Nanoengineered Cobalt Electrocatalyst for Alkaline Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
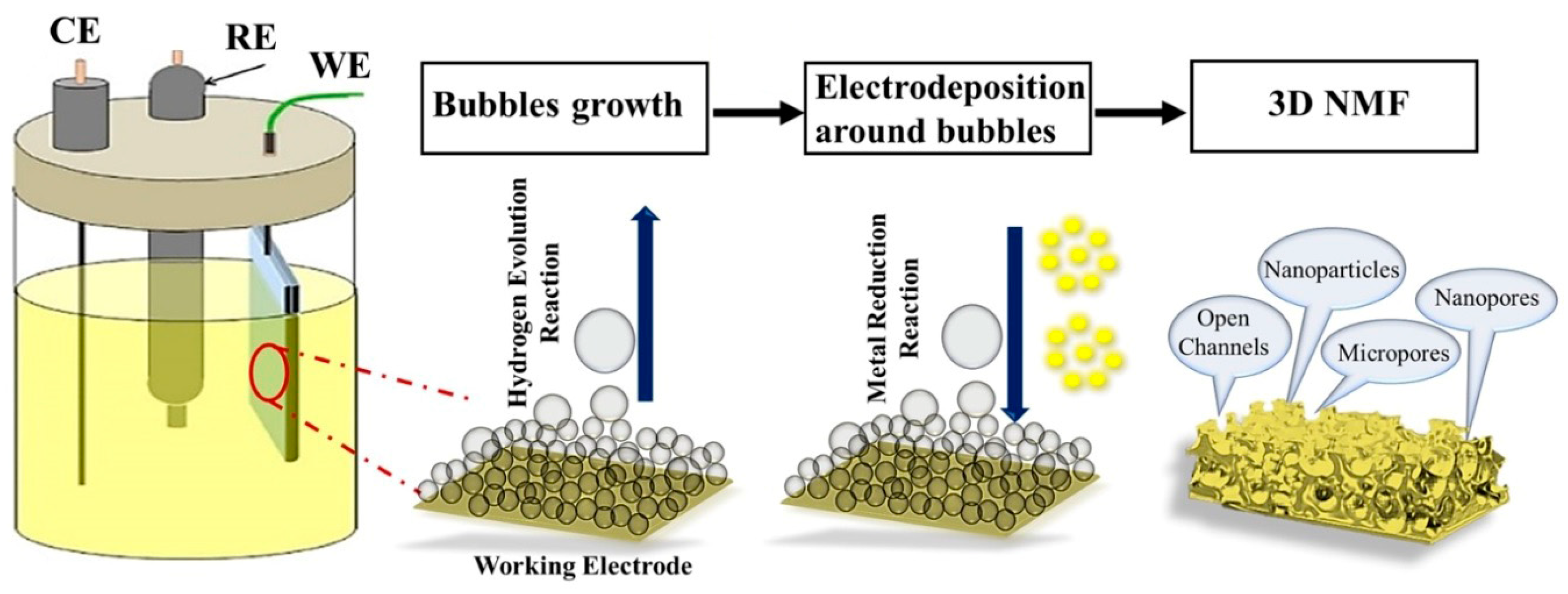
2.2. DHBT Synthesis of Hierarchically Porous Cobalt Electrocatalysts
2.3. Oxygen Evolution Reaction Measurements
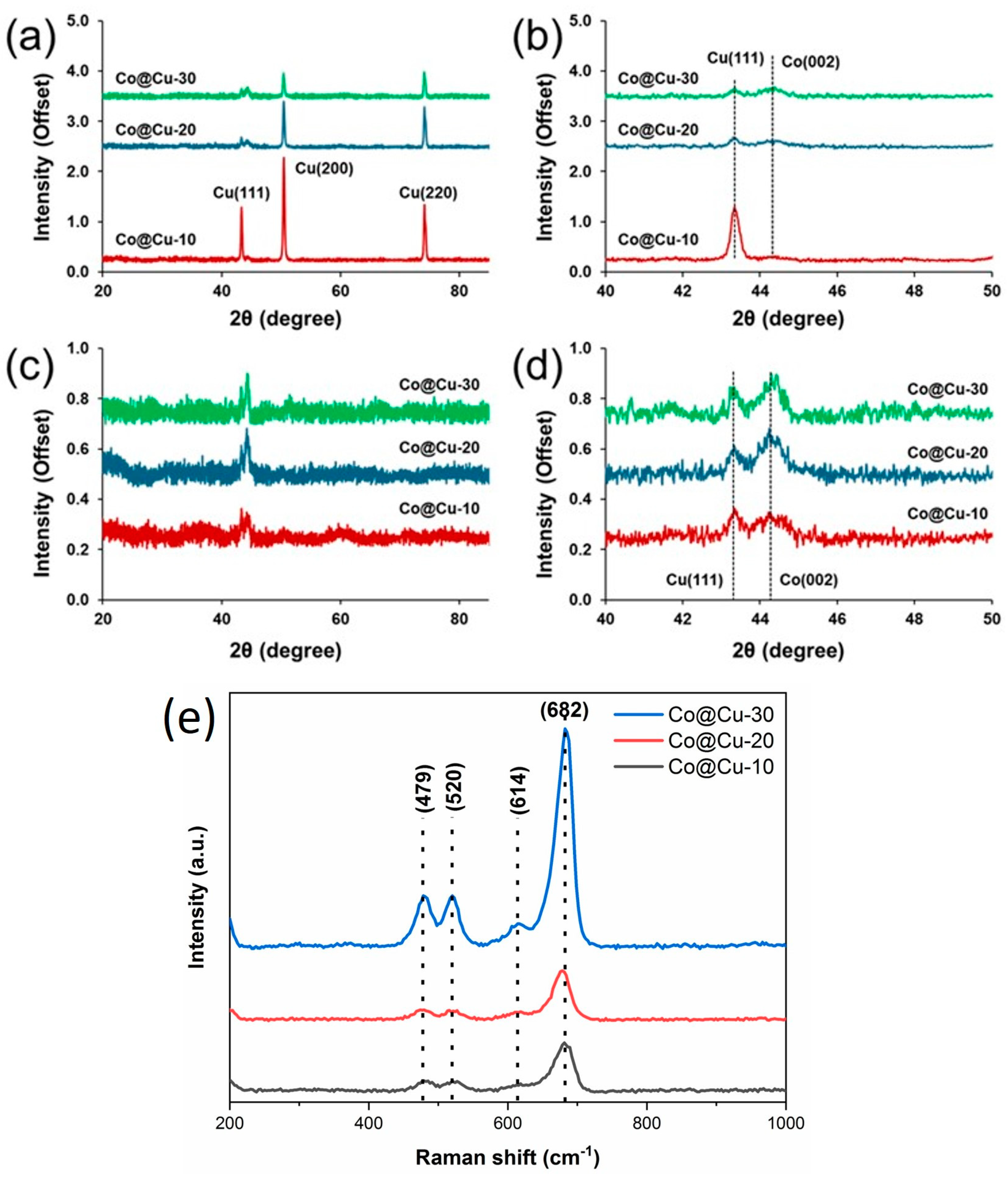
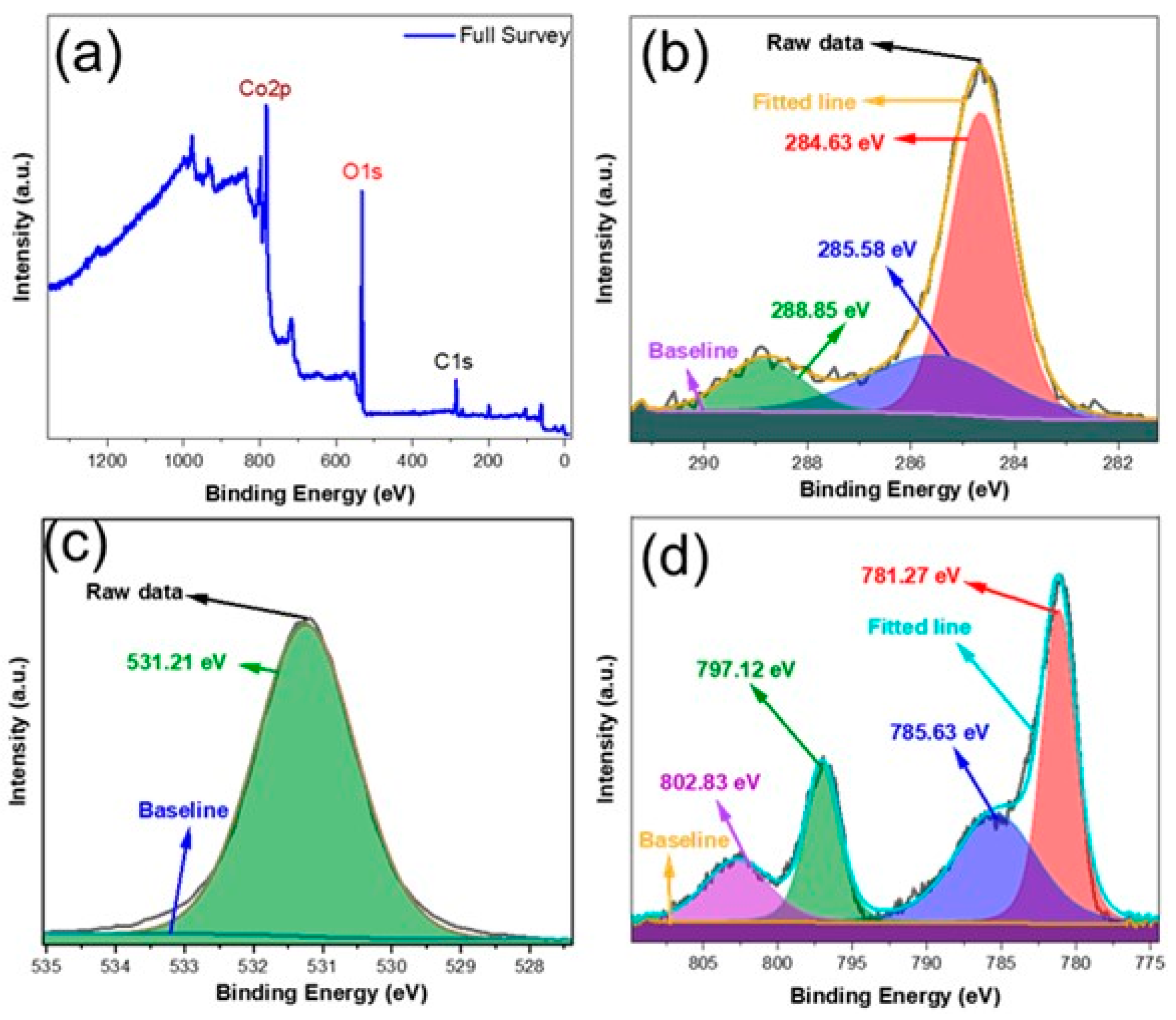
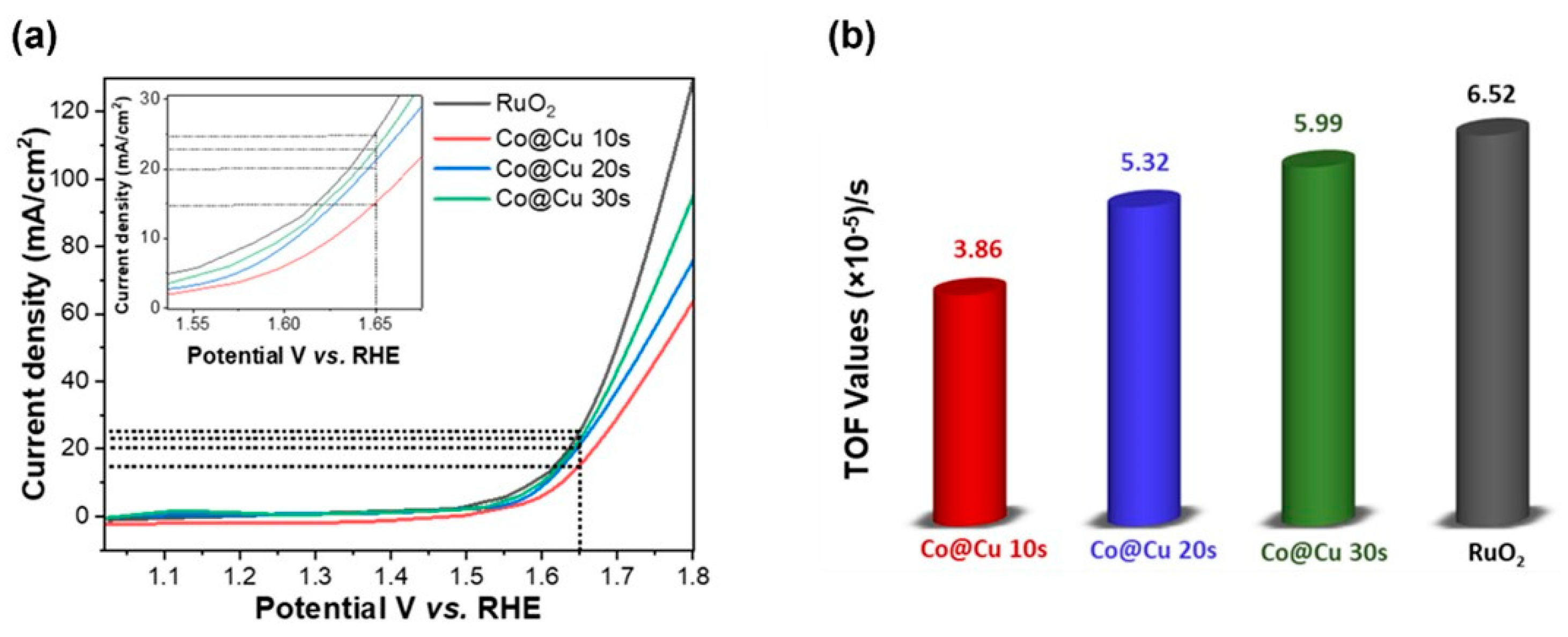
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Shamraiz, U.; Majeed, A.; Raza, B.; Ain, N.u.; Badshah, A. Exploring the potential of cobalt hydroxide and its derivatives as a cost-effective and abundant alternative to noble metal electrocatalysts in oxygen evolution reactions: A review. Sustain. Energy Fuels 2024, 8, 422–459. [Google Scholar] [CrossRef]
- Stojić, D.L.; Marčeta, M.P.; Sovilj, S.P.; Miljanić, Š.S. Hydrogen generation from water electrolysis—Possibilities of energy saving. J. Power Sources 2003, 118, 315–319. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2015; Licence: CC BY 4.0; IEA: Paris, France, 2015; Available online: https://www.iea.org/reports/world-energy-outlook-2015 (accessed on 3 May 2024).
- Mallouk, T.E. Water electrolysis: Divide and conquer. Nat. Chem. 2013, 5, 362–363. [Google Scholar] [CrossRef]
- Lee, W.H.; Han, M.H.; Ko, Y.J.; Min, B.K.; Chae, K.H.; Oh, H.S. Electrode reconstruction strategy for oxygen evolution reaction: Maintaining Fe-CoOOH phase with intermediate-spin state during electrolysis. Nat. Commun. 2022, 13, 605. [Google Scholar] [CrossRef]
- Fabbri, E.; Habereder, A.; Waltar, K.; Kötz, R.; Schmidt, T.J. Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2014, 4, 3800–3821. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.-J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.X.; Ye, S.H.; Xu, H.; Tong, Y.X.; Li, G.R. Design and Synthesis of FeOOH/CeO2 Heterolayered Nanotube Electrocatalysts for the Oxygen Evolution Reaction. Adv. Mater. 2016, 28, 4698–4703. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-Y.; Liu, Z.-Z.; Li, J.; Feng, L.; Yang, M.; Ma, Y.; Liu, D.-P.; Wang, L.; Chai, Y.-M.; Dong, B. Fe-doped CoP core–shell structure with open cages as efficient electrocatalyst for oxygen evolution. J. Energy Chem. 2020, 48, 328–333. [Google Scholar] [CrossRef]
- Frydendal, R.; Paoli, E.A.; Knudsen, B.P.; Wickman, B.; Malacrida, P.; Stephens, I.E.L.; Chorkendorff, I. Benchmarking the Stability of Oxygen Evolution Reaction Catalysts: The Importance of Monitoring Mass Losses. ChemElectroChem 2014, 1, 2075–2081. [Google Scholar] [CrossRef]
- Kötz, R.; Lewerenz, H.J.; Stucki, S. XPS Studies of Oxygen Evolution on Ru and RuO2 Anodes. J. Electrochem. Soc. 1983, 130, 825–829. [Google Scholar] [CrossRef]
- Kötz, R.; Neff, H.; Stucki, S. Anodic Iridium Oxide Films: XPS-Studies of Oxidation State Changes and O2 Evolution. J. Electrochem. Soc. 1984, 131, 72–77. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Wang, B.; Yang, F.; Feng, L. Recent Advances in Co-Based Electrocatalysts for Hydrogen Evolution Reaction. Small 2023, 19, e2302866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cui, L.; Liu, J. Recent advances in cobalt-based electrocatalysts for hydrogen and oxygen evolution reactions. J. Alloys Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]
- Nguyen, A.I.; Van Allsburg, K.M.; Terban, M.W.; Bajdich, M.; Oktawiec, J.; Amtawong, J.; Ziegler, M.S.; Dombrowski, J.P.; Lakshmi, K.V.; Drisdell, W.S.; et al. Stabilization of reactive Co4O4 cubane oxygen-evolution catalysts within porous frameworks. Proc. Natl. Acad. Sci. USA 2019, 116, 11630–11639. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.X.; Xu, H.; Dong, Y.T.; Ye, S.H.; Tong, Y.X.; Li, G.R. FeOOH/Co/FeOOH Hybrid Nanotube Arrays as High-Performance Electrocatalysts for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 3694–3698. [Google Scholar] [CrossRef]
- Yuan, X.; Ge, H.; Wang, X.; Dong, C.; Dong, W.; Riaz, M.S.; Xu, Z.; Zhang, J.; Huang, F. Controlled Phase Evolution from Co Nanochains to CoO Nanocubes and Their Application as OER Catalysts. ACS Energy Lett. 2017, 2, 1208–1213. [Google Scholar] [CrossRef]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin Iron-Cobalt Oxide Nanosheets with Abundant Oxygen Vacancies for the Oxygen Evolution Reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef]
- Koza, J.A.; Hull, C.M.; Liu, Y.-C.; Switzer, J.A. Deposition of β-Co(OH)2 Films by Electrochemical Reduction of Tris(ethylenediamine)cobalt(III) in Alkaline Solution. Chem. Mater. 2013, 25, 1922–1926. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Huang, A.; Wang, Y.; Chu, W.; Luo, W. A copper interface promotes the transformation of nickel hydroxide into high-valent nickel for an efficient oxygen evolution reaction. Inorg. Chem. Front. 2023, 10, 5111–5116. [Google Scholar] [CrossRef]
- Wu, Z.; Gan, Q.; Li, X.; Zhong, Y.; Wang, H. Elucidating Surface Restructuring-Induced Catalytic Reactivity of Cobalt Phosphide Nanoparticles under Electrochemical Conditions. J. Phys. Chem. C 2018, 122, 2848–2853. [Google Scholar] [CrossRef]
- Budiyanto, E.; Ochoa-Hernández, C.; Tüysüz, H. Impact of Highly Concentrated Alkaline Treatment on Mesostructured Cobalt Oxide for the Oxygen Evolution Reaction. Adv. Sustain. Syst. 2023, 7, 2200499. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Wu, Z.-Y.; Adler, Z.; Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- An, L.; Wei, C.; Lu, M.; Liu, H.; Chen, Y.; Scherer, G.G.; Fisher, A.C.; Xi, P.; Xu, Z.J.; Yan, C.H. Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment. Adv. Mater. 2021, 33, e2006328. [Google Scholar] [CrossRef]
- Joo, J.; Kim, T.; Lee, J.; Choi, S.I.; Lee, K. Morphology-Controlled Metal Sulfides and Phosphides for Electrochemical Water Splitting. Adv. Mater. 2019, 31, e1806682. [Google Scholar] [CrossRef]
- Chandran, P.; Bakshi, S.; Chatterjee, D. Study on the characteristics of hydrogen bubble formation and its transport during electrolysis of water. Chem. Eng. Sci. 2015, 138, 99–109. [Google Scholar] [CrossRef]
- Kou, T.; Wang, S.; Shi, R.; Zhang, T.; Chiovoloni, S.; Lu, J.Q.; Chen, W.; Worsley, M.A.; Wood, B.C.; Baker, S.E.; et al. Periodic Porous 3D Electrodes Mitigate Gas Bubble Traffic during Alkaline Water Electrolysis at High Current Densities. Adv. Energy Mater. 2020, 10, 2002955. [Google Scholar] [CrossRef]
- Fan, R.-Y.; Zhou, Y.-N.; Li, M.-X.; Xie, J.-Y.; Yu, W.-L.; Chi, J.-Q.; Wang, L.; Yu, J.-F.; Chai, Y.-M.; Dong, B. In situ construction of Fe(Co)OOH through ultra-fast electrochemical activation as real catalytic species for enhanced water oxidation. Chem. Eng. J. 2021, 426, 131943. [Google Scholar] [CrossRef]
- Xiao, C.; Li, Y.; Lu, X.; Zhao, C. Bifunctional Porous NiFe/NiCo2O4/Ni Foam Electrodes with Triple Hierarchy and Double Synergies for Efficient Whole Cell Water Splitting. Adv. Funct. Mater. 2016, 26, 3515–3523. [Google Scholar] [CrossRef]
- Velev, O.D.; Lenhoff, A.M. Colloidal crystals as templates for porous materials. Curr. Opin. Colloid Interface Sci. 2000, 5, 56–63. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Hu, X.; Zhou, Y.; Chen, S. Three-dimensional ordered macroporous IrO2 as electrocatalyst for oxygen evolution reaction in acidic medium. J. Mater. Chem. 2012, 22, 6010–6016. [Google Scholar] [CrossRef]
- Mehla, S.; Selvakannan, P.R.; Bhargava, S.K. Readily tunable surface plasmon resonances in gold nanoring arrays fabricated using lateral electrodeposition. Nanoscale 2022, 14, 9989–9996. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, H.; Wang, Z.; Mitra, A.; Zhao, D.; Yan, Y. Cuprite Nanowires by Electrodeposition from Lyotropic Reverse Hexagonal Liquid Crystalline Phase. Chem. Mater. 2002, 14, 876–880. [Google Scholar] [CrossRef]
- Seshadri, R.; Meldrum, F.C. Bioskeletons as Templates for Ordered, Macroporous Structures. Adv. Mater. 2000, 12, 1149–1151. [Google Scholar] [CrossRef]
- Shin, H.C.; Dong, J.; Liu, M. Nanoporous Structures Prepared by an Electrochemical Deposition Process. Adv. Mater. 2003, 15, 1610–1614. [Google Scholar] [CrossRef]
- Shin, H.-C.; Liu, M. Copper Foam Structures with Highly Porous Nanostructured Walls. Chem. Mater. 2004, 16, 5460–5464. [Google Scholar] [CrossRef]
- Li, Y.; Jia, W.-Z.; Song, Y.-Y.; Xia, X.-H. Superhydrophobicity of 3D Porous Copper Films Prepared Using the Hydrogen Bubble Dynamic Template. Chem. Mater. 2007, 19, 5758–5764. [Google Scholar] [CrossRef]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with bubbles: The formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem. Commun. 2015, 51, 4331–4346. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Tahir, A.; Haq, T.U.; Munir, A.; Hussain, I.; Sher, F. Bubbles Templated Interconnected Porous Metallic Materials: Synthesis, Surface Modification, and Their Electrocatalytic Applications for Water Splitting and Alcohols Oxidation. ChemistrySelect 2022, 7, e202202774. [Google Scholar] [CrossRef]
- Tsai, W.L.; Hsu, P.C.; Hwu, Y.; Chen, C.H.; Chang, L.W.; Je, J.H.; Lin, H.M.; Groso, A.; Margaritondo, G. Building on bubbles in metal electrodeposition. Nature 2002, 417, 139. [Google Scholar] [CrossRef]
- Ott, A.; Jones, L.A.; Bhargava, S.K. Direct electrodeposition of porous platinum honeycomb structures. Electrochem. Commun. 2011, 13, 1248–1251. [Google Scholar] [CrossRef]
- Najdovski, I.; Selvakannan, P.R.; O’Mullane, A.P.; Bhargava, S.K. Rapid synthesis of porous honeycomb Cu/Pd through a hydrogen-bubble templating method. Chemistry 2011, 17, 10058–10063. [Google Scholar] [CrossRef] [PubMed]
- Plowman, B.J.; Bhargava, S.K.; O’Mullane, A.P. Electrochemical fabrication of metallic nanostructured electrodes for electroanalytical applications. Analyst 2011, 136, 5107–5119. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Bhatt, A.I.; O’Mullane, A.P.; Bhargava, S.K. An investigation of silver electrodeposition from ionic liquids: Influence of atmospheric water uptake on the silver electrodeposition mechanism and film morphology. Electrochim. Acta 2011, 56, 2895–2905. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Connor, P.; Schuch, J.; Kaiser, B.; Jaegermann, W. The Determination of Electrochemical Active Surface Area and Specific Capacity Revisited for the System MnOx as an Oxygen Evolution Catalyst. Z. Phys. Chem. 2020, 234, 979–994. [Google Scholar] [CrossRef]
- Fikry, M.; Tawfik, W.; Omar, M.M. Investigation on the effects of laser parameters on the plasma profile of copper using picosecond laser induced plasma spectroscopy. Opt. Quantum Electron. 2020, 52, 249. [Google Scholar] [CrossRef]
- Gomez, E.; Valles, E. Thick cobalt coatings obtained by electrodeposition. J. Appl. Electrochem. 2002, 32, 693–700. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199. [Google Scholar] [CrossRef]
- Kim, M.G.; Choi, Y.H. Electrocatalytic Properties of Co3O4 Prepared on Carbon Fibers by Thermal Metal-Organic Deposition for the Oxygen Evolution Reaction in Alkaline Water Electrolysis. Nanomaterials 2023, 13, 1021. [Google Scholar] [CrossRef] [PubMed]
- Jirátová, K.; Perekrestov, R.; Dvořáková, M.; Balabánová, J.; Topka, P.; Koštejn, M.; Olejníček, J.; Čada, M.; Hubička, Z.; Kovanda, F. Cobalt Oxide Catalysts in the Form of Thin Films Prepared by Magnetron Sputtering on Stainless-Steel Meshes: Performance in Ethanol Oxidation. Catalysts 2019, 9, 806. [Google Scholar] [CrossRef]
- Xie, P.; Liu, Y.; Feng, M.; Niu, M.; Liu, C.; Wu, N.; Sui, K.; Patil, R.R.; Pan, D.; Guo, Z.; et al. Hierarchically porous Co/C nanocomposites for ultralight high-performance microwave absorption. Adv. Compos. Hybrid. Mater. 2021, 4, 173–185. [Google Scholar] [CrossRef]
- Fettkenhauer, C.; Wang, X.; Kailasam, K.; Antonietti, M.; Dontsova, D. Synthesis of efficient photocatalysts for water oxidation and dye degradation reactions using CoCl2 eutectics. J. Mater. Chem. A 2015, 3, 21227–21232. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp. 1992, 40, 221. [Google Scholar]
- Brundle, C.R.; Chuang, T.J.; Rice, D.W. X-ray photoemission study of the interaction of oxygen and air with clean cobalt surfaces. Surf. Sci. 1976, 60, 286–300. [Google Scholar] [CrossRef]
- Li, S.; Peng, S.; Huang, L.; Cui, X.; Al-Enizi, A.M.; Zheng, G. Carbon-Coated Co3+-Rich Cobalt Selenide Derived from ZIF-67 for Efficient Electrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 20534–20539. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Gu, Y.; Xu, H.; Wang, C.; Shao, Z.; Guo, Y. Tuning synergy between nickel and iron in Ruddlesden–Popper perovskites through controllable crystal dimensionalities towards enhanced oxygen—Evolving activity and stability. Carbon Energy, 2024; early view. [Google Scholar] [CrossRef]
- Singh, A.K.; Prasad, J.; Azad, U.P.; Singh, A.K.; Prakash, R.; Singh, K.; Srivastava, A.; Alaferdov, A.A.; Moshkalev, S.A. Vanadium doped few-layer ultrathin MoS2 nanosheets on reduced graphene oxide for high-performance hydrogen evolution reaction. RSC Adv. 2019, 9, 22232–22239. [Google Scholar] [CrossRef]
- Roy, A.; Tariq, M.Z.; La, M.; Choi, D.; Park, S.J. A comparative study on the oxygen evolution reaction of cobalt and nickel based hydroxide electrodes in alkaline electrolyte. J. Electroanal. Chem. 2022, 920, 116633. [Google Scholar] [CrossRef]
- Liu, T.; Sun, X.; Asiri, A.M.; He, Y. One-step electrodeposition of Ni–Co–S nanosheets film as a bifunctional electrocatalyst for efficient water splitting. Int. J. Hydrogen Energy 2016, 41, 7264–7269. [Google Scholar] [CrossRef]
- Wu, T.; Pi, M.; Wang, X.; Zhang, D.; Chen, S. Three-dimensional metal–organic framework derived porous CoP3 concave polyhedrons as superior bifunctional electrocatalysts for the evolution of hydrogen and oxygen. Phys. Chem. Chem. Phys. 2017, 19, 2104–2110. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, H.; Yu, Z.; Islam, S.M.; He, H.; Yuan, M.; Yue, Y.; Xu, K.; Hao, W.; Sun, G.; et al. Hierarchical Nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a Highly Efficient Electrocatalyst for Overall Water Splitting in a Wide pH Range. J. Am. Chem. Soc. 2019, 141, 10417–10430. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Wu, R.; Yu, Y.; Zhang, B. Anchoring CoO Domains on CoSe2 Nanobelts as Bifunctional Electrocatalysts for Overall Water Splitting in Neutral Media. Adv. Sci. 2016, 3, 1500426. [Google Scholar] [CrossRef]
- Yang, C.C.; Zai, S.F.; Zhou, Y.T.; Du, L.; Jiang, Q. Fe3C-Co Nanoparticles Encapsulated in a Hierarchical Structure of N-Doped Carbon as a Multifunctional Electrocatalyst for ORR, OER, and HER. Adv. Funct. Mater. 2019, 29, 1901949. [Google Scholar] [CrossRef]
- Wu, T.; Pi, M.; Zhang, D.; Chen, S. 3D structured porous CoP3 nanoneedle arrays as an efficient bifunctional electrocatalyst for the evolution reaction of hydrogen and oxygen. J. Mater. Chem. A 2016, 4, 14539–14544. [Google Scholar] [CrossRef]
- Gu, W.; Hu, L.; Zhu, X.; Shang, C.; Li, J.; Wang, E. Rapid synthesis of Co3O4 nanosheet arrays on Ni foam by in situ electrochemical oxidization of air-plasma engraved Co(OH)2 for efficient oxygen evolution. Chem. Commun. 2018, 54, 12698–12701. [Google Scholar] [CrossRef] [PubMed]

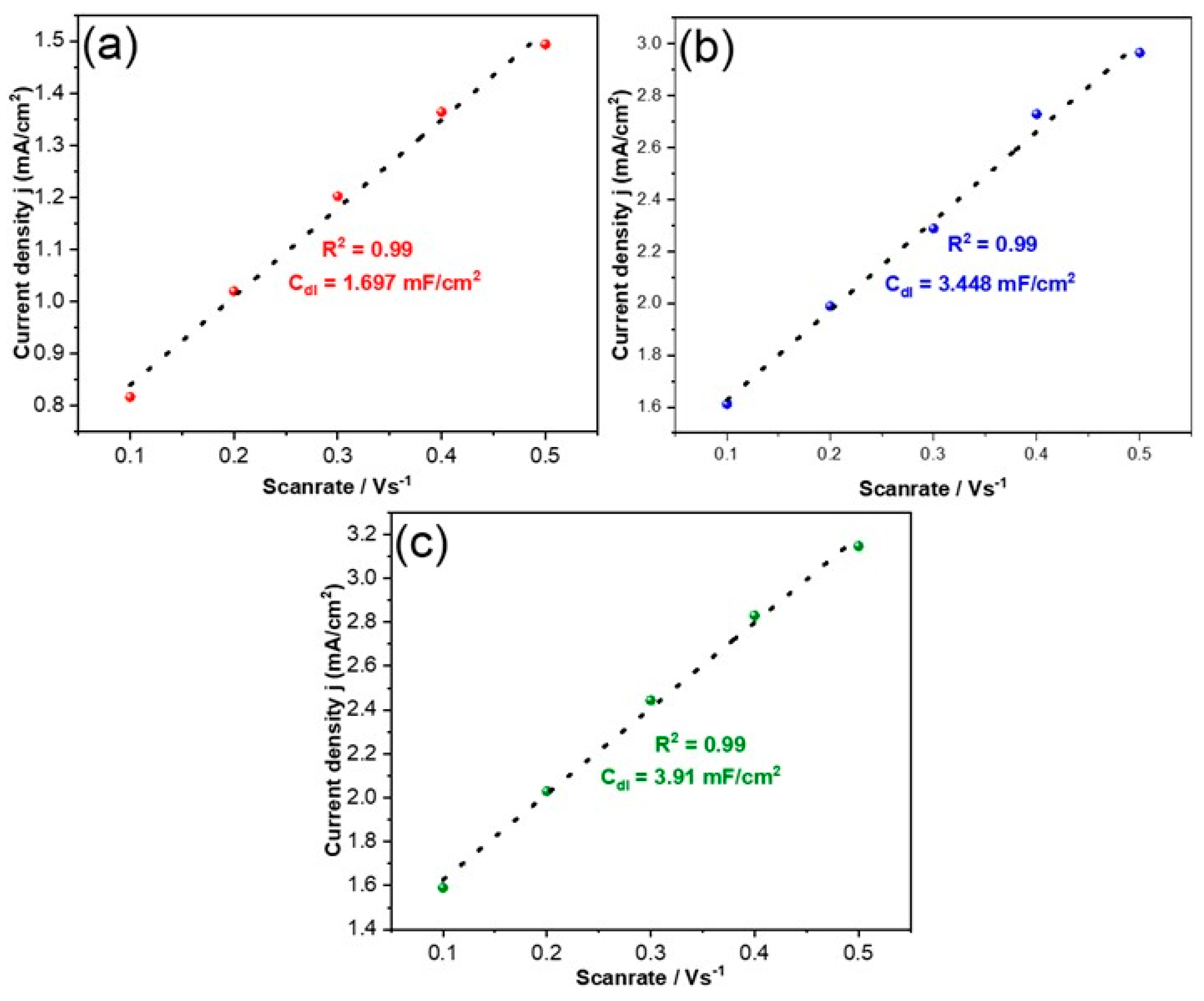

| Electrocatalyst | Co a (wt %) | O a (wt %) | Cu a (wt %) | Cdl b (mF/cm2) |
|---|---|---|---|---|
| Cu | 0 | 0 | 100 | - |
| Co@Cu-10 | 71.0 | 18.3 | 10.7 | 1.69 |
| Co@Cu-20 | 74.1 | 16.1 | 9.8 | 3.44 |
| Co@Cu-30 | 80.0 | 10.9 | 9.1 | 3.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajagopal, V.; Mehla, S.; Jones, L.A.; Bhargava, S.K. Nanoengineered Cobalt Electrocatalyst for Alkaline Oxygen Evolution Reaction. Nanomaterials 2024, 14, 946. https://doi.org/10.3390/nano14110946
Rajagopal V, Mehla S, Jones LA, Bhargava SK. Nanoengineered Cobalt Electrocatalyst for Alkaline Oxygen Evolution Reaction. Nanomaterials. 2024; 14(11):946. https://doi.org/10.3390/nano14110946
Chicago/Turabian StyleRajagopal, Venkatachalam, Sunil Mehla, Lathe A. Jones, and Suresh K. Bhargava. 2024. "Nanoengineered Cobalt Electrocatalyst for Alkaline Oxygen Evolution Reaction" Nanomaterials 14, no. 11: 946. https://doi.org/10.3390/nano14110946






