Oxygen Vacancy-Enhanced Ni3N-CeO2/NF Nanoparticle Catalysts for Efficient and Stable Electrolytic Water Splitting
Abstract
:1. Introduction
2. Material and Methods
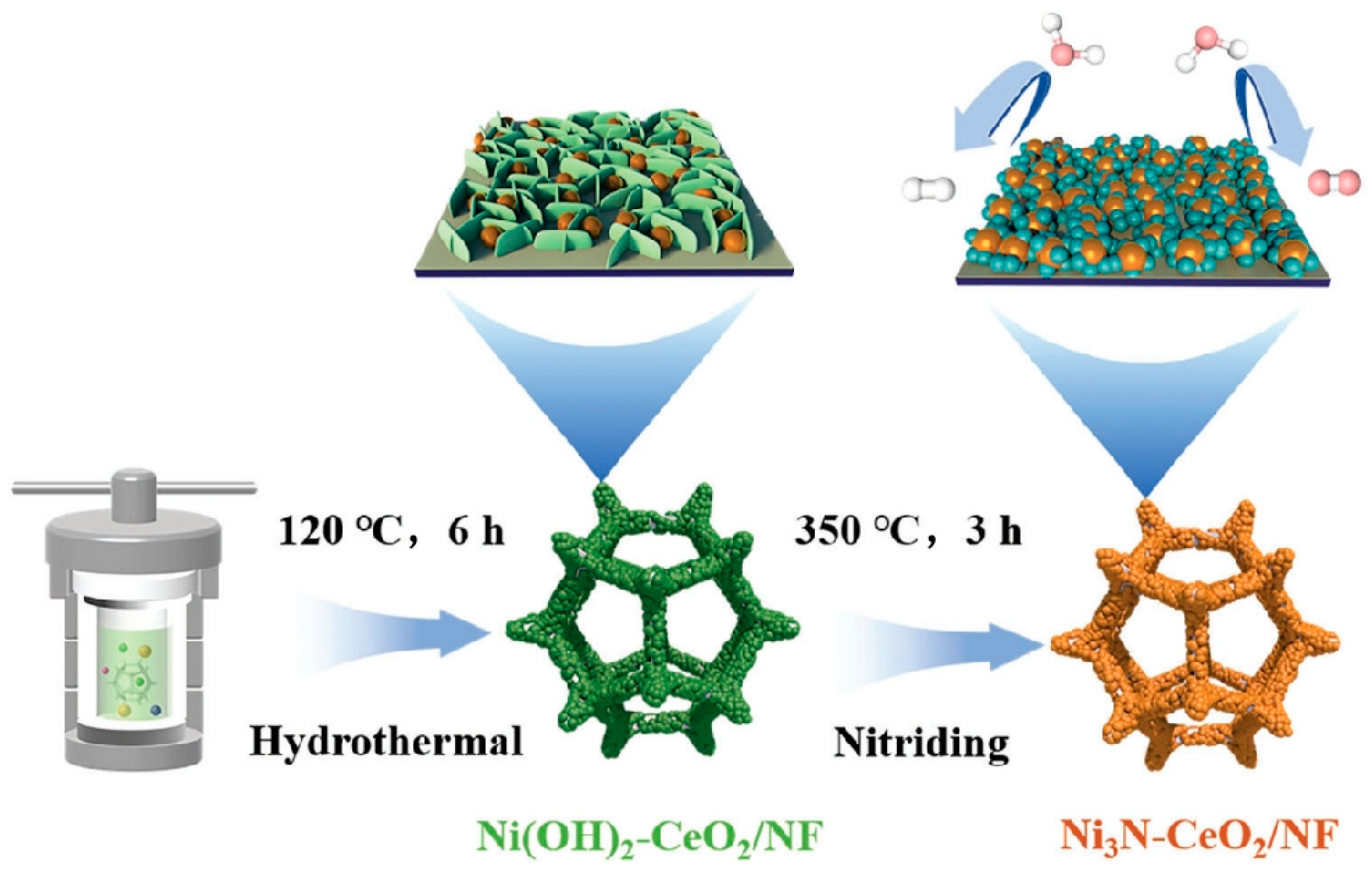
2.1. Synthesis of Composites
2.2. Electrochemical Measurements
2.3. Characterization of Material
3. Results and Discussion
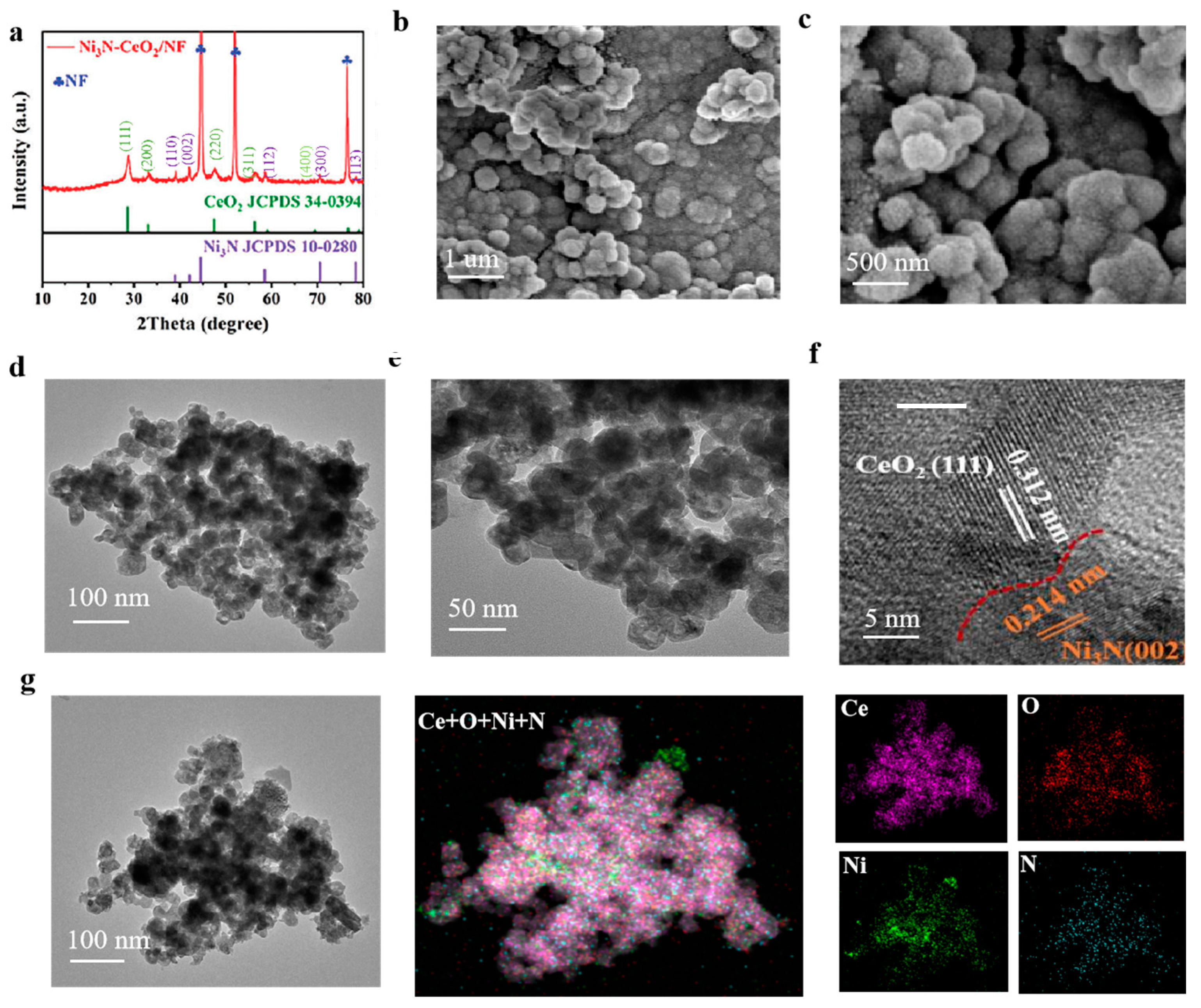
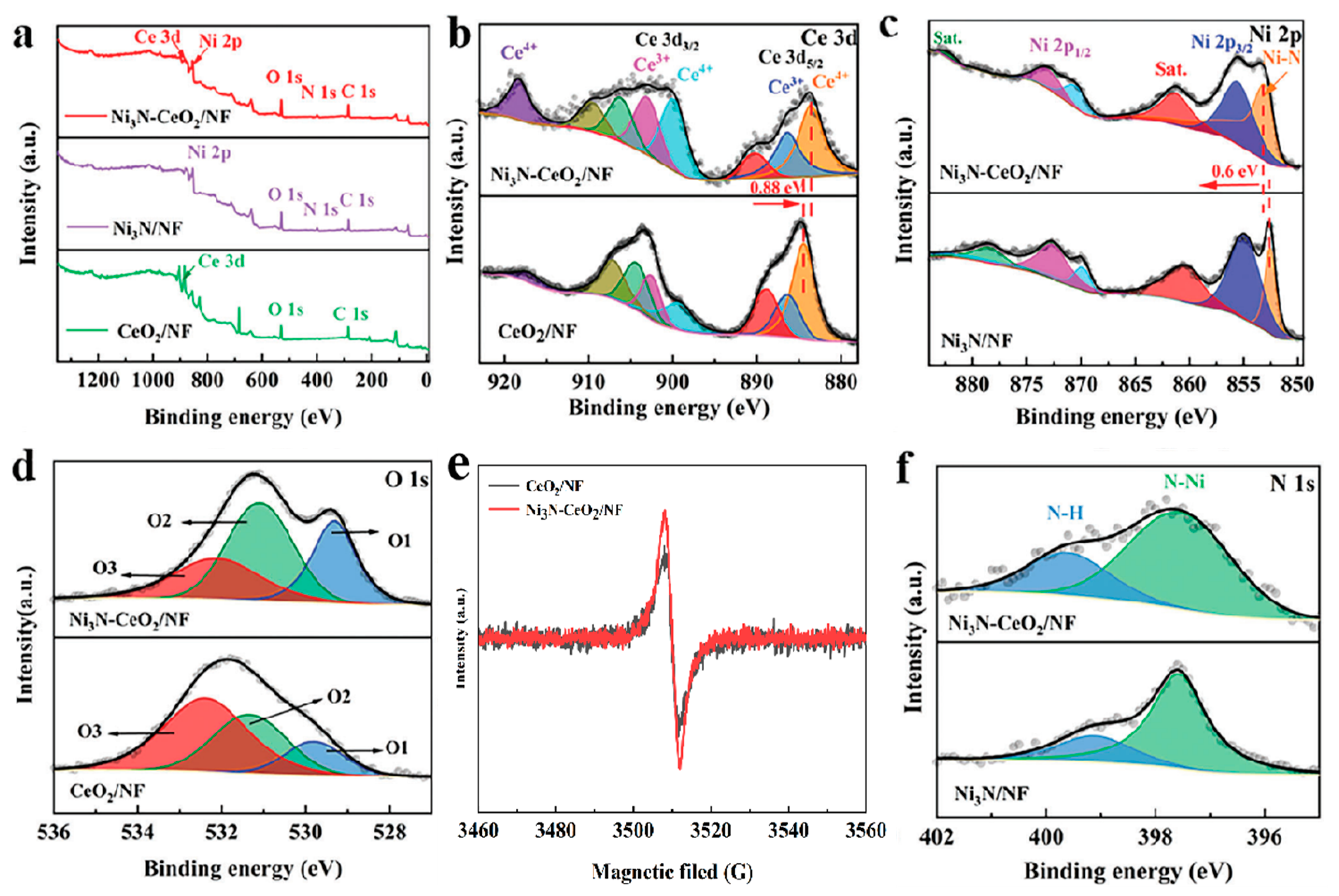
3.1. Characterization of Composites
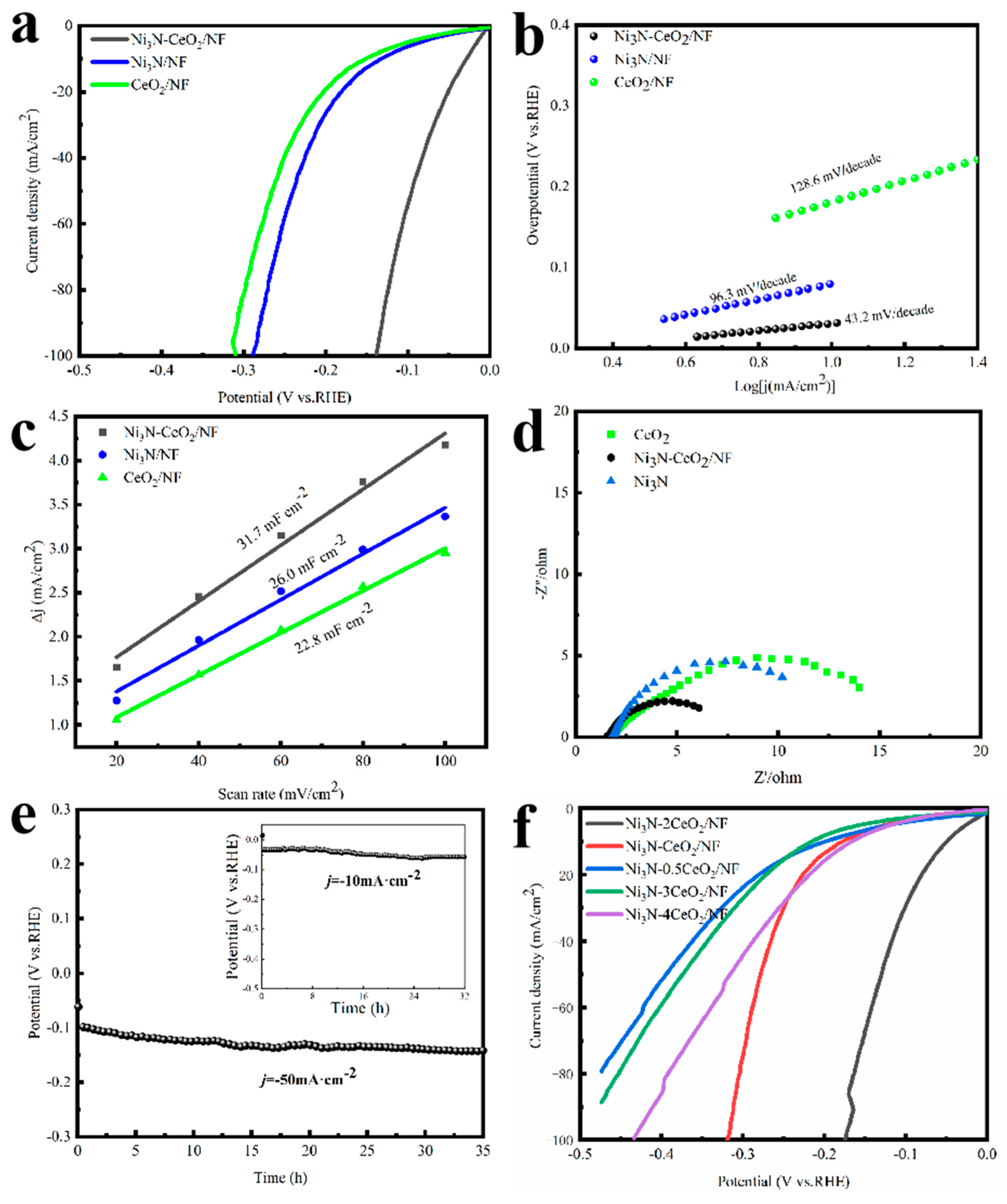
3.2. Electrocatalytic Performance for HER
3.3. Electrocatalytic Performance for OER
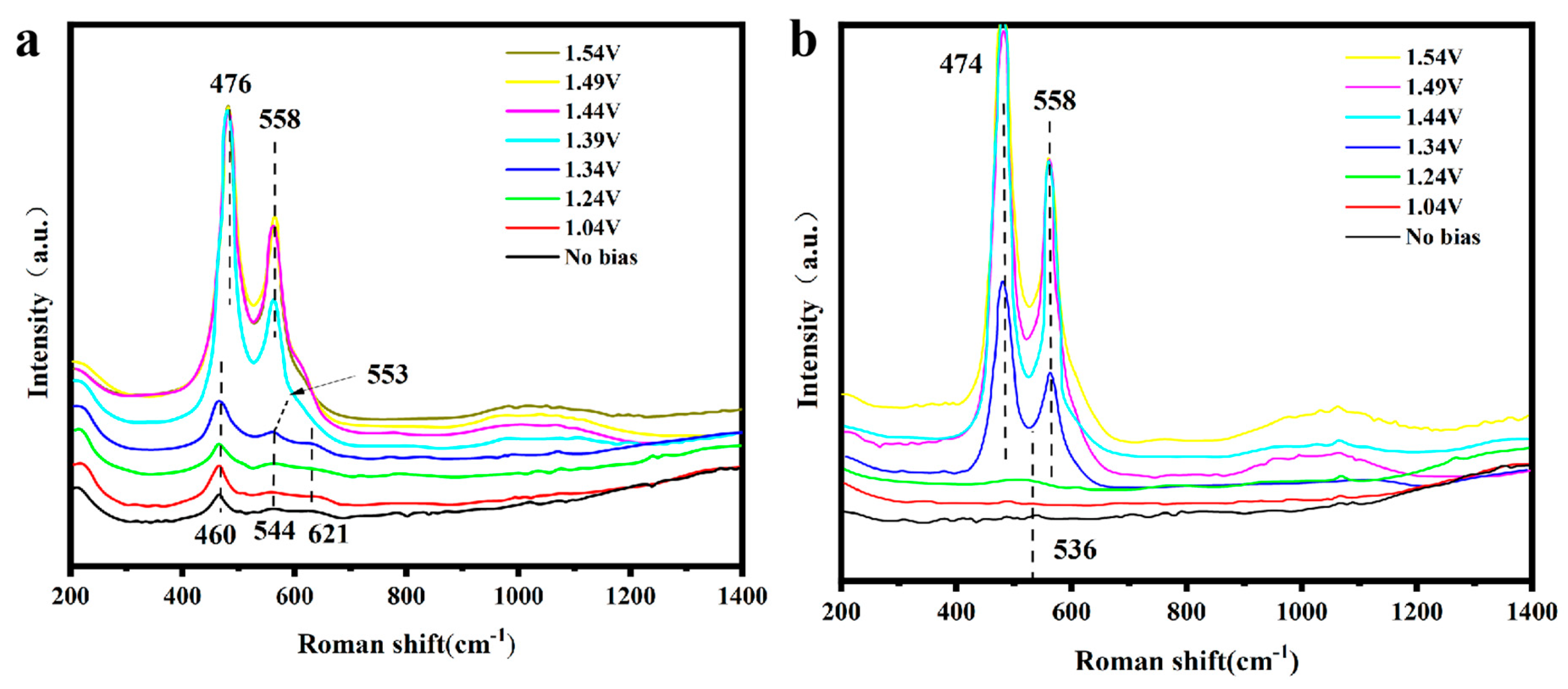
3.4. In Situ Raman Spectroscopy
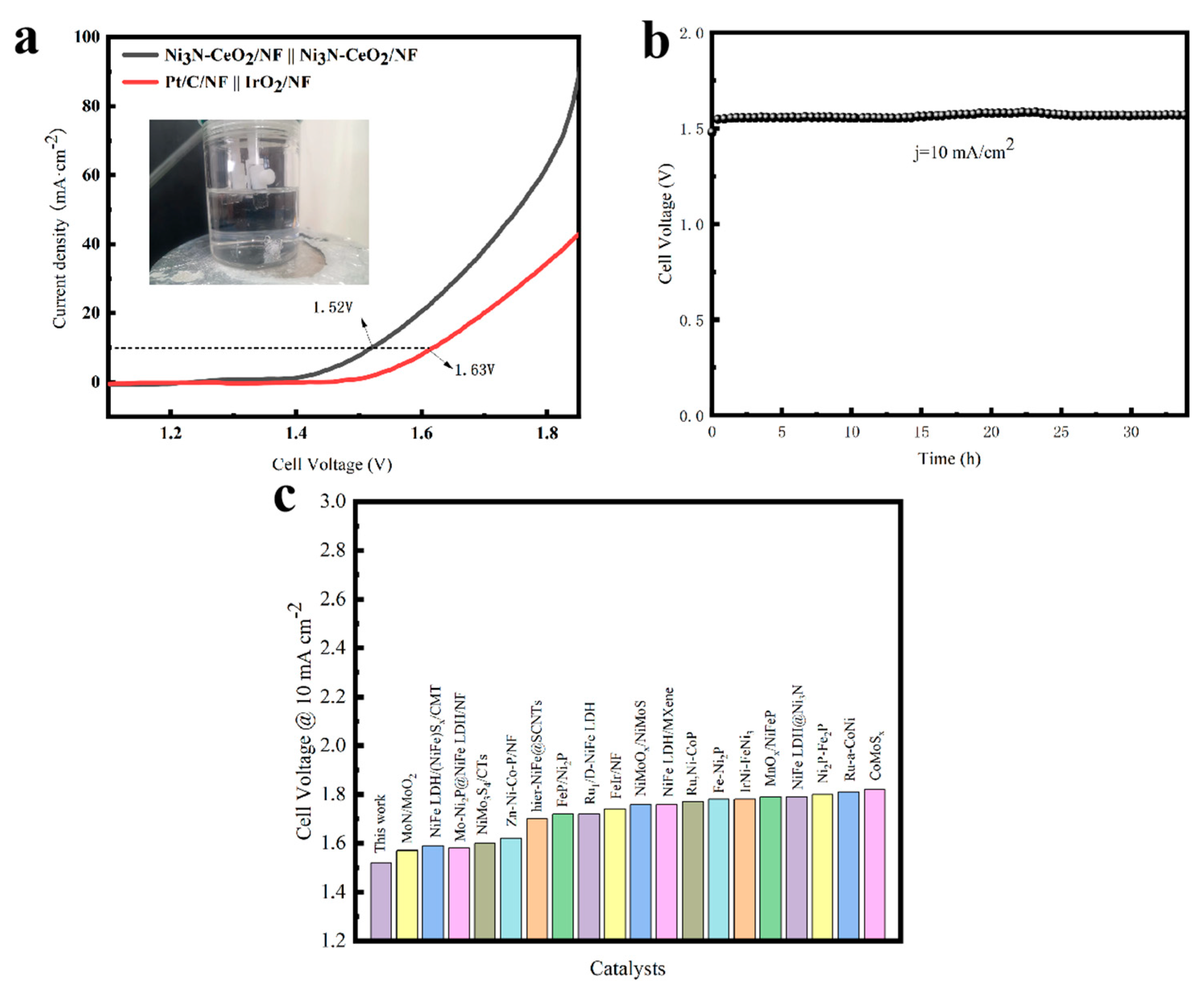
3.5. Overall Water Splitting in an Alkaline Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, Q.; Zhou, Y.; Shou, H.; Wang, X.; Zhang, P.; Xu, W.; Qiao, S.; Wu, C.; Liu, H.; Liu, D.; et al. Synergic Reaction Kinetics over Adjacent Ruthenium Sites for Superb Hydrogen Generation in Alkaline Media. Adv. Mater. 2022, 34, e2110604. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Qu, J.; Xiao, Y.; Zhao, S.; Chen, H.; Dai, L. Carbon Nanomaterials for Energy and Biorelated Catalysis: Recent Advances and Looking Forward. ACS Cent. Sci. 2019, 5, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, T.J.; Fjällström, V.; Sahlberg, M.; Edoff, M.; Edvinsson, T. A monolithic device for solar water splitting based on series interconnected thin film absorbers reaching over 10% solar-to-hydrogen efficiency. Energy Environ. Sci. 2013, 6, 3676–3683. [Google Scholar] [CrossRef]
- Ji, Q.; Kong, Y.; Tan, H.; Duan, H.; Li, N.; Tang, B.; Wang, Y.; Feng, S.; Lv, L.; Wang, C.; et al. Operando Identification of Active Species and Intermediates on Sulfide Interfaced by Fe3O4 for Ultrastable Alkaline Oxygen Evolution at Large Current Density. ACS Catal. 2022, 12, 4318–4326. [Google Scholar] [CrossRef]
- Jiang, M.; Zhai, H.; Chen, L.; Mei, L.; Tan, P.; Yang, K.; Pan, J. Unraveling the Synergistic Mechanism of Bi-Functional Nickel–Iron Phosphides Catalysts for Overall Water Splitting. Adv. Funct. Mater. 2023, 33, 2302621. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Wu, Y.; Zhang, G.; Wu, H.; Lyu, F.; He, J.; Fan, J.; Lu, J.; Li, Y.Y. Identifying Fe as OER Active Sites and Ultralow-Cost Bifunctional Electrocatalysts for Overall Water Splitting. Small 2023, 19, e2301715. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, X.; Huo, J.; Qu, J.; Chen, W.; Liu, C.; Zhao, Y.; Liu, H.; Wang, G. High valence metals engineering strategies of Fe/Co/Ni-based catalysts for boosted OER electrocatalysis. J. Energy Chem. 2023, 76, 195–213. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chen, Q.; Xia, X.; Chen, M. Emerging of Heterostructure Materials in Energy Storage: A Review. Adv. Mater. 2021, 33, 2100855. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced electrocatalysts with unusual active sites for electrochemical water splitting. InfoMat 2024, 6, e12494. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Q.; Xie, L.; Zeng, H.; Zhou, S.; Huang, Y.; Yan, M.; Zhang, X.; Liu, T.; Zeng, J.; et al. Superassembly of Surface-Enriched Ru Nanoclusters from Trapping–Bonding Strategy for Efficient Hydrogen Evolution. ACS Nano 2022, 16, 7993–8004. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, T.; Zhang, J.; Zheng, X.; Mao, J.; Liu, H.; Li, Y.; Hao, Q. Engineering Ni2P-NiSe2 heterostructure interface for highly efficient alkaline hydrogen evolution. Appl. Catal. B Environ. 2020, 262, 118245. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology control and electronic tailoring of CoxAy (A = P, S, Se) electrocatalysts for water splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Liu, X.; Lu, H.; Zhu, S.; Cui, Z.; Li, Z.; Wu, S.; Xu, W.; Liang, Y.; Long, G.; Jiang, H. Alloying-Triggered Phase Engineering of NiFe System via Laser-Assisted Al Incorporation for Full Water Splitting. Angew. Chem. Int. Ed. 2023, 62, e202300800. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Yu, Y.; Wu, Y.; Ma, Z.; Ma, X.; Jiang, Y.; Shen, W.; He, R.; Su, W.; Li, M. Realizing efficient oxygen evolution at low overpotential via dopant-induced interfacial coupling enhancement effect. Appl. Catal. B Environ. 2023, 336, 122928. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Adv. Mater. 2022, 34, 2108133. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Y.; Wu, C.-X.; Feng, X.-J.; Tan, H.-Q.; Yan, L.-K.; Liu, Y.; Kang, Z.-H.; Wang, E.-B.; Li, Y.-G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Xu, X.M.; Pan, Y.L.; Ge, L.; Chen, Y.B.; Mao, X.; Guan, D.Q.; Li, M.R.; Zhong, Y.J.; Hu, Z.W.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Li, F.; Jung, S.-M.; Okyay, M.S.; Ahmad, I.; Kim, S.-J.; Park, N.; Jeong, H.Y.; Baek, J.-B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441–446. [Google Scholar] [CrossRef]
- Mir, Z.M.; Bastos, A.; Höche, D.; Zheludkevich, M.L. Recent Advances on the Application of Layered Double Hydroxides in Concrete—A Review. Materials 2020, 13, 1426. [Google Scholar] [CrossRef]
- Ojha, K.; Saha, S.; Kumar, B.; Hazra, K.S.; Ganguli, A.K. Controlling the Morphology and Efficiency of Nanostructured Molybdenum Nitride Electrocatalysts for the Hydrogen Evolution Reaction. ChemCatChem 2016, 8, 1218–1225. [Google Scholar] [CrossRef]
- Ortel, E.; Reier, T.; Strasser, P.; Kraehnert, R. Mesoporous IrO2 Films Templated by PEO-PB-PEO Block-Copolymers: Self-Assembly, Crystallization Behavior, and Electrocatalytic Performance. Chem. Mater. 2011, 23, 3201–3209. [Google Scholar] [CrossRef]
- Xia, B.; Wang, T.; Chi, X.; Yu, X.; Liu, P.; Zhang, J.; Xi, S.; Du, Y.; Gao, D. Anion vacancy-mediated ferromagnetism in atomic-thick Ni3N nanosheets. Appl. Phys. Lett. 2017, 111, 232402. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Zhao, H.; Zhang, Y.; Huang, Y.; Yin, Z.; Du, Y. Regulating the active species of Ni(OH)2 using CeO2: 3D CeO2/Ni(OH)2/carbon foam as an efficient electrode for the oxygen evolution reaction. Chem. Sci. 2017, 8, 3211–3217. [Google Scholar] [CrossRef]
- Park, S.; Shviro, M.; Hartmann, H.; Besmehn, A.; Mayer, J.; Stolten, D.; Carmo, M. Nickel Structures as a Template Strategy to Create Shaped Iridium Electrocatalysts for Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 13576–13585. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Chen, J.; Yu, T.; Liu, J.; Luo, L.; Yin, S. Three-Phase Heterojunction NiMo-Based Nano-Needle for Water Splitting at Industrial Alkaline Condition. Nano-Micro Lett. 2021, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Dai, R.; Mateen, M.; Hassan, Z.; Zhuang, Z.; Liu, C.; Israr, M.; Cheong, W.C.; Hu, B.; Tu, R.; et al. Cobalt Single Atom Incorporated in Ruthenium Oxide Sphere: A Robust Bifunctional Electrocatalyst for HER and OER. Angew. Chem. Int. Ed. 2021, 61, e202114951. [Google Scholar] [CrossRef]
- Shen, S.; Wang, Z.; Lin, Z.; Song, K.; Zhang, Q.; Meng, F.; Gu, L.; Zhong, W. Crystalline-Amorphous Interfaces Coupling of CoSe2/CoP with Optimized d-Band Center and Boosted Electrocatalytic Hydrogen Evolution. Adv. Mater. 2022, 34, 2110631. [Google Scholar] [CrossRef]
- Singh, B.; Gawande, M.B.; Kute, A.D.; Varma, R.S.; Fornasiero, P.; McNeice, P.; Jagadeesh, R.V.; Beller, M.; Zbořil, R. Single-Atom (Iron-Based) Catalysts: Synthesis and Applications. Chem. Rev. 2021, 121, 13620–13697. [Google Scholar] [CrossRef]
- Su, P.; Pei, W.; Wang, X.; Ma, Y.; Jiang, Q.; Liang, J.; Zhou, S.; Zhao, J.; Liu, J.; Lu, G.Q. Exceptional Electrochemical HER Performance with Enhanced Electron Transfer between Ru Nanoparticles and Single Atoms Dispersed on a Carbon Substrate. Angew. Chem. Int. Ed. 2021, 60, 16044–16050. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3 nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Huang, Z.; Liao, X.; Zhang, W.; Hu, J.; Gao, Q. Ceria-Promoted Reconstruction of Ni-Based Electrocatalysts toward Efficient Oxygen Evolution. ACS Catal. 2022, 12, 13951–13960. [Google Scholar] [CrossRef]
- Tan, X.; Geng, S.; Ji, Y.; Shao, Q.; Zhu, T.; Wang, P.; Li, Y.; Huang, X. Closest Packing Polymorphism Interfaced Metastable Transition Metal for Efficient Hydrogen Evolution. Adv. Mater. 2020, 32, e2002857. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.D.; Chuong, N.D.; Hien, H.V.; Kshetri, T.; Tuan, L.H.; Kim, N.H.; Lee, J.H. Recent advances in two-dimensional transition metal dichalcogenides-graphene heterostructured materials for electrochemical applications. Prog. Mater. Sci. 2018, 96, 51–85. [Google Scholar] [CrossRef]
- Tyndall, D.; Craig, M.J.; Gannon, L.; McGuinness, C.; McEvoy, N.; Roy, A.; García-Melchor, M.; Browne, M.P.; Nicolosi, V. Demonstrating the source of inherent instability in NiFe LDH-based OER electrocatalysts. J. Mater. Chem. A 2023, 11, 4067–4077. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.; Lawrence, M.J.; Han, S.; Cai, C.; Xiang, S.; Rodriguez, P.; Xiang, B.; et al. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018, 53, 458–467. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, M.; Zhang, J.; Chen, Z.; Zheng, X.; Tian, Z.; Zhao, N.; Han, X.; Zaghib, K.; et al. Highly Active and Durable Single-Atom Tungsten-Doped NiS0.5Se0.5 Nanosheet NiS0.5Se0.5 Nanorod Heterostructures for Water Splitting. Adv. Mater. 2022, 34, 2107053. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Gong, Y.; Liu, D.; Liu, S.; Xiao, W.; Xiao, Z.; Li, Z.; Wu, Z.; Wang, L. Amorphous high-valence Mo-doped NiFeP nanospheres as efficient electrocatalysts for overall water-splitting under large-current density. Chem. Eng. J. 2023, 468, 143833. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, B.; Lin, Z.; Xu, Y.; Lin, Y.; Meng, F.; Zhang, Q.; Gu, L.; Fang, B.; Guo, S.; et al. PtSe2/Pt Heterointerface with Reduced Coordination for Boosted Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 23388–23393. [Google Scholar] [CrossRef]
- Wei, C.; Sun, S.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.J. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zou, W.; Tang, H. Nb-Doped nickel nitride-derived catalysts for electrochemical water splitting. Catal. Sci. Technol. 2021, 11, 6455–6461. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L.; et al. Salt-Templated Synthesis of 2D Metallic MoN and Other Nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Zhao, X.; Min, Y.; Li, Q.; Xu, Q. Oxygen Vacancy-Enhanced Ni3N-CeO2/NF Nanoparticle Catalysts for Efficient and Stable Electrolytic Water Splitting. Nanomaterials 2024, 14, 935. https://doi.org/10.3390/nano14110935
Meng X, Zhao X, Min Y, Li Q, Xu Q. Oxygen Vacancy-Enhanced Ni3N-CeO2/NF Nanoparticle Catalysts for Efficient and Stable Electrolytic Water Splitting. Nanomaterials. 2024; 14(11):935. https://doi.org/10.3390/nano14110935
Chicago/Turabian StyleMeng, Xianghao, Xin Zhao, Yulin Min, Qiaoxia Li, and Qunjie Xu. 2024. "Oxygen Vacancy-Enhanced Ni3N-CeO2/NF Nanoparticle Catalysts for Efficient and Stable Electrolytic Water Splitting" Nanomaterials 14, no. 11: 935. https://doi.org/10.3390/nano14110935




