The Incorporated Drug Affects the Properties of Hydrophilic Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Evaluation of Polymer Solutions
2.2.1. Rheology Measurements
2.2.2. Electrical Conductivity Measurements
2.3. Electrospinning of Nanofibers
2.4. Evaluation of Nanofibers
2.4.1. Scanning Electron Microscopy
2.4.2. Fourier-Transform Infrared Analysis
2.4.3. Contact Angle Measurements
2.4.4. Thermogravimetric Analysis
2.4.5. Evaluation of Nanofiber Dispersibility
2.5. Determination of Drug Loading in Nanofibers
2.6. Evaluation of Drug Release In Vitro
2.7. HPLC Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Properties of Polymer Solutions
3.2. Morphology of Electrospun Products
3.3. Chemical Interactions between Components in Nanofibers
3.4. Surface Properties of Nanofiber Mats
3.5. Moisture Content in Nanofibers
3.6. Drug Loading in Nanofibers and Drug Entrapment Efficiency
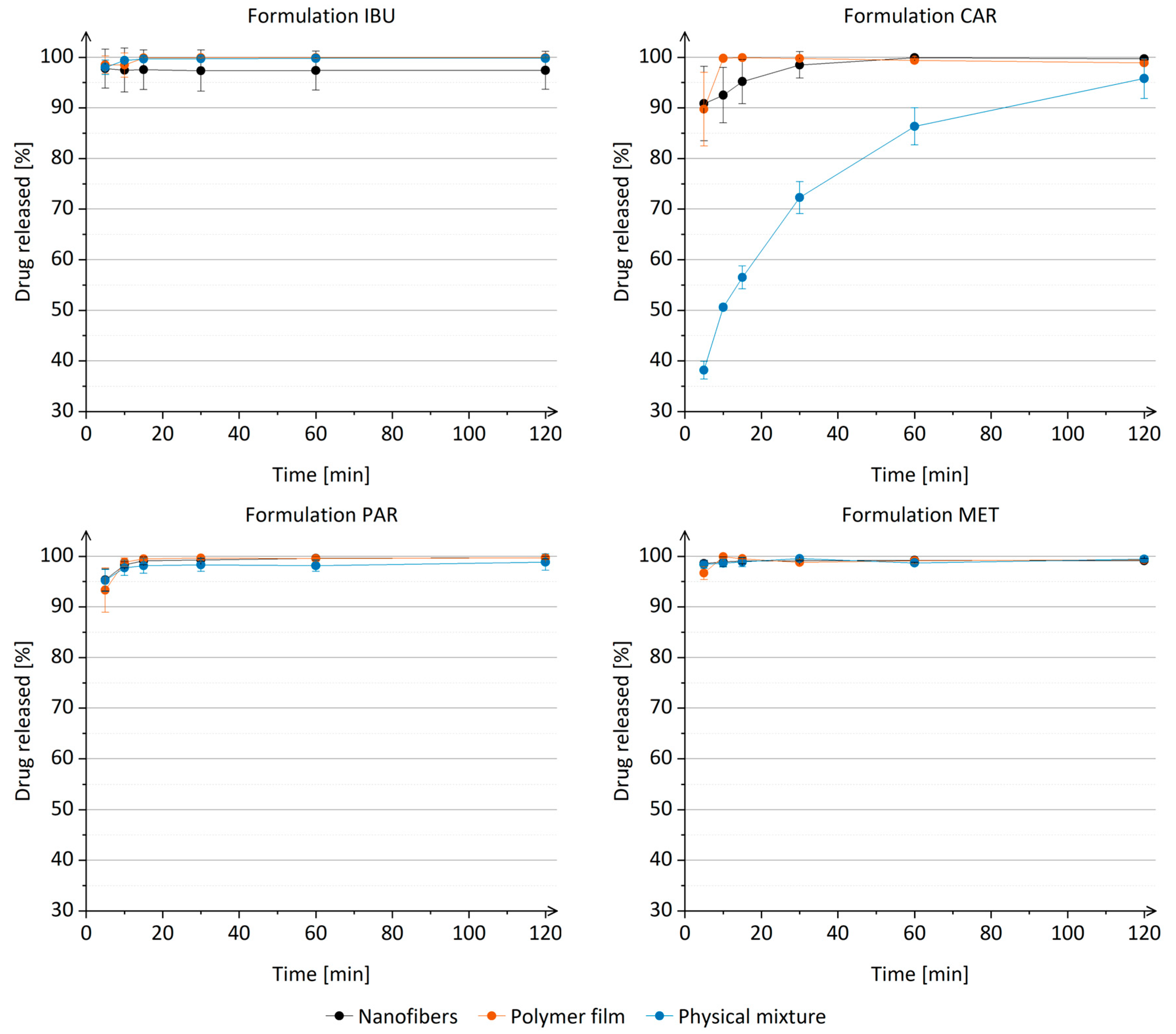
3.7. Drug Release from Nanofibers In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepa, I.; Mudra, E.; Dusza, J. Electrospinning through the Prism of Time. Mater. Today Chem. 2021, 21, 100543. [Google Scholar] [CrossRef]
- Kulkarni, D.; Musale, S.; Panzade, P.; Paiva-Santos, A.C.; Sonwane, P.; Madibone, M.; Choundhe, P.; Giram, P.; Cavalu, S. Surface Functionalization of Nanofibers: The Multifaceted Approach for Advanced Biomedical Applications. Nanomaterials 2022, 12, 3899. [Google Scholar] [CrossRef] [PubMed]
- Kocbek, P. Novosti na področju farmacevtske nanotehnologije. Farm. Vestn. 2012, 63, 75–81. [Google Scholar]
- Rošic, R.; Kocbek, P.; Pelipenko, J.; Kristl, J.; Baumgartner, S. Nanofibers and Their Biomedical Use. Acta Pharm. 2013, 63, 295–304. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical Attributes of Nanofibers: Preparation, Drug Loading, and Tissue Regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun Nanofibers for Customized Drug-Delivery Systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun Cellular Microenvironments: Understanding Controlled Release and Scaffold Structure. Adv. Drug Deliv. Rev. 2011, 63, 209–220. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Zussman, E.; Xu, H. Electrospinning of Nanofibers from Polymer Solutions and Melts. In Advances in Applied Mechanics; Aref, H., van der Giessen, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 41, pp. 43–346. [Google Scholar]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M.K. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Sukowati, R.; Rohman, Y.M.; Agung, B.H.; Hapidin, D.A.; Damayanti, H.; Khairurrijal, K. An Investigation of the Influence of Nanofibers Morphology on the Performance of QCM-Based Ethanol Vapor Sensor Utilizing Polyvinylpyrrolidone Nanofibers Active Layer. Sens. Actuators B Chem. 2023, 386, 133708. [Google Scholar] [CrossRef]
- Scheidt, D.T.; Pellá, M.C.G.; Breitenbach, G.L.; Simões, M.R.; Caetano, J.; Martins, C.V.B.; de S. Rossin, A.R.; Muniz, E.C.; Dragunski, D.C. Blend Composition Effect on the Diameter of Electrospun Chitosan (CHT)/Poly(Ethylene Oxide) (PEO) Nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131516. [Google Scholar] [CrossRef]
- Rosman, N.; Wan Salleh, W.N.; Jamalludin, M.R.; Adam, M.R.; Ismail, N.H.; Jaafar, J.; Harun, Z.; Ismail, A.F. Electrospinning Parameters Evaluation of PVDF-ZnO/Ag2CO3/Ag2O Composite Nanofiber Affect on Porosity by Using Response Surface Methodology. Mater. Today Proc. 2021, 46, 1824–1830. [Google Scholar] [CrossRef]
- Zupančič, Š.; Potrč, T.; Baumgartner, S.; Kocbek, P.; Kristl, J. Formulation and Evaluation of Chitosan/Polyethylene Oxide Nanofibers Loaded with Metronidazole for Local Infections. Eur. J. Pharm. Sci. 2016, 95, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.; Daraei, A.; Lee, H.; Guthold, M. The Effect of Molecular Weight and Fiber Diameter on the Mechanical Properties of Single, Electrospun PCL Nanofibers. Mater. Today Commun. 2023, 35, 105773. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Q.; Geng, J.; Liu, X.; Wei, Q. Fiber Flexibility Reconciles Matrix Recruitment and the Fiber Modulus to Promote Cell Mechanosensing. Nano Lett. 2024, 24, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Dragar, Č.; Ileršič, N.; Potrč, T.; Nemec, S.; Kralj, S.; Kocbek, P. Electrospinning as a Method for Preparation of Redispersible Dry Product with High Content of Magnetic Nanoparticles. Int. J. Pharm. 2022, 629, 122389. [Google Scholar] [CrossRef] [PubMed]
- Beigmoradi, R.; Samimi, A.; Mohebbi-Kalhori, D. Controllability of the Hydrophilic or Hydrophobic Behavior of the Modified Polysulfone Electrospun Nanofiber Mats. Polym. Test. 2021, 93, 106970. [Google Scholar] [CrossRef]
- Zidar, A.; Zupančič, Š.; Kristl, J.; Jeras, M. Development of a Novel in Vitro Cell Model for Evaluation of Nanofiber Mats Immunogenicity. Int. J. Pharm. 2024, 650, 123696. [Google Scholar] [CrossRef]
- Gouda, M.; Khalaf, M.M.; Shaaban, S.; El-Lateef, H.M.A. Fabrication of Chitosan Nanofibers Containing Some Steroidal Compounds as a Drug Delivery System. Polymers 2022, 14, 2094. [Google Scholar] [CrossRef]
- Gelb, M.B.; Punia, A.; Sellers, S.; Kadakia, P.; Ormes, J.D.; Khawaja, N.N.; Wylie, J.; Lamm, M.S. Effect of Drug Incorporation and Polymer Properties on the Characteristics of Electrospun Nanofibers for Drug Delivery. J. Drug Deliv. Sci. Technol. 2022, 68, 103112. [Google Scholar] [CrossRef]
- Zupančič, Š.; Casula, L.; Rijavec, T.; Lapanje, A.; Luštrik, M.; Fadda, A.M.; Kocbek, P.; Kristl, J. Sustained Release of Antimicrobials from Double-Layer Nanofiber Mats for Local Treatment of Periodontal Disease, Evaluated Using a New Micro Flow-through Apparatus. J. Control. Release 2019, 316, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, T.; Goyal, A.K.; Rath, G. Development, Optimization and Evaluation of Polymeric Electrospun Nanofiber: A Tool for Local Delivery of Fluconazole for Management of Vaginal Candidiasis. Artif. Cells Nanomed. Biotechnol. 2016, 44, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Li, C.; Yu, C.; Xie, H.; Shi, S.; Li, Z.; Wang, Q.; Lu, L. A Novel Electrospun Membrane Based on Moxifloxacin Hydrochloride/Poly(Vinyl Alcohol)/Sodium Alginate for Antibacterial Wound Dressings in Practical Application. Drug Deliv. 2016, 23, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Neveling, D.P.; Dicks, L.M.T. Co-Spinning of Silver Nanoparticles with Nisin Increases the Antimicrobial Spectrum of PDLLA: PEO Nanofibers. Curr. Microbiol. 2015, 71, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Karataş, A.; Algan, A.H.; Pekel-Bayramgil, N.; Turhan, F.; Altanlar, N. Ofloxacin Loaded Electrospun Fibers for Ocular Drug Delivery: Effect of Formulation Variables on Fiber Morphology and Drug Release. Curr. Drug Deliv. 2016, 13, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Can Suner, S.; Yildirim, Y.; Yurt, F.; Ozel, D.; Oral, A.; Ozturk, I. Antibiotic Loaded Electrospun Poly (Lactic Acid) Nanofiber Mats for Drug Delivery System. J. Drug Deliv. Sci. Technol. 2022, 71, 103263. [Google Scholar] [CrossRef]
- Tseng, Y.-Y.; Kao, Y.-C.; Liao, J.-Y.; Chen, W.-A.; Liu, S.-J. Biodegradable Drug-Eluting Poly[Lactic- Co -Glycol Acid] Nanofibers for the Sustainable Delivery of Vancomycin to Brain Tissue: In Vitro and in Vivo Studies. ACS Chem. Neurosci. 2013, 4, 1314–1321. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Z.; Cai, Z.; Yu, L.; Lv, Y. Voriconazole Composited Polyvinyl Alcohol/Hydroxypropyl-β-Cyclodextrin Nanofibers for Ophthalmic Delivery. PLoS ONE 2016, 11, e0167961. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of Tetracycline Hydrochloride from Electrospun Poly(Ethylene-Co-Vinylacetate), Poly(Lactic Acid), and a Blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun Curcumin Loaded Poly(ε-Caprolactone)/Gum Tragacanth Nanofibers for Biomedical Application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Baumgartner, S.; Lavrič, Z.; Petelin, M.; Kristl, J. Local Delivery of Resveratrol Using Polycaprolactone Nanofibers for Treatment of Periodontal Disease. J. Drug Deliv. Sci. Technol. 2015, 30, 408–416. [Google Scholar] [CrossRef]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid Electrospun Chitosan-Phospholipids Nanofibers for Transdermal Drug Delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Vrbata, P.; Berka, P.; Stránská, D.; Doležal, P.; Musilová, M.; Čižinská, L. Electrospun Drug Loaded Membranes for Sublingual Administration of Sumatriptan and Naproxen. Int. J. Pharm. 2013, 457, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chang, S.; Bai, Y.; Du, Y.; Yu, D.-G. Electrospun Triaxial Nanofibers with Middle Blank Cellulose Acetate Layers for Accurate Dual-Stage Drug Release. Carbohydr. Polym. 2020, 243, 116477. [Google Scholar] [CrossRef]
- Panda, D.S.; Alruwaili, N.K.; Swain, K.; Pattnaik, S. Ibuprofen Loaded Electrospun Polymeric Nanofibers: A Strategy to Improve Oral Absorption. Acta Chim. Slov. 2022, 69, 483–488. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, D.; Zhang, Z.; Pan, J.; Cui, Z.; Yu, D.-G.; Annie Bligh, S.-W. Testing of Fast Dissolution of Ibuprofen from Its Electrospun Hydrophilic Polymer Nanocomposites. Polym. Test. 2021, 93, 106872. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Drug-Loaded Electrospun Mats of Poly(Vinyl Alcohol) Fibres and Their Release Characteristics of Four Model Drugs. Nanotechnology 2006, 17, 2317–2329. [Google Scholar] [CrossRef]
- Chu, K.; Zhu, Y.; Lu, G.; Huang, S.; Yang, C.; Zheng, J.; Chen, J.; Ban, J.; Jia, H.; Lu, Z. Formation of Hydrophilic Nanofibers from Nanostructural Design in the Co-Encapsulation of Celecoxib through Electrospinning. Pharmaceutics 2023, 15, 730. [Google Scholar] [CrossRef]
- Pisani, S.; Friuli, V.; Conti, B.; Bruni, G.; Maggi, L. Tableted Hydrophilic Electrospun Nanofibers to Promote Meloxicam Dissolution Rate. J. Drug Deliv. Sci. Technol. 2021, 66, 102878. [Google Scholar] [CrossRef]
- Hsu, K.-H.; Fang, S.-P.; Lin, C.-L.; Liao, Y.-S.; Yoon, Y.-K.; Chauhan, A. Hybrid Electrospun Polycaprolactone Mats Consisting of Nanofibers and Microbeads for Extended Release of Dexamethasone. Pharm. Res. 2016, 33, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.S.; Das, A.; Alzhrani, R.M.; Kang, D.; Bhaduri, S.B.; Boddu, S.H.S. Comparison of Electrospun and Solvent Cast Polylactic Acid (PLA)/Poly(Vinyl Alcohol) (PVA) Inserts as Potential Ocular Drug Delivery Vehicles. Mater. Sci. Eng. C 2017, 77, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Strachan, C.; Broadbent, R.; Walker, G.F. A Minitablet Formulation Made from Electrospun Nanofibers. Eur. J. Pharm. Biopharm. 2017, 114, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Ramakrishna, S. Controlled Release of Multiple Epidermal Induction Factors through Core–Shell Nanofibers for Skin Regeneration. Eur. J. Pharm. Biopharm. 2013, 85, 689–698. [Google Scholar] [CrossRef]
- Schneider, A.; Wang, X.Y.; Kaplan, D.L.; Garlick, J.A.; Egles, C. Biofunctionalized Electrospun Silk Mats as a Topical Bioactive Dressing for Accelerated Wound Healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, A.; Rath, G.; Goyal, A.; Mathur, R.B.; Dhakate, S.R. Electrospun Composite Nanofiber-Based Transmucosal Patch for Anti-Diabetic Drug Delivery. J. Mater. Chem. B 2013, 1, 3410. [Google Scholar] [CrossRef] [PubMed]
- Asci, H.; Savran, M.; Cengiz Callıoglu, F.; Sahin, S.; Hasseyid, N.; Kaynak, M.; Izat, N.; Kesici Guler, H. Supralingual Administration of Paracetamol Embedded in Polyvinyl Alcohol Nanofibers: A Pharmacokinetic Study. J. Drug Deliv. Sci. Technol. 2022, 67, 102948. [Google Scholar] [CrossRef]
- Abid, S.; Hussain, T.; Nazir, A.; Zahir, A.; Khenoussi, N. Acetaminophen Loaded Nanofibers as a Potential Contact Layer for Pain Management in Burn Wounds. Mater. Res. Express 2018, 5, 085017. [Google Scholar] [CrossRef]
- Sirc, J.; Hampejsova, Z.; Trnovska, J.; Kozlik, P.; Hrib, J.; Hobzova, R.; Zajicova, A.; Holan, V.; Bosakova, Z. Cyclosporine A Loaded Electrospun Poly(D,L-Lactic Acid)/Poly(Ethylene Glycol) Nanofibers: Drug Carriers Utilizable in Local Immunosuppression. Pharm. Res. 2017, 34, 1391–1401. [Google Scholar] [CrossRef]
- Agrahari, V.; Meng, J.; Ezoulin, M.J.; Youm, I.; Dim, D.C.; Molteni, A.; Hung, W.-T.; Christenson, L.K.; Youan, B.-B.C. Stimuli-Sensitive Thiolated Hyaluronic Acid Based Nanofibers: Synthesis, Preclinical Safety and in Vitro Anti-HIV Activity. Nanomedicine 2016, 11, 2935–2958. [Google Scholar] [CrossRef]
- Kaljević, O.; Djuris, J.; Čalija, B.; Lavrič, Z.; Kristl, J.; Ibrić, S. Application of Miscibility Analysis and Determination of Soluplus Solubility Map for Development of Carvedilol-Loaded Nanofibers. Int. J. Pharm. 2017, 533, 445–454. [Google Scholar] [CrossRef]
- Krstić, M.; Radojević, M.; Stojanović, D.; Radojević, V.; Uskoković, P.; Ibrić, S. Formulation and Characterization of Nanofibers and Films with Carvedilol Prepared by Electrospinning and Solution Casting Method. Eur. J. Pharm. Sci. 2017, 101, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Kajdič, S.; Zupančič, Š.; Roškar, R.; Kocbek, P. The Potential of Nanofibers to Increase Solubility and Dissolution Rate of the Poorly Soluble and Chemically Unstable Drug Lovastatin. Int. J. Pharm. 2020, 573, 118809. [Google Scholar] [CrossRef]
- Casula, L.; Zidar, A.; Kristl, J.; Jeras, M.; Kralj, S.; Fadda, A.M.; Zupančič, Š. Development of Nanofibers with Embedded Liposomes Containing an Immunomodulatory Drug Using Green Electrospinning. Pharmaceutics 2023, 15, 1245. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.-C.; Liao, J.-Y.; Liu, S.-J.; Chan, E.-C. Novel Biodegradable Sandwich-Structured Nanofibrous Drug-Eluting Membranes for Repair of Infected Wounds: An in Vitro and in Vivo Study. Int. J. Nanomed. 2012, 7, 763–771. [Google Scholar] [CrossRef] [PubMed]
- AnjiReddy, K.; Karpagam, S. Chitosan Nanofilm and Electrospun Nanofiber for Quick Drug Release in the Treatment of Alzheimer’s Disease: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2017, 105, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Samadzadeh, S.; Mousazadeh, H.; Ghareghomi, S.; Dadashpour, M.; Babazadeh, M.; Zarghami, N. In Vitro Anticancer Efficacy of Metformin-Loaded PLGA Nanofibers towards the Post-Surgical Therapy of Lung Cancer. J. Drug Deliv. Sci. Technol. 2021, 61, 102318. [Google Scholar] [CrossRef]
- Babazade, R.; Beyzanur Polat, E.; Ertas, B.; Ozcan, G.S.; Kiyak Kirmaci, H.; Tatar, E.; Taskin, T.; Yazir, Y.; Emin Cam, M. Synergistic Anticancer Effects of Metformin and Achillea Vermicularis Trin-Loaded Nanofibers on Human Pancreatic Cancer Cell Line: An in Vitro Study. Eur. Polym. J. 2022, 179, 111565. [Google Scholar] [CrossRef]
- Angkawinitwong, U.; Awwad, S.; Khaw, P.T.; Brocchini, S.; Williams, G.R. Electrospun Formulations of Bevacizumab for Sustained Release in the Eye. Acta Biomater. 2017, 64, 126–136. [Google Scholar] [CrossRef]
- Meng, Z.X.; Xu, X.X.; Zheng, W.; Zhou, H.M.; Li, L.; Zheng, Y.F.; Lou, X. Preparation and Characterization of Electrospun PLGA/Gelatin Nanofibers as a Potential Drug Delivery System. Colloids Surf. B Biointerfaces 2011, 84, 97–102. [Google Scholar] [CrossRef]
- Potrč, T.; Baumgartner, S.; Roškar, R.; Planinšek, O.; Lavrič, Z.; Kristl, J.; Kocbek, P. Electrospun Polycaprolactone Nanofibers as a Potential Oromucosal Delivery System for Poorly Water-Soluble Drugs. Eur. J. Pharm. Sci. 2015, 75, 101–113. [Google Scholar] [CrossRef] [PubMed]
- PubChem Ibuprofen. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3672 (accessed on 8 May 2023).
- PubChem Carvedilol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2585 (accessed on 8 May 2023).
- PubChem Acetaminophen. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1983 (accessed on 8 May 2023).
- PubChem Metformin Hydrochloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/14219 (accessed on 8 May 2023).
- European Pharmacopoeia; 11.2 Ed.; European Directorate for Quality of Medicines & HealthCare Council of Europe: Strasbourg, France, 2023; Available online: https://pheur.edqm.eu/home (accessed on 8 May 2023).
- Azad, M.S.; Trivedi, J.J. Novel Viscoelastic Model for Predicting the Synthetic Polymer’s Viscoelastic Behavior in Porous Media Using Direct Extensional Rheological Measurements. Fuel 2019, 235, 218–226. [Google Scholar] [CrossRef]
- Han, T.; Yarin, A.L.; Reneker, D.H. Viscoelastic Electrospun Jets: Initial Stresses and Elongational Rheometry. Polymer 2008, 49, 1651–1658. [Google Scholar] [CrossRef]
- Rashid, A.; White, E.T.; Howes, T.; Litster, J.D.; Marziano, I. Effect of Solvent Composition and Temperature on the Solubility of Ibuprofen in Aqueous Ethanol. J. Chem. Eng. Data 2014, 59, 2699–2703. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Paracetamol (Acetaminophen). In IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1990. [Google Scholar]
- Potrč, T.; Murnc, K.; Kocbek, P. Hydrophilic Nanofibers as a Supersaturating Delivery System for Carvedilol. Int. J. Pharm. 2021, 603, 120700. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Shetty, S.; Yadav, K.S. Unfolding the Electrospinning Potential of Biopolymers for Preparation of Nanofibers. J. Drug Deliv. Sci. Technol. 2020, 57, 101604. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded Nanofibers Formed during Electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Varnaitė-Žuravliova, S.; Savest, N.; Baltušnikaitė-Guzaitienė, J.; Abraitienė, A.; Krumme, A. The Investigation of the Droduction of Salt-Added Polyethylene Oxide/Chitosan Nanofibers. Materials 2024, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Kajdič, S.; Vrečer, F.; Kocbek, P. Preparation of Poloxamer-Based Nanofibers for Enhanced Dissolution of Carvedilol. Eur. J. Pharm. Sci. 2018, 117, 331–340. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending Instability of Electrically Charged Liquid Jets of Polymer Solutions in Electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Ha, E.-S.; Kim, J.-S.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Kim, M.-S. Equilibrium Solubility and Solute-Solvent Interactions of Carvedilol (Form I) in Twelve Mono Solvents and Its Application for Supercritical Antisolvent Precipitation. J. Mol. Liq. 2019, 294, 111622. [Google Scholar] [CrossRef]
- Sun, X.; Du, S.; Sun, Y.; Li, H.; Yu, C.; Guo, J.; Wang, Y.; Yu, S.; Cheng, Y.; Xue, F. Solubility Measurement and Data Correlation of Metformin Hydrochloride in Four Aqueous Binary Solvents and Three Pure Solvents from 283.15 to 323.15 K. J. Chem. Eng. Data 2021, 66, 3282–3292. [Google Scholar] [CrossRef]
- Hayati, I.; Bailey, A.I.; Tadros, T.F. Investigations into the Mechanisms of Electrohydrodynamic Spraying of Liquids. J. Colloid Interface Sci. 1987, 117, 205–221. [Google Scholar] [CrossRef]
- Asprion, N.; Hasse, H.; Maurer, G. FT-IR Spectroscopic Investigations of Hydrogen Bonding in Alcohol–Hydrocarbon Solutions. Fluid Phase Equilibria 2001, 186, 1–25. [Google Scholar] [CrossRef]
- Furer, V.L.; Vandyukov, A.E.; Zaripov, S.R.; Solovieva, S.E.; Antipin, I.S.; Kovalenko, V.I. FT-IR and FT-Raman Study of Hydrogen Bonding in p-Alkylcalix[8]Arenes. Vib. Spectrosc. 2018, 95, 38–43. [Google Scholar] [CrossRef]
- Zou, S.; Lv, R.; Tong, Z.; Na, B.; Fu, K.; Liu, H. In Situ Hydrogen-Bonding Complex Mediated Shape Memory Behavior of PAA/PEO Blends. Polymer 2019, 183, 121878. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed; Pharmaceutical Press and American Pharmacists Association: London, UK, 2009; ISBN 978-0-85369-792-3. [Google Scholar]
- Wang, H.; She, Y.; Chu, C.; Liu, H.; Jiang, S.; Sun, M.; Jiang, S. Preparation, Antimicrobial and Release Behaviors of Nisin-Poly (Vinyl Alcohol)/Wheat Gluten/ZrO2 Nanofibrous Membranes. J. Mater. Sci. 2015, 50, 5068–5078. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.-J.; Yu, D.-G.; Williams, G.R.; Yang, J.-H.; Wang, X. Influence of the Drug Distribution in Electrospun Gliadin Fibers on Drug-Release Behavior. Eur. J. Pharm. Sci. 2017, 106, 422–430. [Google Scholar] [CrossRef]
- Kenny, P.W. Hydrogen-Bond Donors in Drug Design. J. Med. Chem. 2022, 65, 14261–14275. [Google Scholar] [CrossRef]
- Singh, I.; Kumar, P. Preformulation Studies for Direct Compression Suitability of Cefuroxime Axetil and Paracetamol: A Graphical Representation Using SeDeM Diagram. Acta Pol. Pharm.-Drug Res. 2021, 69, 87–93. [Google Scholar]
- Zhang, Q.; Huang, B.; Xue, H.; Lin, Z.; Zhao, J.; Cai, Z. Preparation, Characterization, and Selection of Optimal Forms of (S)-Carvedilol Salts for the Development of Extended-Release Formulation. Mol. Pharm. 2021, 18, 2298–2310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shi, L.; Marinaro, W.; Lu, Q.; Sun, C.C. Improving Manufacturability of an Ibuprofen Powder Blend by Surface Coating with Silica Nanoparticles. Powder Technol. 2013, 249, 290–296. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, X.; Cai, L. A Drug–Drug Multicomponent Crystal of Metformin and Dobesilate: Crystal Structure Analysis and Hygroscopicity Property. Molecules 2022, 27, 3472. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Branford-White, C.; White, K.; Li, X.-L.; Zhu, L.-M. Dissolution Improvement of Electrospun Nanofiber-Based Solid Dispersions for Acetaminophen. AAPS PharmSciTech 2010, 11, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Khansari, S.; Duzyer, S.; Sinha-Ray, S.; Hockenberger, A.; Yarin, A.L.; Pourdeyhimi, B. Two-Stage Desorption-Controlled Release of Fluorescent Dye and Vitamin from Solution-Blown and Electrospun Nanofiber Mats Containing Porogens. Mol. Pharm. 2013, 10, 4509–4526. [Google Scholar] [CrossRef]
- Zupančič, Š.; Sinha-Ray, S.; Sinha-Ray, S.; Kristl, J.; Yarin, A.L. Long-Term Sustained Ciprofloxacin Release from PMMA and Hydrophilic Polymer Blended Nanofibers. Mol. Pharm. 2016, 13, 295–305. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, Z.; Zhao, H.; Liu, T.; Dong, A.; Zhang, J. Electrospinning of Ibuprofen-Loaded Composite Nanofibers for Improving the Performances of Transdermal Patches. J. Nanosci. Nanotechnol. 2013, 13, 3855–3863. [Google Scholar] [CrossRef]
- Chi, Z.; Zhao, S.; Feng, Y.; Yang, L. On-Line Dissolution Analysis of Multiple Drugs Encapsulated in Electrospun Nanofibers. Int. J. Pharm. 2020, 588, 119800. [Google Scholar] [CrossRef]
- Illangakoon, U.E.; Gill, H.; Shearman, G.C.; Parhizkar, M.; Mahalingam, S.; Chatterton, N.P.; Williams, G.R. Fast Dissolving Paracetamol/Caffeine Nanofibers Prepared by Electrospinning. Int. J. Pharm. 2014, 477, 369–379. [Google Scholar] [CrossRef]
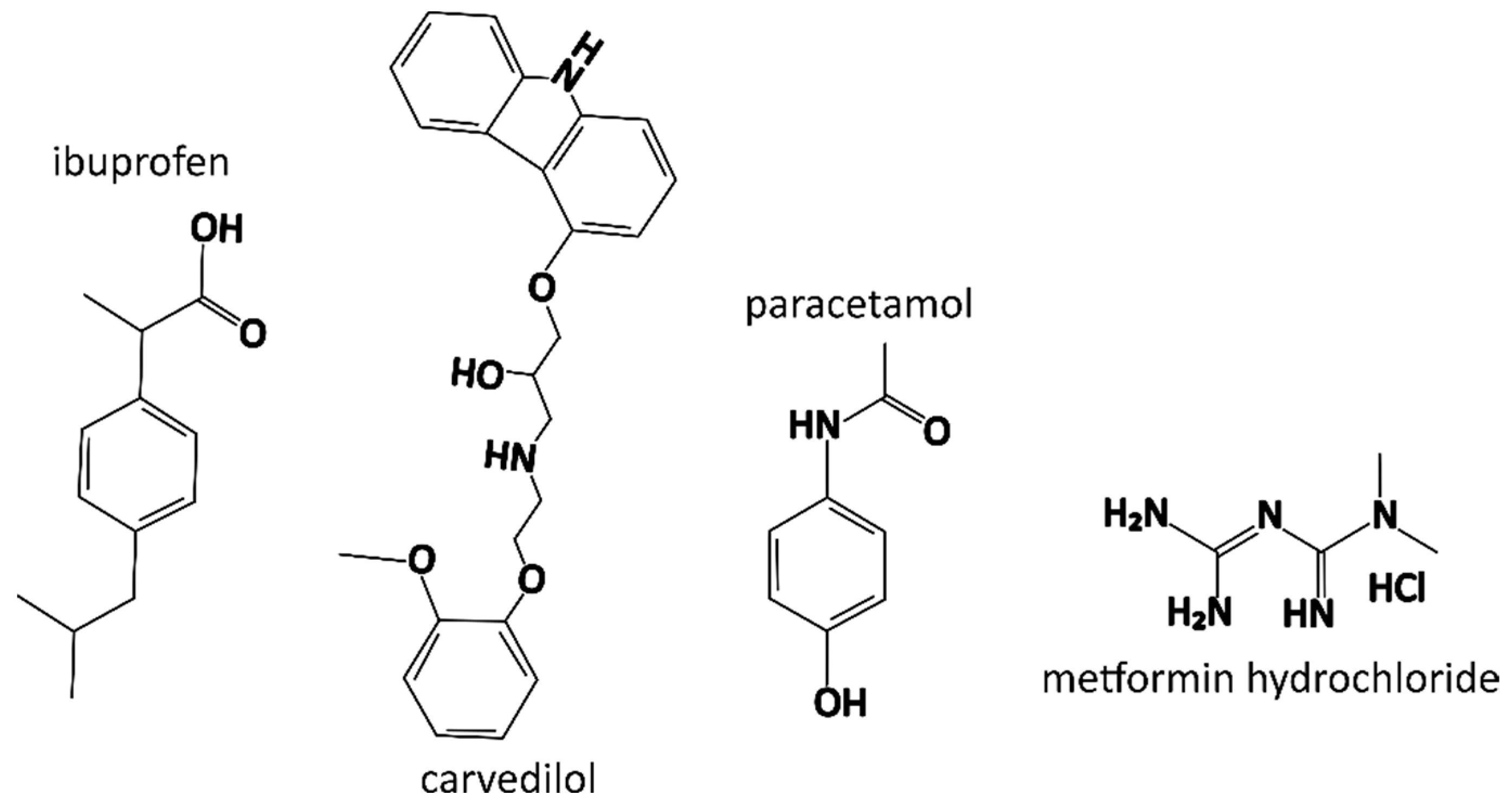

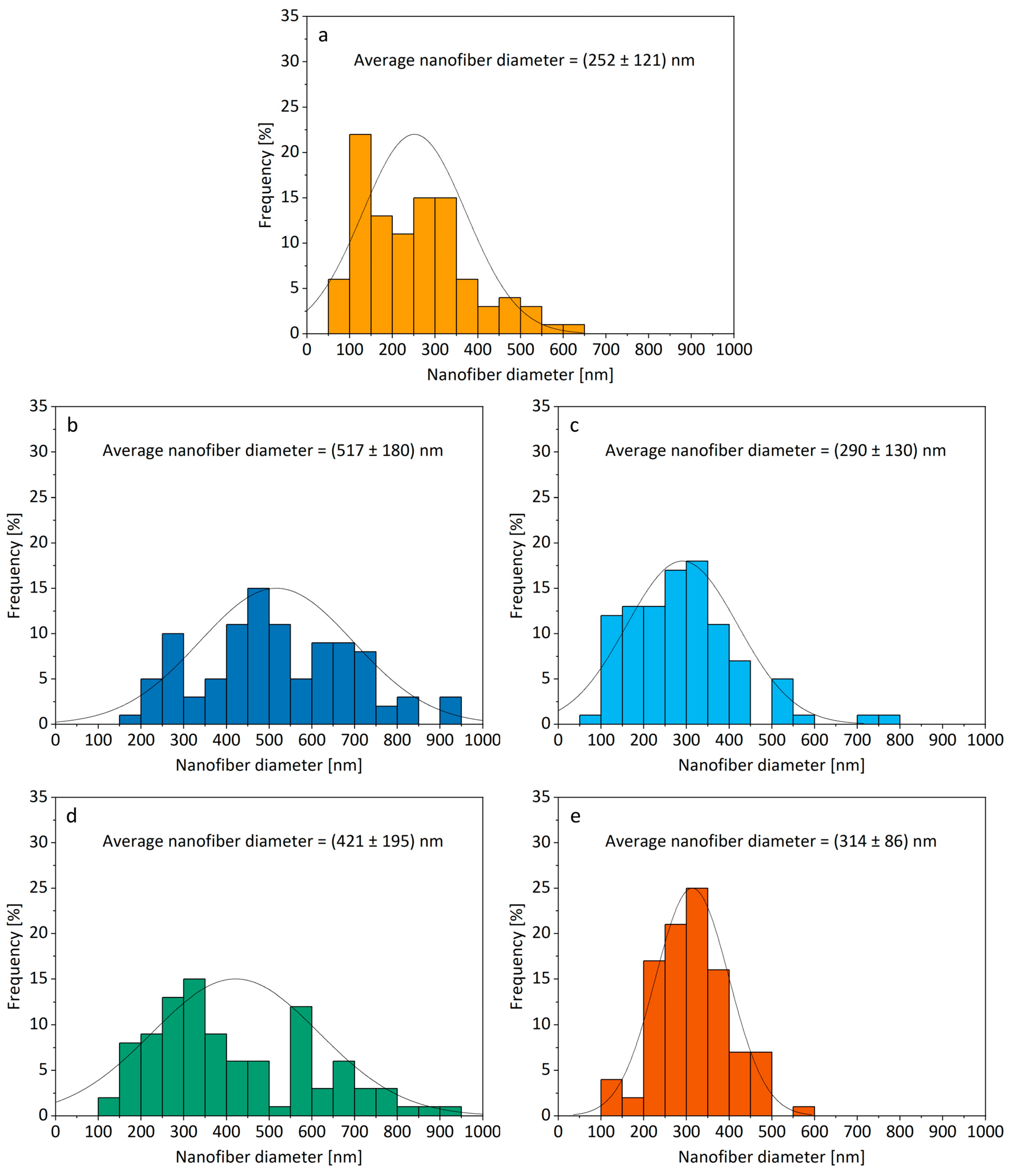
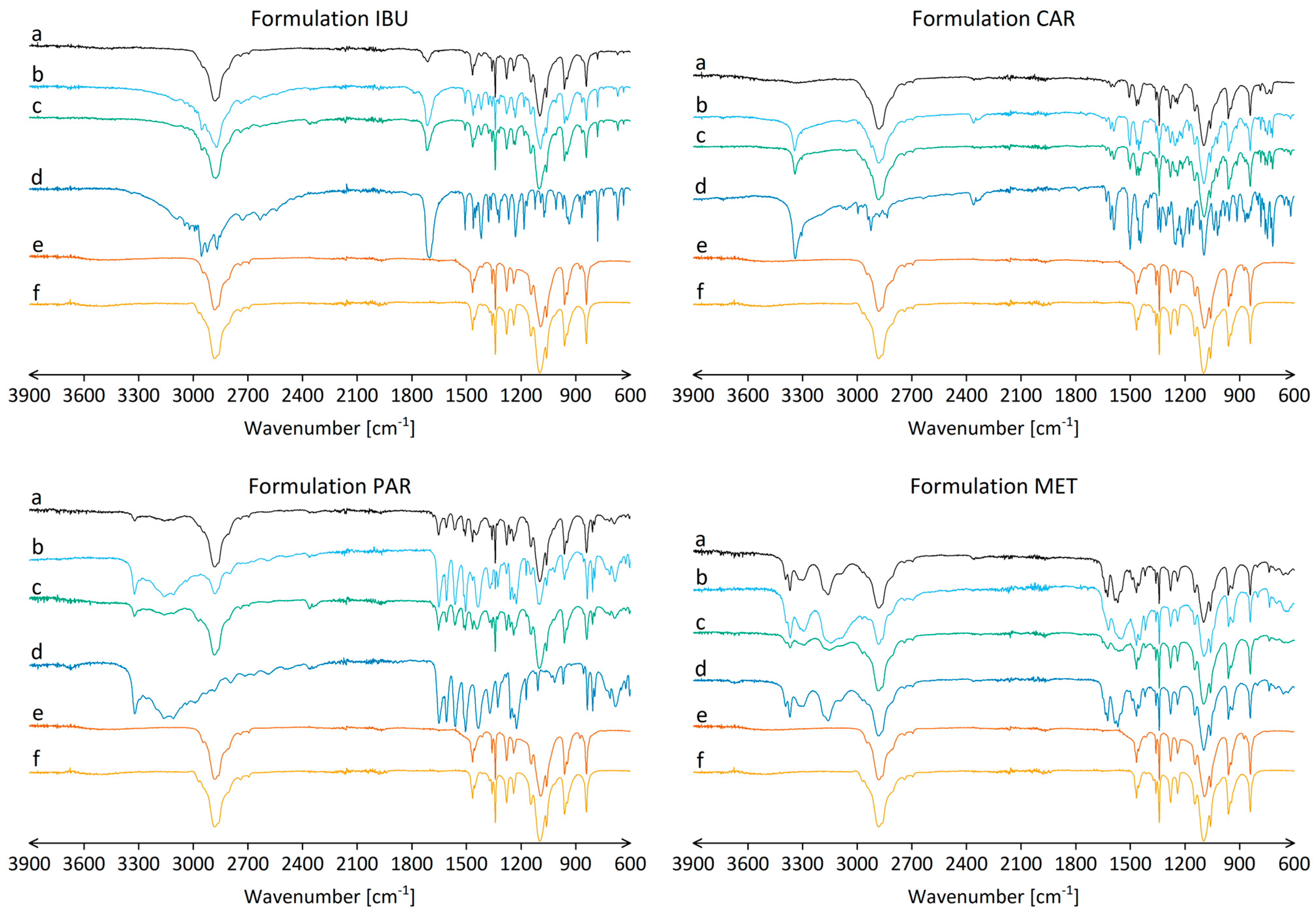
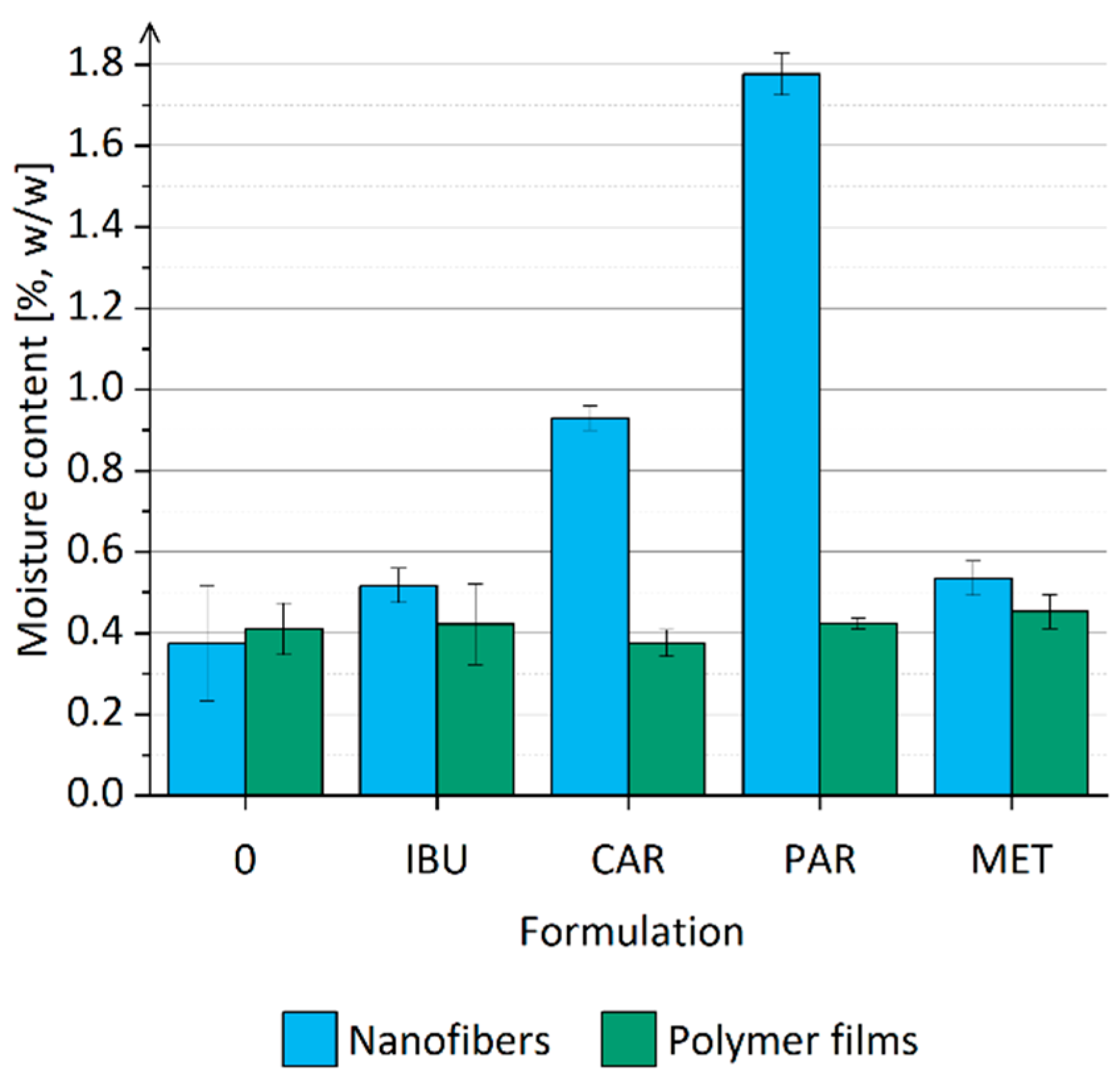
| Formulation | Drug Name | Drug [mg] | P188 [mg] | PEO [mg] | Ethanol [g] |
|---|---|---|---|---|---|
| 0 | / | / | 150 | 150 | 10 |
| IBU | ibuprofen | 75 | 150 | 150 | 10 |
| CAR | carvedilol | 75 | 150 | 150 | 10 |
| PAR | paracetamol | 75 | 150 | 150 | 10 |
| MET | metformin hydrochloride | 75 | 150 | 150 | 10 |
| Parameter/Drug | Ibuprofen | Carvedilol | Paracetamol | Metformin Hydrochloride |
|---|---|---|---|---|
| Injection volume | 20 µL | 20 µL | 2 µL | 1 µL |
| Mobile phase flow rate | 1 mL/min | 1 mL/min | 1 mL/min | 1 mL/min |
| Chromatographic column | Luna® C18 | BetaBasic® C8 | Kinetex® C18 | SynergiTM Hydro-RP |
| Column temperature | 25 °C | 35 °C | 40 °C | 30 °C |
| Detection wavelength | 222 nm | 241 nm | 243 nm | 237 nm |
| Property/Drug | Ibuprofen | Carvedilol | Paracetamol | Metformin Hydrochloride |
|---|---|---|---|---|
| Molecular weight | 206.3 g/mol | 406.5 g/mol | 151.2 g/mol | 165.6 g/mol |
| Appearance | white crystal powder | white crystal powder | white crystal powder | white crystal powder |
| Water solubility * | practically insoluble | practically insoluble | sparingly soluble | freely soluble |
| Ethanol solubility * | freely soluble | slightly soluble | freely soluble | slightly soluble |
| BCS class | II | II | III | III |
| pKa | 4.5 | 14.0 (acid), 8.7 (base) | 9.5 (acid), −4.4 (base) | 12.40 |
| logP (experimental) | 3.7 | 3.8 | 0.5 | −2.6 |
| Formulation | Dynamic Viscosity [mPas] | Electrical Conductivity [μS/cm] |
|---|---|---|
| 0 | 26.8 ± 0.6 | 2.45 ± 0.43 |
| IBU | 25.0 ± 0.2 | 1.53 ± 0.02 |
| CAR | 27.0 ± 0.2 | 2.66 ± 0.23 |
| PAR | 27.0 ± 0.2 | 1.66 ± 0.04 |
| MET | 26.1 ± 0.2 | 687.10 ± 12.60 |
| Formulation | Contact Angle [°] |
|---|---|
| 0 | 53.8 ± 8.8 |
| IBU | 51.8 ± 8.0 |
| CAR | 50.8 ± 10.9 |
| PAR | 29.8 ± 6.4 |
| MET | 29.1 ± 8.4 |
| Formulation | Drug Loading [%, w/w] | Entrapment Efficiency [%] |
|---|---|---|
| IBU | 19.0 ± 0.5 | 94.8 ± 2.7 |
| CAR | 19.5 ± 0.9 | 97.5 ± 4.3 |
| PAR | 18.7 ± 0.1 | 93.4 ± 0.5 |
| MET | 19.9 ± 1.1 | 99.4 ± 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragar, Č.; Roškar, R.; Kocbek, P. The Incorporated Drug Affects the Properties of Hydrophilic Nanofibers. Nanomaterials 2024, 14, 949. https://doi.org/10.3390/nano14110949
Dragar Č, Roškar R, Kocbek P. The Incorporated Drug Affects the Properties of Hydrophilic Nanofibers. Nanomaterials. 2024; 14(11):949. https://doi.org/10.3390/nano14110949
Chicago/Turabian StyleDragar, Črt, Robert Roškar, and Petra Kocbek. 2024. "The Incorporated Drug Affects the Properties of Hydrophilic Nanofibers" Nanomaterials 14, no. 11: 949. https://doi.org/10.3390/nano14110949
APA StyleDragar, Č., Roškar, R., & Kocbek, P. (2024). The Incorporated Drug Affects the Properties of Hydrophilic Nanofibers. Nanomaterials, 14(11), 949. https://doi.org/10.3390/nano14110949






