Encapsulation and Evolution of Polyynes Inside Single-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
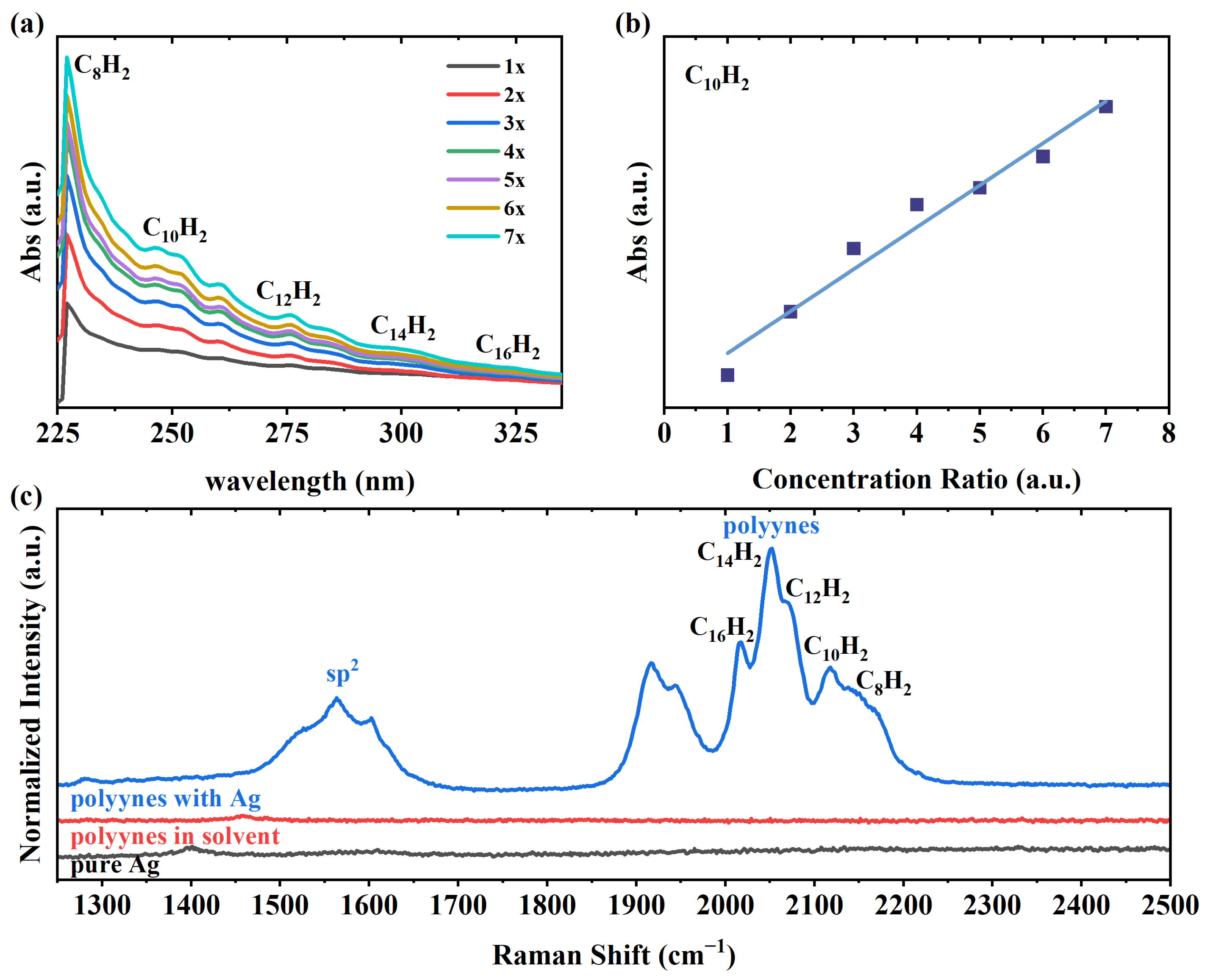
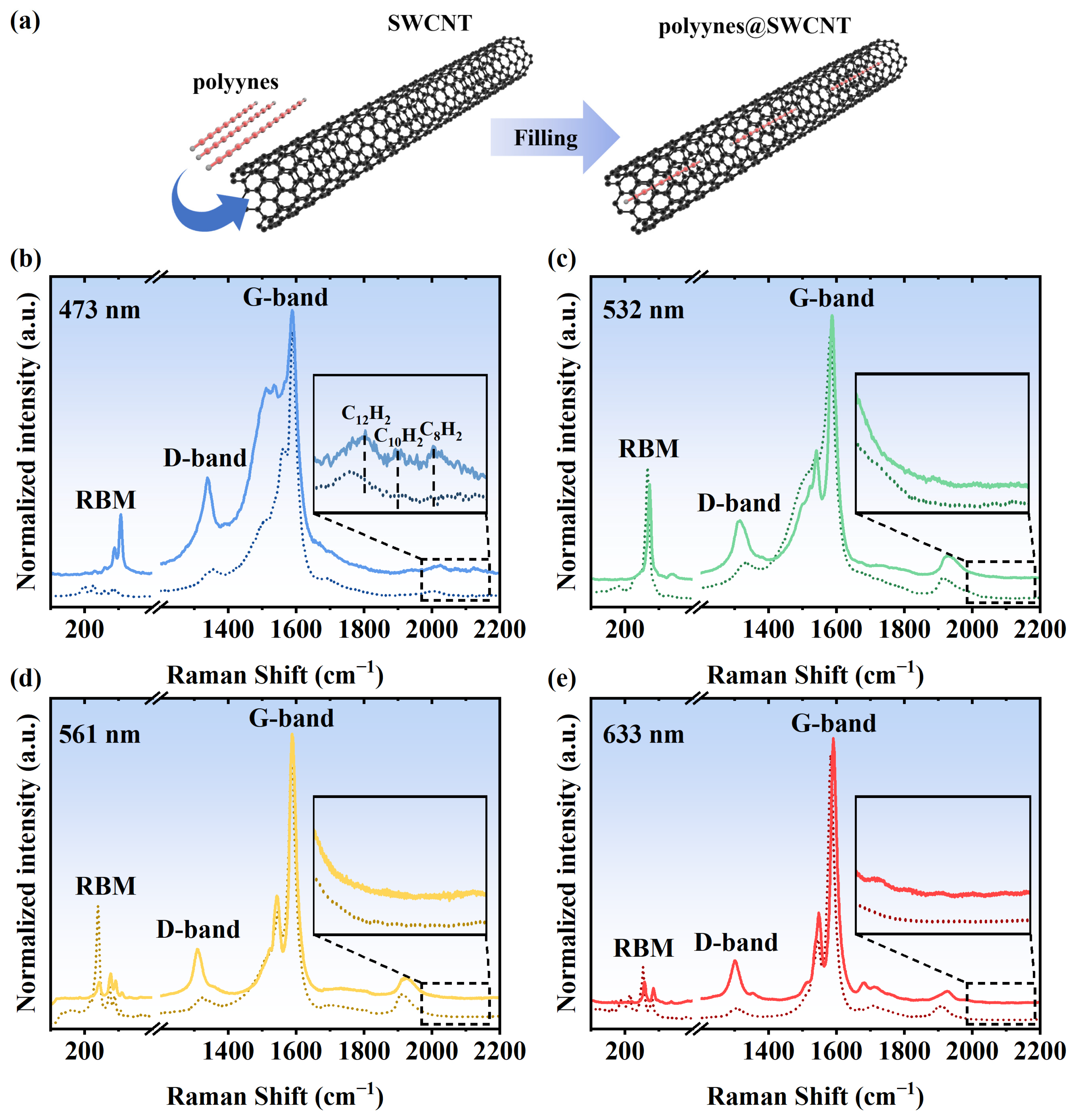
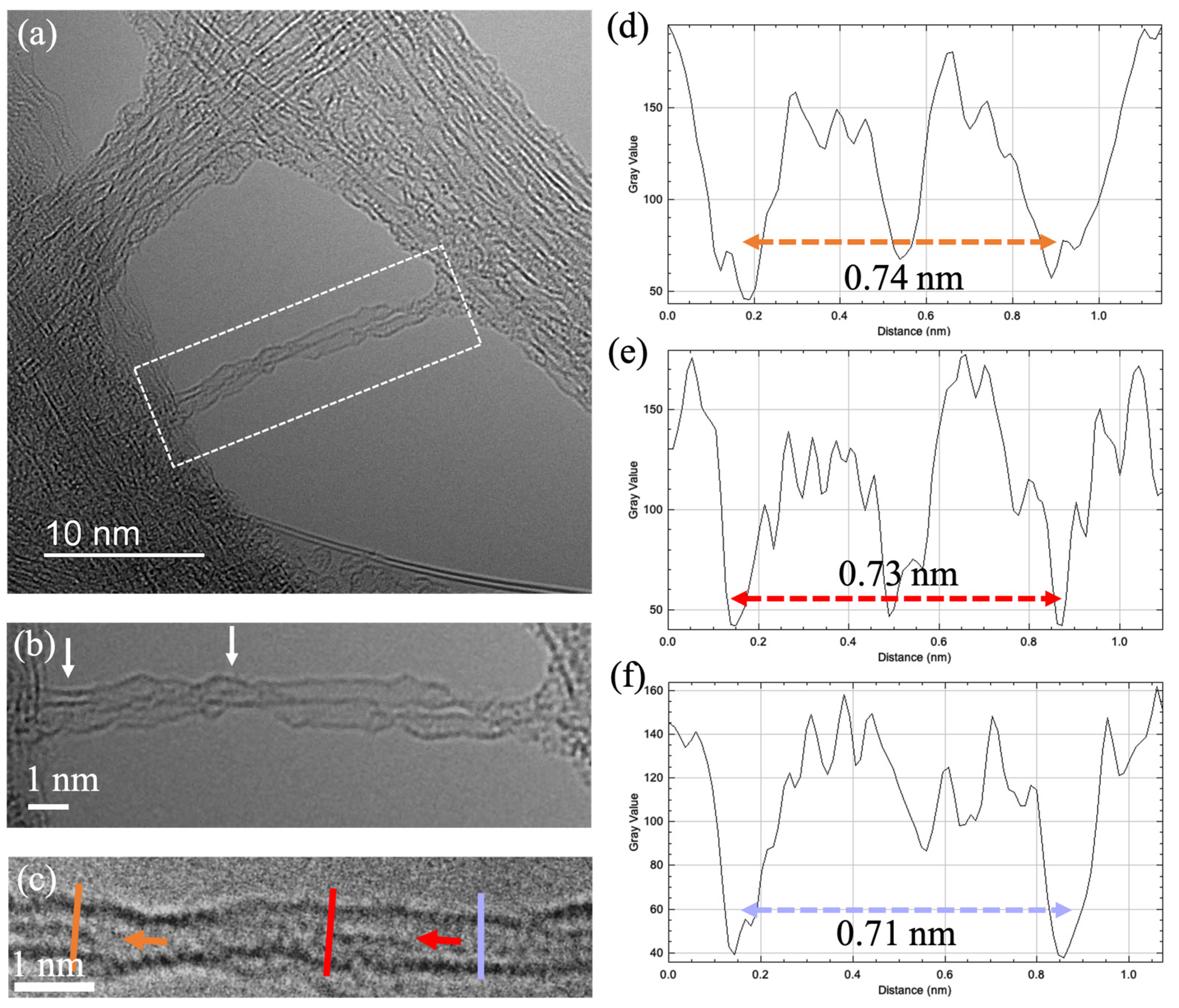
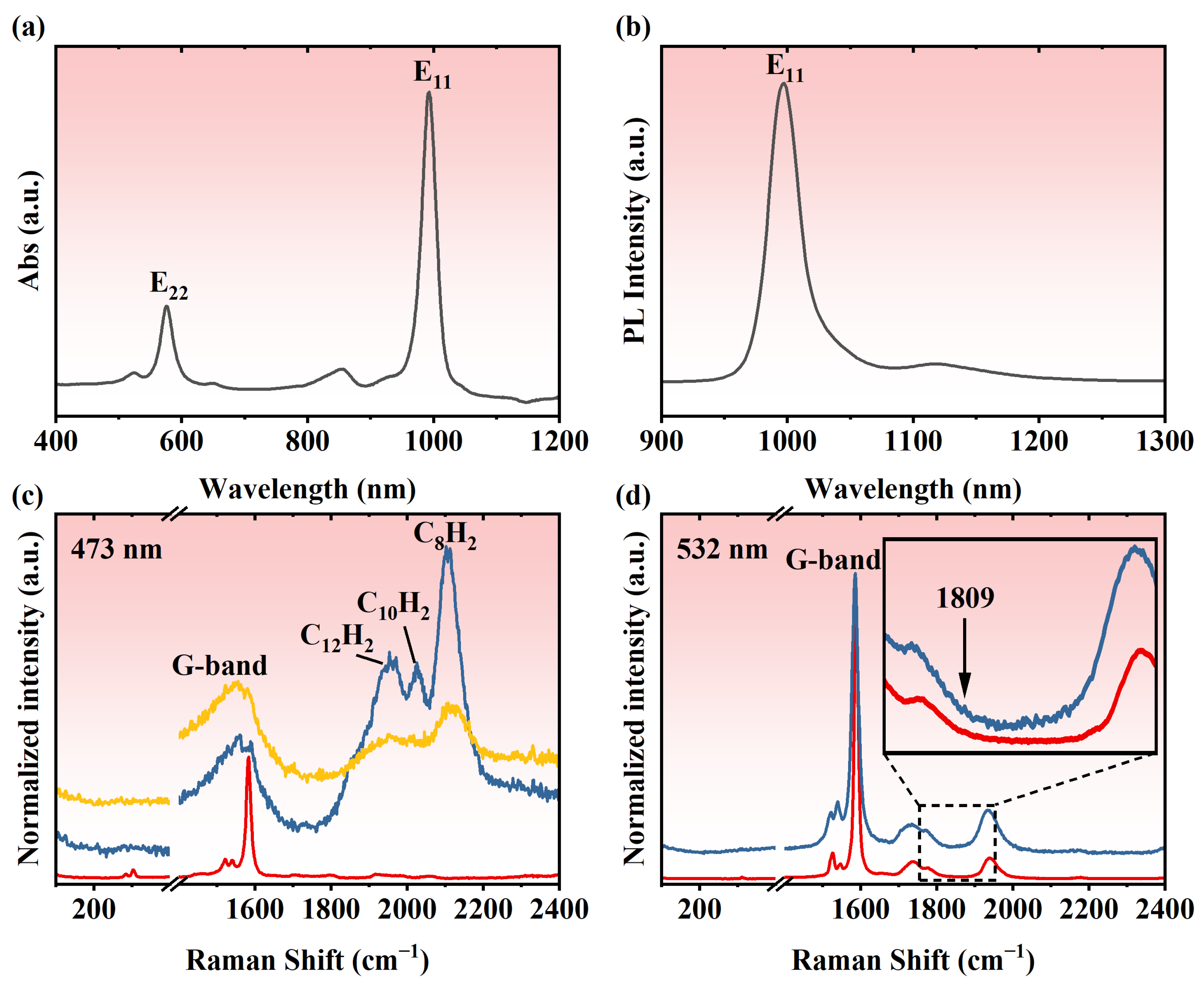
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Artyukhov, V.I.; Lee, H.; Xu, F.; Yakobson, B.I. Carbyne from first principles: Chain of C atoms, a nanorod or a nanorope. ACS Nano 2013, 7, 10075–10082. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F. Polyynes: Synthesis, Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Casari, C.S.; Tommasini, M.; Tykwinski, R.R.; Milani, A. Carbon-atom wires: 1-D systems with tunable properties. Nanoscale 2016, 8, 4414–4435. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Tsuji, T.; Kuboyama, S.; Yoon, S.-H.; Korai, Y.; Tsujimoto, T.; Kubo, K.; Mori, A.; Mochida, I. Formation of hydrogen-capped polyynes by laser ablation of graphite particles suspended in solution. Chem. Phys. Lett. 2002, 355, 101–108. [Google Scholar] [CrossRef]
- Lucotti, A.; Tommasini, M.; Del Zoppo, M.; Castiglioni, C.; Zerbi, G.; Cataldo, F.; Casari, C.; Bassi, A.L.; Russo, V.; Bogana, M.; et al. Raman and SERS investigation of isolated sp carbon chains. Chem. Phys. Lett. 2006, 417, 78–82. [Google Scholar] [CrossRef]
- Cataldo, F. Synthesis of polyynes in a submerged electric arc in organic solvents. Carbon 2004, 42, 129–142. [Google Scholar] [CrossRef]
- Chalifoux, W.A.; Tykwinski, R.R. Synthesis of polyynes to model the sp-carbon allotrope carbyne. Nat. Chem. 2010, 2, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Gibtner, T.; Hampel, F.; Gisselbrecht, J.P.; Hirsch, A. End-cap stabilized oligoynes: Model compounds for the linear sp carbon allotrope carbyne. Chem.–A Eur. J. 2002, 8, 408–432. [Google Scholar] [CrossRef]
- Krempe, M.; Lippert, R.; Hampel, F.; Ivanović-Burmazović, I.; Jux, N.; Tykwinski, R.R. Pyridyl-endcapped polyynes: Stabilized wire-like molecules. Angew. Chem. 2016, 55, 14802–14806. [Google Scholar] [CrossRef]
- Kendall, J.; McDonald, R.; Ferguson, M.J.; Tykwinski, R.R. Synthesis and solid-state structure of perfluorophenyl end-capped polyynes. Org. Lett. 2008, 10, 2163–2166. [Google Scholar] [CrossRef]
- Hoye, T.R.; Baire, B.; Niu, D.; Willoughby, P.H.; Woods, B.P. The hexadehydro-Diels–Alder reaction. Nature 2012, 490, 208–212. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, Y.; Gamez, F.G.; Ferguson, M.J.; Casado, J.; Tykwinski, R.R. The loss of endgroup effects in long pyridyl-endcapped oligoynes on the way to carbyne. Nat. Chem. 2020, 12, 1143. [Google Scholar] [CrossRef] [PubMed]
- Patrick, C.W.; Gao, Y.; Gupta, P.; Thompson, A.L.; Parker, A.W.; Anderson, H.L. Masked alkynes for synthesis of threaded carbon chains. Nat. Chem. 2024, 16, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zheng, W.; Sun, L.; Kang, F.; Zhou, Z.; Xu, W. On-surface synthesis and characterization of polyynic carbon chains. Natl. Sci. Rev. 2024, 11, nwae031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Shi, L. A review of linear carbon chains. Chin. Chem. Lett. 2020, 31, 1746–1756. [Google Scholar] [CrossRef]
- Wang, C.; Batsanov, A.S.; Bryce, M.R.; Martín, S.; Nichols, R.J.; Higgins, S.J.; García-Suárez, V.M.; Lambert, C.J. Oligoyne single molecule wires. J. Am. Chem. Soc. 2009, 131, 15647–15654. [Google Scholar] [CrossRef] [PubMed]
- Eisler, S.; Slepkov, A.D.; Elliott, E.; Luu, T.; McDonald, R.; Hegmann, F.A.; Tykwinski, R.R. Polyynes as a model for carbyne: Synthesis, physical properties, and nonlinear optical response. J. Am. Chem. Soc. 2005, 127, 2666–2676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ando, Y.; Liu, Y.; Jinno, M.; Suzuki, T. Carbon nanowire made of a long linear carbon chain inserted inside a multiwalled carbon nanotube. Phys. Rev. Lett. 2003, 90, 187401. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Vasconcelos, T.; Gouvea, C.; Archanjo, B.; Achete, C.; Kim, Y.; Endo, M.; Fantini, C.; Dresselhaus, M.; Filho, A.S. Linear carbon chains encapsulated in multiwall carbon nanotubes: Resonance Raman spectroscopy and transmission electron microscopy studies. Carbon 2015, 90, 172–180. [Google Scholar] [CrossRef]
- Shi, L.; Sheng, L.; Yu, L.; An, K.; Ando, Y.; Zhao, X. Ultra-thin double-walled carbon nanotubes: A novel nanocontainer for preparing atomic wires. Nano Res. 2011, 4, 759–766. [Google Scholar] [CrossRef]
- Heeg, S.; Shi, L.; Poulikakos, L.V.; Pichler, T.; Novotny, L. Carbon nanotube chirality determines properties of encapsulated linear carbon chain. Nano Lett. 2018, 18, 5426–5431. [Google Scholar] [CrossRef]
- Pham, T.; Oh, S.; Stetz, P.; Onishi, S.; Kisielowski, C.; Cohen, M.L.; Zettl, A. Torsional instability in the single-chain limit of a transition metal trichalcogenide. Science 2018, 361, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.E.; Miyata, Y.; Kitaura, R.; Nishimura, Y.; Nishimoto, Y.; Irle, S.; Warner, J.H.; Kataura, H.; Shinohara, H. Growth of carbon nanotubes via twisted graphene nanoribbons. Nat. Commun. 2013, 4, 2548. [Google Scholar] [CrossRef] [PubMed]
- Khlobystov, A.N. Carbon nanotubes: From nano test tube to nano-reactor. ACS Nano 2011, 5, 9306–9312. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, R.; Nakanishi, R.; Saito, T.; Yoshikawa, H.; Awaga, K.; Shinohara, H. High-yield synthesis of ultrathin metal nanowires in carbon nanotubes. Angew. Chem. Int. Ed. 2009, 48, 8298–8302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Kitaura, R.; Hara, H.; Irle, S.; Shinohara, H. Growth of linear carbon chains inside thin double-wall carbon nanotubes. J. Phys. Chem. C 2011, 115, 13166–13170. [Google Scholar] [CrossRef]
- Chang, W.; Liu, F.; Liu, Y.; Zhu, T.; Fang, L.; Li, Q.; Liu, Y.; Zhao, X. Smallest carbon nanowires made easy: Long linear carbon chains confined inside single-walled carbon nanotubes. Carbon 2021, 183, 571–577. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhu, T.; Li, H.; Liu, Y.; Zhao, X. Effects of precursor molecules on polyyne formation by arc discharge between two copper electrodes. Chem. Phys. Lett. 2019, 730, 64–69. [Google Scholar] [CrossRef]
- Fujimori, T.; Morelos-Gomez, A.; Zhu, Z.; Muramatsu, H.; Futamura, R.; Urita, K.; Terrones, M.; Hayashi, T.; Endo, M.; Young Hong, S.; et al. Conducting linear chains of sulphur inside carbon nanotubes. Nat. Commun. 2013, 4, 2162. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Jagota, A.; Zheng, M. Machine learning-guided systematic search of DNA sequences for sorting carbon nanotubes. ACS Nano 2022, 16, 4705–4713. [Google Scholar] [CrossRef]
- Tabata, H.; Fujii, M.; Hayashi, S.; Doi, T.; Wakabayashi, T. Raman and surface-enhanced Raman scattering of a series of size-separated polyynes. Carbon 2006, 44, 3168–3176. [Google Scholar] [CrossRef]
- Ravagnan, L.; Siviero, F.; Lenardi, C.; Piseri, P.; Barborini, E.; Milani, P.; Casari, C.S.; Bassi, A.L.; Bottani, C.E. Cluster-beam deposition and in situ characterization of carbyne-rich carbon films. Phys. Rev. Lett. 2002, 89, 285506. [Google Scholar] [CrossRef] [PubMed]
- D’urso, L.; Grasso, G.; Messina, E.; Bongiorno, C.; Scuderi, V.; Scalese, S.; Puglisi, O.; Spoto, G.; Compagnini, G. Role of linear carbon chains in the aggregation of copper, silver, and gold nanoparticles. J. Phys. Chem. C 2010, 114, 907–915. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Shi, L.; Rohringer, P.; Wanko, M.; Rubio, A.; Waßerroth, S.; Reich, S.; Cambré, S.; Wenseleers, W.; Ayala, P.; Pichler, T. Electronic band gaps of confined linear carbon chains ranging from polyyne to carbyne. Phys. Rev. Mater. 2017, 1, 075601. [Google Scholar] [CrossRef]
- Cui, W.; Ayala, P.; Pichler, T.; Shi, L. Unraveling the governing properties of confined carbyne through the interaction with its carbon nanotube host. Carbon 2024, 219, 118784. [Google Scholar] [CrossRef]
- Gaufrès, E.; Tang, N.Y.-W.; Lapointe, F.; Cabana, J.; Nadon, M.-A.; Cottenye, N.; Raymond, F.; Szkopek, T.; Martel, R. Giant Raman scattering from J-aggregated dyes inside carbon nanotubes for multispectral imaging. Nat. Photon. 2014, 8, 73–79. [Google Scholar] [CrossRef]
- Nishide, D.; Wakabayashi, T.; Sugai, T.; Kitaura, R.; Kataura, H.; Achiba, Y.; Shinohara, H. Raman spectroscopy of size-selected linear polyyne molecules C2nH2 (n = 4−6) encapsulated in single-wall carbon nanotubes. J. Phys. Chem. C 2007, 111, 5178–5183. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, K.; Li, Y.; Chen, Y.; Cui, W.; Lin, Z.; Zhang, Y.; Shi, L. Encapsulation and Evolution of Polyynes Inside Single-Walled Carbon Nanotubes. Nanomaterials 2024, 14, 966. https://doi.org/10.3390/nano14110966
Tang K, Li Y, Chen Y, Cui W, Lin Z, Zhang Y, Shi L. Encapsulation and Evolution of Polyynes Inside Single-Walled Carbon Nanotubes. Nanomaterials. 2024; 14(11):966. https://doi.org/10.3390/nano14110966
Chicago/Turabian StyleTang, Kunpeng, Yinong Li, Yingzhi Chen, Weili Cui, Zhiwei Lin, Yifan Zhang, and Lei Shi. 2024. "Encapsulation and Evolution of Polyynes Inside Single-Walled Carbon Nanotubes" Nanomaterials 14, no. 11: 966. https://doi.org/10.3390/nano14110966
APA StyleTang, K., Li, Y., Chen, Y., Cui, W., Lin, Z., Zhang, Y., & Shi, L. (2024). Encapsulation and Evolution of Polyynes Inside Single-Walled Carbon Nanotubes. Nanomaterials, 14(11), 966. https://doi.org/10.3390/nano14110966





