Effects of Composition and Polymerization Conditions on the Electro-Optic Performance of Liquid Crystal–Polymer Composites Doped with Ferroelectric Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoparticles and Dispersions of the Particles in the Liquid Crystal
2.2. Preparation of Polymer-Stabilized Liquid Crystals
2.3. Preparation of Polymer-Stabilized Liquid Crystal/Nanoparticle Dispersions
2.4. Thermal and Electro-Optic Characterization of the Samples
3. Results
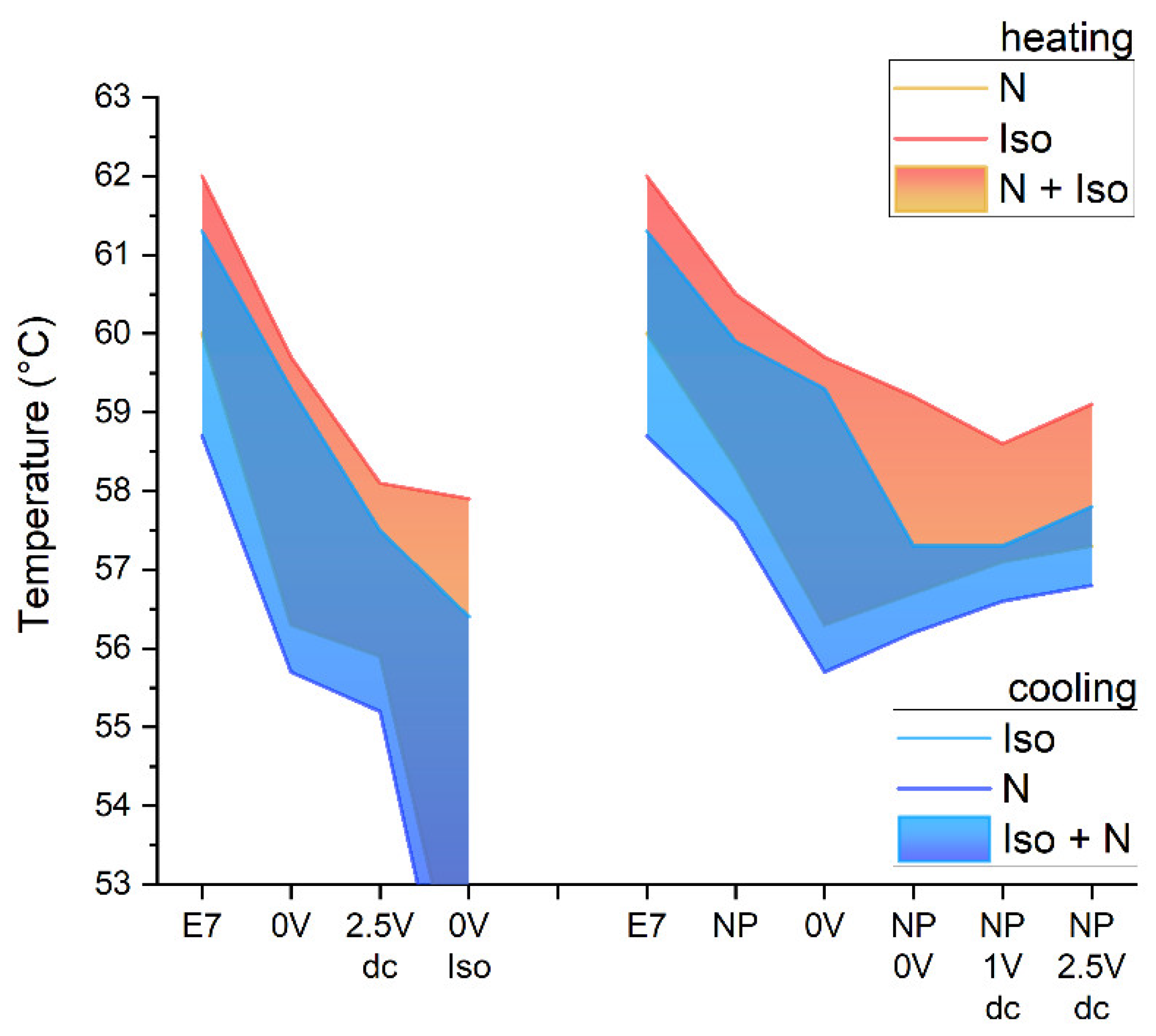
3.1. Phase Transition Temperatures of the Composites
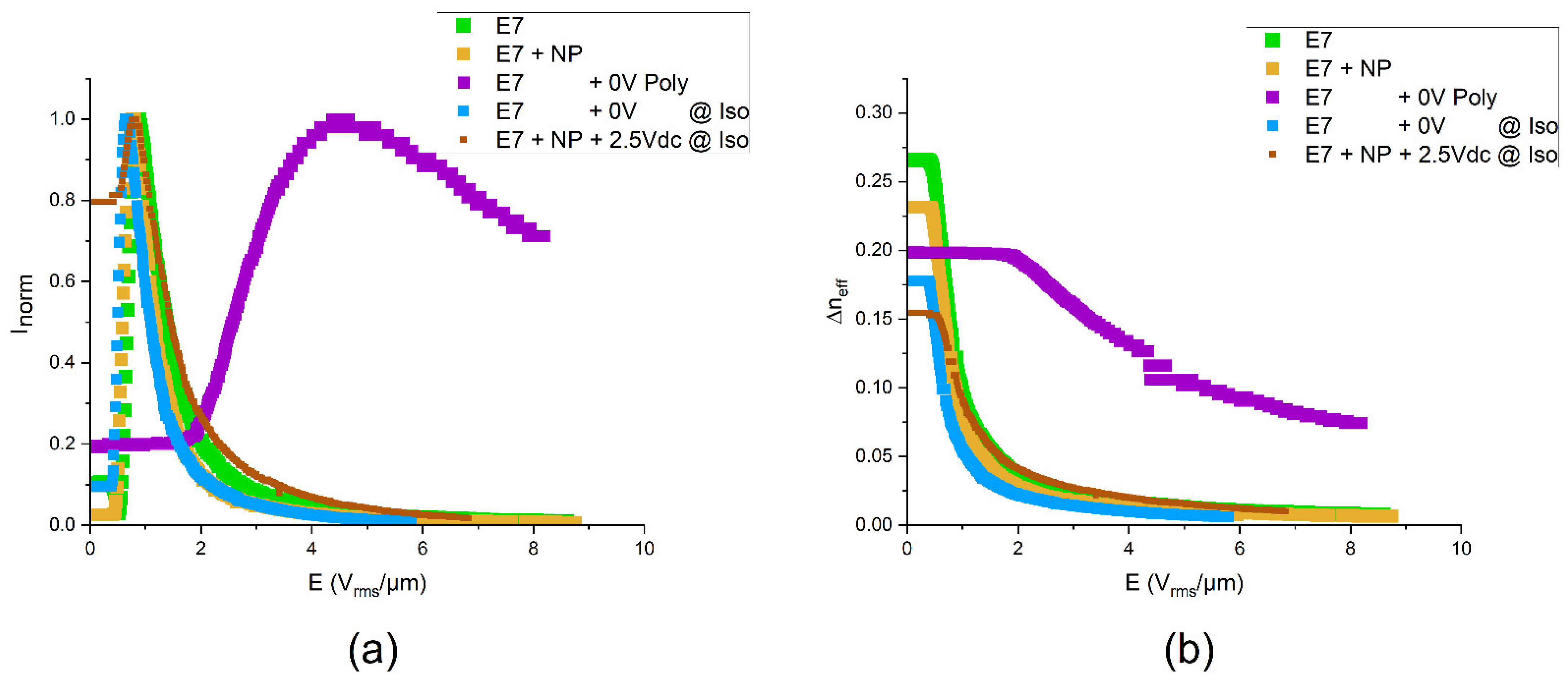
3.2. Electro-Optic Properties
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Gennes, P.-G.; Prost, J. The Physics of Liquid Crystals, 2nd ed.; Clarendon Press: Oxford, UK, 1993. [Google Scholar]
- Stamatoiu, O.; Mirzaei, J.; Feng, X.; Hegmann, T. Nanoparticles in Liquid Crystals and Liquid Crystalline Nanoparticles. In Liquid Crystals; Topics in Current Chemistry; Tschierske, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 318. [Google Scholar]
- Lorenz, A.; Zimmermann, N.; Kumar, S.; Evans, D.R.; Cook, G.; Kitzerow, H.-S. Doping the nematic liquid crystal 5CB with milled BaTiO3 nanoparticles. Phys. Rev. E 2012, 86, 051704. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; Tschudi, T.; de Jeu, W.H.; Eidenschink, R. New liquid crystal display with bistability and selective erasure using scattering in filled nematics. Appl. Phys. Lett. 1993, 62, 1712–1714. [Google Scholar] [CrossRef]
- Yakovkin, I.; Reshetnyak, V. Controlling Plasmon Resonance of Gold and Silver Nanoparticle Arrays with Help of Liquid Crystal. Photonics 2023, 10, 1088. [Google Scholar] [CrossRef]
- Urbanski, M.; Kinkead, B.; Qi, H.; Hegmann, T.; Kitzerow, H.-S. Electroconvection in nematic liquid crystals via nanoparticle doping. Nanoscale 2010, 2, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, J.; Reznikov, M.; Hegmann, T. Quantum dots as liquid crystal dopants. J. Mater. Chem. 2012, 22, 22350–22365. [Google Scholar] [CrossRef]
- Rault, J.; Cladis, P.E.; Burger, J.P. Ferronematics. Phys. Lett. A 1970, 32, 199–200. [Google Scholar] [CrossRef]
- Garbovskiy, Y.; Glushchenko, A. Ferroelectric Nanoparticles in Liquid Crystals: Recent Progress and Current Challenges. Nanomaterials 2017, 7, 361. [Google Scholar] [CrossRef] [PubMed]
- Fergason, J.L. Polymer Encapsulated Nematic Liquid Crystals for Display and Light Control Applications. SID Int. Symp. Dig. Technol. 1985, 16, 68–70. [Google Scholar]
- Drzaic, P.S. Polymer Dispersed Nematic Liquid Crystal for Large Area Displays and Light Valves. J. Appl. Phys. 1986, 60, 2142–2148. [Google Scholar] [CrossRef]
- Doane, J.W.; Vaz, N.A.; Wu, B.G.; Zumer, S. Field Controlled Light Scattering from Nematic Microdroplets. Appl. Phys. Lett. 1986, 48, 269–271. [Google Scholar] [CrossRef]
- Doane, J.W.; Golemme, A.; West, J.L.; Whitehead, J.B., Jr.; Wu, G.-G. Polymer Dispersed Liquid Crystals for Display Application. Mol. Cryst. Liq. Cryst. 1988, 165, 511–532. [Google Scholar] [CrossRef]
- Broer, D.J.; Boven, J.; Mol, G.N.; Challa, G. In-situ photopolymerization of oriented liquid-crystalline acrylates, 3.—Oriented polymer networks from a mesogenic diacrylate. Makromol. Chem. 1989, 190, 2255. [Google Scholar] [CrossRef]
- Hikmet, R.A.M. Electrically induced light scattering from anisotropic gels. J. Appl. Phys. 1990, 68, 4406–4412. [Google Scholar] [CrossRef]
- Bao, R.; Liu, C.M.; Yang, D.K. Smart bistable polymer stabilized cholesteric texture light shutter. Appl. Phys. Express 2009, 2, 112401. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Polymer-stabilized liquid crystal blue phases. Nat. Mater. 2002, 1, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Fung, R.X.; Lin, Y.G.; Hsieh, C.T. Fast switching of polymer-stabilized liquid crystal pi cells. Appl. Phys. Lett. 2007, 90, 171918. [Google Scholar] [CrossRef]
- Dierking, I. A Review of Polymer-Stabilized Ferroelectric Liquid Crystals. Materials 2014, 7, 3568–3587. [Google Scholar] [CrossRef] [PubMed]
- De Baets, J.; Capon, J.; De Rycke, I.; De Smet, H.; Doutreloigne, J.; Van Calster, A.; Vanfleteren, J.; Fujisawa, T.; Ogawa, H.; Takatsu, H.A. Polymer Network Liquid Crystal Poly-CdSe TFT Active Matrix Display. In Proceedings of the Conference Record of the 1991 International Display Research Conference, San Diego, CA, USA, 15–17 October 1991; pp. 215–218. [Google Scholar]
- Choi, C.H.; Kim, S.H.; Hong, E.Y.; Kim, B.K. Electro-Optic Properties and Wavelength Effect in Polymer Network Liquid Crystal. Eur. Polym. J. 1997, 33, 565–569. [Google Scholar] [CrossRef]
- Dierking, I. Polymer Network–Stabilized Liquid Crystals. Adv. Mater. 2000, 12, 167–181. [Google Scholar] [CrossRef]
- Jahanbakhsh, F.; Lorenz, A. Fast copolymer network liquid crystals for tunable birefringence colors. Appl. Opt. 2019, 58, 5587. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, L.; Zhong, T. A Review of Developments in Polymer Stabilized Liquid Crystals. Polymers 2023, 15, 2962. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, C.V.; Hudson, S.D. Effect of Polymerization Temperature on the Morphology and Electrooptic Properties of Polymer-Stabilized Liquid Crystals. Chem. Mater. 1996, 8, 2451–2460. [Google Scholar] [CrossRef]
- Du, F.; Gauza, S.; WU, S.T. Influence of curing temperature and high birefringence on the properties of polymer-stabilized liquid crystals. Opt. Express 2003, 11, 2891–2896. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Fung, R.X.; Lin, Y.G. Effects of Curing Conditions on Electrooptical Properties of Polymer-Stabilized Liquid Crystal Pi Cells. Jpn. J. Appl. Phys. 2007, 46, 5230–5232. [Google Scholar] [CrossRef]
- Jeon, B.G.; Choi, T.H.; Do, S.M.; Woo, J.H.; Yoon, T.H. Effects of Curing Temperature on Switching Between Transparent and Translucent States in a Polymer-Stabilized Liquid-Crystal Cell. IEEE Trans. Electron Devices 2018, 65, 4387–4393. [Google Scholar] [CrossRef]
- Dierking, I. Relationship Between the Electro-Optic Performance of Polymer-Stabilized Liquid-Crystal Devices and the Fractal Dimension of Their Network Morphology. Adv. Mater. 2003, 15, 152–156. [Google Scholar] [CrossRef]
- Dierking, I. Fractal and Non-Fractal Structure-Property Relationships of Polymer-Stabilized Liquid Crystals. Adv. Funct. Mater. 2004, 14, 883–890. [Google Scholar] [CrossRef]
- Choi, W.K.; Li, Y.M. Vertically-Aligned Polymer Stabilized Liquid Crystals (VA-PSLC) with a Curing Voltage for Fast-Repose Wavelength-Tuning Applications. Mol. Cryst. Liq. Cryst. 2015, 613, 45–50. [Google Scholar] [CrossRef]
- Strauss, J.; Kitzerow, H.-S. Antiferroelectric gels. Ber. Bunsenges. 2012, 102, 1609–1614. [Google Scholar] [CrossRef]
- Hsu, C.-J.; Kuo, C.-C.; Hsieh, C.-D.; Huang, C.-Y. Effects of silica nanoparticles on electro-optical properties of polymer-stabilized liquid crystals. Opt. Express 2014, 22, 18513–18518. [Google Scholar] [CrossRef]
- Yan, X.; Liu, W.; Zhou, Y.; Yuan, D.; Hu, X.; Zhao, W.; Zhou, G. Improvement of Electro-Optical Properties of PSLC Devices by Silver Nanowire Doping. Appl. Sci. 2019, 9, 145. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, Y.; Liu, W.; Liu, S.; Hu, X.; Zhao, W.; Zhou, G.; Yuan, D. Effects of silver nanoparticle doping on the electro-optical properties of polymer stabilized liquid crystal devices. Liq. Cryst. 2020, 47, 1131–1138. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Xu, L.; Li, J.; Yang, L.; Yang, Y.; Bolshakov, A.; Zhu, J. Visible and infrared optical modulation of PSLC smart films doped with ATO nanoparticles. Dalton Trans. 2021, 50, 10033–100040. [Google Scholar] [CrossRef] [PubMed]
- Habibpourmoghadam, A.; Jiao, L.; Reshetnyak, V.; Evans, D.R.; Lorenz, A. Optical manipulation and defect creation in a liquid crystal on a photo responsive surface. Phys. Rev. E 2017, 96, 022701. [Google Scholar] [CrossRef] [PubMed]
- Habibpourmoghadam, A.; Lucchetti, L.; Evans, D.R.; Reshetnyak, V.Y.; Lorenz, A. Laser-induced erasable patterns in a N* liquid crystal on an iron doped lithium niobate surface. Opt. Express 2017, 25, 26148. [Google Scholar] [CrossRef] [PubMed]
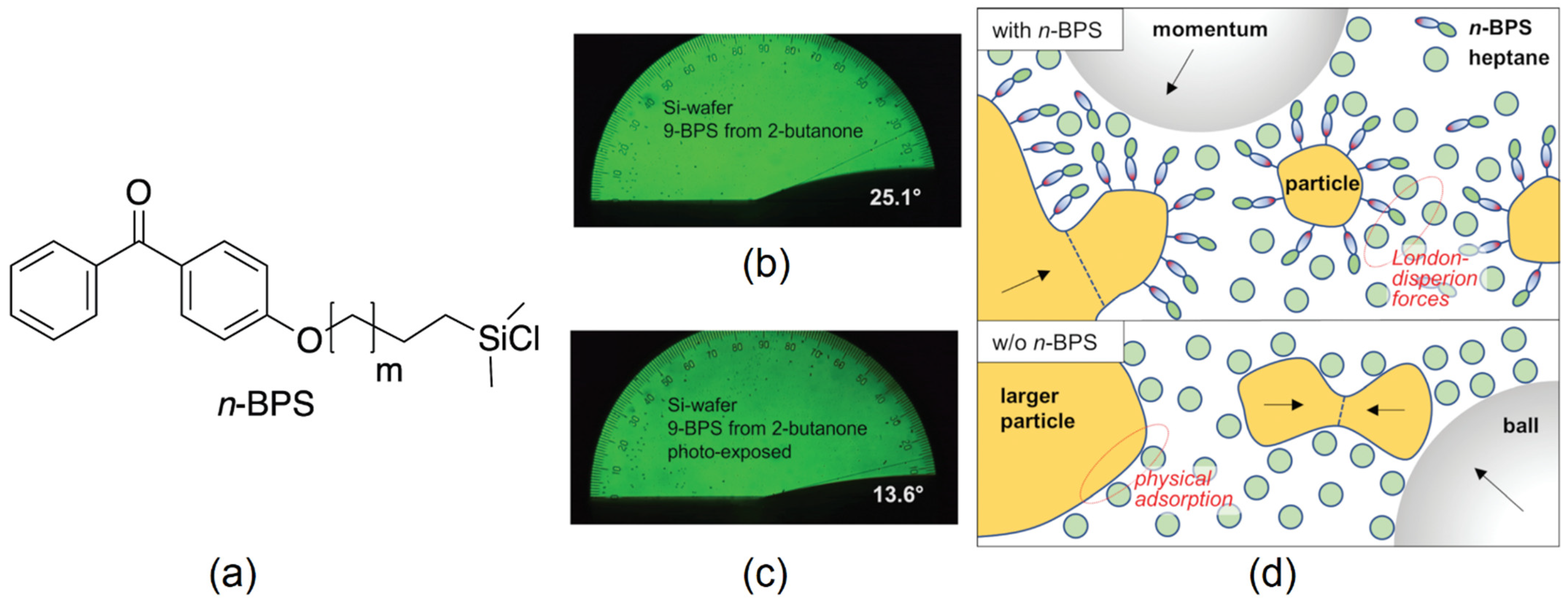
- Braun, L.; Schafforz, S.L.; Lorenz, A. Surface grafted crosslinker in polymer network liquid crystals. J. Mol. Liq. 2018, 267, 109. [Google Scholar] [CrossRef]
- Nordendorf, G.; Schafforz, S.L.; Käkel, E.B.; Miao, S.; Lorenz, A. Surface grafted agents with various molecular lengths and photochemically active benzophenone moiety. Phys. Chem. Chem. Phys. 2020, 22, 1774. [Google Scholar] [CrossRef] [PubMed]
- Mouquinho, A.; Saavedra, M.; Maiau, A.; Petrova, K.; Teresa Barros, M.; Figueirinhas, J.L.; Sotomayor, J. Films Based on New Methacrylate Monomers: Synthesis, Characterisation and Electro-Optical Properties. Mol. Cryst. Liq. Cryst. 2011, 542, 132–140. [Google Scholar] [CrossRef]
- Fréedericksz, V.; Zolina, V. Forces causing the orientation of an anisotropic liquid. Trans. Faraday Soc. 1933, 29, 919–930. [Google Scholar] [CrossRef]
- Shurcliff, W.A. Polarized Light. Production and Use; Harvard University Press: Cambridge, MA, USA, 1966. [Google Scholar]

| Composite | Concentration of the Components [% by Weight] | ||||
|---|---|---|---|---|---|
| E7 | LiNbO3:Fe | EHA | RM 257 | IRG 819 | |
| Pure LC | 100% | - | - | - | - |
| LC/NP dispersion | 99% | 1% | - | - | - |
| PSLC | 89% | - | 5% | 5% | 1% |
| PSLC/NP dispersion | 88% | 1% | 5% | 5% | 1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordendorf, G.; Jünnemann-Held, G.; Lorenz, A.; Kitzerow, H.-S. Effects of Composition and Polymerization Conditions on the Electro-Optic Performance of Liquid Crystal–Polymer Composites Doped with Ferroelectric Nanoparticles. Nanomaterials 2024, 14, 961. https://doi.org/10.3390/nano14110961
Nordendorf G, Jünnemann-Held G, Lorenz A, Kitzerow H-S. Effects of Composition and Polymerization Conditions on the Electro-Optic Performance of Liquid Crystal–Polymer Composites Doped with Ferroelectric Nanoparticles. Nanomaterials. 2024; 14(11):961. https://doi.org/10.3390/nano14110961
Chicago/Turabian StyleNordendorf, Gaby, Gisela Jünnemann-Held, Alexander Lorenz, and Heinz-Siegfried Kitzerow. 2024. "Effects of Composition and Polymerization Conditions on the Electro-Optic Performance of Liquid Crystal–Polymer Composites Doped with Ferroelectric Nanoparticles" Nanomaterials 14, no. 11: 961. https://doi.org/10.3390/nano14110961





