Advance of Microemulsion and Application for Enhanced Oil Recovery
Abstract
1. Introduction
2. Research on the Introduction, Types, and Formation Mechanisms of Microemulsions
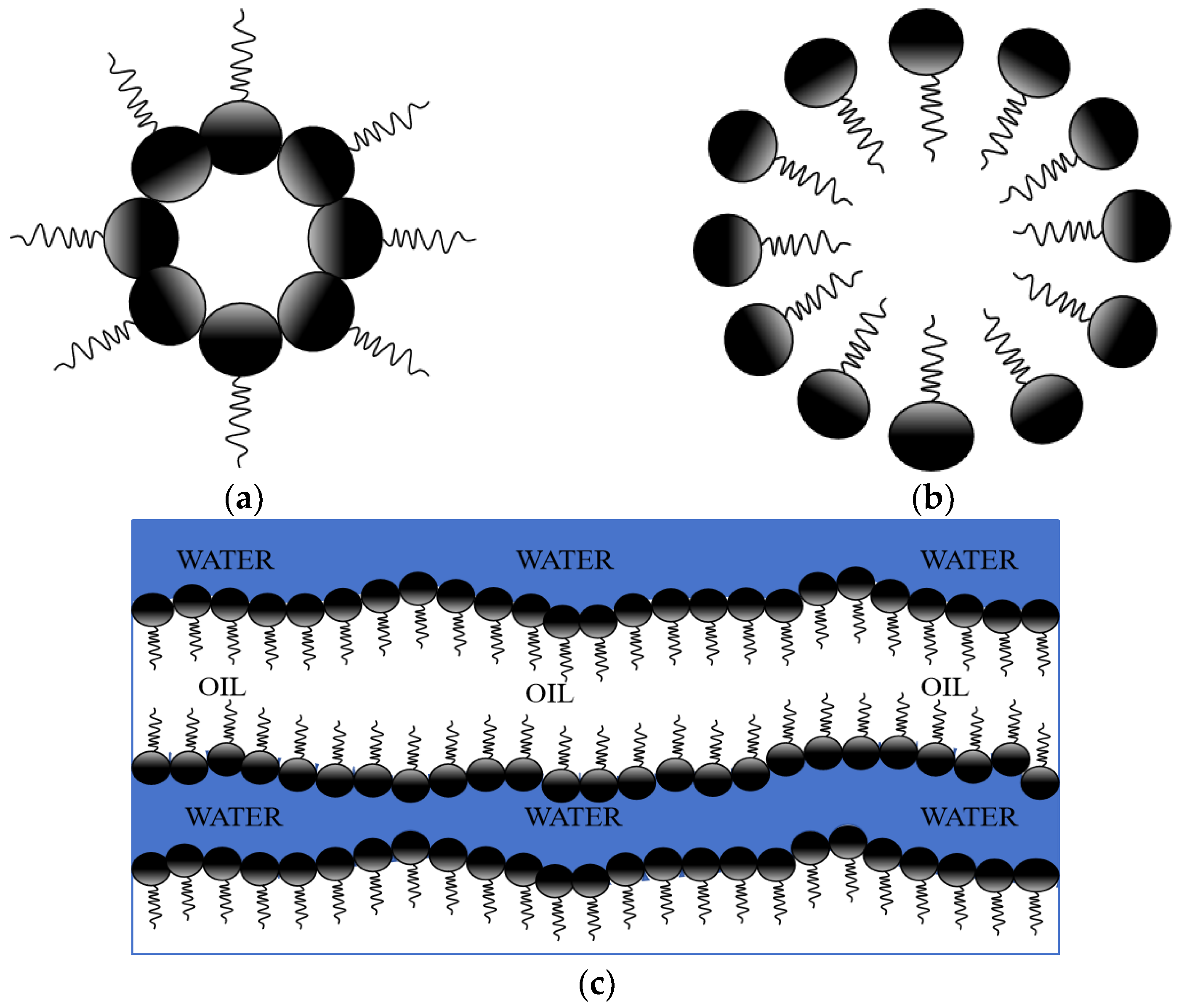
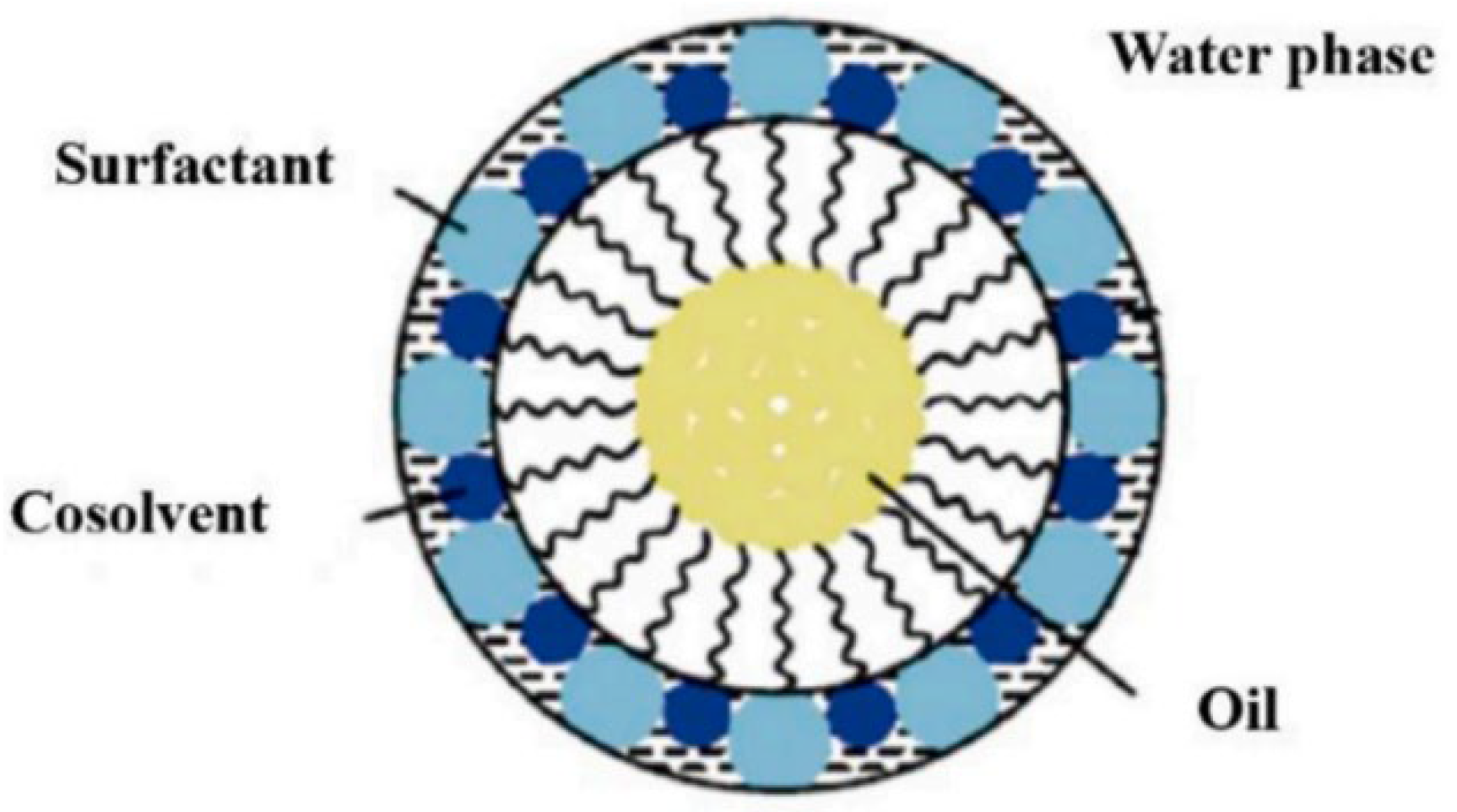
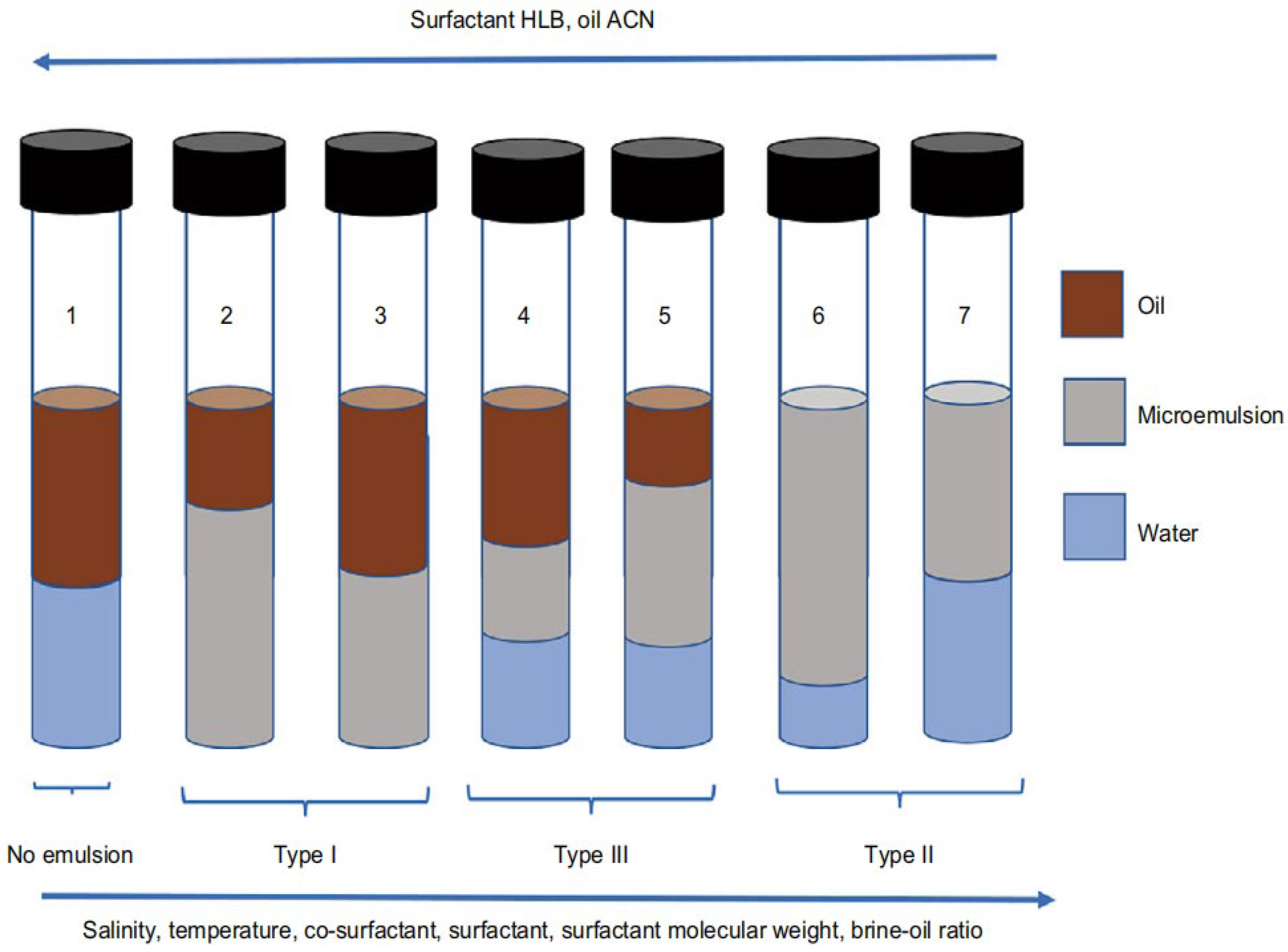
2.1. Profile and Types of Microemulsions
2.2. Characteristics of Microemulsion
| Emulsion Type | Macroemulsion | Nanoemulsion | Microemulsion | Swelling Micelle Solution |
|---|---|---|---|---|
| Particle size | 1~10 | 20~500 | 10~100 | <50 nm |
| Emulsifier concentration | Low | Middle | High | More than the critical micelle concentration |
| Appearance | Opacity | Transparent, translucent, or milky white | Transparent, translucent | Transparent |
| Stability | Instability | Long-term dynamic stability | Thermodynamic stability | Thermodynamic stability |
| Type | O/W and W/O | O/W and W/O | O/W, W/O, and bicontinuous structures | Micelle or reverse micelle solution |
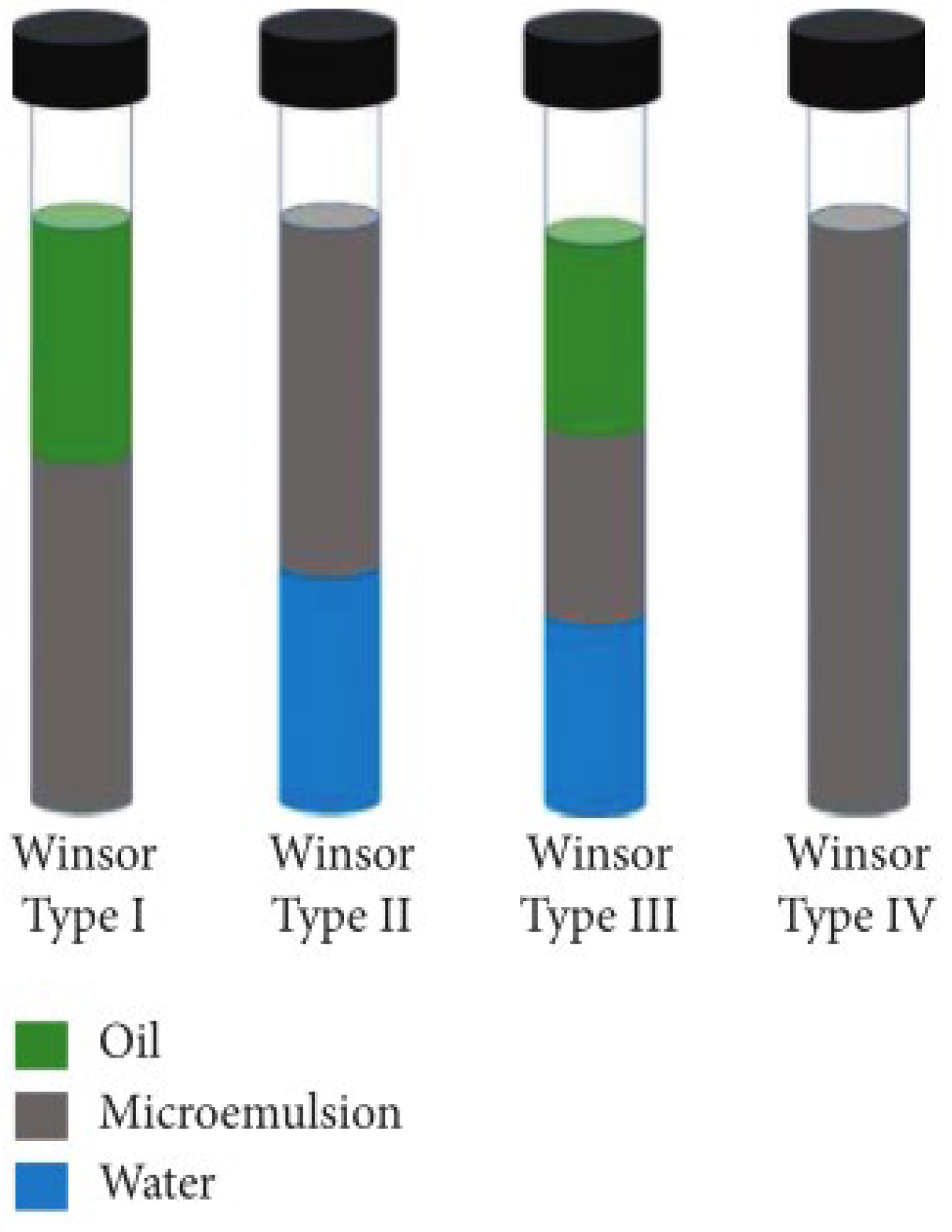
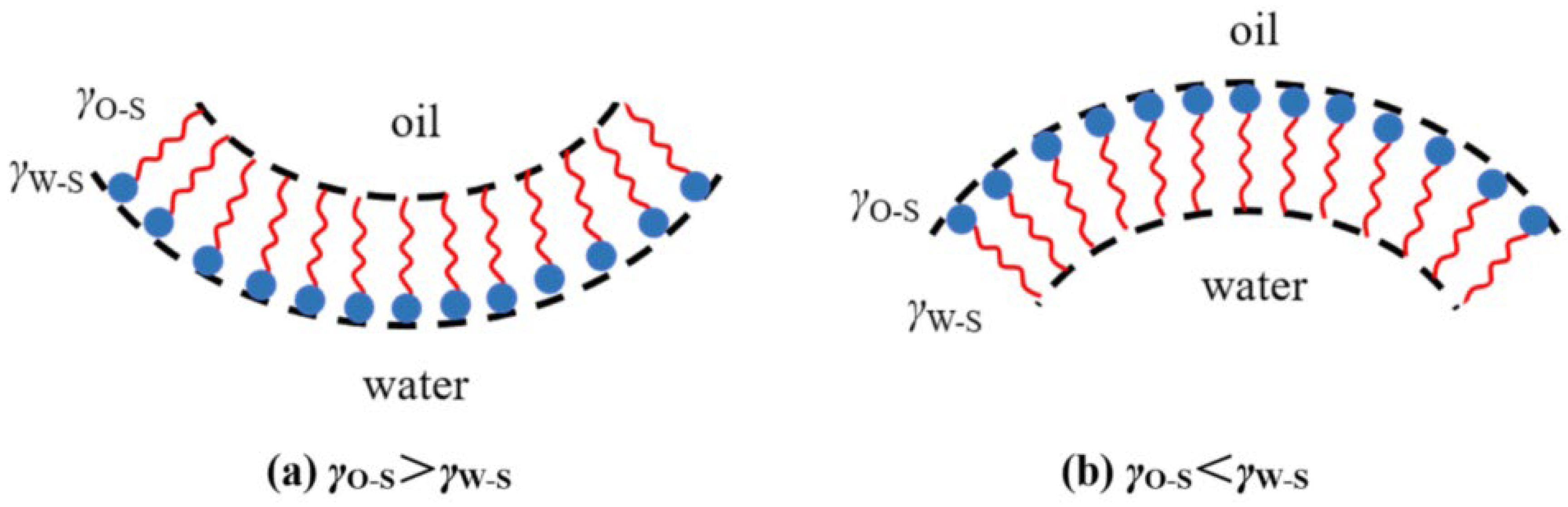
2.3. Formation Mechanisms of Microemulsion
- (1)
- Instantaneous negative interfacial tension theory
- (2)
- Dual-membrane theory
- (3)
- R-ratio theory
- (4)
- Micelle solubilization theory
- (5)
- Geometric arrangement theory
2.4. Research on the Components of Microemulsion
2.4.1. Effect of Surfactant Selection on the Properties of Microemulsion
2.4.2. Effect of the Choice of Cosurfactant on the Properties of Microemulsion
2.4.3. Effect of Oil Phase Selection on the Properties of Microemulsions
2.4.4. Effect of Inorganic Salt Selection on the Properties of Microemulsion
2.4.5. Studies on the Costs of Microemulsion Systems
2.5. Preparation Method of Microemulsion
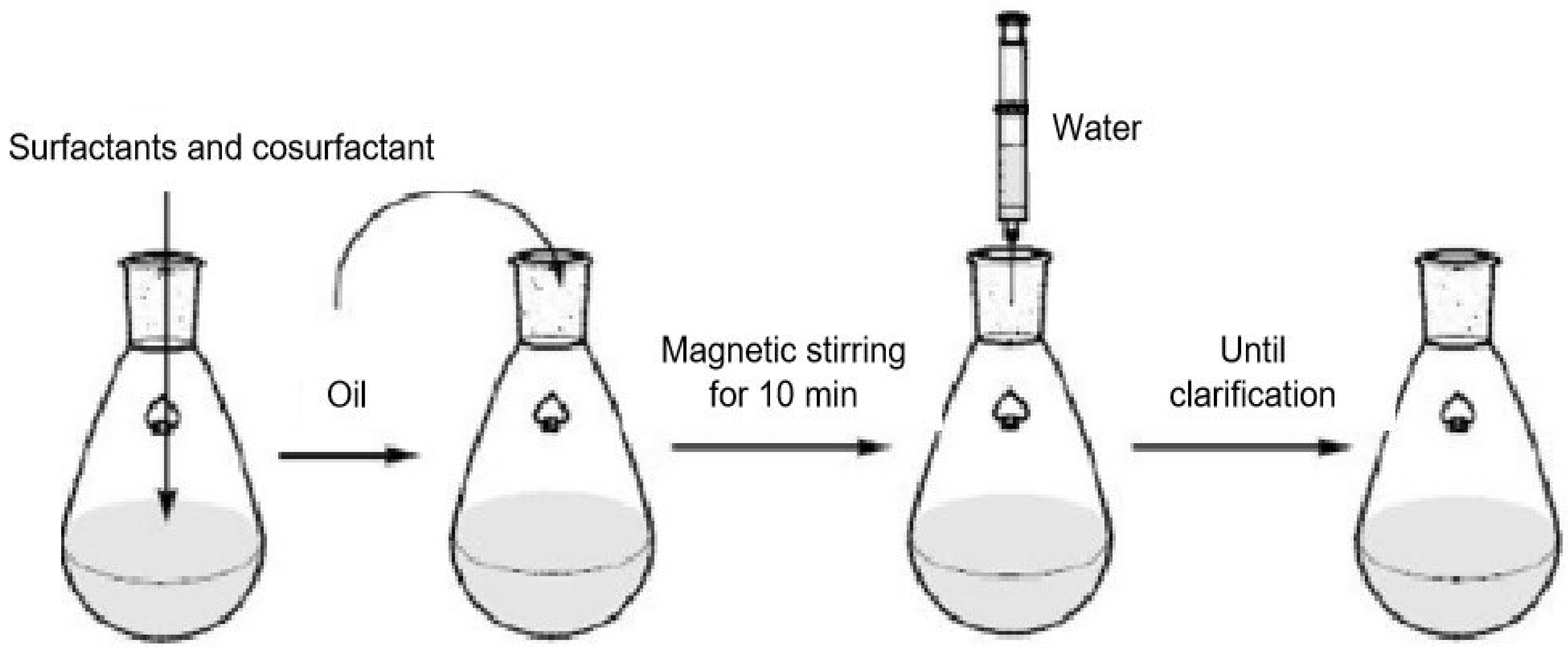
2.5.1. Schulman Method
2.5.2. Shah Method
2.6. Characterization Method of Microemulsion
2.6.1. Phase Behavior
2.6.2. Particle Size Analysis
2.6.3. Interfacial Tension
2.6.4. Viscosity Test
2.6.5. Zeta Potential Test
3. The Basic Method to Enhance Oil Recovery by Microemulsion
3.1. Imbibition Displacement
3.1.1. Influence of Rock Wettability
3.1.2. Influence of Oil–Water Interfacial Tension
3.1.3. Influence of the Core Pore Size
3.2. Conventional Displacement Replacement
3.2.1. Influence of Microscopic Pore Structure
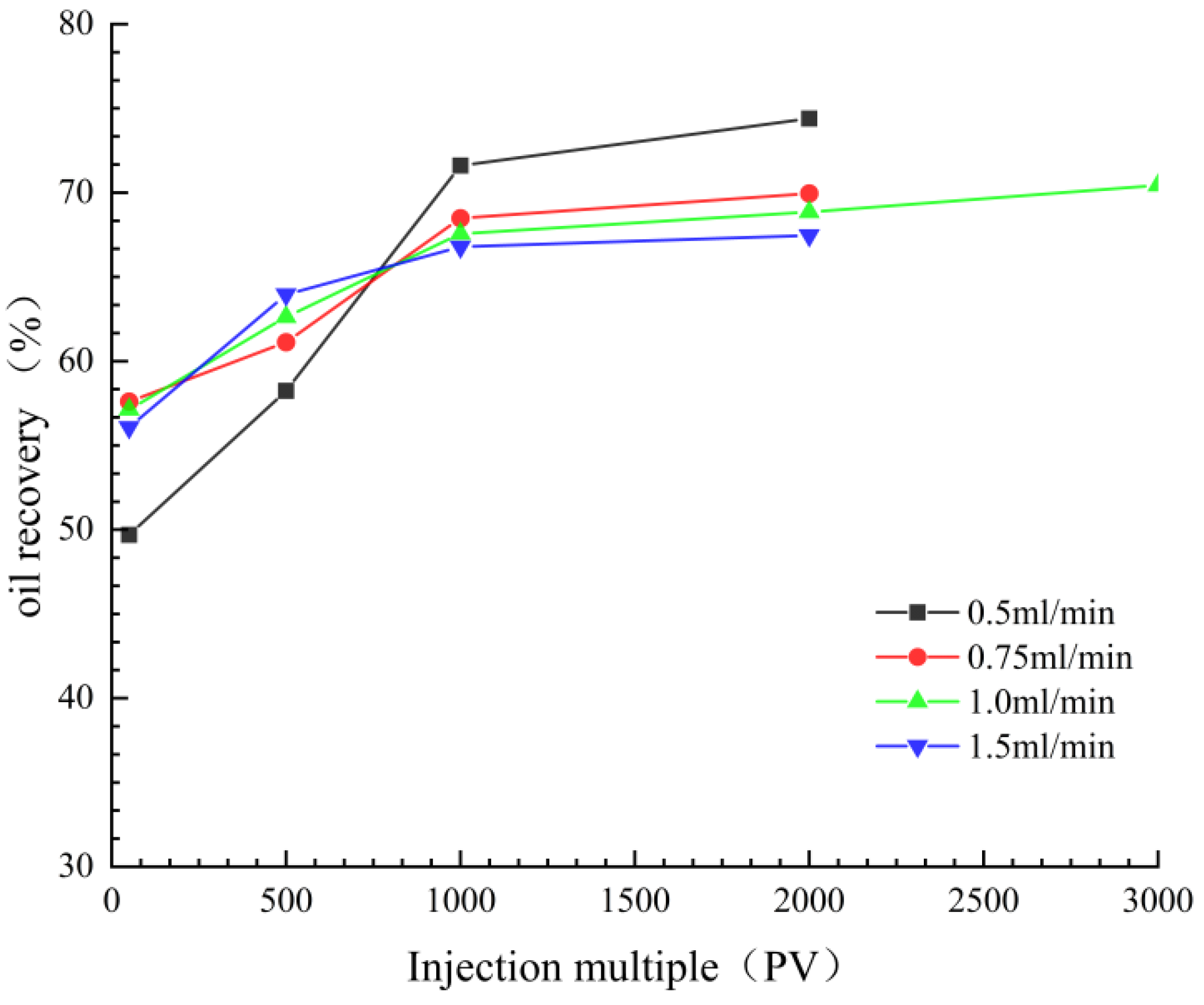
3.2.2. Influence of Displacement Speed
3.2.3. Net Burden Pressure (Confining Pressure)
4. Application of Microemulsion in Enhanced Oil Recovery
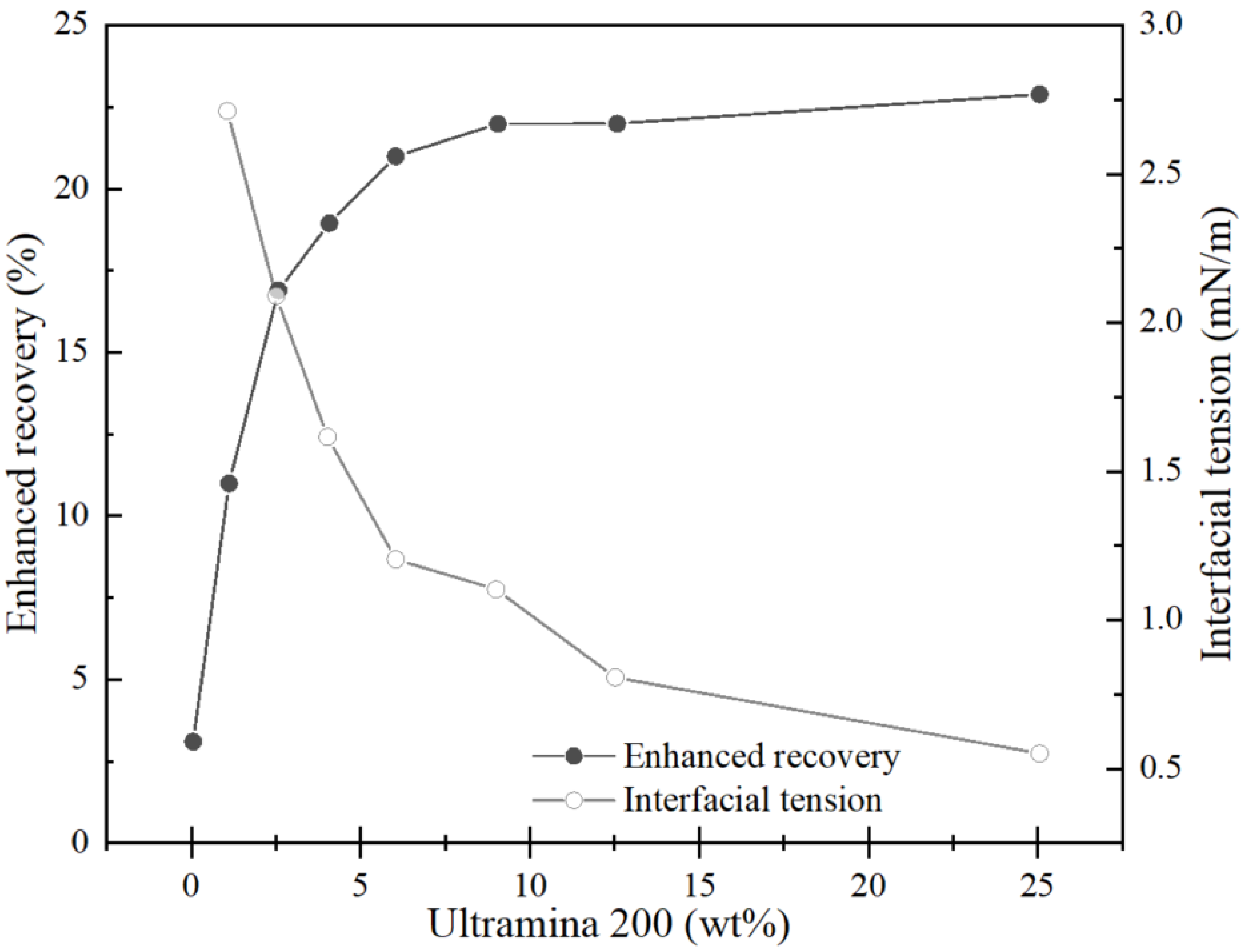
4.1. Microemulsion Indoor Enhanced Oil Recovery Effect
4.2. Effect of Microemulsion on Oilfield Field Production Increase
5. Micro-Remaining Oil Characterization Method of Microemulsion Flooding
5.1. Micro-Etching Glass Model
5.2. Magnetic Resonance Imaging Technology (MRI)
5.3. CT Scan Technique
6. Summary, Challenges, and Development Trends
6.1. Summary
6.2. Challenge
- (1)
- As tertiary oil recovery operations progress, the reservoir environment becomes increasingly harsh, particularly in high-temperature and high-salinity oilfields where the quality of crude oil deteriorates. The existing microemulsion formulations are no longer suitable for these conditions. It is necessary to identify and develop new formulations that can adapt to the changing characteristics of the reservoir environment to maintain and enhance oil recovery efficiency.
- (2)
- The formulation of microemulsions commonly employs short-chain alcohols as cosurfactants, which typically have low flash points (generally below 60 °C). This characteristic can be inadequate for the current reservoir conditions, where the formation temperatures are often higher than 60 °C, potentially leading to safety incidents.
- (3)
- The application of microemulsions in the field exhibits a lack of universality; the same formulation, when utilized in different oil reservoirs, may yield varying degrees of enhanced oil recovery (EOR) effectiveness. Concurrently, there is significant debate regarding whether microemulsions should be prepared ex situ and then injected into the subsurface (non-in situ injection), or whether the individual components, such as surfactants, should be injected into the reservoir where they interact with the crude oil to form microemulsions in situ (in situ injection).
- (4)
- The efficacy of microemulsions in oil reservoirs with varying permeabilities is not yet well-defined, and there is a need for further in-depth investigation into the mechanisms of crude oil mobilization and the migration patterns within such formations.
6.3. Development Trends
- (1)
- Transitioning from microemulsion systems based on single surfactants to those employing blended surfactants in research can lead to a reduction in the chemical cost per ton of oil. This approach also aims to enhance the fundamental properties of microemulsions, such as their thermal and salinity resistance, as well as their ability to withstand shear forces.
- (2)
- The consolidation of numerous existing performance evaluation methods is essential to establish new standards or criteria for the assessment of microemulsion performance.
- (3)
- By integrating with the field of molecular simulation, the study of the interaction mechanisms between microemulsions and various rock formations can be conducted.
- (4)
- At present, research often focuses on either the macroscopic phase behavior or the microscopic structure of microemulsions in isolation. Moving forward, it is of paramount importance to investigate the impact mechanisms of different factors on the phase behavior of microemulsions by combining the study of their macroscopic phase states with their microscopic structures. This integrated approach will provide a more comprehensive understanding of the complex interactions and behaviors of microemulsions in various conditions.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.F. Analysis of the development status of chemical flooding technology for tertiary oil recovery. Chem. Eng. Equip. 2021, 6, 99–100. [Google Scholar]
- Zhu, W.Y.; Yue, M.; Liu, Y.F.; Liu, K.; Song, Z.Y. Research progress on tight oil explortion in China. Adv. Eng. Sci. 2019, 41, 1103–1114. [Google Scholar]
- Li, G.X.; Lei, Z.D.; Dong, W.H.; Wong, H.Y.; Zheng, X.F. Progress, challenges and prospects of unconventional oil and gas development of CNPC. China Pet. Explor. 2022, 27, 1–11. [Google Scholar]
- Shen, A.Q.; Liu, Y.K.; Wang, X.; Cai, B.; He, C.M.; Liang, S. The geological characteristics and exploration of continental tight oil: An investigation in China. J. Pet. Explor. Prod. Technol. 2019, 9, 1651–1658. [Google Scholar] [CrossRef]
- Zhou, Q.F. Changes in tight oil and shale gas production in the United States over the past decade. Oil Gas Geol. 2020, 41, 446. [Google Scholar]
- Zhou, Q.F. Development trend of world tight oil production. Oil Gas Geol. 2021, 42, 2. [Google Scholar]
- Yuan, S.Y.; Wang, Q. New progress and prospect of oilfield development technologies in China. Pet. Explor. Dev. 2018, 45, 657–668. [Google Scholar] [CrossRef]
- Zhu, T.Y.; Kang, W.L.; Yang, H.B.; Li, Z.; Zhou, B.B.; He, Y.Q.; Wang, J.Q.; Aidarova, S.; Sarsenbekuly, B. Advances of microemulsion and its applications for improved oil recovery. Adv. Colloid Interface Sci. 2021, 299, 102527. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.J. What type of surfactants should be used to enhance spontaneous imbibition in shale and tight reservoirs? J. Pet. Sci. Eng. 2017, 159, 635–643. [Google Scholar] [CrossRef]
- Schechter, D.S.; Zhou, D.Q.; Orr, F.M., Jr. Capillary Imbibition and Gravity Segregation in Low IFT Systems. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 6–9 October 1991; SPE: Matteson, IL, USA, 1991. [Google Scholar]
- Liu, J.L.; Luo, M.L.; Jia, Z.L.; Sun, H.T.; Liao, L.J.; Ren, B. Research progress of the microemulsion additives applied to reservior stimulation. Appl. Chem. Ind. 2011, 40, 1440–1443. [Google Scholar]
- Bera, A.; Mandal, A. Microemulsions: A novel approach to enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2015, 5, 255–268. [Google Scholar] [CrossRef]
- Hematpur, H.; Abdollahi, R.; Safari-Beidokhti, M.; Esfandyari, H. Experimental Microemulsion Flooding Study to Increase Low Viscosity Oil Recovery Using Glass Micromodel. Math. Probl. Eng. 2021, 2021, 5021868. [Google Scholar] [CrossRef]
- Qin, C.K.; Chai, J.L.; Chen, J.F. Progress of Research and Application of microemulsion. Shanxi Chem. Ind. 2006, 26, 21–25. [Google Scholar]
- Cayias, J.; Schechter, R.; Wade, W. The utilization of petroleum sulfonates for producing low interfacial tensions between hydrocarbons and water. J. Colloid Interface Sci. 1977, 59, 31–38. [Google Scholar] [CrossRef]
- Glinsmann, G.R. Aqueous Surfactant Systems for in Situ Multiphase Microemulsion Formation. U.S. Patent No. 4,125,156, 14 November 1978. [Google Scholar]
- Sheng, J.J. Investigation of Alkaline-Crude Oil Reaction. Petroleum 2015, 1, 31–39. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, B.S.; Liu, Y.T.; Liang, L.; Liu, P. Research and application progress of the microemulsion additives applied to Oil & Gas stimulation. Appl. Chem. Ind. 2020, 49, 3230–3236. [Google Scholar]
- Bai, W.; Shen, Q. Theory, preparation and application of structured emulsion I. Continuous phase structured emulsion. Polym. Bull. 2009, 11, 49–56. [Google Scholar]
- Hatzopoulos, M.H.; James, C.; Rogers, S.; Grillo, I.; Dowding, P.J.; Eastoe, J. Effects of small ionic amphiphilic additives on reverse microemulsion morphology. J. Colloid. Interface Sci. 2014, 421, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, H.; Liu, X.B.; Li, K.H.; Luo, J.S.; Sun, D.J. Progress of application of Nano emulsion and micro emulsion in oil and gas production. Drill. Fluid. Complet. Fluid 2014, 31, 79–84+101–102. [Google Scholar]
- Prince, L. Microemulsions Theory and Practice; Academic Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Paunov, V.N.; Sandler, S.I.; Kaler, E.W. A Simple Molecular Model for the Spontaneous Curvature and the Bending Constants of Nonionic Surfactant Monolayers at the Oil/Water Interface. Langmuir 2000, 16, 8917–8925. [Google Scholar] [CrossRef]
- Huh, C. Equilibrium of a Microemulsion That Coexists with Oil or Brine. Soc. Pet. Eng. J. 1983, 23, 829–847. [Google Scholar] [CrossRef]
- Miller Clarence, A.; Neogi, P. Surfactant science series. In Interfacial Phenomena: Equilibrium and Dynamic Effects; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Guan, D.; Que, T.L.; Cao, Q.; Tang, W.J.; Luan, H.X. Analysis and application of solubilization and emulsification of sodium naphthenic petroleum sulfonate micelles. Oilfield Chem. 2021, 38, 95–100. [Google Scholar]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Lawson, J.B. The Adsorption of Non-Ionic and Anionic Surfactants on Sandstone and Carbonate. In Proceedings of the SPE Symposium on Improved Methods of Oil Recovery, Tulsa, OK, USA, 16–17 April 1978. [Google Scholar]
- Qing, Y.; Wang, Y.; Lin, F.R.; Peng, Y.G.; Li, S.; Ma, J.Q. Discussion on Measurement of Krafft Point of surfactants. Chin. J. Chem. Educ. 2016, 37, 74–76. [Google Scholar]
- Xue, X.M.; Chen, Y.P.; Yan, G.L. Compounding and application of cationic-anionic-nonionic surfactant ternary system. Text. Aux. 2022, 39, 37–41. [Google Scholar]
- Qu, M.; Hou, R.J.; Wen, Y.C.; Wen, Y.C.; Liang, T. Anionic-nonionic/anionic foaming agents synergistically enhance the salt tolerance of foams. Oilfield Chem. 2019, 36, 501–507. [Google Scholar]
- Standnes, D.C.; Austad, T. Wettability alteration in chalk. J. Pet. Sci. Eng. 2000, 28, 123–143. [Google Scholar] [CrossRef]
- Shen, X. Study on the Retardance Mechanism of Cationic Surfactants. Ph.D. Thesis, Southwest Petroleum University, Sichuan, China, 2019. [Google Scholar]
- Cao, Y.P.; Yang, W.G.; Jiang, Y.J.; Wang, Y.G.; Ju, H.B.; Geng, T. Synthesis and properties of zwitterionic gemini surfactants. Fine Chem. 2021, 38, 335–340. [Google Scholar]
- Mao, X.Q.; Hu, F.T.; Zhu, X.Y.; Dang, Y.B.; Du, C.B.; Wu, Y. Study on synergistic effect of amphoteric and anionic surfactant in compound flooding of low-permeability and high-salt oil reservoirs. J. Xi’an Shiyou Univ. Nat. Sci. Ed. 2022, 37, 100–106. [Google Scholar]
- Li, H.E.; Zhao, H.J.; Yu, P.F.; Zou, W.D.; Pang, H.M. Research on the interface performance of anionic/zwitterionic surfactant compound system. Petrochem. Technol. 2021, 50, 236–241. [Google Scholar]
- Su, L.; Zheng, L.Q. Aggregation behavior of zwitterionic/anionic surfactants mixed systems at the decane/water interface: Experimental and theoretical study. In Proceedings of the 17th National Colloid and Interface Chemistry Symposium of Chinese Chemical Society (Abstract), Wu’xi, China, 29–31 July 2019; Volume 1, pp. 212–213. [Google Scholar]
- Dong, Y.C.; Li, L.; Fang, Y. Progresses in research of sulfobetaine used in tertiary oil recovery. Petrochem. Technol. 2013, 42, 577–581. [Google Scholar]
- Butt, H.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; Wiley-VCH GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Wu, S.H. Report on the present situation of nonionic surfactant production at home. Fine Chem. 2000, 9, 497–500. [Google Scholar]
- Shi, Q.B. Experimental study on the adsorption resistance of non-ionic surfactants in binary systems to oil sands. J. Yangtze Univ. Nat. Sci. Ed. 2010, 7, 510–512. [Google Scholar]
- Myers, D. Surfactant Science and Technology, 3rd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Yan, Q.F. The Characteristic and Application of Nonionic Surfactants. Guizhou Chem. Ind. 2005, 5, 4–7+22. [Google Scholar]
- Liu, Z.H.; Chen, W.Y. Study on foam property of non-ionic surfactant compound system. Mod. Chem. Ind. 2022, 42, 329–331+337. [Google Scholar]
- Xuan, Y.N.; Ni, X.F.; Yu, J.T. The effect of common binary surfactant compound system on foaming ability. J. East. China Univ. Sci. Technol. 2022, 48, 449–455. [Google Scholar]
- Dai, C.Y.; Zheng, Y.C.; Li, C.Y.; Cheng, Z.; Lan, L.F.; Tang, S.F. Study on the interaction and interfacial activity of alcohol ether sulfonate and nonionic surfactant. Spec. Petrochem. 2020, 37, 48–53. [Google Scholar]
- Zhang, H.X. Effect of surfactant chain branch on the formation of W/O microemulsion system by mesoscopic simulation. Chem. Res. Appl. 2016, 28, 71–75. [Google Scholar]
- Yuwanti, S.; Raharjo, S.; Hastuti, P.; Supriyadi, S. Stable O/W Microemulsion Formulation Using Combination of Three Nonionic Surfactants with Low, High and Medium HLB Values. Agritech 2011, 31, 21–29. [Google Scholar]
- Zhou, Y.Z.; Yin, D.Y.; Wang, D.Q.; Zhang, C.L.; Yang, Z.H. Experiment investigation of microemulsion enhanced oil recovery in low permeability reservoir. J. Mater. Res. Technol. 2020, 9, 8306–8313. [Google Scholar] [CrossRef]
- Li, J.C.; Qiu, X.Q.; Feng, Y.H.; Lin, Q. Effects of Pure and Mixed Surfactants on Bifenthrin Microemulsion. Chin. J. Appl. Chem. 2008, 8, 890–894. [Google Scholar]
- Vo, T.V.; Chou, Y.Y.; Chen, B.H. Preparation of Microemulsion from an Alkyl Polyglycoside Surfactant and Tea Tree Oil. Molecules 2021, 26, 1971. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Fletcher, P.D.I.; Macnab, J.R. The effect of temperature on the adsorption of dodecane onto nonionic and ionic surfactant monolayers at the air-water surface. Berichte Der Bunsen-Ges. 1996, 100, 224–231. [Google Scholar] [CrossRef]
- Wu, J.; Mei, P.; Wu, J.; Fu, J.W.; Cheng, L.; Lai, L. Surface properties and microemulsion of anionic/nonionic mixtures based on sulfonate Gemini surfactant in the presence of NaCl. J. Mol. Liq. 2020, 317, 113907. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, Y.; Liao, G.; Liu, W.D. Visualizing in-situ emulsification in porous media during surfactant flooding: A microfluidic study. J. Colloid. Interface Sci. 2020, 578, 629–640. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, H.E.; Xu, M.M.; Ding, C.Q.; Cheng, S.; Qi, X.L. Effect of Inorganic Salts on the Phase Behavior of Sodium Dodecyl Bezene Sulfonate Microemulsion System. Petrochem. Technol. 2014, 43, 1277–1283. [Google Scholar]
- Ali, R.; Hassan, G.; Fatemeh, E. An in-depth investigation of the impact of salt nature on the formulation of microemulsion systems. Sci. Rep. 2023, 13, 14362. [Google Scholar]
- Zamula, Y.S.; Afanasyev MOBatirshin, E.S. Characterization of microemulsion structure using atomic force microscopy. Lett. Mater. 2023, 13, 286–291. [Google Scholar]
- Pal, N.; Alzahid, Y.; Alsofi, A.M.; Ali, M.; Yekeen, N.; Hoteit, H. An experimental workflow to assess the applicability of microemulsions for conformance improvement in oil-bearing reservoir. Heliyon 2023, 9, 17667. [Google Scholar] [CrossRef]
- Pal, N.; Kumar, S.; Bera, A.; Mandal, A. Phase behaviour and characterization of microemulsion stabilized by a novel synthesized surfactant: Implications for enhanced oil recovery. Fuel 2019, 235, 995–1009. [Google Scholar] [CrossRef]
- Bera, A.; Ojha, K.; Mandal, A.; Kumar, T. Interfacial tension and phase behavior of surfactant-brine–oil system. Colloids Surf. A Physicochem. Eng. Asp. 2011, 383, 114–119. [Google Scholar] [CrossRef]
- Zhang, H.X.; Xia, J.H.; Ding, C.G.; Xu, B.; Wang, S.L.; Wu, Z.X.; Zhao, W.M. The formation and temperature stability of microemulsion emulsified by polyoxyethylene ether surfactant. Prog. Nat. Sci. Mater. Int. 2023, 33, 295–300. [Google Scholar] [CrossRef]
- Schmidt, R.F.; Prevost, S.; Gradzielski, M.; Zemb, T. Structure of microemulsions in the continuous phase channel. Eur. Phys. J. E 2023, 46, 76. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Zhou, F.J.; Su, H.; Yao, E.D.; Hu, J.G.; Chen, Z.X. Adsorption characteristics, isotherm, kinetics, and diffusion of nanoemulsion in tight sandstone reservoir. Chem. Eng. J. 2023, 470, 144070. [Google Scholar] [CrossRef]
- Maria, L.D.O.; Kelly, C.B.M.; Walner, C.S.; Arthur, C.L.; Agatha, D.D.S.F.; Thiago, L.V.; Regina, S.V.N.; Daniel, G. Fe3O4 Nanoparticles as Surfactant Carriers for Enhanced Oil Recovery and Scale Prevention. Nano Mater. 2020, 3, 5762–5772. [Google Scholar]
- Fattahi, R.; Lashkarbolooki, M.; Abedini, R.; Younesi, H. Investigating the Synergistic/Antagonistic Effects of Mixing SiO2 Nanoparticles and Ionic Liquid, Nonionic Emulsifier, and Gemini Surfactants on the Main Mechanisms of Crude Oil Production. Energy Fuels 2023, 37, 14741–14751. [Google Scholar] [CrossRef]
- Phaodee, P.; Sabatini, D. Anionic and Cationic Surfactant Synergism: Minimizing Precipitation, Microemulsion Formation, and Enhanced Solubilization and Surface Modification. J. Surfactants Deterg. 2021, 24, 551–562. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Liu, H.E.; Ning, T.F.; Yu, Y.F.; Liu, Y.R. Effect of cosurfactant and oil/water volume ratio on the phase behavior of microemulsion. Fine Chem. 2020, 37, 1645–1652. [Google Scholar]
- Li, Y.J. Study on Phase Behavior of Biodiesel Microemulsion and Remediation of Petroleum Contaminated Soil by Washing. Ph.D. Thesis, Zhejiang Ocean University, Zhoushan, China, 2022. [Google Scholar]
- Chen, S.; Liu, S.W.; Gu, X.L.; Wang, M.; Liu, H.E. Influence of inorganic salt content and temperature on phase behavior of microemulsion system. Chem. Res. Appl. 2022, 34, 1284–1293. [Google Scholar]
- Phaodee, P.; Sabatini, D. Synergy of anionic and cationic surfactants: Minimize the formation of precipitation and micro lotion, and enhance solubilization and surface modification. J. Am. Oil Chem. Soc. 2022, 99, 189. [Google Scholar]
- Wu, T.J.; Lv, W.; Li, C.N.; Zhao, Y.H. Screening of middle-phase microemulsion flooding system in low permeability reservoir. Appl. Chem. Ind. 2023, 52, 2551–2555. [Google Scholar]
- Badu, K.; Maurya, N.K.; Mandal, A.; Saxena, V.K. Synthesis and characterization of sodium methyl ester sulfonate for chemically-enhanced oil recovery. Braz. J. Chem. Eng. 2015, 32, 795–803. [Google Scholar]
- Tarkars, H.; Rokade, A.; Jadkar, S. Pioneering method for the synthesis of lead sulfide (PbS) nanoparticles using a surfactant-free microemulsion scheme. RSC Adv. 2024, 14, 4352–4361. [Google Scholar] [CrossRef] [PubMed]
- Karasunghe, A.; Pathma, J.L.; Cai, J.J.; Lu, J.; Sung, H.J.; Upali, P.W.; Gary, A.P. New Surfactants and Cosolvents Increase Oil Recovery and Reduce Cost. In Proceedings of the SPE Improved Oil Recovery Conference, SPE-179702-MS, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Jin, C.; Geng, Z.L.; Liu, X.; Ampah, J.D.; Ji, J.; Wang, G.; Niu, K.; Hu, N.; Liu, H.F. Effects of water content on the solubility between Isopropanol-Butanol-Ethanol (IBE) and diesel fuel under various ambient temperatures. Fuel 2021, 286, 119492. [Google Scholar] [CrossRef]
- Lucas, C.R.S.; Aum, Y.K.P.G.; Dantas, T.N.D.C.; Silva, D.C.D.; Oliveira, K.C.D.; Aum, P.T.P. Experimental study of microemulsion systems applied on formation damage remediation. Energy Sources 2020, 42, 807–814. [Google Scholar] [CrossRef]
- Huang, K. Low Permeability Reservoirs Microemulsion Oil Displacement System Optimized Formulation and Performance Evaluation. Ph.D. Thesis, Northeast Petroleum University, Heilongjiang, China, 2017. [Google Scholar]
- Zhou, B.L. The Preparation of Microemulsion and Evaluation on Oil Displacement Effect. Ph.D. Thesis, Northeast Petroleum University, Heilongjiang, China, 2016. [Google Scholar]
- Lv, T. Study on Mechanism of Enhanced Oil Recovery by Microemulsion Flooding in low Permeability Reservoirs. Ph.D. Thesis, Northeast Petroleum University, Heilongjiang, China, 2017. [Google Scholar]
- Karambeigi, M.S.; Abbassi, R.; Roayaei, E.; Emadi, M.A. Emulsion flooding for enhanced oil recovery: Interactive optimization of phase behavior, microvisual and core-flood experiments. J. Ind. Eng. Chem. 2015, 29, 382–391. [Google Scholar] [CrossRef]
- Pal, S.; Mushtaq, M.; Banat, F.; Sumaiti, A.M. Review of surfactant-assisted chemical enhanced oil recovery for carbonate reservoirs: Challenges and future perspectives. Pet. Sci. 2018, 15, 77–102. [Google Scholar] [CrossRef]
- Peyrelasse, J.; Boned, C.; Heil, J.; Clausse, M. Electrical properties of quaternary Winsor IV phases (the so-called microemulsions), as correlated to phase diagram features. Influence of the chemical structure of the alcohol cosurfactant. J. Phys. C Solid. State Phys. 2000, 15, 7099. [Google Scholar] [CrossRef]
- Heil, J.; Clausse, M.; Peyrelasse, J.; Boned, C. Etude de phases quaternaires Winsor IV. Diagrammes de phases et propriétés electriques. Colloid. Polym. Sci. 1982, 260, 93–95. [Google Scholar] [CrossRef]
- Fan, W.N.; Li, G.H.; Wang, T.T.; Song, J.; Zhao, D.D. Study on the phase behavior of (AEO-9/SAS60)/petroleum ether/alcohol/water microemulsion. Fine Chem. 2016, 33, 7. [Google Scholar]
- Marija, L.; Haegel, F.H.; Pavelkic, V.M.; Zlatanovic, D. The influence of alkyl polyglucosides (and highly ethoxylated alcohol boosters) on the phase behavior of a water/toluene/technical alkyl polyethoxylate microemulsion system. Chem. Ind. Chem. Eng. Q. 2016, 22, 27–32. [Google Scholar]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Nicoli, D.F.; Buzzaccarini, F.D.; Romsted, L.S.; Bunton, C.A. Measurement of the correlation radius in oil-in-water microemulsions by dynamic light scattering. Chem. Phys. Lett. 1981, 80, 422–426. [Google Scholar] [CrossRef]
- Geyer, A.D.; Molle, B.; Lartigue, C.; Guillermo, A.; Farago, B. Dynamics of caged microemulsion droplets: A neutron spin echo and dynamic light scattering study. Phys. B Phys. Condens. Matter 2004, 350, 200–203. [Google Scholar] [CrossRef]
- Mohagheghpour, E.; Moztarzadeh, F.; Rabiee, M.; Tahriri, M.; Ashuri, M.; Sameie, H.; Salimi, R.; Moghadas, S. Micro-Emulsion Synthesis, Surface Modification, and Photophysical Properties of Zn1−x MnxS Nanocrystals for Biomolecular Recognition. IEEE Trans. Nanobiosci. 2012, 11, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.G.; Wang, R.; Feng, Q.; Zheng, Y. Synthesis of betaine surfactant and its application in microemulsion flushing fluid. J. Dispers. Sci. Technol. 2024, 1–15. [Google Scholar] [CrossRef]
- Sun, M.; Yu, L.; Hao, Z.F.; Sun, J. Preparation and characterization of SnO2 nano-particles. Chin. J. Inorg. Chem. 2005, 21, 925–928. [Google Scholar]
- Yang, J.; Jiang, W.; Guan, B.S.; Qiu, X.H.; Lu, H.J. Preparation of D-limonene Oil-in-Water Nanoemulsion from an Optimum Formulation. J. Oleo Sci. 2014, 63, 1133–1140. [Google Scholar] [CrossRef][Green Version]
- Dantas, C.T.N.; Souza, T.T.C.; Rodrigue, M.A.F.; Neto, A.A.D.; Aum, P.T.P. Experimental study of combined microemulsion/brine flooding to EOR in carbonate reservoirs. J. Dispers. Sci. Technol. 2020, 43, 827–837. [Google Scholar] [CrossRef]
- Li, Y.H. Optimization of Mixed Microemulsion Formulation for a Low Permeability Reservoir. In Proceedings of the 2019 International Conference on Exploration and Development of Oil and Gas Fields, Xi’an, China, 16–18 October 2019; pp. 133–141. [Google Scholar]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar]
- Zhou, F.J.; Su, H.; Liang, X.Y.; Meng, L.F.; Yuan, L.S.; Li, X.H.; Liang, T.B. Integrated hydraulic fracturing techniques to enhanced oil recovery from tight rocks. Pet. Explor. Dev. 2019, 46, 1007–1014. [Google Scholar] [CrossRef]
- Liu, W.D.; Yao, T.Y.; Xiao, H.M.; Zheng, D.W.; Yang, Y. Study on adsorption characteristics of wettability reversal agent. In Proceedings of the Chinese Society of Mechanics Academic Conference’ 2005 Abstracts (II), Beijing, China, 7–12 August 2005; p. 767. [Google Scholar]
- Morrow, N.R.; Mason, G. Recovery of oil by spontaneous imbibition. Curr. Opin. Colloid. Interface Sci. 2001, 6, 321–337. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Hou, J.R.; Qu, M.; Xiao, L.X.; Liu, Y.B. Performance evaluation and oil displacement mechanism analysis of nano-microemulsion using in tight oil reservoir. Oilfield Chem. 2022, 39, 338–342+354. [Google Scholar]
- Lowry, E.; Sedghi, M.; Goual, L. Molecular simulations of NAPL removal from mineral surfaces using microemulsions and surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 485–494. [Google Scholar] [CrossRef]
- Xiao, L.X.; Hou, J.R.; Wen, Y.C.; Qu, M.; Wang, W.J.; Wu, W.P.; Liang, T. Imbibition mechanisms of high temperature resistant microemulsion system in ultra-low permeability and tight reservoirs. Pet. Explor. Dev. 2022, 49, 1206–1216. [Google Scholar] [CrossRef]
- Al-Attar, H.H. Experimental study of spontaneous capillary imbibition in selected carbonate core samples. J. Pet. Sci. Eng. 2010, 70, 320–326. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, H.; Zhang, Y.; Lyu, Y.F.; Fang, J.C.; Dai, C.L. Experimental study on spontaneous imbibition of recycled fracturing flow-back fluid to enhance oil recovery in low permeability sandstone reservoirs. J. Pet. Sci. Eng. 2018, 166, 375–380. [Google Scholar]
- Yang, X.; Liao, R.Q.; Yuan, X. Spontaneous imbibition experiment in high-temperature and high-pressure of tight core based on NMR technology. Pet. Geol. Oilfield Dev. Daqing 2022, 42, 1–8. [Google Scholar]
- Cheng, Z.; Wang, Q.; Ning, Z.F.; Li, M.Q.; Lin, C.H. Experimental investigation of countercurrent spontaneous imbibition in tight sandstone using nuclear magnetic resonance. Energy Fuels 2018, 32, 6507–6517. [Google Scholar] [CrossRef]
- Zhao, H.P.; Jiang, G.P. Optimization of microemulsion formulation of fractured reservoirs and oil displacement effect. J. Petrochem. Univ. 2020, 33, 42–47. [Google Scholar]
- Zhao, M.; Wu, d.; Wang, J. Microemulsion formation of petroleum sulfonate flooding system and solubilization properties. Sci. Technol. Eng. 2015, 15, 144–150. [Google Scholar]
- Ma, K.Q.; Cai, H.; Sun, Z.B. Nuclear magnetic resonance-based experiment on effect of displacement velocity and multiple on pore throat characteristics and recovery factor of unconsolidated sandstone reservoirs. China Offshore Oil Gas. 2019, 31, 86–91. [Google Scholar]
- Liu, J.Q.; Zhou, K.; Ding, L.; Wang, B.Y.; Li, Y.Y. Displacemen experiments of gas and water with sandstone micro-models of HE-8 reservoir in the east of oreos basin and the major factors affecting the gas displacement efficiency. Xinjiang Oil Gas 2012, 8, 46–49+3. [Google Scholar]
- Ding, B.; Xiong, C.M.; Geng, X.F.; Guan, B.S.; Pan, J.J.; Xu, J.G.; Dong, J.F.; Zheng, C.M. Characteristics and EOR mechanisms of nanofluids permeation flooding for tight oil. Pet. Explor. Dev. 2020, 47, 810–819. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Wettability alteration of sandstone rock by surfactant stabilized nanoemulsion for enhanced oil recovery—A mechanistic study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 125043. [Google Scholar] [CrossRef]
- Dantas, C.T.N.; Oliveira, A.C.; Souza, T.T.C.; Santos, L.C.R.; Andrade, A.E.; Aum, T.P. Experimental study of the effects of acid microemulsion flooding to enhancement of oil recovery in carbonate reservoirs. J. Pet. Explor. Prod. Technol. 2020, 10, 1127–1135. [Google Scholar] [CrossRef]
- Pandava, A.P.T.; Yanne, G.A.K.; Edson, P.A.A.; Luyara, D.A.C.; Silva, D.N.D.; Lucas, C.R.S.; Dantas, T.N.C. Evaluation of oil-in-water microemulsion base ethoxylated surfactant under acid conditions. Fuel 2021, 290, 120045. [Google Scholar]
- Dantas, C.T.N.; Freitas, V.F.; Souza, T.C.T.; Neto, A.V.D.; Aum, P.T.P. Study of single-phase polymer-alkaline-microemulsion flooding for enhancing oil recovery in sandstone reservoirs. Fuel 2021, 302, 121176. [Google Scholar] [CrossRef]
- Karambeigi, M.S.; Nasiri, M.; Asl, A.H.; Emadi, M. Enhanced oil recovery in high temperature carbonates using microemulsions formulated with a new hydrophobic component. J. Ind. Eng. Chem. 2016, 39, 136–148. [Google Scholar] [CrossRef]
- Nourafkan, E.; Gardy, J.; Asachi, M.; Ahmed, W.; Wen, D.S. Nanoparticle Formation in Stable Microemulsions for Enhanced Oil Recovery Application. Ind. Eng. Chem. Res. 2019, 58, 12664–12677. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Xia, L.J. Formulation optimization and performance evaluation of internal olefin sulfonate microemulsion system used in low permeability reservoir. Oilfield Chem. 2020, 37, 102–108. [Google Scholar]
- Yin, D.Y.; Lv, T. Formulation optimization of surfactant mixture system of microemulsion flooding in low permeability reservoir. Chem. Eng. 2017, 31, 39–42+62. [Google Scholar]
- Song, H.Z.; Li, T.L.; Sun, Y.B.; Li, T.; Sun, Y.T.; Lin, X. Development and application exploration of heavy oil microemulsion based on dodecylbenzene sulfonate. China Pet. Chem. Stand. Qual. 2021, 41, 103–105. [Google Scholar]
- Lv, Q.C.; Zhang, H.S.; Zuo, B.W.; Zhang, X.; Zhang, Z.; Li, Q.; Zhou, T.K. Micro-visualization study of microemulsion flooding law in ultra-high water cut stage. J. Xi’an Shiyou Univ. Nat. Sci. Ed. 2020, 35, 71–77+119. [Google Scholar]
- Qin, T.Z.; Goual, L.; Piri, M.; Hu, Z.L.; Wen, D.S. Nanoparticle-stabilized microemulsions for enhanced oil recovery from heterogeneous rocks. Fuel 2020, 274, 117830. [Google Scholar] [CrossRef]
- Hu, Z.L.; Nourafkan, E.; Gao, H.; Wen, D.S. Microemulsions stabilized by in-situ synthesized nanoparticles for enhanced oil recovery. Fuel 2017, 210, 272–281. [Google Scholar] [CrossRef]
- Xu, F.; Jiang, H.Q.; Liu, M.; Zhang, M.; Yu, F.W.; Zang, C.Z.; Li, J.J. Microfluidic-Based Analysis on the In Situ Microemulsion Formation and Transport in Fractured Porous Media. Energy Fuels 2024, 38, 150–159. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J. Interaction of Foam and Microemulsion Components in Low-Tension-Gas Flooding. Fluid. Dyn. Mater. Process. 2023, 19, 1951–1961. [Google Scholar] [CrossRef]
- Oliveira, G.V.B.; Araujo, J.D.C.D.; Meneses, M.C.D.L.; Paula, T.A.F.; Marcos, A.F.R.; Dantas, T.N.C.; Neto, A.O.W.; Haas, D.A. Study on Steam and Microemulsion Alternate Injection for Enhanced Productivity in Heavy Oil Fields. Energy Fuels 2023, 37, 7111–7121. [Google Scholar] [CrossRef]
- Hon, V.Y.; Saaid, I.M.; Chai, I.C.H.; Fauzi, N.A.A.M.; Deguillard, E.; Male, J.V.; Handgraaf, J.W. Microemulsion interface model for chemical enhanced oil recovery design. J. Pet. Sci. Eng. 2022, 212, 110279. [Google Scholar] [CrossRef]
- Lu, M.; Lindman, B.; Holmberg, K. Effect of polymer addition on the phase behavior of oil–water–surfactant systems of Winsor III type. Phys. Chem. Chem. Phys. 2024, 26, 3699–3710. [Google Scholar] [CrossRef]
- Yomi, A.; Dagogo, J.C.; Okogbue, I.; Ogweda, M.; Afoaku, A.; Dinyelu, T. Production Enhancement Using Microemulsion Technology in Rona Field. In Proceedings of the Paper Presented at the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 1–2 August 2019; SPE: Matteson, IL, USA. [Google Scholar]
- Putz, A.; Chevalier, J.P.; Stock, G.; Philippot, J. A Field Test of Microemulsion Flooding, Chateaurenard Field, France. J. Pet. Technol. 1981, 33, 710–718. [Google Scholar] [CrossRef]
- Bouabboune, M.; Hammouch, N.; Benhadid, S. Comparison Between Micro-Emulsion and Surfactant Solution Flooding Efficiency for Enhanced Oil Recovery in TinFouye Oil Field. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 13–15 June 2006. [Google Scholar]
- Li, G.; Zhai, L.; Zheng, L. Tertiary Oil Recovery in China. Deterg. Cosmet. 1999, 21, 367–408. [Google Scholar]
- Li, Y.F.; Li, D.S.; Shan, L. Development and application of microemulsified thermo-chemical compound blocking remover. Spec. Oil Gas. Reserv. 2003, 10, 67–70+112. [Google Scholar]
- Liu, J.T.; Li, J.Y.; Qian, Q.; Liu, J.; Zhang, L. Application of microemulsion active agent in low permeability reservoir. Inn. Mong. Petrochem. Ind. 2016, 42, 122–123+133. [Google Scholar]
- Wang, W.L.; Li, Y.C.; Xu, Y.G.; Zhang, W.D.; Zhu, J.J.; Bao, X.N. Development of in-situ microemulsion surfactant system and its application in pressure decrease and injection increase. Pet. Geol. Oilfield Dev. Daqing 2021, 40, 116–122. [Google Scholar]
- Yan, W.C.; Sun, J.M. Analysis of research present situation of microscopic remaining oil. Prog. Geophys. 2016, 31, 2198–2211. [Google Scholar]
- Liang, B.; Wang, X.M.; Zeng, J.Q.; Wang, Q.G.; Wei, Y.H.; Tang, W.Y. Application of CT scanning technology in the analysis of core microscopic oil displacement characteristics. Mud Logging Eng. 2019, 30, 34–37+133. [Google Scholar]
- Wang, Z.L.; Zhang, J.H.; Zhang, Z.H.; Pan, M.; Shi, Y.M.; Zhang, Z.Q.; Wang, H.; Shi, S.Y. Study on ultra-micro remaining oil based on field emission environmental scanning electron microscopy and energy spectrum analysis. In Proceedings of the 2018 China Earth Science Joint Academic Annual Meeting, Beijing, China, 21 October 2018; pp. 62–64. [Google Scholar]
- Wang, J.; Meng, X.H.; Wang, W.M.; Liu, N.G. 2D NMR distribution function for microscopic remaining oil. Pet. Geol. Exp. 2015, 37, 654–659. [Google Scholar]
- Tong, Y.; Jia, Y.Y. Remaining oil Microscopic Features under the control of high permeability Zone Based on Etching Model. Sci. Technol. Eng. 2018, 18, 80–86. [Google Scholar]
- Zhou, Y.; Wang, D.; Wang, Z.; Cao, R. The formation and viscoelasticity of pore-throat scale emulsion in porous media. Pet. Explor. Dev. 2017, 44, 111–118. [Google Scholar] [CrossRef]
- Maaref, S.; Ayatollahi, S.; Rezaei, N.; Masihi, M. The Effect of Dispersed Phase Salinity on Water-in-Oil Emulsion Flow Performance: A Micromodel Study. Ind. Eng. Chem. Res. 2017, 56, 4549–4561. [Google Scholar] [CrossRef]
- Wu, M.; Lu, X.; Zhou, X.; Lin, Z.Y.; Zeng, F.H. Experimental study on the temperature effects of foamy oil flow in porous media. Fuel 2020, 271, 117649. [Google Scholar] [CrossRef]
- Wu, F.P.; Li, N.; Yang, W.; Chen, J.H.; Ding, B.J.; Xia, L.; Liu, J.; Wang, C.; Wang, L.S. Experimental characterization and mechanism of hydraulic pulsation waves driving microscopic residual oil. Pet. Explor. Dev. 2022, 49, 1411–1422. [Google Scholar] [CrossRef]
- Xiong, C.M.; Ding, B.; Geng, X.F.; Guan, B.S.; Dong, J.F.; Huang, B.; Yan, Y.G. Quantitative analysis on distribution of microcosmic residual oil in reservoirs by frozen phase and nuclear magnetic resonance (NMR) technology. J. Pet. Sci. Eng. 2020, 192, 107256. [Google Scholar] [CrossRef]
- Zhang, P.F.; Lu, S.F.; Li, J.Q.; Chang, X.C.; Lin, Z.Z.; Li, J.J.; Liu, J.Z.; Tian, S.S. Evaluating microdistribution of adsorbed and free oil in a lacustrine shale using nuclear magnetic resonance: A theoretical and experimental study. J. Pet. Sci. Eng. 2022, 212, 110208. [Google Scholar] [CrossRef]
- Jin, G.; Xie, R.; Xiao, L. Quantitative characterization of bound and movable fluid microdistribution in porous rocks using nuclear magnetic resonance. J. Pet. Sci. Eng. 2021, 196, 107677. [Google Scholar] [CrossRef]
- Cao, Y.N. Application CT scanning technology analysis micro-flooding experiment and the residual oil. Comput. Tomogr. Theory Appl. 2015, 24, 47–56. [Google Scholar]
- Zhang, J.C.; Wang, M.X.; Dong, J.F.; Shi, S.Z.; Wang, C.C.; Yang, Y.; Izuchukwu, O.N. Micro-mechanism analysis of enhanced oil recovery for positive-negative drive. Fuel 2020, 260, 1163321–1163327. [Google Scholar] [CrossRef]
- Iglauer, S.; Fern, M.A.; Shearing, P.; Blunt, M.J. Comparison of residual oil cluster size distribution, morphology and saturation in oil-wet and water-wet sandstone. J. Colloid. Interface 2012, 375, 187–192. [Google Scholar] [CrossRef]



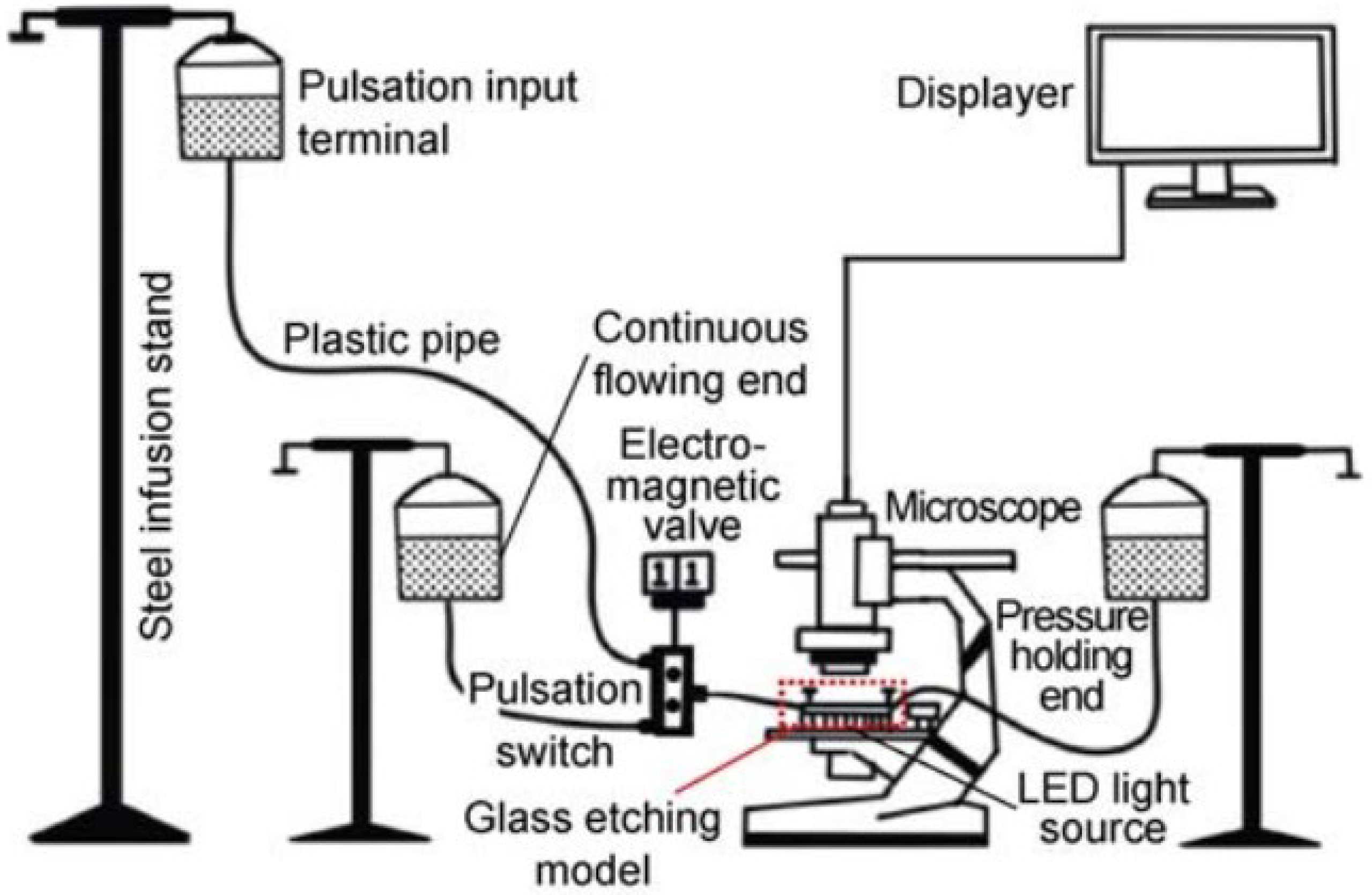
| Type | The Surfactants in the Microemulsion Formulation | Researchers | Results |
|---|---|---|---|
| Anionic surfactants | Sodium dodecylbenzenesulfonate | Zhao Xuezhi [54] | In situ injection forms the following two types of emulsions: smaller droplets enhance the fluidity of oil, while larger droplets block the main pathways, improving sweep efficiency. Compared to water flooding, the recovery rate can be increased by 30%. |
| Yuan Ying [55] | The identification of a single inorganic salt within the sodium dodecylbenzene sulfonate system predicted the mixed salt effect, providing a new method to simplify mixed salt microemulsion systems. | ||
| Ali Rezaie [56] | Accurately assessing the influence of each salt on the phase behavior of microemulsions, the optimal salinity and solubilization parameters for different types of salts are consistent with Hofmeister series. | ||
| Sodium dodecyl sulfate | Zamula YS [57] | Through atomic force microscopy, the microscopic structure of microemulsions was studied. By comparing differences in surface morphology, single particles or complex structures with well-defined shapes corresponded to the bicontinuous structure of microemulsions. | |
| Pal N [58] | Through physical-chemical evaluation studies to assess the suitability of microemulsions for consistency improvement techniques, the potential of the formulation’s use was demonstrated. | ||
| Fatty Acid Methyl Ester Sulfonate | Pal N [59] | The interfacial tension values between microemulsion systems and hydrocarbon systems were found to be significantly lower than the interfacial tension values between surfactant and hydrocarbon systems. After injection of this system, the recovery rate was close to around 30% of conventional secondary water flooding. | |
| Nonionic Surfactants | Tergitol | Achinta Bera [60] | The interfacial tension between oil and the microemulsion phase is a strong function of surfactant concentration and salinity. |
| Polyoxyethylene ether | Zhang Haixia [61] | Longer chain length and fewer ethylene oxide units in long-chain polyoxyethylene ether surfactants make it easier to form W/O microemulsions. In this case, microemulsions exhibit excellent high-temperature and low-temperature stability, as well as lower interfacial tension. | |
| Pentaethylene glycol monododecyl ether | Schmidt RF [62] | Compared to the ionic surfactant AOT, Pentaethylene glycol monododecyl ether is best described using a symmetrically disordered lamellar model. | |
| Fatty alcohol polyoxyethylene ether 9 (AEO 9) | Xu H [63] | It is capable of forming stable microemulsions, and the oil phase in this system can control the release of adhered surfactants. Additionally, there exists a competitive adsorption relationship between the oil phase and the rock surface for free surfactants in the solution, which offers the potential to reduce surfactant adsorption losses and extend the penetration distance of surfactants into the reservoir matrix. | |
| Cationic surfactants | Cetyltrimethylammonium bromide | Maria LDO [64] | Improving the migration rate of nanoparticles in porous limestone media and their stability in saline solutions containing high concentrations of divalent cations, oil displacement tests were conducted in uncemented porous media, achieving a recovery rate of up to 60%. |
| Imidazoliumbased ionic liquid | Fattahi R [65] | The addition of nanoparticles to cationic surfactants enables the formation of stable microemulsions, with the ability to reduce interfacial tension similar to microemulsions formed by anionic surfactants. | |
| Benzethonium chloride | Phaodee P [66] | It can promote the formation of the middle phase in microemulsions when copolymerized with different anionic surfactants, while also reducing or eliminating the demand for electrolytes, enhancing solubilization or cosolvency. |
| Compound Category | Property |
|---|---|
| Homoelectric surfactant compounding | For the compounding of homologous surfactants, the performance is among various components. Different types of compounding also have synergistic effects (anionic-anionic, nonionic-nonionic). |
| Anion + cation | It has the strongest synergistic effect, but it is easy to precipitate in aqueous solution, so it needs to be designed reasonably. |
| Anion + nonion | It can reduce the repulsion of the hydrophilic head group, increase the surface activity, and make up for the shortcomings of nonionic temperature resistance and anion salt resistance. |
| Cationic + nonionic | There is a synergistic effect, but the effect is not as good as that of anionic-nonionic compound (the oxygen atom on the ethoxy group of the nonionic surfactant can be combined with water by hydrogen bond, which is positively charged and repelled with the cationic surfactant). |
| Displacement Methods | Mechanism | Technical Characteristics | Advantage | Disadvantage | Research Progress |
|---|---|---|---|---|---|
| Polymer flooding (P) | Increase the sweep coefficient | Increase water phase viscosity and improve fluidity ratio | Good oil increasing effect, cost saving, and simple operation | Narrow applicability and low polymer stability | industrial application |
| Surfactant flooding (S) | Improve oil washing efficiency and increase sweep coefficient | Reduce the interfacial tension between oil and water; changing the surface wettability of porous media | Significant effect and convenient use | High cost, difficult to treat produced liquid | Pilot Test |
| Alkaline waterflooding (A) | Same as above | Reduce the interfacial tension between oil and water; changing the surface wettability of porous media | Low-cost and simple operation | Corroded pipelines, scaling, and difficult to treat produced liquids | Pilot Test |
| Ternary composite flooding (ASP) | Same as above | Improving the fluidity ratio and altering the surface wettability of porous media | The advantages of combining polymers and surfactants | High cost, complex operation, corrosion of pipelines, scaling | field test |
| Binary compound flooding (SP) | Same as above | The increase in oil recovery rate is significant and has a wide range of applications | Similar to ternary composite flooding | High cost and complex operation | field test |
| Gas flooding | Same as above | Reduce crude oil viscosity, eliminate Jamin effect, reduce interfacial tension and capillary pressure | Suitable for various reservoir conditions, with a significant increase in oil recovery rate | High requirements for ground gas injection systems and equipment, high injection pressure, and difficulty in continuous injection | Continuously optimizing and improving in practical applications |
| Nano chemical flooding | Same as above | Utilizing the special properties of nanomaterials | Expected to significantly increase oil recovery rate | Currently still in the interior research stage | Laboratory Study |
| Microemulsion flooding | Same as above | Ultralow oil–water interfacial tension; changing the surface wettability of porous media; strong solubilizing oil and emulsifying ability | Expected to maximize oil recovery | The system has multiple components and high costs | Continuously optimizing and improving in practical applications |
| Researchers | Formulation |
|---|---|
| Xu [123] | 4 wt% Sodium dodecyl sulfate + 8 wt%n-butanol + Pentane + 6%KCl |
| Zhao [124] | 0.5 wt% Internal olefin sulfonate + 3.5%Sodium dodecyl sulfate + 5 wt%2-butanol + 1-dodecanol + 6.5 wt%NaCl |
| Oliveira [125] | Nonylphenol ethoxylate 100 + n-butanol + kerosene + Synthesis of Produced Water |
| Hon [126] | Sodium C14-16 olefin sulfonate + intermediate oil + NaCl |
| Lu [127] | Ethylene glycol + decane + water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, K.; Guan, B.; Liu, W.; Jiang, C.; Cong, S.; Peng, B.; Tao, Y. Advance of Microemulsion and Application for Enhanced Oil Recovery. Nanomaterials 2024, 14, 1004. https://doi.org/10.3390/nano14121004
Leng K, Guan B, Liu W, Jiang C, Cong S, Peng B, Tao Y. Advance of Microemulsion and Application for Enhanced Oil Recovery. Nanomaterials. 2024; 14(12):1004. https://doi.org/10.3390/nano14121004
Chicago/Turabian StyleLeng, Kaiqi, Baoshan Guan, Weidong Liu, Chen Jiang, Sunan Cong, Baoliang Peng, and Yufan Tao. 2024. "Advance of Microemulsion and Application for Enhanced Oil Recovery" Nanomaterials 14, no. 12: 1004. https://doi.org/10.3390/nano14121004
APA StyleLeng, K., Guan, B., Liu, W., Jiang, C., Cong, S., Peng, B., & Tao, Y. (2024). Advance of Microemulsion and Application for Enhanced Oil Recovery. Nanomaterials, 14(12), 1004. https://doi.org/10.3390/nano14121004






