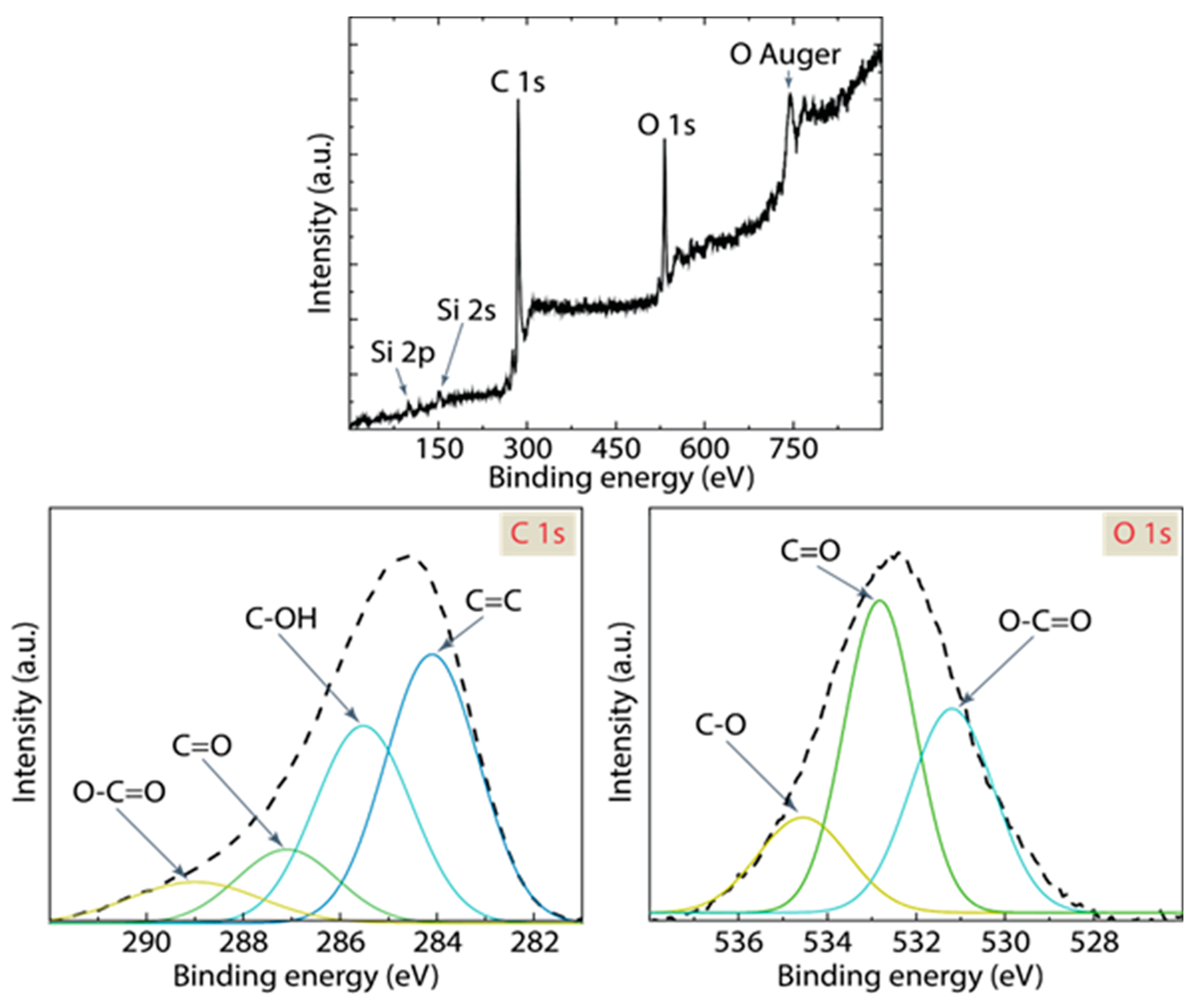
3.3. Spectroscopic Analysis
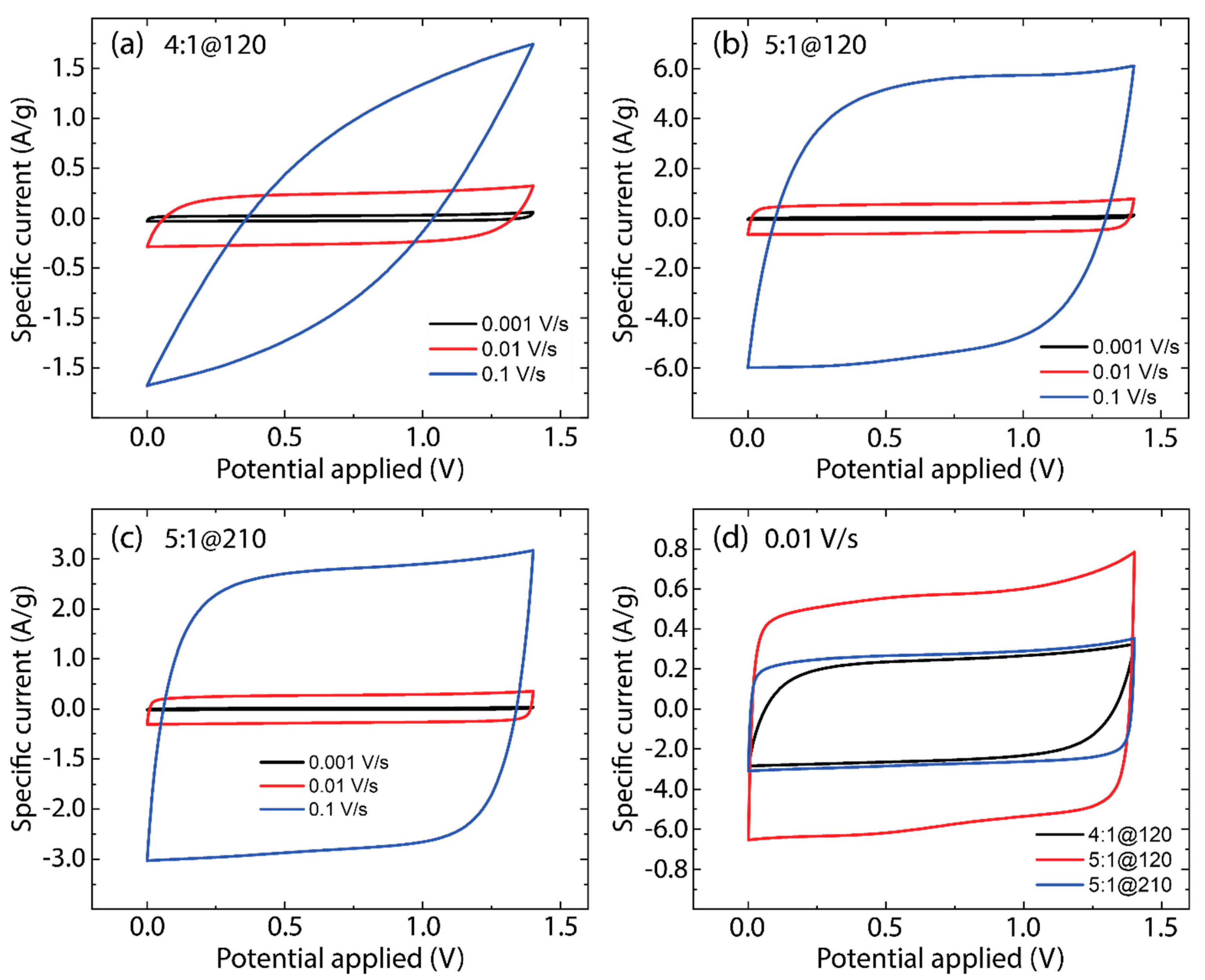
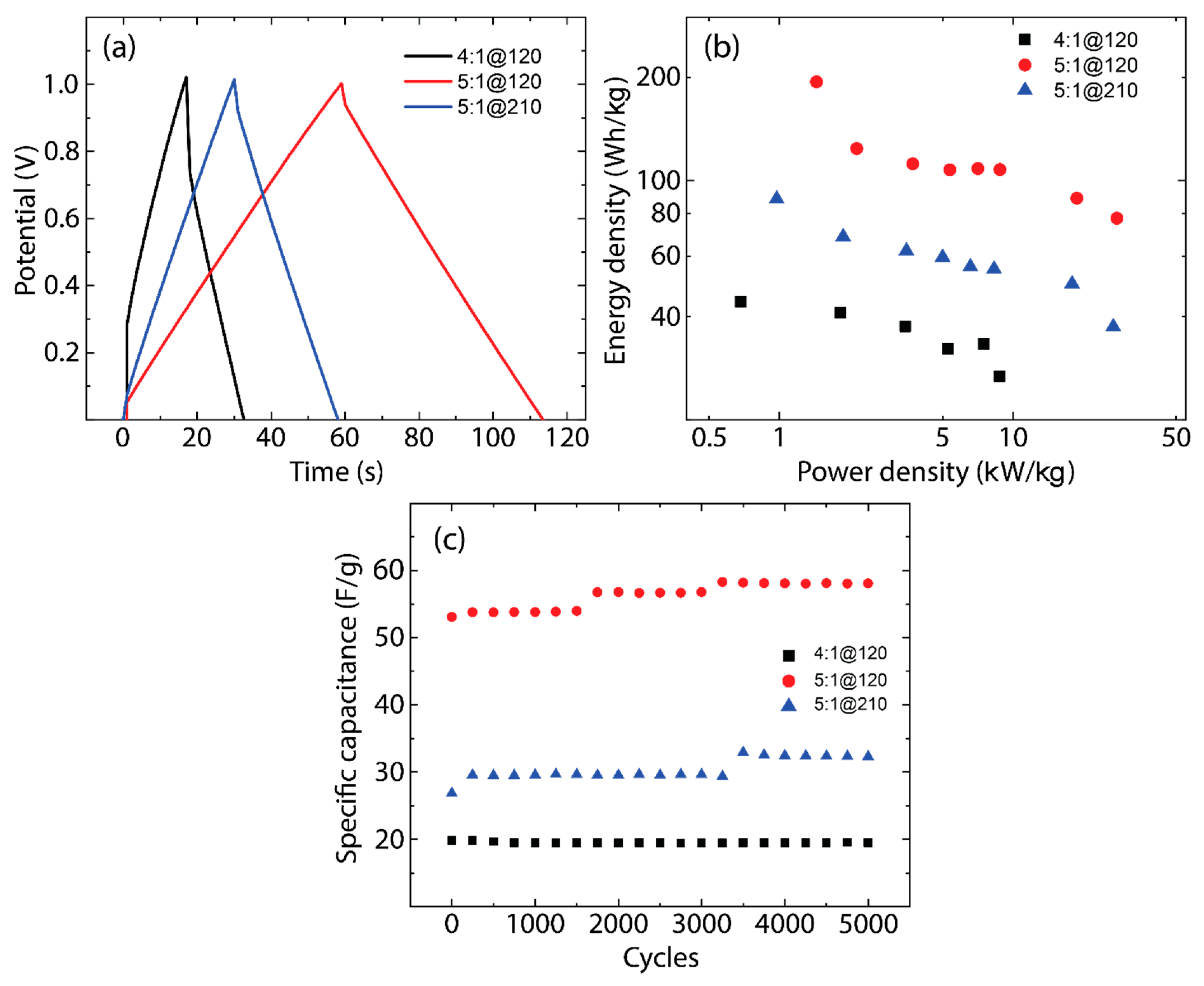
3.4. Electrochemical Analysis
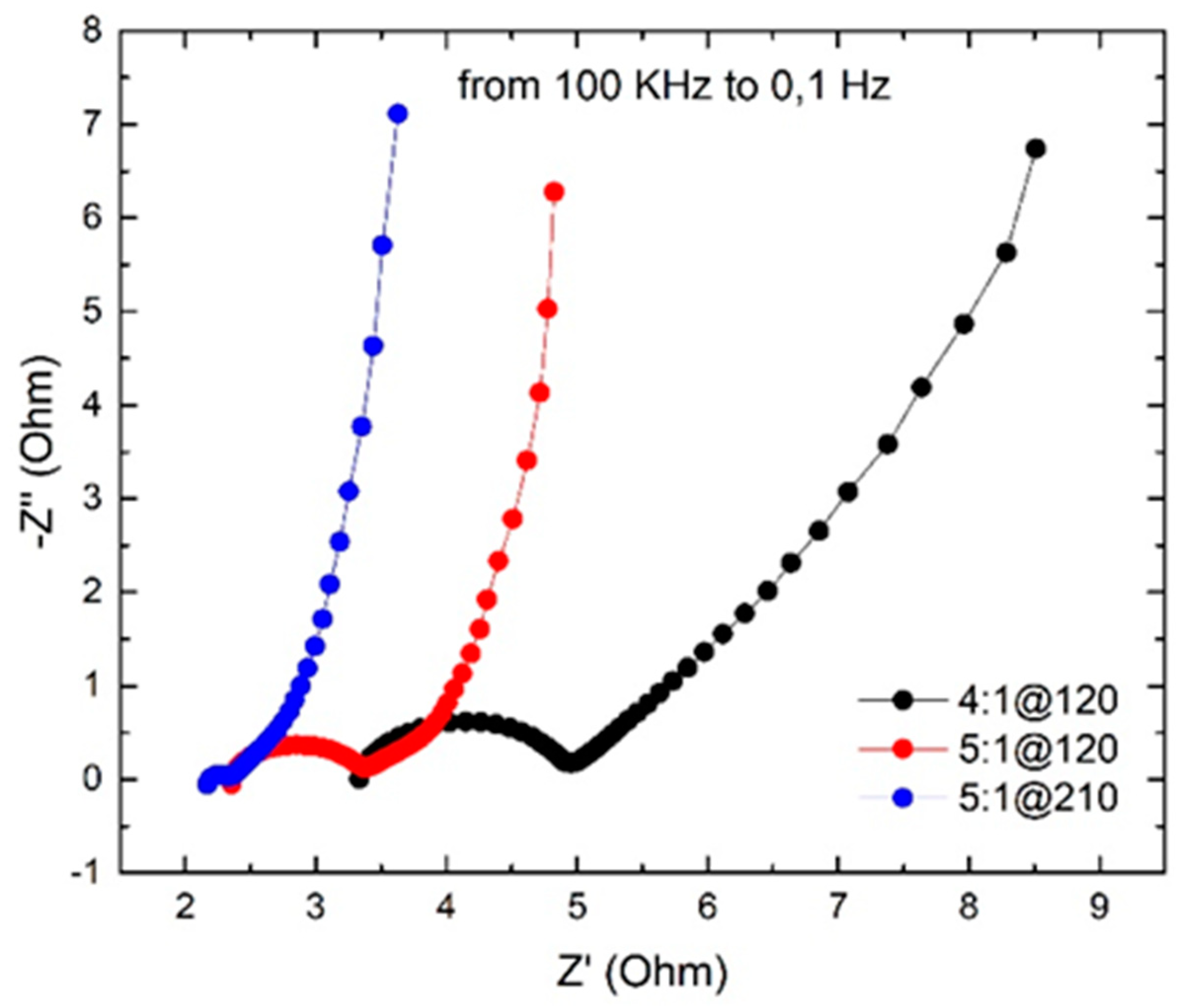
3.5. Electrochemical Impedance Spectroscopy (EIS)
4. A Comparison with Previous Literature
| Precursor | SSA [m2/g] | Electrolyte | Cell Type | [F/g] | Ref. |
|---|---|---|---|---|---|
| Waste compact disc | 1390 | 1 M EMIMBF4 | Two electrodes | 51 | [52] |
| Rice Husk | 770 | 1 M Et4NBF4 | Two electrodes | 19 | [53] |
| Cotton stalk | 1481 | 1 M Et4NBF4 | Two electrodes | 28.5 (114 normalized to 4 factor) | [50] |
| Palm oil | 1704 | 1 M H2S04 | Two electrodes | 37.25 (149 normalized to 4 factor) | [54] |
| Pinecone | 1515 | 1 M Na2SO4 | Two electrodes | 34.25 (137 normalized to 4 factor) | [55] |
| Cellulose | 1198 | 1 M Na2SO4 | Two electrodes | 26 (96 normalized to 4 factor) | [56] |
| Poultry litter | 3035 | 1 M Na2SO4 | Two electrodes | 41 (164 normalized to 4 factor) | [51] |
| Orange Juice | 2203 | 1 M Na2SO4 | Two electrodes | 56 | This work |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gouda, M.S.; Shehab, M.; Helmy, S.; Soliman, M.; Salama, R.S. Nickel and cobalt oxides supported on activated carbon derived from willow catkin for efficient supercapacitor electrode. J. Energy Storage 2023, 61, 106806. [Google Scholar] [CrossRef]
- Bhat, S.; Uthappa, U.; Sadhasivam, T.; Altalhi, T.; Han, S.S.; Kurkuri, M.D. Abundant cilantro derived high surface area activated carbon (AC) for superior adsorption performances of cationic/anionic dyes and supercapacitor application. Chem. Eng. J. 2023, 459, 141577. [Google Scholar] [CrossRef]
- Said, B.; Bacha, O.; Rahmani, Y.; Harfouche, N.; Kheniche, H.; Zerrouki, D.; Belkhalfa, H.; Henni, A. Activated carbon prepared by hydrothermal pretreatment-assisted chemical activation of date seeds for supercapacitor application. Inorg. Chem. Commun. 2023, 155, 111012. [Google Scholar] [CrossRef]
- Sk, M.; Pradhan, P.; Patra, B.; Guria, A. Green biomass derived porous carbon materials for electrical double-layer capacitors (EDLCs). Mater. Today Chem. 2023, 30, 101582. [Google Scholar] [CrossRef]
- You, W.; Li, M.; Li, Q.; Jiang, J.; Xiang, K.; Xie, M. Oxygen defect-mediated NiCo2O4 nanosheets as the electrode for pseudocapacitors with improved rate capability. Phys. Chem. Chem. Phys. 2023, 25, 24862–24870. [Google Scholar] [CrossRef]
- Dutta, A.; Mitra, S.; Basak, M.; Banerjee, T. A comprehensive review on batteries and supercapacitors: Development and challenges since their inception. Energy Storage 2022, 5, e339. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Devi, S.B.; Vignesh, V.; Kumar, P.V.; Oh, M.S.; Navamathavan, R. Transport supercapacitors. In Smart Supercapacitors; Elsevier: Amsterdam, The Netherlands, 2023; pp. 503–534. [Google Scholar] [CrossRef]
- Satpathy, S.; Misra, N.K.; Shukla, D.K.; Goyal, V.; Bhattacharyya, B.K.; Yadav, C.S. An in-depth study of the electrical characterization of supercapacitors for recent trends in energy storage system. J. Energy Storage 2023, 57, 106198. [Google Scholar] [CrossRef]
- Ma, T.; Yang, H.; Lu, L. Development of hybrid battery–supercapacitor energy storage for remote area renewable energy systems. Appl. Energy 2015, 153, 56–62. [Google Scholar] [CrossRef]
- Popoola, I.K.; Gondal, M.A.; Popoola, A.; Oloore, L.E.; Younas, M. Inorganic perovskite photo-assisted supercapacitor for single device energy harvesting and storage applications. J. Energy Storage 2023, 73, 108828. [Google Scholar] [CrossRef]
- Al-Furjan, M.; Qi, Z.; Shan, L.; Farrokhian, A.; Shen, X.; Kolahchi, R. Nano supercapacitors with practical application in aerospace technology: Vibration and wave propagation analysis. Aerosp. Sci. Technol. 2023, 133, 108082. [Google Scholar] [CrossRef]
- Reddy, P.H.; Amalraj, J.; Ranganatha, S.; Patil, S.S.; Chandrasekaran, S. A review on effect of conducting polymers on carbon-based electrode materials for electrochemical supercapacitors. Synth. Met. 2023, 298, 117447. [Google Scholar] [CrossRef]
- Deng, W.; Liu, W.; Zhu, H.; Chen, L.; Liao, H.; Chen, H. Click-chemistry and ionic cross-linking induced double cross-linking ionogel electrolyte for flexible lithium-ion batteries. J. Energy Storage 2023, 72, 108509. [Google Scholar] [CrossRef]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2018, 101, 123–145. [Google Scholar] [CrossRef]
- Schütter, C.; Pohlmann, S.; Balducci, A. Industrial Requirements of Materials for Electrical Double Layer Capacitors: Impact on Current and Future Applications. Adv. Energy Mater. 2019, 9, 1900334. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, Y.; Lou, X.; Chen, H.; Jiang, S.; Zhou, W. Vanadium oxide/carbonized chestnut needle composites as cathode materials for advanced aqueous zinc-ion batteries. J. Energy Storage 2024, 77, 109859. [Google Scholar] [CrossRef]
- Yao, Y.; Ge, D.; Yu, Y.; Zhang, Y.; Du, C.; Ye, H.; Wan, L.; Chen, J.; Xie, M. Filling macro/mesoporosity of commercial activated carbon enables superior volumetric supercapacitor performances. Microporous Mesoporous Mater. 2023, 350, 112446. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumar, S.; Shaalan, N.M.; Arshi, N.; Dalela, S.; Chae, K.H. Fabrication of High-Performance Asymmetric Supercapacitors Using Rice Husk-Activated Carbon and MnFe2O4 Nanostructures. Nanomaterials 2023, 13, 1870. [Google Scholar] [CrossRef]
- Yue, Z.; Economy, J. Carbonization and activation for production of activated carbon fibers. In Activated Carbon Fiber and Textiles; Woodhead Publishing: Sawston, UK, 2017; pp. 61–139. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N. Activated carbon from biomass: Preparation, factors improving basicity and surface properties for enhanced CO2 capture capacity—A review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Hussain, O.A.; Hathout, A.S.; Abdel-Mobdy, Y.E.; Rashed, M.; Rahim, E.A.; Fouzy, A. Preparation and characterization of activated carbon from agricultural wastes and their ability to remove chlorpyrifos from water. Toxicol. Rep. 2023, 10, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Chung, E.; Shah, H.H. Effect of different activation agents for activated carbon preparation through characterization and life cycle assessment. Int. J. Environ. Sci. Technol. 2022, 20, 7645–7656. [Google Scholar] [CrossRef]
- Veltri, F.; Alessandro, F.; Scarcello, A.; Beneduci, A.; Polanco, M.A.; Perez, D.C.; Gomez, C.V.; Tavolaro, A.; Giordano, G.; Caputi, L.S. Porous carbon materials obtained by the hydrothermal carbonization of orange juice. Nanomaterials 2020, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Negro, V.; Ruggeri, B.; Fino, D.; Tonini, D. Life cycle assessment of orange peel waste management. Resour. Conserv. Recycl. 2017, 127, 148–158. [Google Scholar] [CrossRef]
- Tardiolo, G.; Nicolò, M.S.; Drago, C.; Genovese, C.; Fava, G.; Gugliandolo, C.; D’antona, N. Orange Peel Waste as Feedstock for the Production of Glycerol-Free Biodiesel by the Microalgae Nannochloropsis oculata. Molecules 2023, 28, 6846. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, A.; Agovino, M.; Ferraro, A.; Mariani, A. Household food waste: A case study in southern Italy. Sustainability 2020, 12, 1495. [Google Scholar] [CrossRef]
- Carota, E.; Petruccioli, M.; D’annibale, A.; Gallo, A.M.; Crognale, S. Orange peel waste–based liquid medium for biodiesel production by oleaginous yeasts. Appl. Microbiol. Biotechnol. 2020, 104, 4617–4628. [Google Scholar] [CrossRef]
- Fazal-ur-Rehman, M. Methodological trends in preparation of activated carbon from local sources and their impacts on production: A review. Chem. Int. 2018, 4, 109–119. [Google Scholar]
- Angin, D. Utilization of activated carbon produced from fruit juice industry solid waste for the adsorption of Yellow 18 from aqueous solutions. Bioresour. Technol. 2014, 168, 259–266. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Santhosh, R.; Jeniffer, S.; Raghavan, V.; Jacob, G.; Nanaji, K.; Kollu, P.; Jeong, S.K.; Grace, A.N. Natural biomass derived hard carbon and activated carbons as electrochemical supercapacitor electrodes. Sci. Rep. 2019, 9, 16315. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2014, 8, 702–730. [Google Scholar] [CrossRef]
- Du, X.; Qin, Z.; Li, Z. Free-standing rGO-CNT nanocomposites with excellent rate capability and cycling stability for Na2SO4 aqueous electrolyte supercapacitors. Nanomaterials 2021, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Ding, Z.; Wei, Q.; Wang, Z.; Yang, X.; Qiu, J. Biomass-based hierarchical porous carbon for supercapacitors: Effect of aqueous and organic electrolytes on the electrochemical performance. ChemSusChem 2019, 12, 5099–5110. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Jin, B.; Tang, H.; Ma, L.; Zhang, R.; Ran, J.; Zhang, H. Optimizing porous structure of carbon electrodes for temperature-independent capacitance at sub-zero temperatures. Chem. Eng. J. 2022, 441, 136053. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, N.; Razal, J.M.; Zheng, Y.; Zhou, X.; Ding, J.; Cho, K.; Ge, S.; Zhang, R.; Gogotsi, Y.; et al. Temperature-independent capacitance of carbon-based supercapacitor from −100 to 60 °C. Energy Storage Mater. 2019, 22, 323–329. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Wang, D.; Pan, Z.; Lu, Z. From starch to porous carbon nanosheets: Promising cathodes for high-performance aqueous Zn-ion hybrid supercapacitors. Microporous Mesoporous Mater. 2020, 306, 110445. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem.–Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Tene, T.; Guevara, M.; Valarezo, A.; Salguero, O.; Arias, F.A.; Arias, M.; Scarcello, A.; Caputi, L.S.; Gomez, C.V. Drying-time study in graphene oxide. Nanomaterials 2021, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Shroder, R.E.; Nemanich, R.J.; Glass, J.T. Analysis of the composite structures in diamond thin films by Raman spectroscopy. Phys. Rev. B 1990, 41, 3738–3745. [Google Scholar] [CrossRef] [PubMed]
- Kratochvílová, I.; Škoda, R.; Škarohlíd, J.; Ashcheulov, P.; Jäger, A.; Racek, J.; Taylor, A.; Shao, L. Nanosized polycrystalline diamond cladding for surface protection of zirconium nuclear fuel tubes. J. Am. Acad. Dermatol. 2014, 214, 2600–2605. [Google Scholar] [CrossRef]
- Strange, L.E.; Engelhard, M.H.; Easton, C.D.; Kim, J.; Baer, D.R. Surface analysis insight note: X-ray photoelectron spectroscopy analysis of battery electrodes—Challenges with nickel–manganese–cobalt and Li examples using an Al Kα X-ray source. Surf. Interface Anal. 2023, 55, 715–729. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Orange-peel-derived carbon: Designing sustainable and high-performance supercapacitor electrodes. C 2017, 3, 25. [Google Scholar] [CrossRef]
- Chen, M.; Kang, X.; Wumaier, T.; Dou, J.; Gao, B.; Han, Y.; Xu, G.; Liu, Z.; Zhang, L. Preparation of activated carbon from cotton stalk and its application in supercapacitor. J. Solid State Electrochem. 2012, 17, 1005–1012. [Google Scholar] [CrossRef]
- Pontiroli, D.; Scaravonati, S.; Magnani, G.; Fornasini, L.; Bersani, D.; Bertoni, G.; Milanese, C.; Girella, A.; Ridi, F.; Verucchi, R.; et al. Super-activated biochar from poultry litter for high-performance supercapacitors. Microporous Mesoporous Mater. 2019, 285, 161–169. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Bhat, B.R.; Sahajwalla, V. Performance of an activated carbon supercapacitor electrode synthesised from waste Compact Discs (CDs). J. Ind. Eng. Chem. 2018, 65, 387–396. [Google Scholar] [CrossRef]
- Kuratani, K.; Okuno, K.; Iwaki, T.; Kato, M.; Takeichi, N.; Miyuki, T.; Awazu, T.; Majima, M.; Sakai, T. Converting rice husk activated carbon into active material for capacitor using three-dimensional porous current collector. J. Power Sources 2011, 196, 10788–10790. [Google Scholar] [CrossRef]
- Farma, R.; Deraman, M.; Awitdrus, A.; Talib, I.; Taer, E.; Basri, N.; Manjunatha, J.; Ishak, M.; Dollah, B.; Hashmi, S. Preparation of highly porous binderless activated carbon electrodes from fibres of oil palm empty fruit bunches for application in supercapacitors. Bioresour. Technol. 2013, 132, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.; Manyala, N.; Barzegar, F.; Khaleed, A.A.; Momodu, D.Y.; Dangbegnon, J.K. Renewable pine cone biomass derived carbon materials for supercapacitor application. RSC Adv. 2015, 6, 1800–1809. [Google Scholar] [CrossRef]
- Fischer, J.; Pohle, B.; Dmitrieva, E.; Thümmler, K.; Fischer, S.; Mikhailova, D. Symmetric supercapacitors with cellulose-derived carbons and Na2SO4 electrolytes operating in a wide temperature range. J. Energy Storage 2022, 55, 105725. [Google Scholar] [CrossRef]
- Fan, Z.-S.; Kaneti, Y.V.; Chowdhury, S.; Wang, X.; Karim, M.R.; Alnaser, I.A.; Zhang, F.-B. Weak base-modulated synthesis of bundle-like carbon superstructures from metal-organic framework for high-performance supercapacitors. Chem. Eng. J. 2023, 462, 142094. [Google Scholar] [CrossRef]
- Ahmad, F.; Zahid, M.; Jamil, H.; Khan, M.A.; Atiq, S.; Bibi, M.; Shahbaz, K.; Adnan, M.; Danish, M.; Rasheed, F.; et al. Advances in graphene-based electrode materials for high-performance supercapacitors: A review. J. Energy Storage 2023, 72, 108731. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Strategies to enhance the performance of electrochemical capacitors based on carbon materials. Front. Mater. 2019, 6, 115. [Google Scholar] [CrossRef]
- Hou, P.; Gao, C.; Wang, J.; Zhang, J.; Liu, Y.; Gu, J.; Huo, P. A semi-transparent polyurethane/porous wood composite gel polymer electrolyte for solid-state supercapacitor with high energy density and cycling stability. Chem. Eng. J. 2023, 454, 139954. [Google Scholar] [CrossRef]
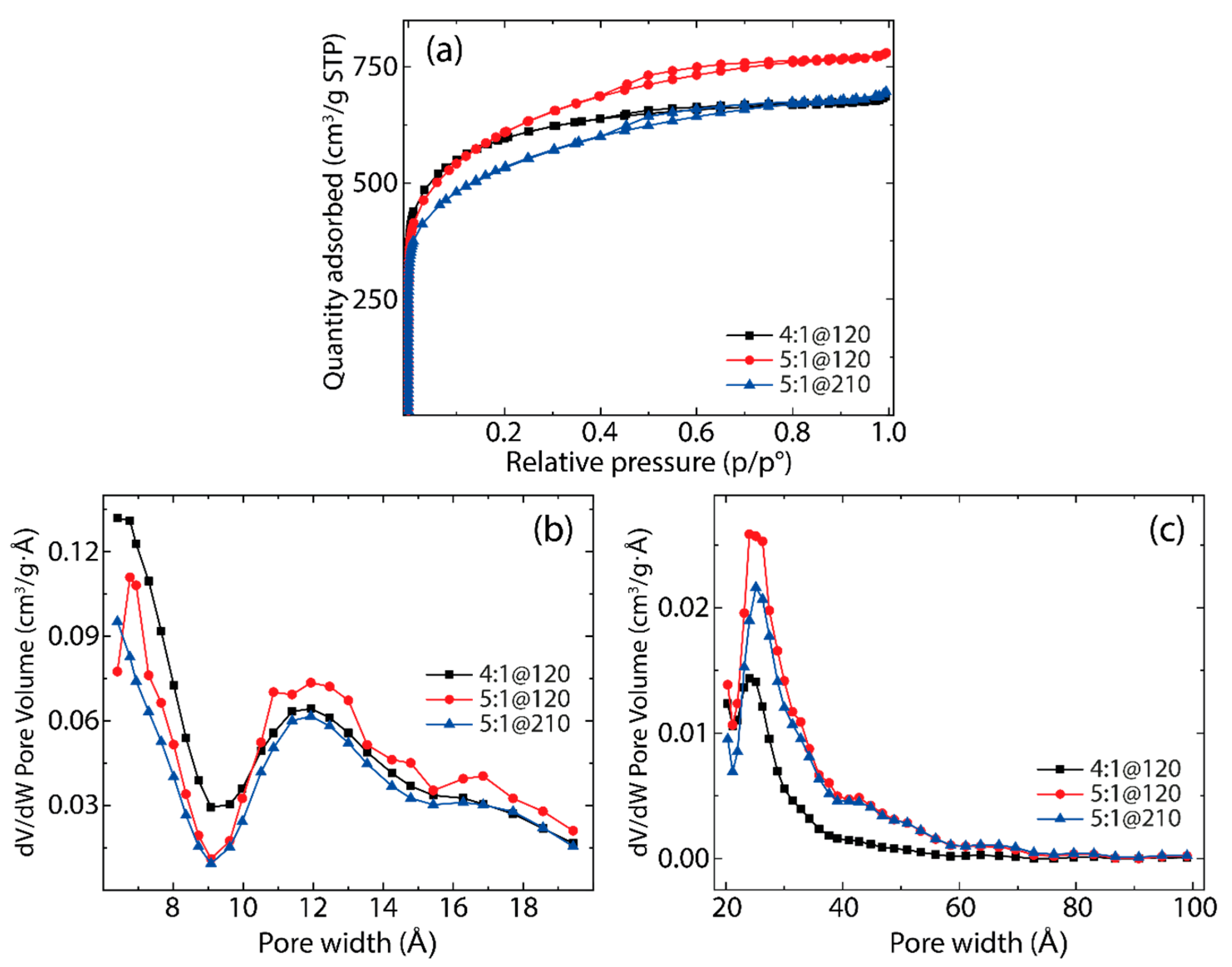
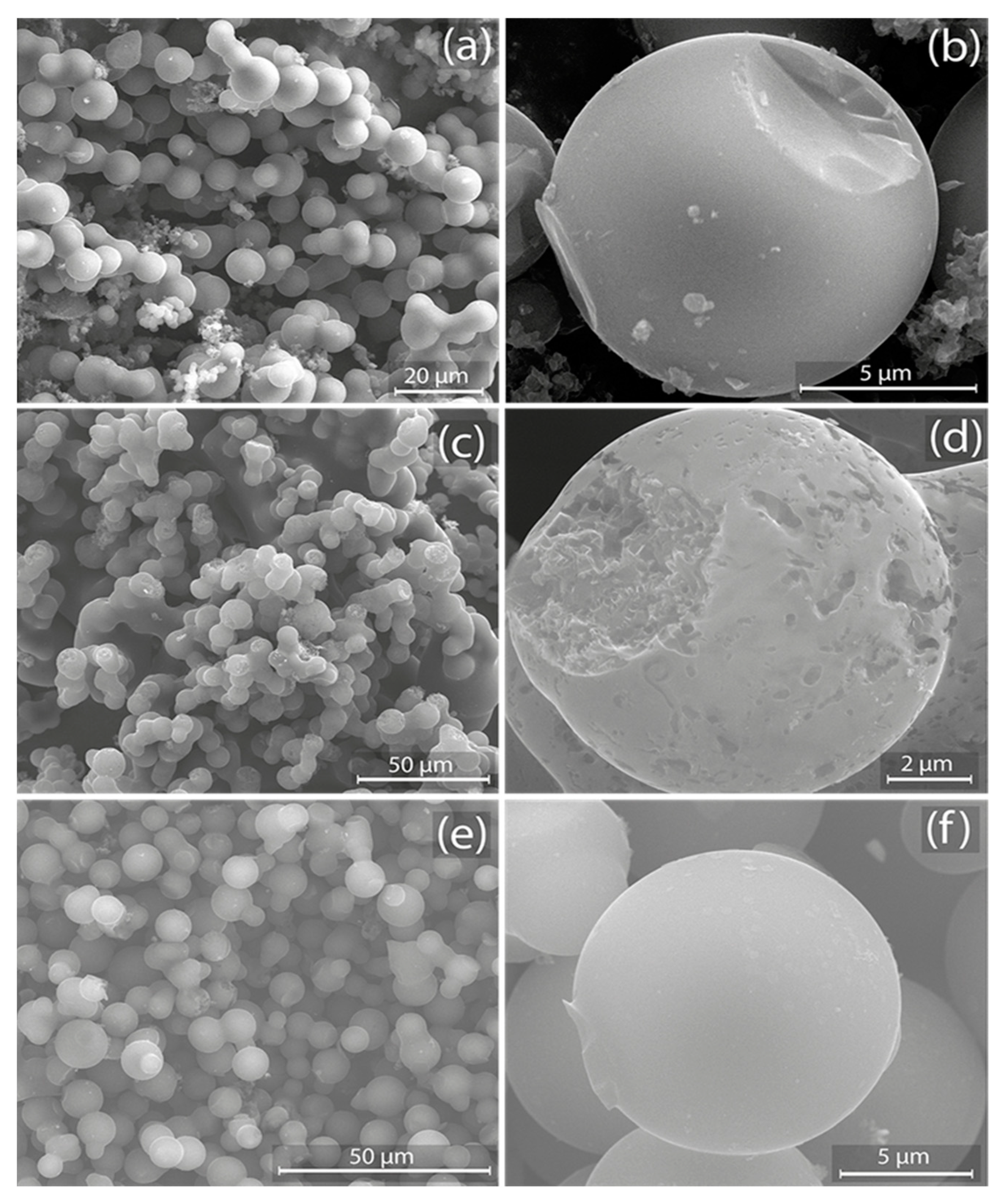

| Activation Time (min) | |||
|---|---|---|---|
| Ratio (w%) | 30 | 120 | 210 |
| 3:1 | 1271.58 ± 14.22 | 1590.11 ± 7.75 | 1779.68 ± 8.91 |
| 4:1 | 1865.69 ± 12.95 | 2195.85 ± 7.66 | 1573.91 ± 6.26 |
| 5:1 | 1309.97 ± 12.64 | 2202.81 ± 7.43 | 1926.69 ± 7.42 |
| Sample | ID/IG | I2D/IG |
|---|---|---|
| 4:1@120 | 0.97 | --- |
| 5:1@120 | 0.85 | 0.44 |
| 5:1@210 | 0.68 | 0.60 |
| C 1s | |||
| C=C | 284.10 | 46% | R2 = 0.999 |
| C-OH | 285.52 | 34% | |
| C=O | 287.10 | 13% | |
| O-C=O | 289.00 | 7% | |
| O 1s | |||
| O-C=O | 531.19 | 15% | R2 = 0.999 |
| C=O | 532.82 | 30% | |
| C-O | 534.55 | 55% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarcello, A.; Alessandro, F.; Cruz Salazar, Y.; Arias Polanco, M.; Vacacela Gomez, C.; Tene, T.; Guevara, M.; Bellucci, S.; Straface, S.; Caputi, L.S. Stable Supercapacitors Based on Activated Carbon Prepared from Italian Orange Juice. Nanomaterials 2024, 14, 71. https://doi.org/10.3390/nano14010071
Scarcello A, Alessandro F, Cruz Salazar Y, Arias Polanco M, Vacacela Gomez C, Tene T, Guevara M, Bellucci S, Straface S, Caputi LS. Stable Supercapacitors Based on Activated Carbon Prepared from Italian Orange Juice. Nanomaterials. 2024; 14(1):71. https://doi.org/10.3390/nano14010071
Chicago/Turabian StyleScarcello, Andrea, Francesca Alessandro, Yolenny Cruz Salazar, Melvin Arias Polanco, Cristian Vacacela Gomez, Talia Tene, Marco Guevara, Stefano Bellucci, Salvatore Straface, and Lorenzo S. Caputi. 2024. "Stable Supercapacitors Based on Activated Carbon Prepared from Italian Orange Juice" Nanomaterials 14, no. 1: 71. https://doi.org/10.3390/nano14010071
APA StyleScarcello, A., Alessandro, F., Cruz Salazar, Y., Arias Polanco, M., Vacacela Gomez, C., Tene, T., Guevara, M., Bellucci, S., Straface, S., & Caputi, L. S. (2024). Stable Supercapacitors Based on Activated Carbon Prepared from Italian Orange Juice. Nanomaterials, 14(1), 71. https://doi.org/10.3390/nano14010071












