Electrochemical Detection of Glyphosate in Surface Water Samples Based on Modified Screen-Printed Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
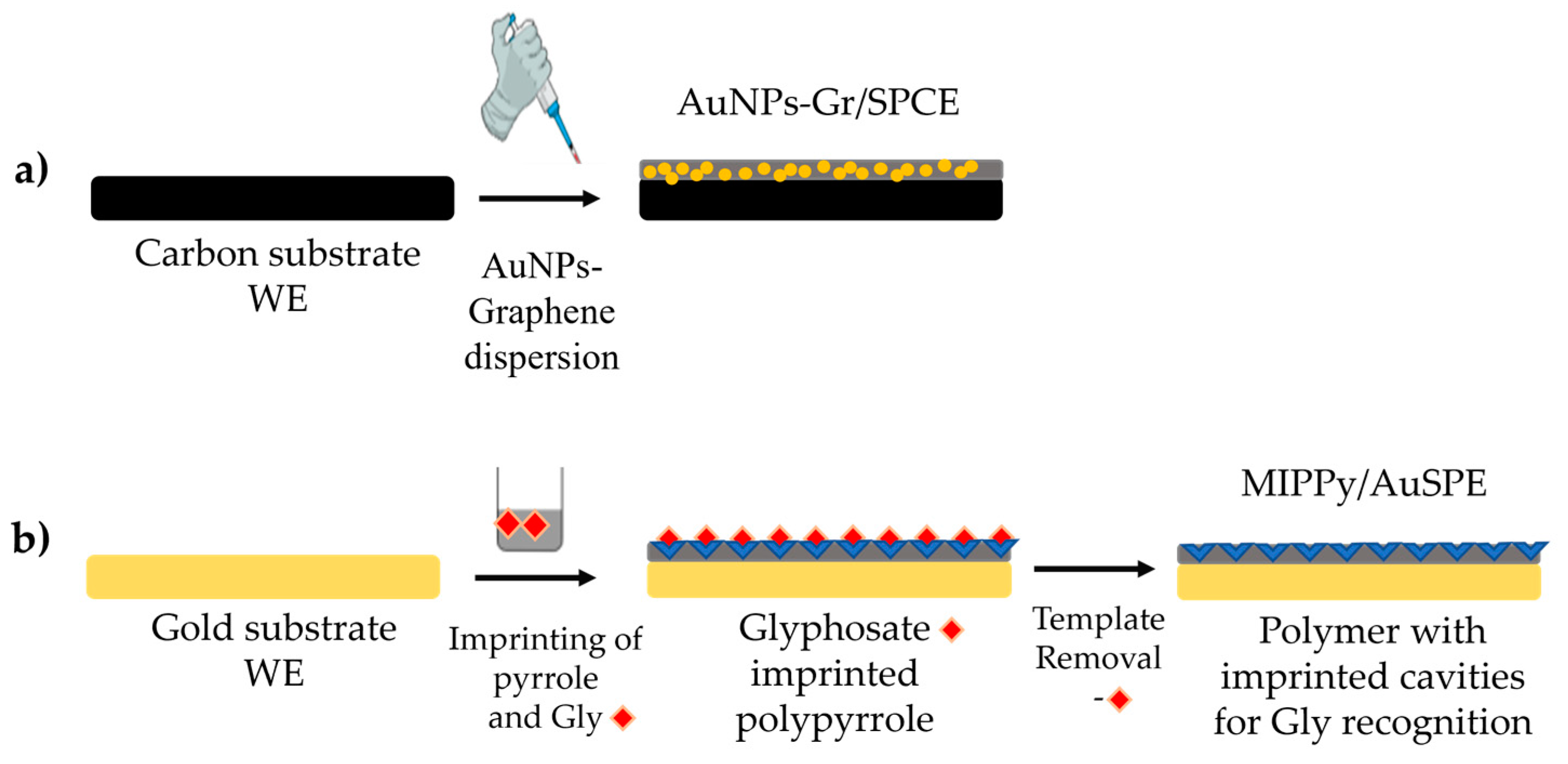
2.2. Electrochemical Apparatus and the Functionalization Procedures
3. Results
3.1. Structural and Chemical Characterization of AuNPS-Gr Nanohybrid Material
3.2. Morphological Characterization of the Molecularly Imprinted Polypyrrole
3.3. Electrochemical Characterization of the Modified SPEs
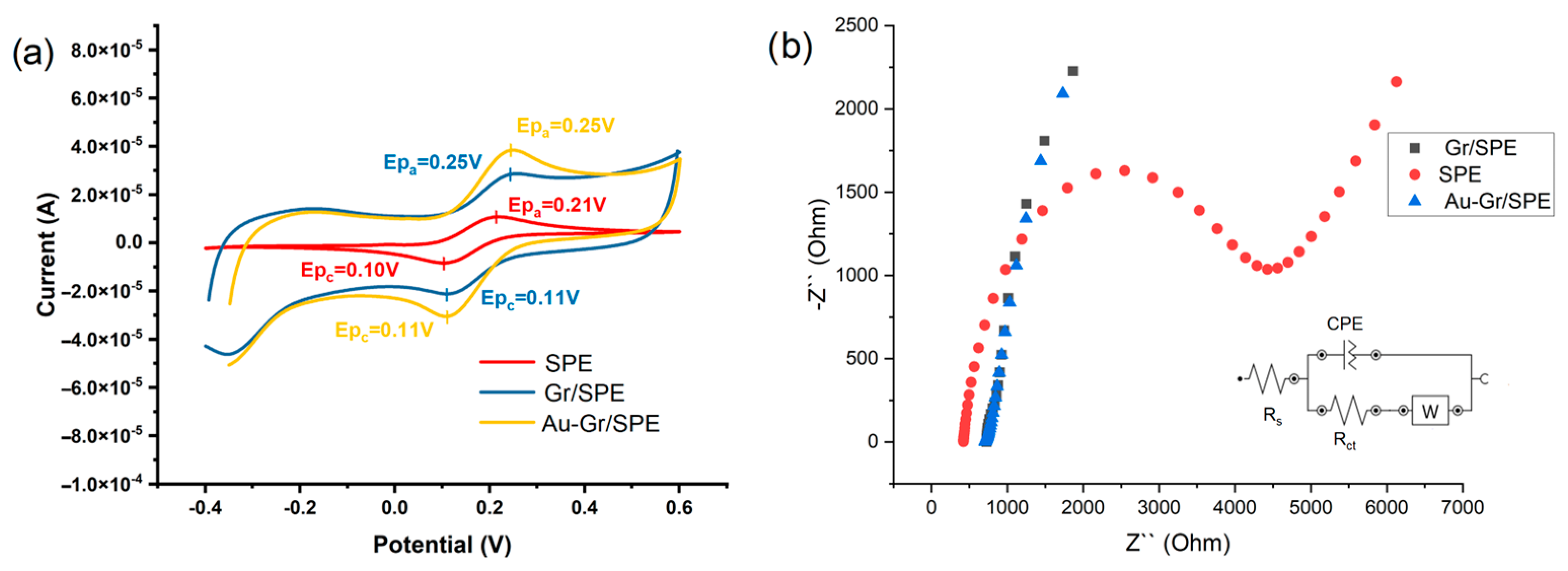
3.3.1. Electrochemical Characterization of the AuNPs-Gr/SPCE
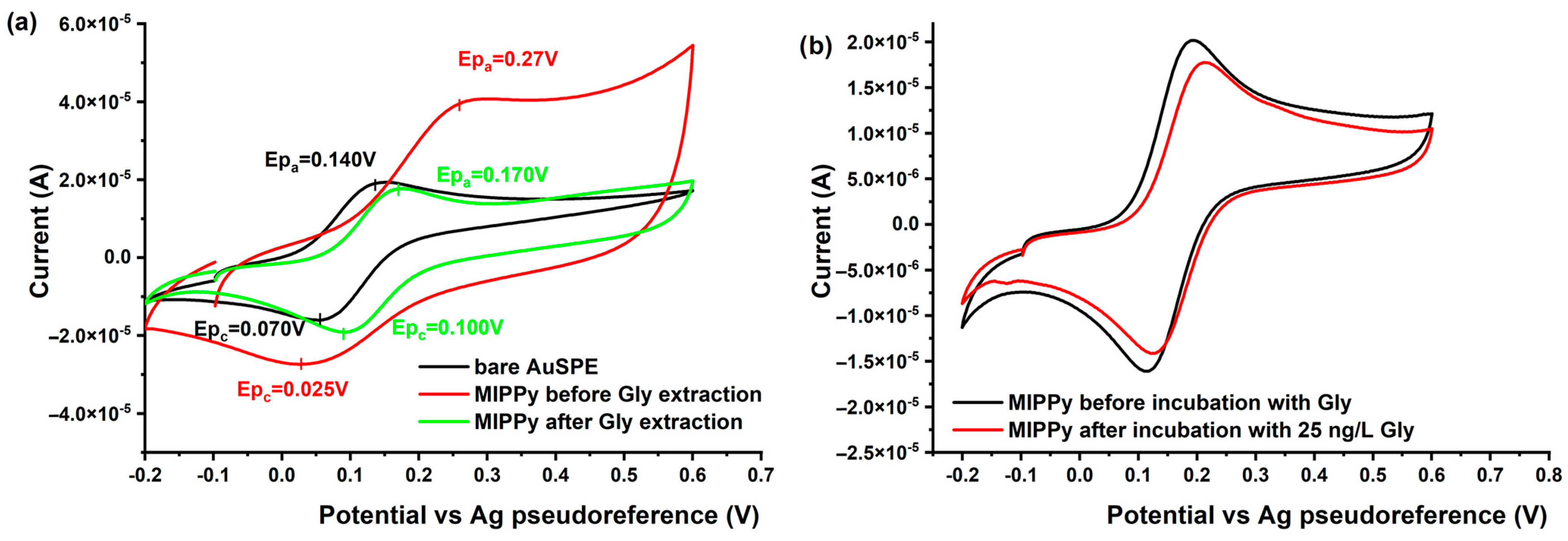
3.3.2. Electrochemical Characterization of the MIPPy/AuSPE
3.4. Voltammetric Detection of Gly Using Modified SPE
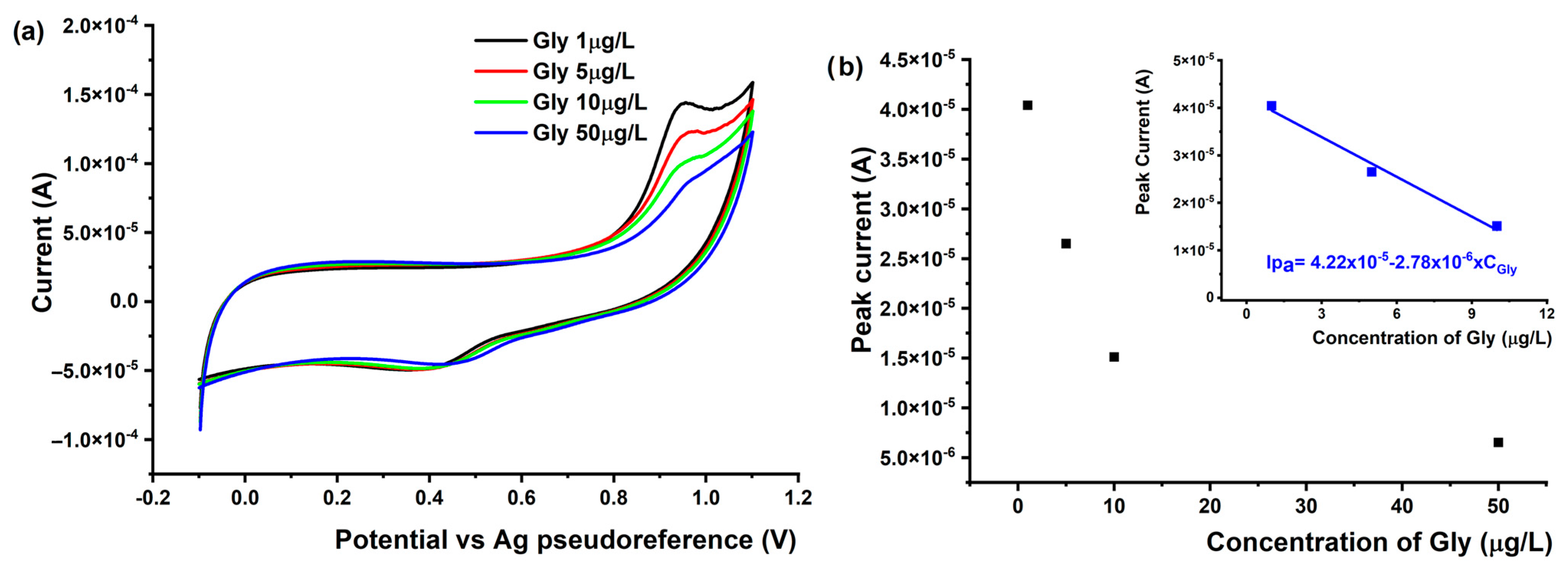
3.4.1. Voltammetric Detection of Gly Using AuNPs-Gr/SPCE
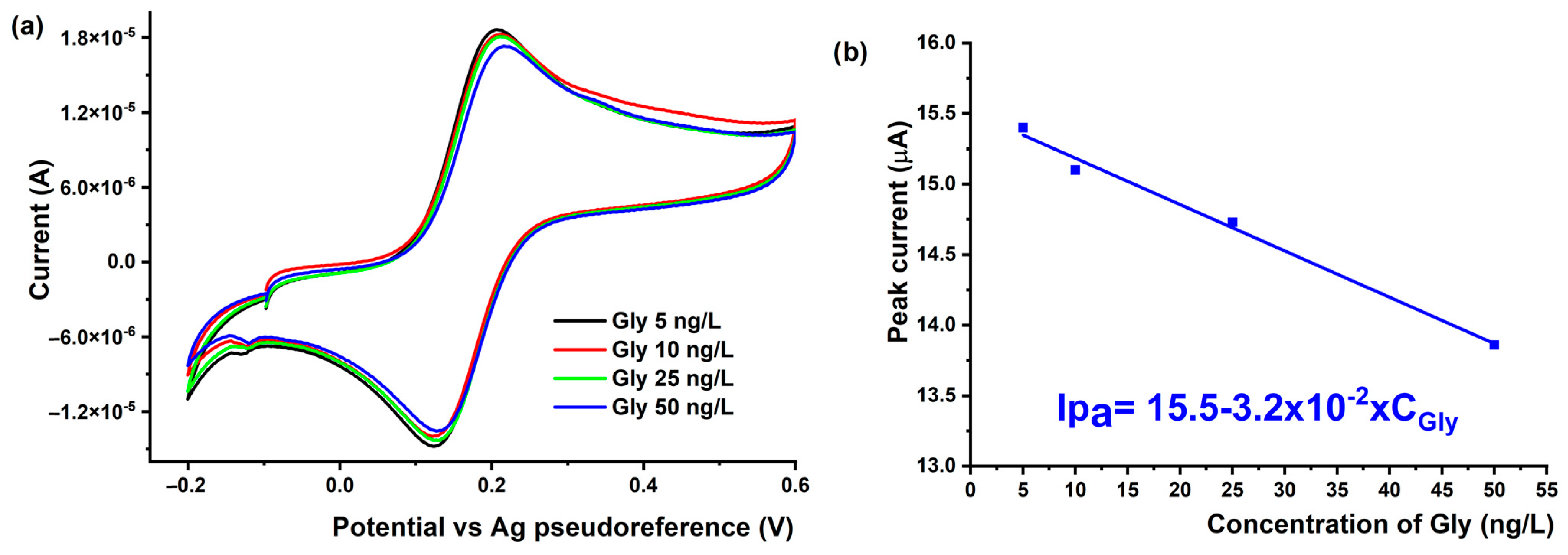
3.4.2. Voltammetric Detection of Gly Using MIPPy/AuSPE
3.4.3. Selectivity Studies and Analytical Application of Gly Using MIPPy/AuSPE
4. Conclusions
5. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Avino, P.; Notardonato, I.; Russo, M.V. A Review of the Analytical Methods Based on Chromatography for Analyzing Glyphosate in Foods. In Pests, Weeds and Diseases in Agricultural Crop and Animal Husbandry Production; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Rigobello-Masini, M.; Pereira, E.A.O.; Abate, G.; Masini, J.C. Solid-Phase Extraction of Glyphosate in the Analyses of Environmental, Plant, and Food Samples. Chromatographia 2019, 82, 1121–1138. [Google Scholar] [CrossRef]
- Saunders, L.E.; Pezeshki, R. Glyphosate in Runoff Waters and in the Root-Zone: A Review. Toxics 2015, 3, 462–480. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, Y.S.; Xiao, G.T.; Chiu, T.C.; Hu, C.C. A Highly Selective and Sensitive Nanosensor for the Detection of Glyphosate. Talanta 2016, 161, 94–98. [Google Scholar] [CrossRef]
- Hamilton, D.J.; Ambrus, Á.; Dieterle, R.M.; Felsot, A.S.; Harris, C.A.; Holland, P.T.; Katayama, A.; Kurihara, N.; Linders, J.; Unsworth, J.; et al. Regulatory limits for pesticide residues in water (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 1123–1155. [Google Scholar] [CrossRef]
- US EPA. Drinking Water Regulations and Contaminants. Available online: https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants (accessed on 23 December 2023).
- Arkan, T.; Molnár-Perl, I. The Role of Derivatization Techniques in the Analysis of Glyphosate and Aminomethyl-Phosphonic Acid by Chromatography. Microchem. J. 2015, 121, 99–106. [Google Scholar] [CrossRef]
- Fang, F.; Wei, R.; Liu, X. Novel Pre-Column Derivatisation Reagent for Glyphosate by High-Performance Liquid Chromatography and Ultraviolet Detection. Int. J. Environ. Anal. Chem. 2014, 94, 661–667. [Google Scholar] [CrossRef]
- Sun, L.; Kong, D.; Gu, W.; Guo, X.; Tao, W.; Shan, Z.; Wang, Y.; Wang, N. Determination of Glyphosate in Soil/Sludge by High Performance Liquid Chromatography. J. Chromatogr. A 2017, 1502, 8–13. [Google Scholar] [CrossRef]
- Campanale, C.; Triozzi, M.; Massarelli, C.; Uricchio, V. Development of a UHPLC-MS/MS Method to Enhance the Detection of Glyphosate, AMPA and Glufosinate at Sub-Microgram/L Levels in Water Samples. J. Chromatogr. A 2022, 1672, 463028. [Google Scholar] [CrossRef]
- Liao, Y.; Berthion, J.M.; Colet, I.; Merlo, M.; Nougadère, A.; Hu, R. Validation and Application of Analytical Method for Glyphosate and Glufosinate in Foods by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2018, 1549, 31–38. [Google Scholar] [CrossRef]
- Dovidauskas, S.; Okada, I.; dos Santos, F. Validation of a Simple Ion Chromatography Method for Simultaneous Determination of Glyphosate, Aminomethylphosphonic Acid and Ions of Public Health Concern in Water Intended for Human Consumption. J. Chromatogr. A 2020, 1632, 461603. [Google Scholar] [CrossRef]
- Connolly, A.; Koslitz, S.; Bury, D.; Bruning, T.; Conrad, A.; Kolossa-Gehring, M.; Coggins, M.; Koch, H. Sensitive and Selective Quantification of Glyphosate and Aminomethylphosphonic Acid (AMPA) in Urine of the General Population by Gas Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2020, 1158, 122348. [Google Scholar] [CrossRef]
- Wimmer, B.; Pattky, M.; Zada, L.G.; Meixner, M.; Haderlein, S.B.; Zimmermann, H.P.; Huhn, C. Capillary Electrophoresis-Mass Spectrometry for the Direct Analysis of Glyphosate: Method Development and Application to Beer Beverages and Environmental Studies. Anal. Bioanal. Chem. 2020, 412, 4967–4983. [Google Scholar] [CrossRef]
- Duffy, G.; Regan, F. Recent Developments in Sensing Methods for Eutrophying Nutrients with a Focus on Automation for Environmental Applications. Analyst 2017, 142, 4355–4372. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.L.; Mello, F.C.C.; Alves-Balvedi, R.P.; Rodrigues, L.P.; Goulart, L.R. Glyphosate Detection: Methods, Needs and Challenges. Environ. Chem. Lett. 2019, 17, 291–317. [Google Scholar] [CrossRef]
- Gauglitz, G.; Wimmer, B.; Melzer, T.; Huhn, C. Glyphosate Analysis Using Sensors and Electromigration Separation Techniques as Alternatives to Gas or Liquid Chromatography. Anal. Bioanal. Chem. 2018, 410, 725–746. [Google Scholar] [CrossRef]
- Noori, J.S.; Mortensen, J.; Geto, A. Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review. Sensors 2020, 20, 2221. [Google Scholar] [CrossRef]
- Zambrano-Intriago, L.A.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Araújo, A.N.; Montenegro, M.C.B.S.M. Challenges in the Design of Electrochemical Sensor for Glyphosate-Based on New Materials and Biological Recognition. Sci. Total Environ. 2021, 793, 148496. [Google Scholar] [CrossRef]
- Cahuantzi-Muñoz, S.L.; González-Fuentes, M.A.; Ortiz-Frade, L.A.; Torres, E.; Ţălu, Ş.; Trejo, G.; Méndez-Albores, A. Electrochemical Biosensor for Sensitive Quantification of Glyphosate in Maize Kernels. Electroanalysis 2019, 31, 927–935. [Google Scholar] [CrossRef]
- Oliveira, P.C.; Maximiano, E.M.; Oliveira, P.A.; Camargo, J.S.; Fiorucci, A.R.; Arruda, G.J. Direct Electrochemical Detection of Glyphosate at Carbon Paste Electrode and Its Determination in Samples of Milk, Orange Juice, and Agricultural Formulation. J. Environ. Sci. Health Part B 2018, 53, 817–823. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Akbari, A.; Norouzi, L. Development of a Novel Hollow Fiber- Pencil Graphite Modified Electrochemical Sensor for the Ultra-Trace Analysis of Glyphosate. Sens. Actuators B Chem. 2018, 272, 415–424. [Google Scholar] [CrossRef]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.S.; Brett, C.M.A. Direct Electrochemical Determination of Glyphosate at Copper Phthalocyanine/Multiwalled Carbon Nanotube Film Electrodes. Electroanalysis 2010, 22, 1586–1591. [Google Scholar] [CrossRef]
- Noori, J.S.; Dimaki, M.; Mortensen, J.; Svendsen, W.E. Detection of Glyphosate in Drinking Water: A Fast and Direct Detection Method without Sample Pretreatment. Sensors 2018, 18, 2961. [Google Scholar] [CrossRef] [PubMed]
- Songa, E.A.; Waryo, T.; Jahed, N.; Baker, P.G.L.; Kgarebe, B.V.; Iwuoha, E.I. Electrochemical Nanobiosensor for Glyphosate Herbicide and Its Metabolite. Electroanalysis 2009, 21, 671–674. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Shen, C.; Wang, C.; Hu, X.; Wang, G. An Electrochemical Sensor on the Hierarchically Porous Cu-BTC MOF Platform for Glyphosate Determination. Sens. Actuators B Chem. 2019, 283, 487–494. [Google Scholar] [CrossRef]
- Poorahong, S.; Thammakhet, C.; Thavarungkul, P.; Kanatharana, P. One-Step Preparation of Porous Copper Nanowires Electrode for Highly Sensitive and Stable Amperometric Detection of Glyphosate. Chem. Pap. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Teófilo, R.F.; Reis, E.L.; Reis, C.; Da Silva, G.A.; Paiva, J.F.; Kubota, L.T. Glyphosate Determination in Soil, Water and Vegetables Using DPV Optimized by Response Surface Methodology. Port. Electrochim. Acta 2008, 26, 325–337. [Google Scholar] [CrossRef]
- Khenifi, A.; Derriche, Z.; Forano, C.; Prevot, V.; Mousty, C.; Scavetta, E.; Ballarin, B.; Guadagnini, L.; Tonelli, D. Glyphosate and Glufosinate Detection at Electrogenerated NiAl-LDH Thin Films. Anal. Chim. Acta 2009, 654, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Bahamon-Pinzon, D.; Moreira, G.; Obare, S.; Vanegas, D. Development of a Nanocopper-Decorated Laser-Scribed Sensor for Organophosphorus Pesticide Monitoring in Aqueous Samples. Microchim. Acta 2022, 189, 254. [Google Scholar] [CrossRef] [PubMed]
- Butmee, P.; Tumcharern, G.; Songsiriritthigul, C.; Durand, M.; Thouand, G.; Kerr, M.; Kalcher, K.; Samphao, A. Enzymatic Electrochemical Biosensor for Glyphosate Detection Based on Acid Phosphatase Inhibition. Anal. Bioanal. Chem. 2021, 413, 5859–5869. [Google Scholar] [CrossRef]
- Setznagl, S.; Cesarino, I. Copper Nanoparticles and Reduced Graphene Oxide Modified a Glassy Carbon Electrode for the Determination of Glyphosate in Water Samples. Int. J. Environ. Anal. Chem. 2022, 102, 293–305. [Google Scholar] [CrossRef]
- Habekost, A. Rapid and Sensitive Spectroelectrochemical and Electrochemical Detection of Glyphosate and AMPA with Screen-Printed Electrodes. Talanta 2017, 162, 583–588. [Google Scholar] [CrossRef]
- del Carmen Aguirre, M.; Urreta, S.E.; Gomez, C.G. A Cu2+-Cu/Glassy Carbon System for Glyphosate Determination. Sens. Actuators B Chem. 2019, 284, 675–683. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Wang, C.; Hu, X.; Liu, Y.; Wang, G. Sensitive Detection of Glyphosate Based on a Cu-BTC MOF/g-C3N4 Nanosheet Photoelectrochemical Sensor. Electrochim. Acta 2019, 317, 341–347. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, L.; Miao, F.; Tao, B.; Li, H.; Qiu, Z.; Zang, Y. Development of a Sensitive ZnO/CuO/Au Electrochemical Sensor for Measuring Glyphosate. Vacuum 2023, 214, 112138. [Google Scholar] [CrossRef]
- Shrivastava, S.; Kumar, A.; Verma, N.; Chen, B.-Y.; Chang, C.-T. Voltammetric Detection of Aqueous Glyphosate on a Copper and Poly(Pyrrole)-Electromodified Activated Carbon Fiber. Electroanalysis 2021, 33, 916–924. [Google Scholar] [CrossRef]
- Jiang, R.; Pang, Y.-H.; Yang, Q.-Y.; Wan, C.-Q.; Shen, X.-F. Copper Porphyrin Metal-Organic Framework Modified Carbon Paper for Electrochemical Sensing of Glyphosate. Sens. Actuators B Chem. 2022, 358, 131492. [Google Scholar] [CrossRef]
- Wong, A.; de Lima, D.; Ferreira, P.; Khan, S.; da Silva, R.; de Faria, J.; Sotomayor, M. Voltammetric Sensing of Glyphosate in Different Samples Using Carbon Paste Electrode Modified with Biochar and Copper(II) Hexadecafluoro-29H,31 Phtalocyanine Complex. J. Appl. Electrochem. 2021, 51, 761–768. [Google Scholar] [CrossRef]
- Balciunas, D.; Plausinaitis, D.; Ratautaite, V.; Ramanaviciene, A.; Ramanavicius, A. Towards Electrochemical Surface Plasmon Resonance Sensor Based on the Molecularly Imprinted Polypyrrole for Glyphosate Sensing. Talanta 2022, 241, 123252. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Li, S.; Ruan, X.; Fei, M.; Zhou, Y.; Niu, X.; Zhu, W.; Du, D.; Lin, Y. Molecularly Imprinted Polypyrrole Nanotubes Based Electrochemical Sensor for Glyphosate Detection. Biosens. Bioelectron. 2021, 191, 113434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; She, Y.; Li, T.; Zhao, F.; Jin, M.; Guo, Y.; Zheng, L.; Wang, S.; Jin, F.; Shao, H.; et al. A Highly Selective Electrochemical Sensor Based on Molecularly Imprinted Polypyrrole-Modified Gold Electrode for the Determination of Glyphosate in Cucumber and Tap Water. Anal. Bioanal. Chem. 2017, 409, 7133–7144. [Google Scholar] [CrossRef] [PubMed]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Abroa-Nemeir, I.; Ben Halima, H.; Gallardo-Gonzalez, J.; El Alami El Hassani, N.; Alcacer, A.; Bausells, J.; Jaffrezic-Renault, N.; et al. Electrochemical Impedance Spectroscopy Determination of Glyphosate Using a Molecularly Imprinted Chitosan. Sens. Actuators B Chem. 2020, 309, 127753. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Alcacer, A.; Bausells, J.; Jaffrezic-Renault, N.; Zine, N.; Errachid, A. Experimental Study and Mathematical Modeling of a Glyphosate Impedimetric Microsensor Based on Molecularly Imprinted Chitosan Film. Chemosensors 2020, 8, 104. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Alcacer, A.; Bausells, J.; Jaffrezic-Renault, N.; Zine, N.; Errachid, A. Electrochemical Impedance Spectroscopy Microsensor Based on Molecularly Imprinted Chitosan Film Grafted on a 4-Aminophenylacetic Acid (CMA) Modified Gold Electrode, for the Sensitive Detection of Glyphosate. Front. Chem. 2021, 9, 621057. [Google Scholar] [CrossRef] [PubMed]
- Dinu, L.A.; Kurbanoglu, S. Enhancing Electrochemical Sensing through the Use of Functionalized Graphene Composites as Nanozymes. Nanoscale 2023, 15, 16514–16538. [Google Scholar] [CrossRef] [PubMed]
- Raji, M.; Zari, N.; el kacem Qaiss, A.; Bouhfid, R. Chapter 1—Chemical Preparation and Functionalization Techniques of Graphene and Graphene Oxide. In Functionalized Graphene Nanocomposites and Their Derivatives; Jawaid, M., Bouhfid, R., el Kacem Qaiss, A., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–20. ISBN 978-0-12-814548-7. [Google Scholar]
- Kader, M.A.; Suhaity Azmi, N.; Kafi, A.K.M. Recent Advances in Gold Nanoparticles Modified Electrodes in Electrochemical Nonenzymatic Sensing of Chemical and Biological Compounds. Inorg. Chem. Commun. 2023, 153, 110767. [Google Scholar] [CrossRef]
- Geana, E.-I.; Baracu, A.M.; Stoian, M.C.; Brincoveanu, O.; Pachiu, C.; Dinu, L.A. Hybrid Nanomaterial-Based Indirect Electrochemical Sensing of Glyphosate in Surface Water: A Promising Approach for Environmental Monitoring. Environ. Sci. Process. Impacts 2023, 25, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, C.; Wen, Y.; Wang, Y.; Yang, Y. Adsorption Performance and Mechanism of Magnetic Reduced Graphene Oxide in Glyphosate Contaminated Water. Environ. Sci. Pollut. Res. 2018, 25, 21036–21048. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly Imprinted Polymer-Based Electrochemical Sensors for Environmental Analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef] [PubMed]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Molecularly Imprinted Polypyrrole-Based Synthetic Receptor for Direct Detection of Bovine Leukemia Virus Glycoproteins. Biosens. Bioelectron. 2004, 20, 1076–1082. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly Imprinted Polypyrrole Based Sensor for the Detection of SARS-CoV-2 Spike Glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef]
- Kang, M.S.; Cho, E.; Choi, H.E.; Amri, C.; Lee, J.-H.; Kim, K.S. Molecularly Imprinted Polymers (MIPs): Emerging Biomaterials for Cancer Theragnostic Applications. Biomater. Res. 2023, 27, 45. [Google Scholar] [CrossRef] [PubMed]
- Mazouz, Z.; Rahali, S.; Fourati, N.; Zerrouki, C.; Aloui, N.; Seydou, M.; Yaakoubi, N.; Chehimi, M.M.; Othmane, A.; Kalfat, R. Highly Selective Polypyrrole MIP-Based Gravimetric and Electrochemical Sensors for Picomolar Detection of Glyphosate. Sensors 2017, 17, 2586. [Google Scholar] [CrossRef] [PubMed]
- Parlak, O. Portable and Wearable Real-Time Stress Monitoring: A Critical Review. Sens. Actuators Rep. 2021, 3, 100036. [Google Scholar] [CrossRef]
- Mariani, F.; Gualandi, I.; Schuhmann, W.; Scavetta, E. Micro- and Nano-Devices for Electrochemical Sensing. Microchim Acta 2022, 189, 459. [Google Scholar] [CrossRef] [PubMed]
- Marinoiu, A.; Andrei, R.; Vagner, I.; Niculescu, V.; Bucura, F.; Constantinescu, M.; Carcadea, E. One Step Synthesis of Au Nanoparticles Supported on Graphene Oxide Using an Eco-Friendly Microwave-Assisted Process. Mater. Sci. 2020, 26, 249–254. [Google Scholar] [CrossRef]
- Lazar, O.-A.; Marinoiu, A.; Raceanu, M.; Pantazi, A.; Mihai, G.; Varlam, M.; Enachescu, M. Reduced Graphene Oxide Decorated with Dispersed Gold Nanoparticles: Preparation, Characterization and Electrochemical Evaluation for Oxygen Reduction Reaction. Energies 2020, 13, 4307. [Google Scholar] [CrossRef]
- Oliveira Pereira, E.A.; Freitas Melo, V.; Abate, G.; Masini, J.C. Determination of Glyphosate and Aminomethylphosphonic Acid by Sequential-Injection Reversed-Phase Chromatography: Method Improvements and Application in Adsorption Studies. Anal. Bioanal. Chem. 2019, 411, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, H.T.; Xie, T.J.; Yang, X.; Dong, A.J.; Zhang, H.; Wang, J.; Wang, Z.Y. Molecularly Imprinted Polymer on Graphene Surface for Selective and Sensitive Electrochemical Sensing Imidacloprid. Sens. Actuators B Chem. 2017, 252, 991–1002. [Google Scholar] [CrossRef]
- Zhao, P.; Yan, M.; Zhang, C.; Peng, R.; Ma, D.; Yu, J. Determination of Glyphosate in Foodstuff by One Novel Chemiluminescence-Molecular Imprinting Sensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1482–1486. [Google Scholar] [CrossRef]
- Prasad, B.B.; Jauhari, D.; Tiwari, M.P. Doubly Imprinted Polymer Nanofilm-Modified Electrochemical Sensor for Ultra-Trace Simultaneous Analysis of Glyphosate and Glufosinate. Biosens. Bioelectron. 2014, 59, 81–88. [Google Scholar] [CrossRef] [PubMed]

| Analytical Parameter | AuNPs-Gr(DMF)/SPCE | AuNPs-Gr(NAF)/SPCE |
|---|---|---|
| Linear concentration range | 1.0–50.0 μg/L | 20.0–100.0 ng/L |
| Detection limit | 0.3 μg/L | 6.6 ng/L |
| Quantification limit | 1.0 μg/L | 1.0 ng/L |
| Sensitivity | −2.78 μA/μg/L | −5.52 × 10−3 μA/ng/L |
| Correlation coefficient (r) | 0.9854 | 0.9956 |
| Analytical Parameter | MIPPy/AuSPE |
|---|---|
| Linear concentration range (ng/L) | 5.0–50.0 |
| Detection limit (ng/L) | 1.6 |
| Quantification limit (ng/L) | 5.0 |
| Sensitivity (μA/ng/L) | −3.27 × 10−2 |
| Correlation coefficient (r) | 0.9913 |
| Type of Sensor | Linear Range | LOD | Reference |
|---|---|---|---|
| Sensors using nanomaterials for non-enzymatic indirect detection | |||
| AuNPs-Gr(DMF)/SPCE | 1–50 μg/L | 0.3 μg/L | This study |
| AuNPs-Gr(NAF)/SPCE | 20–100 ng/L | 6.6 ng/L | This study |
| MWCNTs—IL/CuO | 5 Nm–1.1 μM | 1.3 nM | [22] |
| CPE | 4.40 × 10−8–2.8 × 10−6 mol/L | 2 × 10−9 mol/L | [61] |
| Cu-BTC MOF | - | 1.4 × 10−13 mol/L | [26] |
| GCE/MWCNT/CuPc | 0.83–9.90 μM | 12.20 nM | [23] |
| Electrode with porous copper nano-wires | 0.010–5.0 μmol/L | 10.0 nmol/L | [27] |
| Sensors using MIP-for indirect detection | |||
| MIP-Ppy/AuSPE | 5.0–50.0 ng/L | 1.6 ng/L | This study |
| AuSPE | 5.91 nM–5.91 μM | 4.73 nM | [24] |
| MIPPy/AuE | 5–800 ng/mL | 0.27 ng/mL | [62] |
| MIM/CL | 0.024–1.04 μM | 2 nM | [63] |
| MIP/GNPs-PGE | 3.98–176.23 ng/mL | 0.353 ng/mL | [64] |
| PPY-MIP/AuE | 0.10 pM–10.0 μM | 1 pM | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geana, E.-I.; Ciucure, C.T.; Soare, A.; Enache, S.; Ionete, R.E.; Dinu, L.A. Electrochemical Detection of Glyphosate in Surface Water Samples Based on Modified Screen-Printed Electrodes. Nanomaterials 2024, 14, 948. https://doi.org/10.3390/nano14110948
Geana E-I, Ciucure CT, Soare A, Enache S, Ionete RE, Dinu LA. Electrochemical Detection of Glyphosate in Surface Water Samples Based on Modified Screen-Printed Electrodes. Nanomaterials. 2024; 14(11):948. https://doi.org/10.3390/nano14110948
Chicago/Turabian StyleGeana, Elisabeta-Irina, Corina Teodora Ciucure, Amalia Soare, Stanica Enache, Roxana Elena Ionete, and Livia Alexandra Dinu. 2024. "Electrochemical Detection of Glyphosate in Surface Water Samples Based on Modified Screen-Printed Electrodes" Nanomaterials 14, no. 11: 948. https://doi.org/10.3390/nano14110948
APA StyleGeana, E.-I., Ciucure, C. T., Soare, A., Enache, S., Ionete, R. E., & Dinu, L. A. (2024). Electrochemical Detection of Glyphosate in Surface Water Samples Based on Modified Screen-Printed Electrodes. Nanomaterials, 14(11), 948. https://doi.org/10.3390/nano14110948









