Amorphous Fe2O3 Anchored on N-Doped Graphene with Internal Micro-Channels as an Active and Durable Anode for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Combined Film of GO Flakes Containing Iron Acetylacetonate and Nylon Nanofibers
2.3. Preparation of Fe2O3@N-GIMC and Fe2O3@G
2.4. Characterization
2.5. Electrochemical Experiments
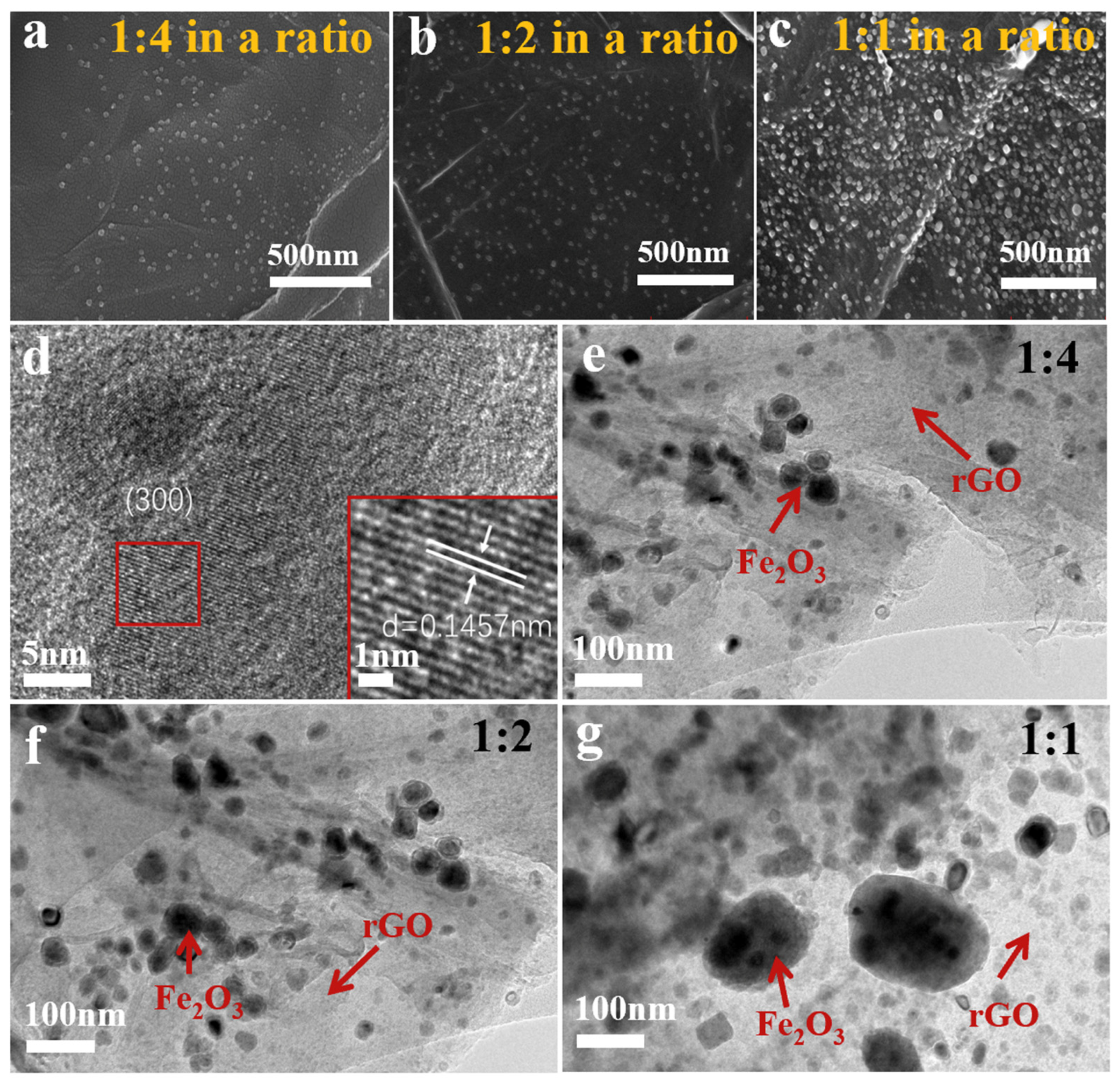
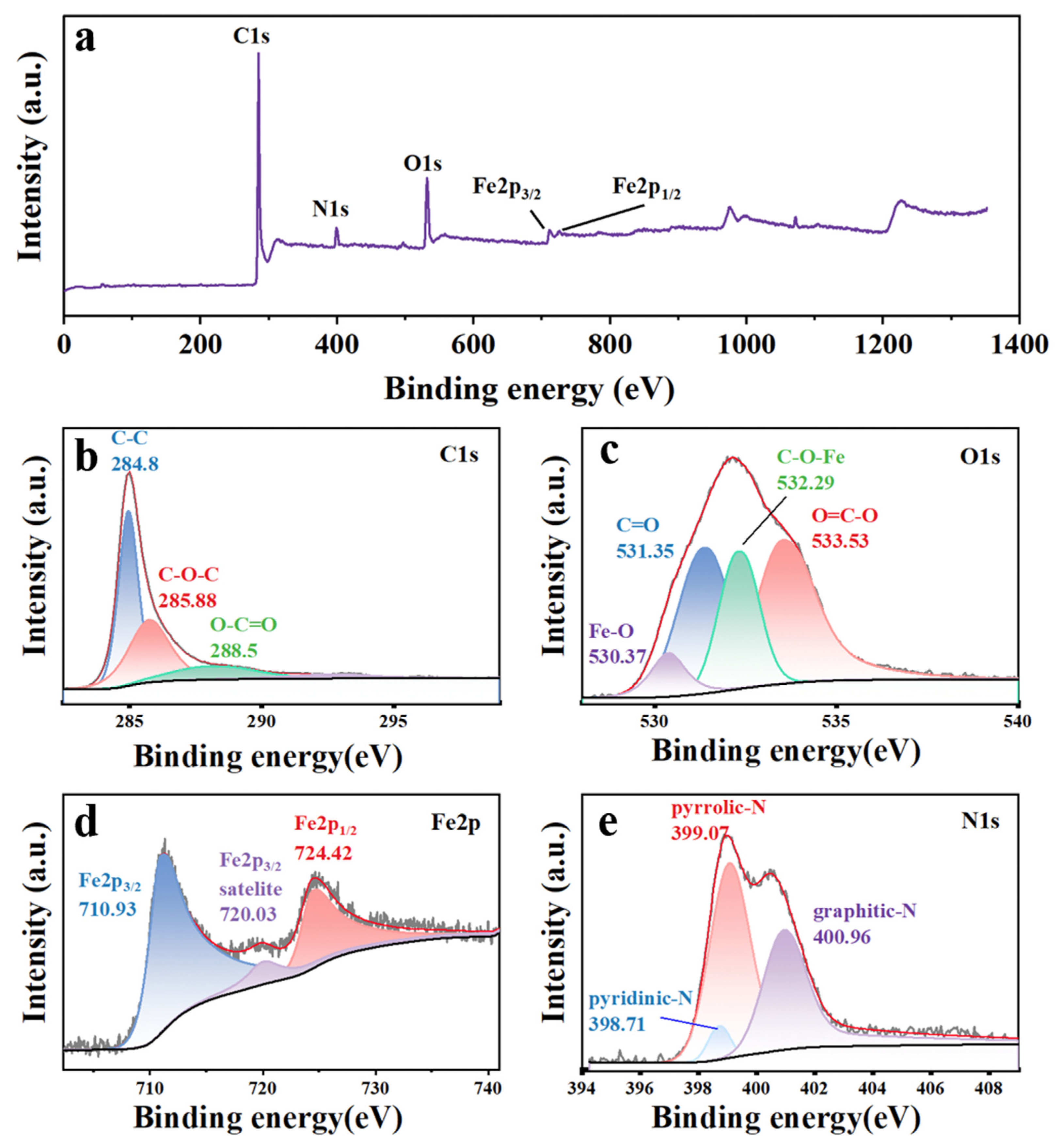
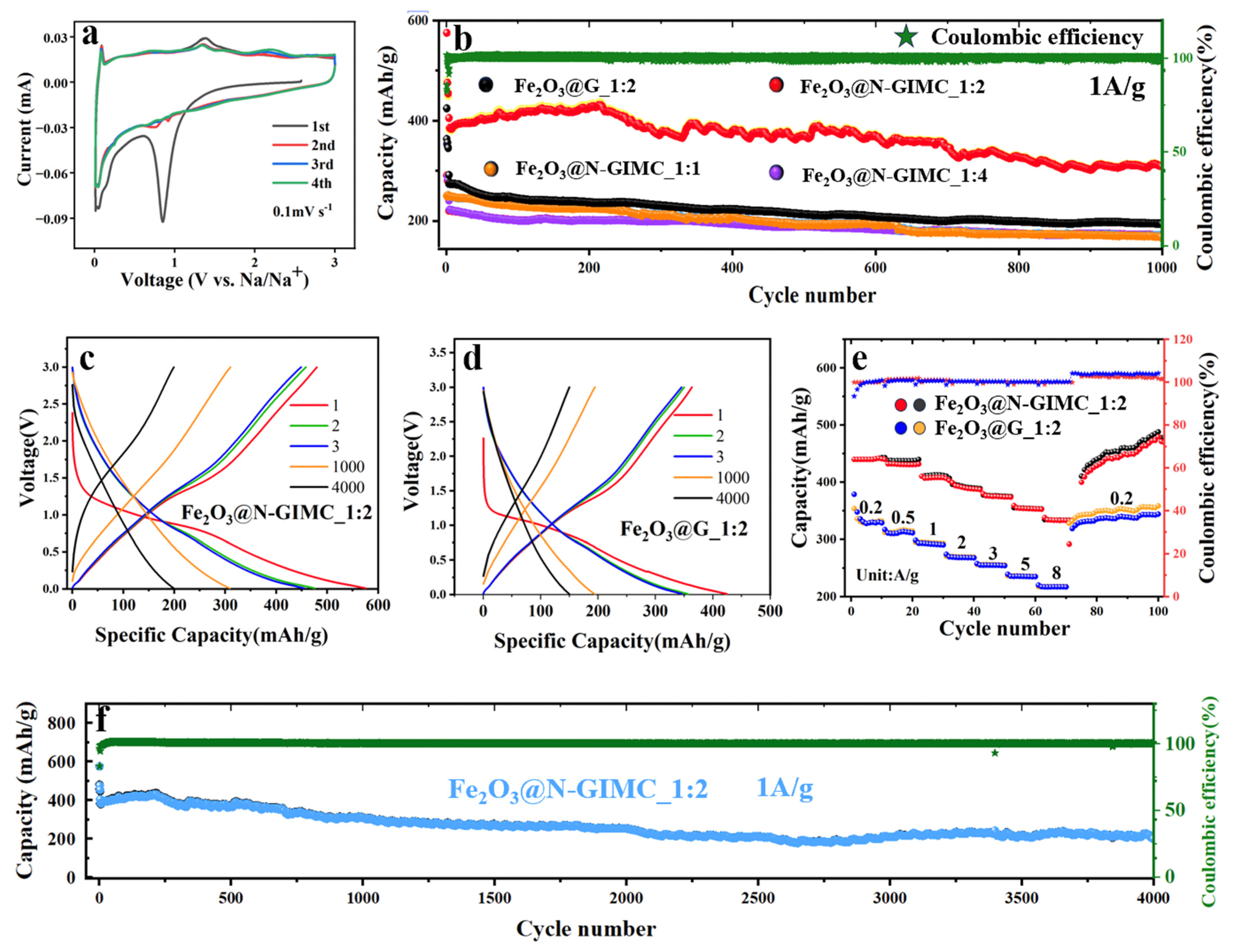
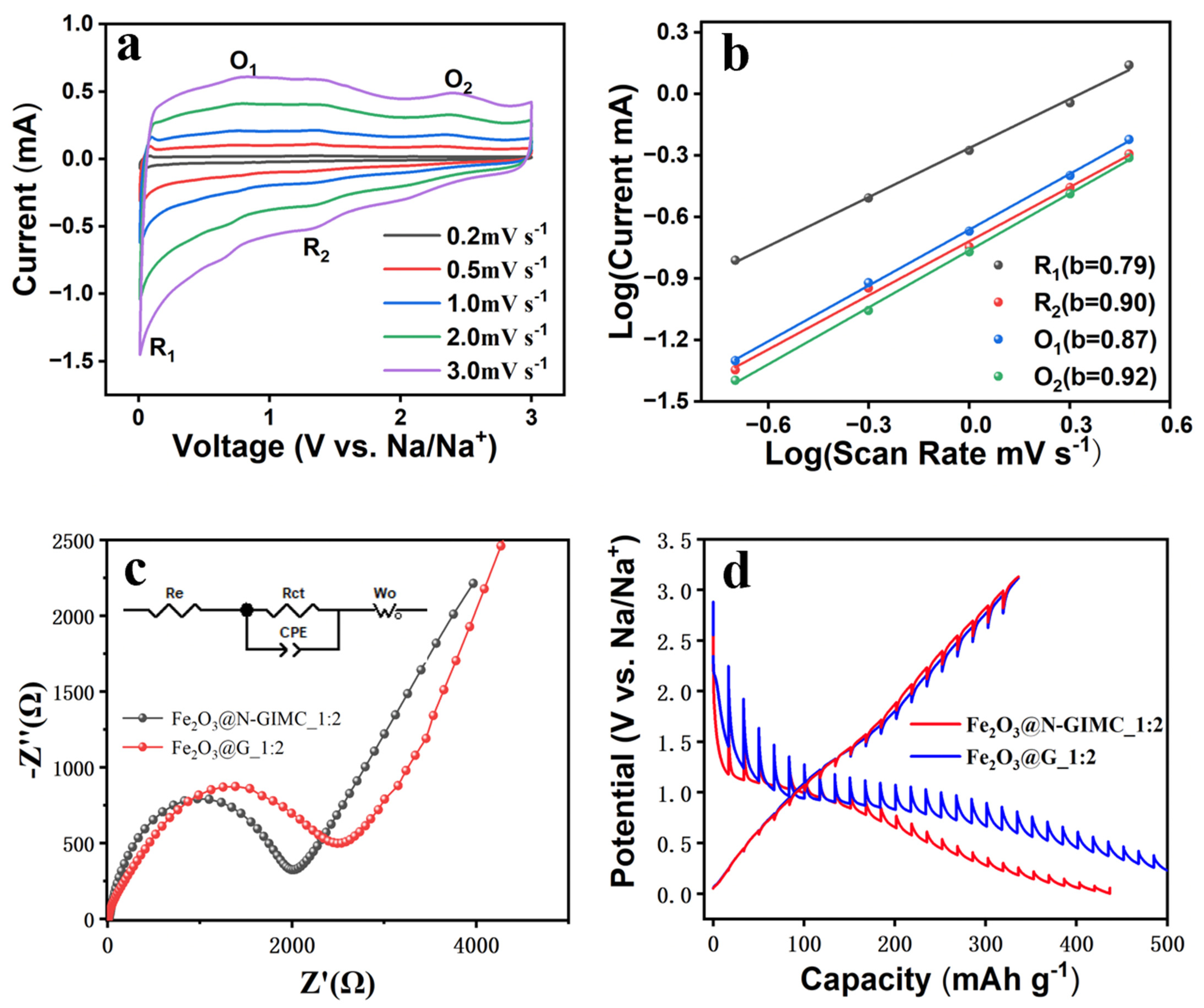
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage†. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Wickramaratne, N.P.; Jaroniec, M.; Dou, S.; Dai, L. High-Performance Sodium Ion Batteries Based on a 3D Anode from Nitrogen-Doped Graphene Foams. Adv. Mater. 2015, 27, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.; Pan, L.; Lu, T.; Yao, Y.; Sun, Z.; Chua, D.H.C.; Chen, Q. Electrospun carbon nanofibers as anode materials for sodium ion batteries with excellent cycle performance. J. Mater. Chem. A 2014, 2, 4117–4121. [Google Scholar] [CrossRef]
- Ould, D.M.C.; Menkin, S.; O'Keefe, C.A.; Coowar, F.; Barker, J.; Grey, C.P.; Wright, D.S. Beyond the Norm: Synthesis and Electrochemical Study of High Concentrated NaPF6 Electrolytes. ECS Meet. Abstr. 2022; MA2022-01, 498. [Google Scholar]
- Xiong, X.; Yang, C.; Wang, G.; Lin, Y.; Ou, X.; Wang, J.-H.; Zhao, B.; Liu, M.; Lin, Z.; Huang, K. SnS nanoparticles electrostatically anchored on three-dimensional N-doped graphene as an active and durable anode for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1757–1763. [Google Scholar] [CrossRef]
- Kang, J.; Lee, J.-I.; Choi, S.; Choi, Y.; Park, S.; Ryu, J. Nonporous Oxide-Terminated Multicomponent Bulk Anode Enabling Energy-Dense Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 26576–26584. [Google Scholar] [CrossRef]
- Dimogiannis, K.; Bhaskar, A.; Johnson, L.R. The Sodium-Ion Battery: Effect of Electrolyte Additives on the SEI Layer of Hard Carbon Anodes. ECS Meet. Abstr. 2020; MA2020-02, 767. [Google Scholar]
- Wu, Z.; Tiefeng, L.; Yao, W.; Yujing, L.; Jianwei, N.; Liang, Z.; Ouwei, S.; Xinyong, T. Strategies to improve the performance of phosphide anodes in sodium-ion batteries. Nano Energy 2021, 90, 106475. [Google Scholar]
- Chang, G.; Zhao, Y.; Dong, L.; Wilkinson, D.P.; Zhang, L.; Shao, Q.; Yan, W.; Sun, X.; Zhang, J. A review of phosphorus and phosphides as anode materials for advanced sodium-ion batteries. J. Mater. Chem. A 2020, 10, 4996–5048. [Google Scholar] [CrossRef]
- Wang, N.; Bai, Z.; Qian, Y.; Yang, J. Double-Walled Sb@TiO2−x Nanotubes as a Superior High-Rate and Ultralong-Lifespan Anode Material for Na-Ion and Li-Ion Batteries. Adv. Mater. 2016, 28, 4126–4133. [Google Scholar] [CrossRef]
- Vejpravová, J. Mixed sp2–sp3 Nanocarbon Materials: A Status Quo Review. Nanomaterials 2021, 11, 2469. [Google Scholar] [CrossRef]
- Vozniakovskii, A.A.; Smirnova, E.A.; Apraksin, R.V.; Kidalov, S.V.; Voznyakovskii, A.P. Use of Few-Layer Graphene Synthesized under Conditions of Self-Propagating High-Temperature Synthesis for Supercapacitors Applications. Nanomaterials 2023, 13, 2368. [Google Scholar] [CrossRef]
- Iannazzo, D.; Celesti, C.; Giofrè, S.V.; Ettari, R.; Bitto, A. Theranostic Applications of 2D Graphene-Based Materials for Solid Tumors Treatment. Nanomaterials 2023, 13, 2380. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, H.; Gao, C. Advanced Graphene Materials for Sodium/Potassium/Aluminum-Ion Batteries. ACS Mater. Lett. 2021, 3, 1221–1237. [Google Scholar] [CrossRef]
- Lai, L.; Zhu, J.; Li, Z.; Yu, D.Y.W.; Jiang, S.; Cai, X.; Yan, Q.; Lam, Y.M.; Shen, Z.; Lin, J. Co3O4/nitrogen modified graphene electrode as Li-ion battery anode with high reversible capacity and improved initial cycle performance. Nano Energy 2013, 3, 134–143. [Google Scholar] [CrossRef]
- Hu, M.; Song, J.; Fan, H.; Bai, L.; Wang, Y.; Liu, S.; Jin, Y.; Cui, Y.; Liu, W. Pseudocapacitance-rich carbon nanospheres with graphene protective shield achieving favorable capacity-cyclability combinations of K-ion storage. Chem. Eng. J. 2022, 451, 138452. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The Chemistry and Promising Applications of Graphene and Porous Graphene Materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Huang, L. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon 2019, 143, 610–640. [Google Scholar] [CrossRef]
- Liang, K.; Li, M.; Hao, Y.; Yan, W.; Cao, M.; Fan, S.; Han, W.; Su, J. Reduced graphene oxide with 3D interconnected hollow channel architecture as high-performance anode for Li/Na/K-ion storage. Chem. Eng. J. 2020, 394, 124956. [Google Scholar] [CrossRef]
- Liu, L.; Yan, X.; Li, L.; Su, J.; Ramakrishna, S.; Long, Y.-Z.; Han, W. Preparation of high-performance graphene materials by adjusting internal micro-channels using a combined electrospray/electrospinning technique. J. Alloys Compd. 2023, 940, 168882. [Google Scholar] [CrossRef]
- Chen, W.; Wan, M.; Liu, Q.; Xiong, X.; Yu, F.; Huang, Y. Heteroatom-Doped Carbon Materials: Synthesis, Mechanism, and Application for Sodium-Ion Batteries. Small Methods 2018, 3, 1800323. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y. 2D material as anode for sodium ion batteries: Recent progress and perspectives. Energy Storage Mater. 2019, 16, 323–343. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhang, L.; Xie, F.; Vasileff, A.; Qiao, S.Z. Graphitic Carbon Nitride (g-C3N4)-Derived N-Rich Graphene with Tuneable Interlayer Distance as a High-Rate Anode for Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1901261. [Google Scholar] [CrossRef] [PubMed]
- Quan, B.; Jin, A.; Yu, S.-H.; Kang, S.M.; Jeong, J.; Abruña, H.D.; Jin, L.; Piao, Y.; Sung, Y.-E. Solvothermal-Derived S-Doped Graphene as an Anode Material for Sodium-Ion Batteries. Adv. Sci. 2018, 5, 1700880. [Google Scholar] [CrossRef]
- Sha, M.; Zhang, H.; Nie, Y.; Nie, K.; Lv, X.; Sun, N.; Xie, X.; Ma, Y.; Sun, X. Sn nanoparticles@nitrogen-doped carbon nanofiber composites as high-performance anodes for sodium-ion batteries†. J. Mater. Chem. A 2017, 5, 6277–6283. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Yang, W.-D. Boosting the Capacitive Performance of Supercapacitors by Hybridizing N, P-Codoped Carbon Polycrystalline with Mn3O4-Based Flexible Electrodes. Nanomaterials 2023, 13, 2060. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, Y.; Mi, H.; Luo, S.; Zhang, P.; Lin, Z.; Li, J.; Zhang, H. Robust SnO2−x Nanoparticle-Impregnated Carbon Nanofibers with Outstanding Electrochemical Performance for Advanced Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 8901–8905. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Huang, D.; Pang, W.; Sun, D.; Wang, Q.; Tang, Y.; Ji, X.; Guo, Z.; Wang, H. Plasma-Induced Amorphous Shell and Deep Cation-Site S Doping Endow TiO2 with Extraordinary Sodium Storage Performance. Adv. Mater. 2018, 30, 1801013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, F.; Wang, C.; Wang, S.; Wang, C.; Zhao, Z.; Duan, L.; Liu, Y.; Wu, Y.; Li, W.; et al. Encapsulating highly crystallized mesoporous Fe3O4 in hollow N-doped carbon nanospheres for high-capacity long-life sodium-ion batteries. Nano Energy 2018, 56, 426–433. [Google Scholar] [CrossRef]
- Jing, P.; Wang, Q.; Wang, B.; Gao, X.; Zhang, Y.; Wu, H. Encapsulating yolk-shell FeS2@carbon microboxes into interconnected graphene framework for ultrafast lithium/sodium storage. Carbon 2020, 159, 366–377. [Google Scholar] [CrossRef]
- Li, T.; Qin, A.; Yang, L.; Chen, J.; Wang, Q.; Zhang, D.; Yang, H. In Situ Grown Fe2O3 Single Crystallites on Reduced Graphene Oxide Nanosheets as High Performance Conversion Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 19900–19907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ye, L.; Wei, A.; Xu, G.; Cao, Z.; Zhu, P.; Chen, Y. Constructing SnS/Fe2O3 heterostructure anchored on few-layered graphene as an ion-adsorption/diffusion enhancer for ultrafast and cycle-stable sodium storage. Chem. Eng. J. 2022, 457, 141243. [Google Scholar] [CrossRef]
- Zheng, C.; Xu, X.; Lin, Q.; Chen, Y.; Guo, Z.; Jian, B.; Li, N.; Zhang, H.; Lv, W. Confined growth of Fe2O3 nanoparticles by holey graphene for enhanced sodium-ion storage. Carbon 2021, 176, 31–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. Edge sites-driven accelerated kinetics in ultrafine Fe2O3 nanocrystals anchored graphene for enhanced alkali metal ion storage. Chem. Eng. J. 2021, 428, 131204. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Chen, X.; Song, H. Amorphous Fe2O3/Graphene Composite Nanosheets with Enhanced Electrochemical Performance for Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 30899–30907. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, T.; Gracia-Espino, E.; Reza Barzegar, H.; Jia, X.; Nitze, F.; Hu, G.; Nordblad, P.; Tai, C.-W.; Wågberg, T. Formation of nitrogen-doped graphene nanoscrolls by adsorption of magnetic γ-Fe2O3 nanoparticles. Nat. Commun. 2013, 4, 2319. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, Z.; Mao, M.; Wang, H.; Li, Y.; Ma, J. Metal oxide/graphene composite anode materials for sodium-ion batteries. Energy Storage Mater. 2019, 16, 434–454. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Zhang, P.; Sun, N.; Anasori, B.; Zhu, Q.-Z.; Liu, H.; Gogotsi, Y.; Xu, B. Self-Assembly of Transition Metal Oxide Nanostructures on MXene Nanosheets for Fast and Stable Lithium Storage. Adv. Mater. 2018, 30, 1707334. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xu, W.; Wang, Y.; Zhang, B.; Luo, S.; Zhou, X.; Zhang, C.; Gu, X.; Hu, C. Porous Fe2O3 nanospheres anchored on activated carbon cloth for high-performance symmetric supercapacitors. Nano Energy 2018, 57, 379–387. [Google Scholar] [CrossRef]
- Yan, J.-X.; Leng, Y.-C.; Guo, Y.-N.; Wang, G.-Q.; Gong, H.; Guo, P.-Z.; Tan, P.-H.; Long, Y.-Z.; Liu, X.-L.; Han, W.-P. Highly Conductive Graphene Paper with Vertically Aligned Reduced Graphene Oxide Sheets Fabricated by Improved Electrospray Deposition Technique. ACS Appl. Mater. Interfaces 2019, 11, 10810–10817. [Google Scholar] [CrossRef]
- Gong, H.; Li, M.-F.; Yan, J.-X.; Lin, M.-L.; Liu, X.-L.; Sun, B.; Tan, P.-H.; Long, Y.-Z.; Han, W.-P. Highly conductive, flexible and functional multi-channel graphene microtube fabricated by electrospray deposition technique. J. Mater. Sci. 2019, 54, 14378–14387. [Google Scholar] [CrossRef]
- Han, W.; Wu, Y.; Gong, H.; Liu, L.; Yan, J.; Li, M.; Long, Y.; Shen, G. Reliable sensors based on graphene textile with negative resistance variation in three dimensions. Nano Res. 2021, 14, 2810–2818. [Google Scholar] [CrossRef]
- Yijun, W.; Linxin, L.; Sai, W.; Tong, Z.; Yun-Ze, L.; Wenpeng, H. Preparation of stretchable and porous graphene paper via functionalized with diol oligomer. J. Mater. Sci. Mater. Electron. 2023, 34, 636. [Google Scholar]
- Nai, J.; Kang, J.; Guo, L. Tailoring the shape of amorphous nanomaterials: Recent developments and applications. Sci. China Mater. 2015, 58, 44–59. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Xu, J.; Zhong, H.; Yang, Y.; Chen, W. Self-assembly of α-Fe2O3 nanoparticles into rice ball shaped microstructures via a pyrolytic route. Superlattices Microstruct. 2010, 47, 253–258. [Google Scholar] [CrossRef]
- Nair, A.K.; Elizabeth, I.; Gopukumar, S.; Thomas, S.; M. S, K.; Kalarikkal, N. Nitrogen doped graphene—Silver nanowire hybrids: An excellent anode material for Lithium ion batteries. Appl. Surf. Sci. 2018, 428, 1119–1129. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Yao, J.; Park, J. Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon 2009, 47, 2049–2053. [Google Scholar] [CrossRef]
- Kong, D.; Cheng, C.; Wang, Y.; Liu, B.; Huang, Z.; Yang, H.Y. Seed-assisted growth of α-Fe2O3 nanorod arrays on reduced graphene oxide: A superior anode for high-performance Li-ion and Na-ion batteries†. J. Mater. Chem. A 2016, 4, 11800–11811. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, W.; Lee, D.C.; Bell, C.; Drexler, M.; Ma, Z.F.; Magasinski, A.; Yushin, G.; Alamgir, F.M. Porous FeP/C composite nanofibers as high-performance anodes for Li-ion/Na-ion batteries. Mater. Today Energy 2020, 16, 100410. [Google Scholar] [CrossRef]

| Model Number | Single Layer Rate | Transverse Size Distribution | Particle Size (D50) | Viscosity (cp, 10 mg/g) |
|---|---|---|---|---|
| GO2-DMF | >99% | 20–30 μm | 1.9 ± 0.2 μm | 4800 ± 8000 |
| Re/Ω | Rct/Ω | CPE1-T/Ω | CPE1-P/Ω | W1-R/Ω | W1-T/Ω | W1-P/Ω | |
|---|---|---|---|---|---|---|---|
| Fe2O3@N-GIMC_1:2 | 15.4 | 1842 | 0.000000087 | 0.87 | 1182 | 18.11 | 0.52 |
| Fe2O3@G_1:2 | 14.1 | 2087 | 0.000000045 | 0.64 | 1803 | 17.20 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, H.; Liu, L.; Yan, X.; Long, Y.; Han, W. Amorphous Fe2O3 Anchored on N-Doped Graphene with Internal Micro-Channels as an Active and Durable Anode for Sodium-Ion Batteries. Nanomaterials 2024, 14, 937. https://doi.org/10.3390/nano14110937
Li L, Li H, Liu L, Yan X, Long Y, Han W. Amorphous Fe2O3 Anchored on N-Doped Graphene with Internal Micro-Channels as an Active and Durable Anode for Sodium-Ion Batteries. Nanomaterials. 2024; 14(11):937. https://doi.org/10.3390/nano14110937
Chicago/Turabian StyleLi, Lin, Hui Li, Linxin Liu, Xunchang Yan, Yunze Long, and Wenpeng Han. 2024. "Amorphous Fe2O3 Anchored on N-Doped Graphene with Internal Micro-Channels as an Active and Durable Anode for Sodium-Ion Batteries" Nanomaterials 14, no. 11: 937. https://doi.org/10.3390/nano14110937
APA StyleLi, L., Li, H., Liu, L., Yan, X., Long, Y., & Han, W. (2024). Amorphous Fe2O3 Anchored on N-Doped Graphene with Internal Micro-Channels as an Active and Durable Anode for Sodium-Ion Batteries. Nanomaterials, 14(11), 937. https://doi.org/10.3390/nano14110937






