Design Polyaniline/α-Zirconium Phosphate Composites for Achieving Self-Healing Anti-Corrosion of Carbon Steel
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of α-ZrP
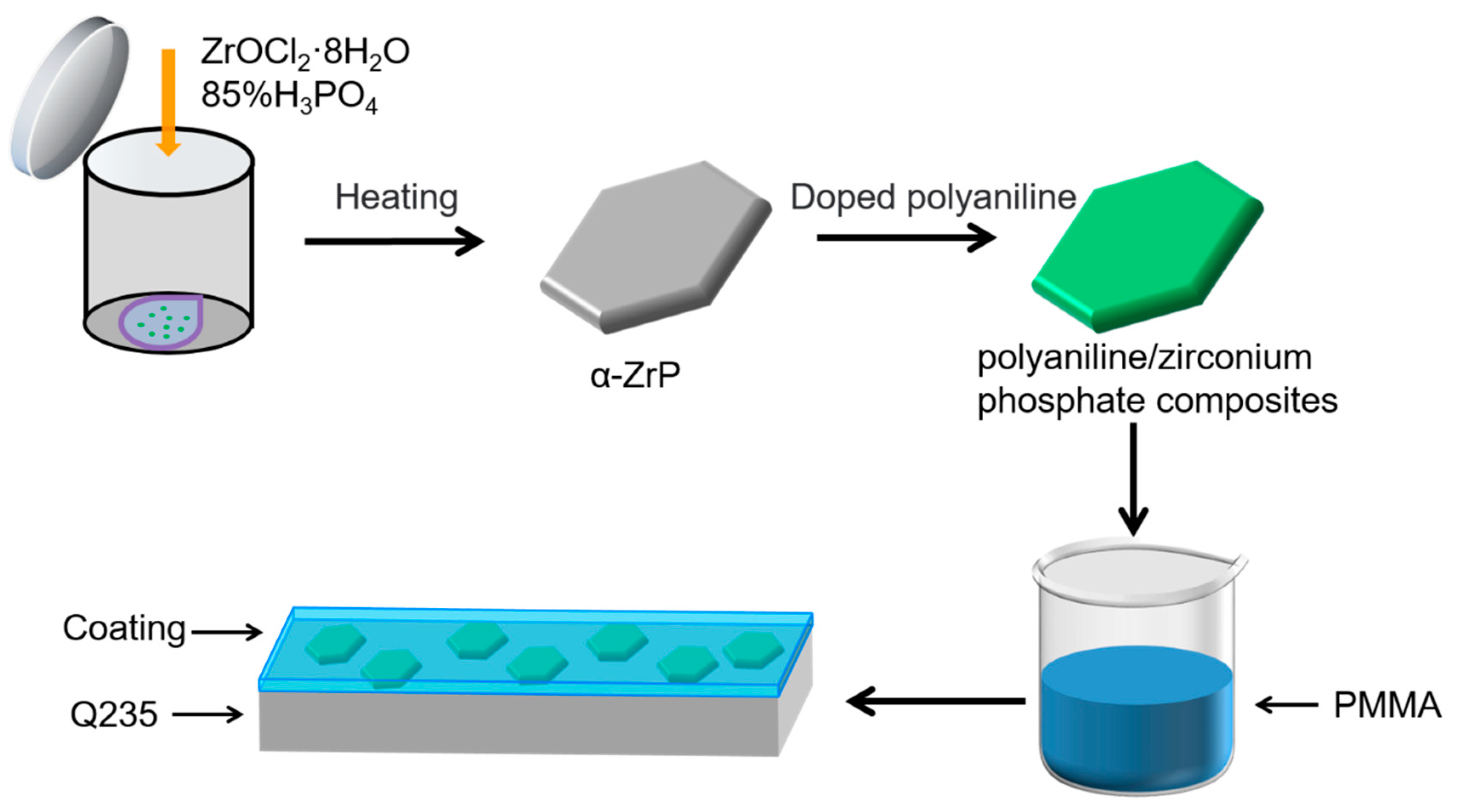
2.3. Preparation of Polyaniline/Zirconium Phosphate Composites
2.4. Preparation of Composite Coatings
2.5. Characterizations
3. Results and Discussions
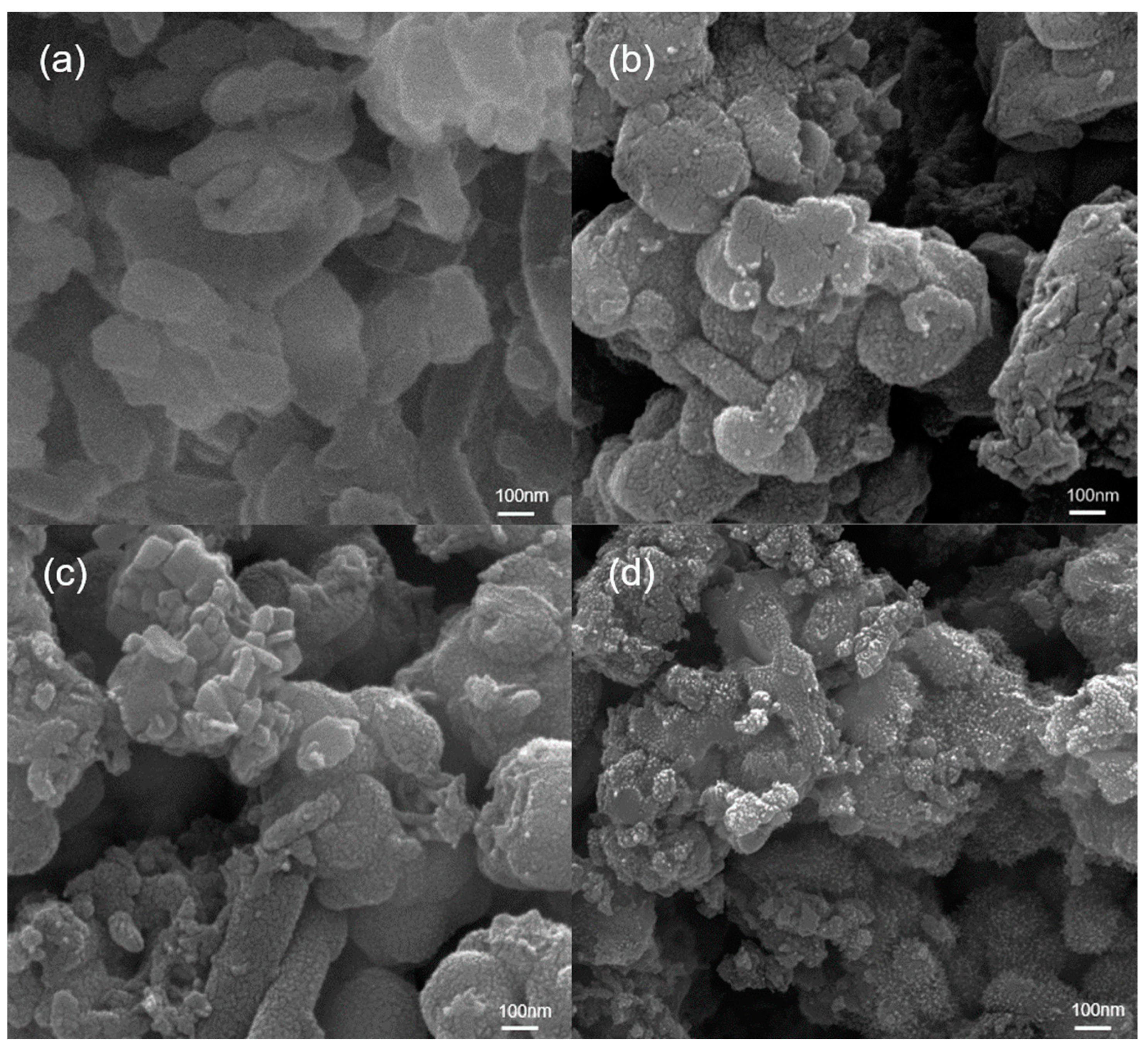
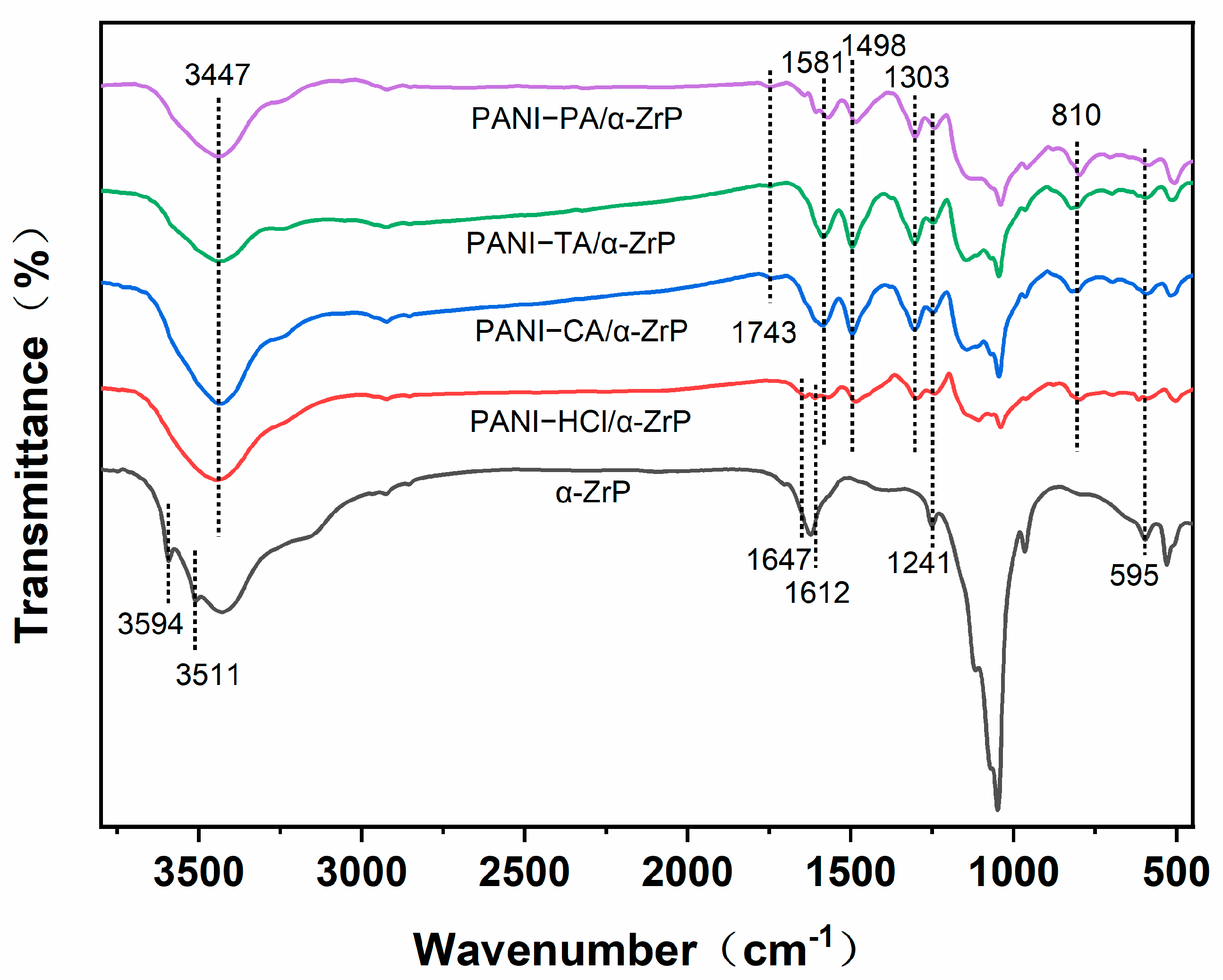
3.1. Morphological and Structure Characteristics of Polyaniline/Zirconium Phosphate Composites
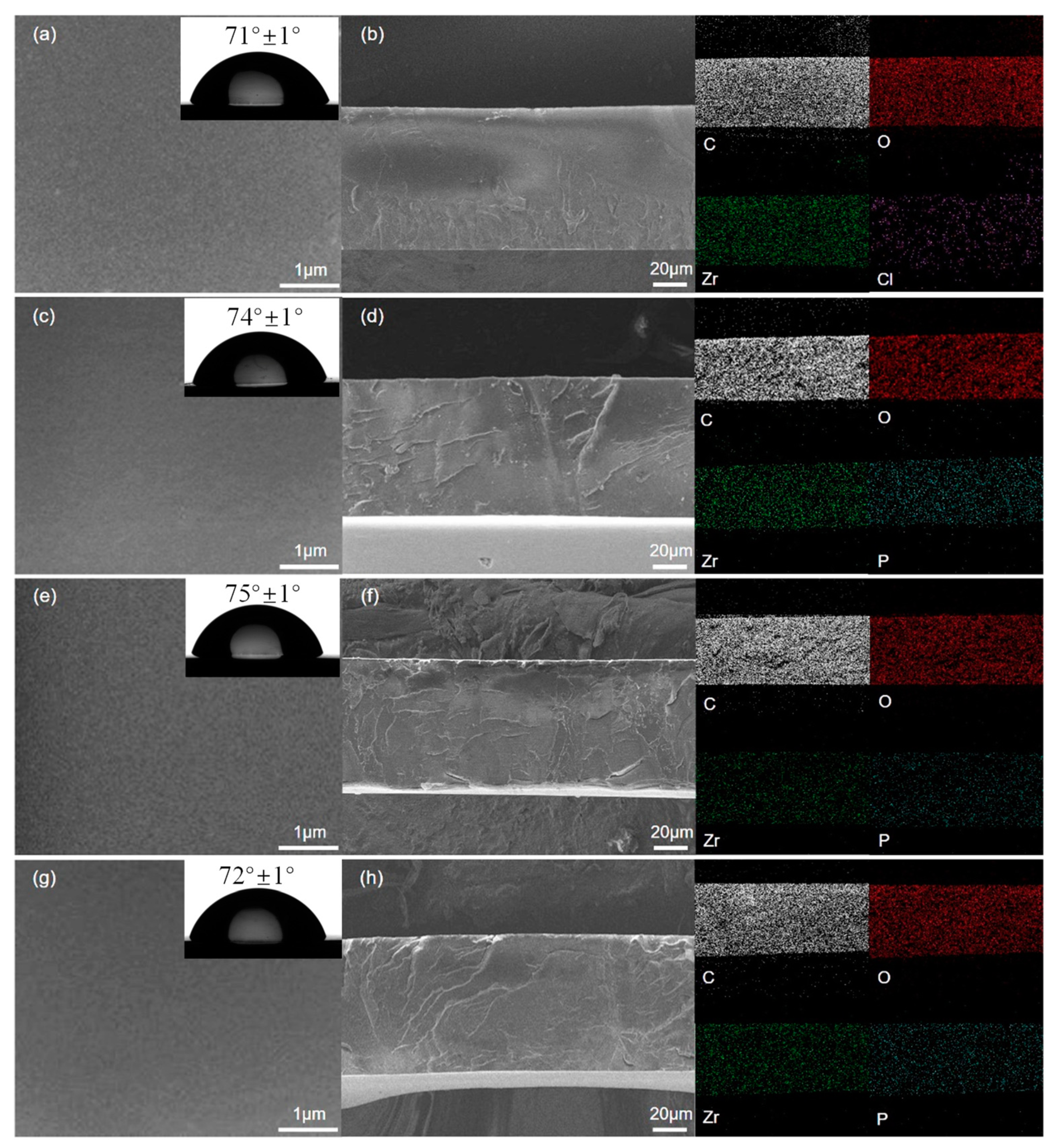
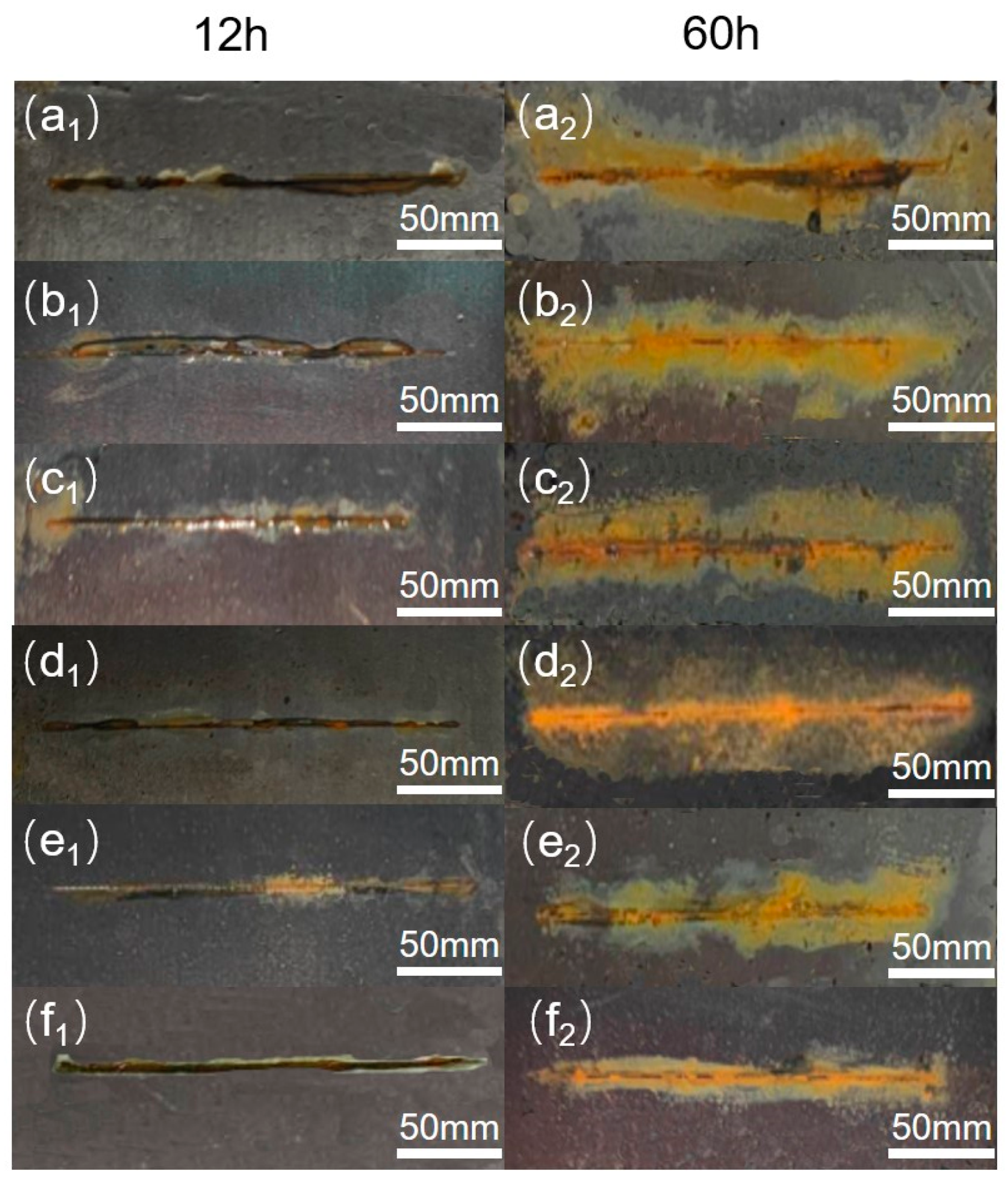
3.2. Anti-Corrosion Performance Composite Coating
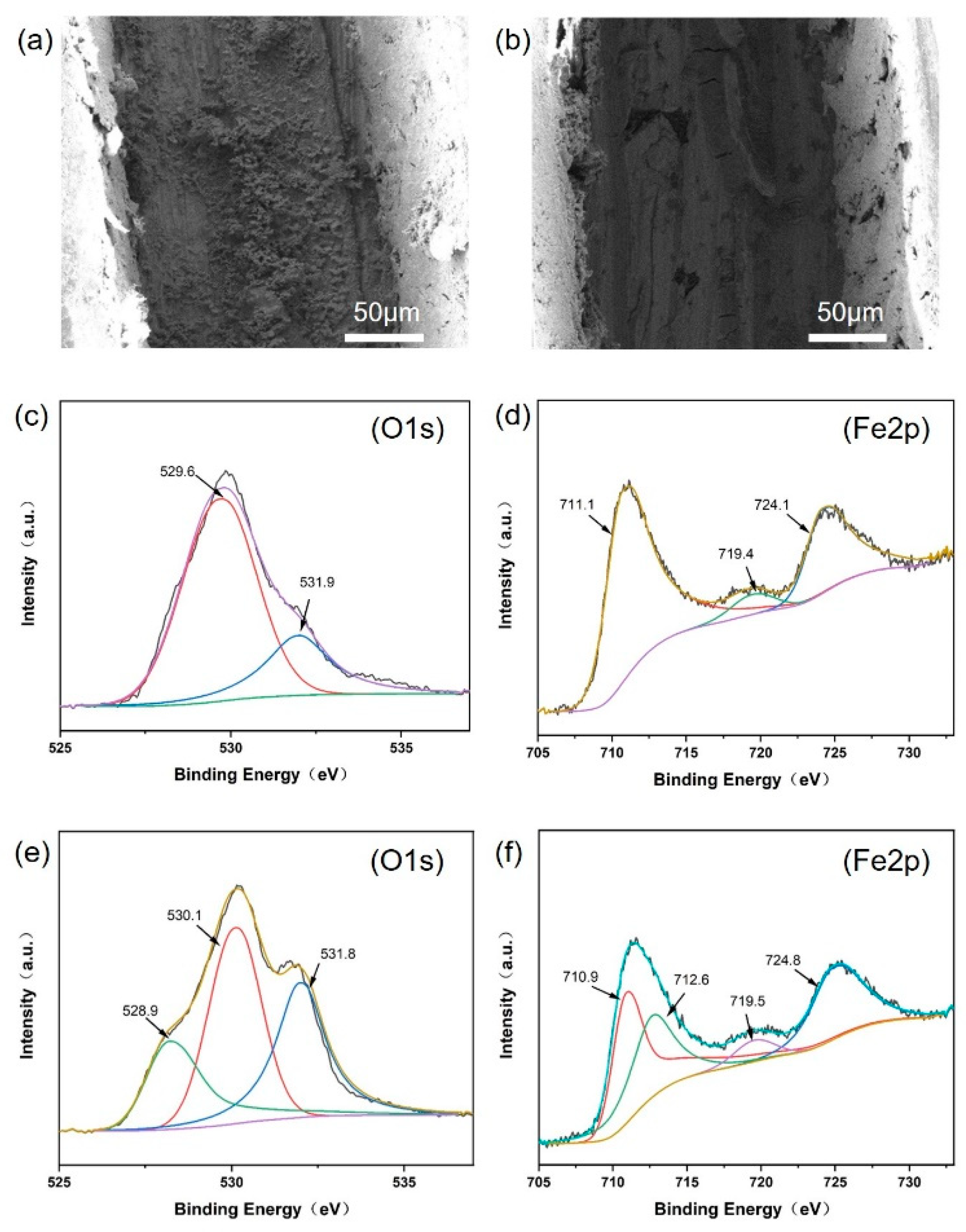
3.3. Anti-Corrosion Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- George, J.S.; Paduvilan, J.K.; Salim, N.; Sunarso, J.; Kalarikkal, N.; Hameed, N.; Thomas, S. Advances and future outlook in epoxy/graphene composites for anticorrosive applications. Prog. Org. Coat. 2022, 162, 106571. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Ansari, K.R.; Saleh, T.A. Graphene and graphene oxide as new class of materials for corrosion control and protection: Present status and future scenario. Prog. Org. Coat. 2020, 147, 105741. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, Y.; Yuan, Y.; Zhou, X.; Liu, Y.; Meng, X. Recent advances of metal–organic frameworks in corrosion protection: From synthesis to applications. Chem. Eng. J. 2022, 430, 132823. [Google Scholar] [CrossRef]
- Youh, M.-J.; Huang, Y.-R.; Peng, C.-H.; Lin, M.-H.; Chen, T.-Y.; Chen, C.-Y.; Liu, Y.-M.; Pu, N.-W.; Liu, B.-Y.; Chou, C.-H.; et al. Using Graphene-Based Composite Materials to Boost Anti-Corrosion and Infrared-Stealth Performance of Epoxy Coatings. Nanomaterials 2021, 11, 1603. [Google Scholar] [CrossRef]
- Nawaz, M.; Shakoor, R.; Kahraman, R.; Montemor, M. Cerium oxide loaded with Gum Arabic as environmentally friendly anti-corrosion additive for protection of coated steel. Mater. Des. 2021, 198, 109361. [Google Scholar] [CrossRef]
- Xiang, T.; Ren, H.; Zhang, Y.; Qiang, Y.; Yang, Y.; Li, C.; Wang, P.; Zhang, S. Rational design of PDMS/paraffin infused surface with enhanced corrosion resistance and interface erosion mechanism. Mater. Des. 2022, 215, 110450. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Tian, Y.; Xie, Y.; Sheng, X.; Jiang, X.; Zhang, X. Exfoliation and functionalization of α-zirconium phosphate in one pot for waterborne epoxy coatings with enhanced anticorrosion performance. Prog. Org. Coat. 2019, 138, 105390. [Google Scholar] [CrossRef]
- Sheng, X.; Mo, R.; Ma, Y.; Zhang, X.; Zhang, L.; Wu, H. Waterborne Epoxy Resin/Polydopamine Modified Zirconium Phosphate Nanocomposite for Anticorrosive Coating. Ind. Eng. Chem. Res. 2019, 58, 16571–16580. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Luo, P.; Fan, Y.; Gou, L.; Li, Z.; Zhang, H. Preparation of laminar α-ZrP nanosheets enhanced NiW nanocomposite coating and investigation of its mechanical and anti-corrosion properties. Surf. Coat. Technol. 2021, 423, 127590. [Google Scholar] [CrossRef]
- Kim, H.; Ra, H.N.; Kim, M.; Kim, H.G.; Kim, S.S. Enhancement of barrier properties by wet coating of epoxy-ZrP nanocomposites on various inorganic layers. Prog. Org. Coat. 2017, 108, 25–29. [Google Scholar] [CrossRef]
- Li, M.L.; Huang, H.W.; Mo, R.B.; Sheng, X.X.; Jiang, X.; Zhang, X.Y. Single-step exfoliation, acidification and covalent functionalization of α-zirconium phosphate for enhanced anticorrosion of waterborne epoxy coatings. Surf. Interfaces 2021, 23, 100887. [Google Scholar]
- Fang, K.; Shen, Y.; Yie, K.H.R.; Zhou, Z.; Cai, L.; Wu, S. Preparation of zirconium hydrogen phosphate coatings on sandblasted/acid-etched titanium for enhancing its osteoinductivity and friction/corrosion resistance. Int. J. Nanomed. 2021, 16, 8265–8277. [Google Scholar] [CrossRef]
- Huang, T.-C.; Lai, G.-H.; Li, C.-E.; Tsai, M.-H.; Wan, P.-Y.; Chung, Y.-H.; Lin, M.-H. Advanced anti-corrosion coatings prepared from α-zirconium phosphate/polyurethane nanocomposites. RSC Adv. 2017, 7, 9908–9913. [Google Scholar] [CrossRef]
- Zhu, Z.; Baker, J.; Liu, C.; Zhao, M.; Kotaki, M.; Sue, H.-J. High performance epoxy nanocomposites based on dual epoxide modified α-Zirconium phosphate nanoplatelets. Polymer 2021, 212, 123154. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, Y.; Wang, Z.; Zhu, Y.; Wang, H. Fabrication of “wooden ear”-like filler and anti-corrosion of composite coating under high temperature environment. Prog. Org. Coat. 2023, 183, 107788. [Google Scholar] [CrossRef]
- Cai, Z.; Li, C.; Zhang, D.; Li, J.; Gao, L. An anti-corrosion coating with self-healing function of polyurethane modified by lipoic acid. J. Coat. Technol. Res. 2023, 20, 713–724. [Google Scholar] [CrossRef]
- Hu, C.; Kwan, K.; Xie, X.; Zhou, C.; Ren, K. Superhydrophobic polyaniline/TiO2 composite coating with enhanced anticorrosion function. React. Funct. Polym. 2022, 179, 105381. [Google Scholar] [CrossRef]
- Gao, F.; Mu, J.; Bi, Z.; Wang, S.; Li, Z. Recent advances of polyaniline composites in anticorrosive coatings: A review. Prog. Org. Coat. 2021, 151, 106071. [Google Scholar] [CrossRef]
- Najmi, P.; Keshmiri, N.; Ramezanzadeh, M.; Ramezanzadeh, B. Synthesis and application of Zn-doped polyaniline modified multi-walled carbon nanotubes as stimuli-responsive nanocarrier in the epoxy matrix for achieving excellent barrier-self-healing corrosion protection potency. Chem. Eng. J. 2021, 412, 128637. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, S.; Lin, D.; Zhang, Z.; Li, G. Functional anti-corrosive and anti-bacterial surface coatings based on cuprous oxide/polyaniline microcomposites. Mater. Des. 2022, 216, 110589. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, H.; Xu, W.; Duan, J. Thermally triggered self-healing epoxy coating towards sustained anti-corrosion. J. Mater. Res. Technol. 2022, 17, 2684–2689. [Google Scholar] [CrossRef]
- Sun, W.; Luo, N.; Liu, Y.; Li, H.; Wang, D. A New Self-Healing Triboelectric Nanogenerator Based on Polyurethane Coating and Its Application for Self-Powered Cathodic Protection. ACS Appl. Mater. Interfaces 2022, 14, 10498–10507. [Google Scholar] [CrossRef]
- Motamedi, M.; Mohammadkhah, S.; Ramezanzadeh, M.; Mohammadloo, H.E.; Ramezanzadeh, B. Designing hybrid mesoporous Pr/Tannate-inbuilt ZIF8-decorated MoS2 as novel nanoreservoirs toward smart pH-triggered anti-corrosion/robust thermomechanical epoxy nanocoatings. ACS Appl. Mater. Interfaces 2022, 14, 31170–31193. [Google Scholar] [CrossRef]
- Kong, H.; Luo, X.; Zhang, P.; Feng, J.; Li, P.; Hu, W.; Wang, X.; Liu, X. Self-Healing, Solvent-Free, Anti-Corrosion Coating Based on Skin-like Polyurethane/Carbon Nanotubes Composites with Real-Time Damage Monitoring. Nanomaterials 2022, 13, 124. [Google Scholar] [CrossRef]
- Zheng, N.; Qiao, L.; Liu, J.; Lu, J.; Li, W.; Li, C.; Liu, Q.; Xue, Y.; Zhang, Q. Microcapsules of multilayered shell structure synthesized via one-part strategy and their application in self-healing coatings. Compos. Commun. 2018, 12, 26–32. [Google Scholar] [CrossRef]
- Dieleman, C.D.; Denissen, P.J.; Garcia, S.J. Long-term active corrosion protection of damaged Coated-AA2024-T3 by embedded electrospun inhibiting nanonetworks. Adv. Mater. Interfaces 2018, 5, 1800176. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Fu, J. Self-healing anti-corrosion coatings based on micron-nano containers with different structural morphologies. Prog. Org. Coat. 2023, 175, 107381. [Google Scholar] [CrossRef]
- Neupane, S.; Losada-Pérez, P.; Tiringer, U.; Taheri, P.; Desta, D.; Xie, C.; Crespo, D.; Mol, A.; Milošev, I.; Kokalj, A.; et al. Study of mercaptobenzimidazoles as inhibitors for copper corrosion: Down to the molecular scale. J. Electrochem. Soc. 2021, 168, 051504. [Google Scholar] [CrossRef]
- Valentini, F.; Pezzato, L.; Dabalà, M.; Brunelli, K. Study of the effect of functionalization with inhibitors on the corrosion properties of PEO-coated additive manufactured AlSi10Mg alloy. J. Mater. Res. Technol. 2023, 27, 3595–3609. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Lamaka, S.V.; Blawert, C.; Serdechnova, M.; Scharnagl, N.; Karlova, P.; Wieland, D.; Zheludkevich, M.L. Exploring the corrosion inhibition mechanism of 8-hydroxyquinoline for a PEO-coated magnesium alloy. Corros. Sci. 2022, 203, 110344. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, C.; Wang, A.; Zhou, X.; Xing, X.; Liu, J.; Lu, Q. Research progress on self-healing polymer/graphene anticorrosion coatings. Prog. Org. Coat. 2021, 155, 106231. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Jaenicke, S.; Chuah, G.K. Minimalistic liquid-assisted route to highly crystalline α-zirconium phosphate. ChemSusChem 2017, 10, 3235–3242. [Google Scholar] [CrossRef]
- Kokalj, A.; Lozinšek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Stavber, S.; Crespo, D.; Rennerc, F.U.; et al. Simplistic correlations between molecular electronic properties and inhibition efficiencies: Do they really exist? Corros. Sci. 2021, 179, 108856. [Google Scholar] [CrossRef]
- Li, Z.; Wu, H.; Bai, Y.; Cong, D.; Huang, A.; Song, K.; Zhang, M.; Wei, Z.; Ding, X.; Wang, X.; et al. Study on damage behavior of NiCoCrAlY/MSZ plasma-sprayed coating in neutral salt spray environment. Coatings 2022, 12, 1611. [Google Scholar] [CrossRef]
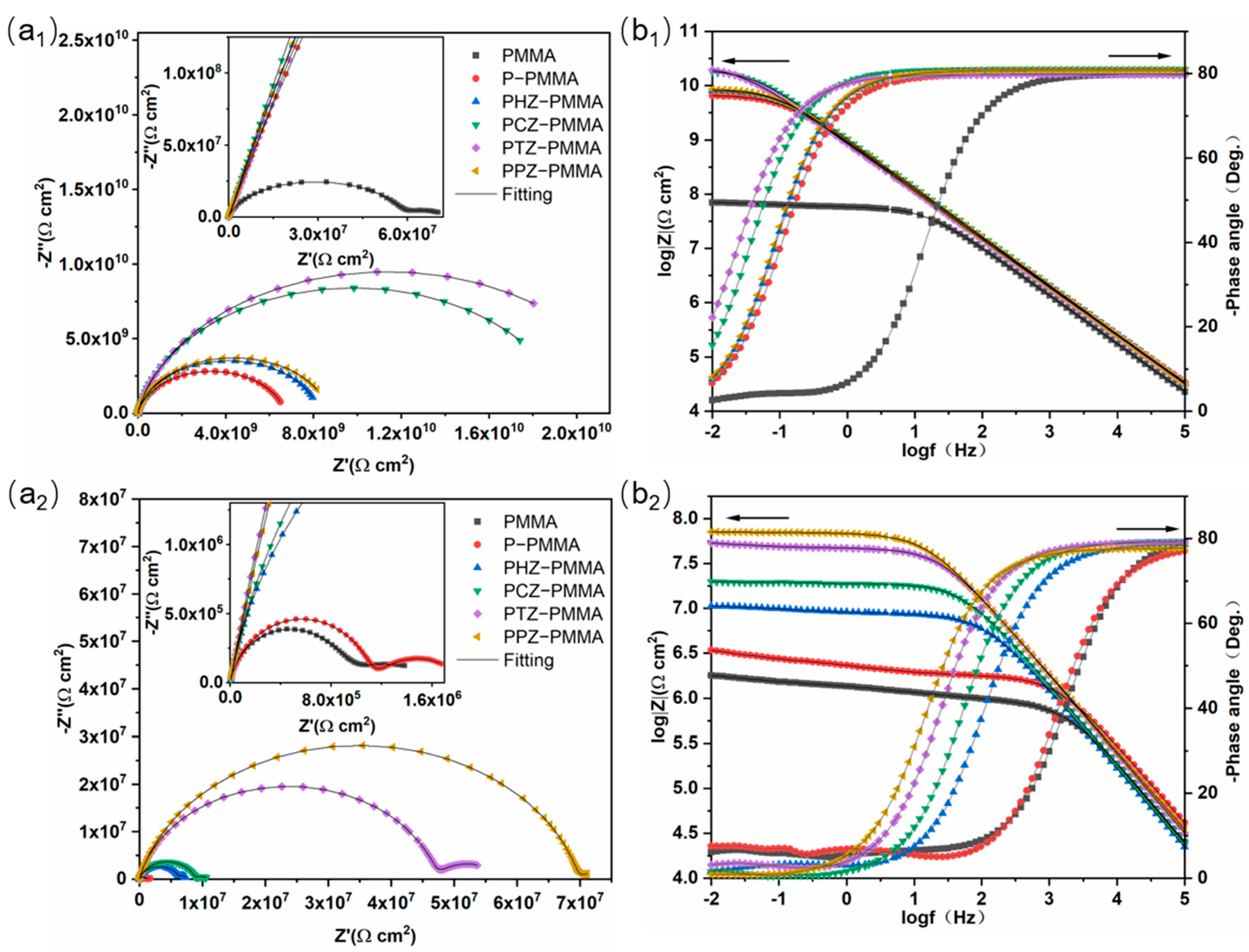

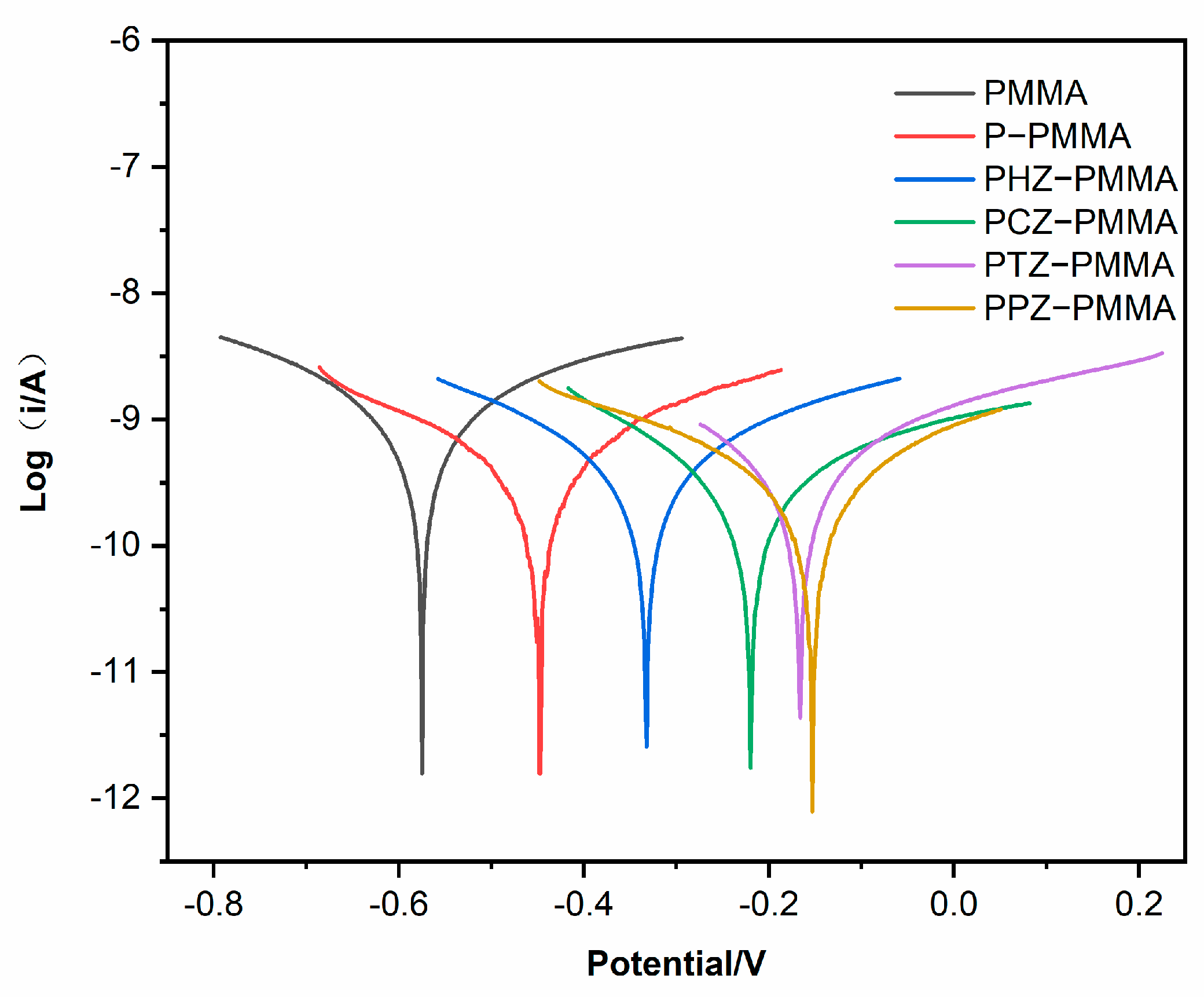

| Time | Samples | CPEC | Rc (Ω cm2) | CPEdl | Rct (Ω cm2) | IE(%) | ||
|---|---|---|---|---|---|---|---|---|
| (Ω−1cm−2Sn) | nf | (Ω−1cm−2Sn) | nf | |||||
| 24 h | PMMA | 3.143 × 10−10 | 0.8879 | 5.8338 × 107 | 1.403 × 10−7 | 0.6376 | 2.067 × 107 | - |
| P-PMMA | 2.111 × 10−10 | 0.8875 | 6.718 × 109 | - | - | - | - | |
| PHZ-PMMA | 2.005 × 10−10 | 0.8967 | 8.287 × 109 | - | - | - | - | |
| PCZ-PMMA | 1.830 × 10−10 | 0.9006 | 1.965 × 1010 | - | - | - | - | |
| PTZ-PMMA | 2.124 × 10−10 | 0.8889 | 1.975 × 1010 | - | - | - | - | |
| PPZ-PMMA | 1.986 × 10−10 | 0.8971 | 8.754 × 109 | - | - | - | - | |
| 168 h | PMMA | 7.653 × 10−10 | 0.8105 | 2.031 × 107 | 6.879 × 10−6 | 0.4376 | 1.119 × 107 | - |
| P-PMMA | 2.237 × 10−10 | 0.8978 | 2.621 × 107 | 9.504 × 10−7 | 0.4362 | 2.089 × 107 | 46.43 | |
| PHZ-PMMA | 2.312 × 10−10 | 0.9023 | 3.906 × 107 | 4.135 × 10−7 | 0.4341 | 2.281 × 107 | 50.99 | |
| PCZ-PMMA | 3.806 × 10−10 | 0.8784 | 6.406 × 107 | 5.122 × 10−8 | 0.4821 | 2.531 × 107 | 55.79 | |
| PTZ-PMMA | 2.823 × 10−10 | 0.8635 | 6.987 × 107 | 2.811 × 10−8 | 0.5631 | 3.056 × 107 | 63.38 | |
| PPZ-PMMA | 2.833 × 10−10 | 0.8591 | 7.671 × 107 | 8.795 × 10−8 | 0.4161 | 5.820 × 107 | 80.78 | |
| Samples | Icorr (A/cm2) | Ecorr (V) | IE (%) | RP (Ω/cm2) |
|---|---|---|---|---|
| PMMA | 1.298 × 10−9 | −0.5768 | 3.383 × 107 | |
| P-PMMA | 6.863 × 10−10 | −0.4486 | 47.12% | 6.105 × 107 |
| PHZ-PMMA | 6.106 × 10−10 | −0.3315 | 52.96% | 7.072 × 107 |
| PCZ-PMMA | 5.595 × 10−10 | −0.2214 | 56.89% | 7.583 × 107 |
| PTZ-PMMA | 4.676 × 10−10 | −0.1674 | 63.97% | 8.913 × 107 |
| PPZ-PMMA | 2.416 × 10−10 | −0.1553 | 81.38% | 1.826 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Z.; Ren, K.; Liu, T.; Zhao, Y.; Zhang, Z.; Li, G. Design Polyaniline/α-Zirconium Phosphate Composites for Achieving Self-Healing Anti-Corrosion of Carbon Steel. Nanomaterials 2024, 14, 76. https://doi.org/10.3390/nano14010076
Lv Z, Ren K, Liu T, Zhao Y, Zhang Z, Li G. Design Polyaniline/α-Zirconium Phosphate Composites for Achieving Self-Healing Anti-Corrosion of Carbon Steel. Nanomaterials. 2024; 14(1):76. https://doi.org/10.3390/nano14010076
Chicago/Turabian StyleLv, Ziqi, Kai Ren, Tao Liu, Yunyan Zhao, Zhonghua Zhang, and Guicun Li. 2024. "Design Polyaniline/α-Zirconium Phosphate Composites for Achieving Self-Healing Anti-Corrosion of Carbon Steel" Nanomaterials 14, no. 1: 76. https://doi.org/10.3390/nano14010076
APA StyleLv, Z., Ren, K., Liu, T., Zhao, Y., Zhang, Z., & Li, G. (2024). Design Polyaniline/α-Zirconium Phosphate Composites for Achieving Self-Healing Anti-Corrosion of Carbon Steel. Nanomaterials, 14(1), 76. https://doi.org/10.3390/nano14010076






