Unveiling the Role of Sulfur Vacancies in Enhanced Photocatalytic Activity of Hybrids Photocatalysts
Abstract
:1. Introduction
2. Characterization and Engineering Strategies of Sulfur Vacancies
2.1. Characterization of Sulfur Vacancies in Photocatalysts
2.1.1. High-Resolution Transmission Electron Microscopy
2.1.2. Scanning Transmission Electron Microscopy
2.1.3. Electron Spin Resonance
2.1.4. X-ray Photoelectron Spectroscopy
2.1.5. X-ray Absorption Fine Structure
2.2. Engineering Strategies for Sulfur Vacancies Generation
2.2.1. Lithiation-Chemistry Approach
2.2.2. Thermal Treatment

2.2.3. Chemical Reduction
3. The Role of Sulfur Vacancies in Photocatalysis
3.1. Defect Energy Levels
3.2. Electrons Reservoir
3.3. Adsorption and Activate Sites
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.; Navid, I.A.; Ma, Y.J.; Xiao, Y.X.; Wang, P.; Ye, Z.W.; Zhou, B.W.; Sun, K.; Mi, Z.T. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, X.J.; Xu, J.; Gong, Y.; Shen, S.L.; Fan, J.C.; Zhang, X.J.; Xue, Y.H. Surface structural transformation of Ni2P@C electrocatalysts for overall alkaline water splitting. Prog. Nat. Sci. Mater. Int. 2024, 34, 102–107. [Google Scholar] [CrossRef]
- Li, Y.; He, N.N.; Chen, X.H.; Fang, B.; Liu, X.J.; Li, H.B.; Gong, Z.W.; Lu, T.; Pan, L.K. Interface regulation of Zr-MOF/Ni2P@nickel foam as high-efficient electrocatalyst for pH-universal hydrogen evolution reaction. J. Colloid Interface Sci. 2024, 656, 289–296. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y.J. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Chen, R.T.; Ren, Z.F.; Liang, Y.; Zhang, G.H.; Dittrich, T.; Liu, R.Z.; Liu, Y.; Zhao, Y.; Pang, S.; An, H.Y.; et al. Spatiotemporal imaging of charge transfer in photocatalyst particles. Nature 2022, 610, 296–301. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Zhou, L.N.; Robatjazi, H.; Bao, J.L.; Zhou, J.Y.; Bayles, A.; Yuan, L.; Lou, M.H.; Lou, M.H.; Khatiwada, S.; et al. Earth-abundant photocatalyst for H2 generation from NH3 with light-emitting diode illumination. Science 2022, 378, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Yang, W.W.; Liu, Y.Q.; Yu, Y.S. Engineering sulfur vacancies in basal plane of MoS2 for enhanced hydrogen evolution reaction. J. Catal. 2020, 391, 91–97. [Google Scholar] [CrossRef]
- Zhang, W.W.; Chen, L.J.; Dai, S.; Zhao, C.X.; Ma, C.; Wei, L.; Zhu, M.H.; Chong, S.Y.; Yang, H.F.; Liu, L.J.; et al. Reconstructed covalent organic frameworks. Nature 2022, 604, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Pan, C.S.; Bian, G.M.; Xu, J.; Dong, Y.M.; Zhang, Y.; Lou, Y.; Liu, W.X.; Zhu, Y.F. H2O2 generation from O2 and H2O on a near-infrared absorbing porphyrin supramolecular photocatalyst. Nat. Energy 2023, 8, 361–371. [Google Scholar] [CrossRef]
- Alvi, N.U.H.; Sandberg, M. Sustainable and Low-Cost Electrodes for Photocatalytic Fuel Cells. Nanomaterials 2024, 14, 636. [Google Scholar] [CrossRef]
- Qian, Y.Y.; Han, Y.L.; Zhang, X.Y.; Yang, G.; Zhang, G.Z.; Jiang, H.L. Computation-based regulation of excitonic effects in donor-acceptor covalent organic frameworks for enhanced photocatalysis. Nat. Commun. 2023, 14, 3083. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.C.; Wu, X.D.; Tang, L.; Chen, X.H.; Li, M.; Mou, Y.; Su, B.; Wang, S.B.; Feng, C.Y.; Liu, J.W.; et al. Dual donor-acceptor covalent organic frameworks for hydrogen peroxide photosynthesis. Nat. Commun. 2023, 14, 5238. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Dai, C.; Wang, X.; Hu, J.Y.; Zhang, J.Y.; Zheng, L.X.; Mao, L.; Zheng, H.J.; Zhu, M.S. Protruding Pt single-sites on hexagonal ZnIn2S4 to accelerate photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 1287. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.M.; Li, P.X.; Liu, B.B.; Gong, Y.; Luo, C.L.; Yang, K.; Liu, X.J.; Fan, J.C.; Xue, Y.H. Interface Coordination Engineering of P-Fe3O4/Fe@C Derived from an Iron-Based Metal Organic Framework for pH-Universal Water Splitting. Nanomaterials 2023, 13, 1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Xia, Y.G.; Li, H.P.; Wang, X.; Yu, Y.; Jiao, X.L.; Chen, D.R. Highly active deficient ternary sulfide photoanode for photoelectrochemical water splitting. Nat. Commun. 2020, 11, 3078. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.L.; Da, Y.M.; Wang, M.; Dou, X.Y.; Cui, X.H.; Chen, J.; Jiang, R.; Xi, S.B.; Cui, B.H.; Luo, Y.N.; et al. Selective photoelectrochemical oxidation of glucose to glucaric acid by single atom Pt decorated defective TiO2. Nat. Commun. 2023, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhao, M.Y.; Chen, X.; Sun, G.W.; Wang, C.; Song, Q.J. Unraveling the dual defect effects in C3N5 for piezo-photocatalytic degradation and H2O2 generation. Appl. Catal. B Environ. 2023, 332, 122752. [Google Scholar] [CrossRef]
- Shen, S.L.; Chu, Y.; Xu, Y.L.; Liu, X.J.; Xiu, H.X.; Li, J.; Tang, Z.H.; Xu, J.C.; Xiao, S.N. Cu doping induced synergistic effect of S-vacancies and S-scheme Cu: Mn0.5Cd0.5S@CuS heterojunction for enhanced H2 evolution from photocatalytic seawater splitting. Int. J. Hydrog. Energy 2024, 61, 734–742. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Liu, X.; Liu, C.B.; Luo, S.L.; Wang, L.L.; Cai, T.; Zeng, Y.X.; Yuan, J.L.; Dong, W.Y.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef]
- Chen, X.; Denninger, P.; Stimpel-Lindner, T.; Spiecker, E.; Duesberg, G.S.; Backes, C.; Knirsch, K.C.; Hirsch, A. Defect Engineering of Two-Dimensional Molybdenum Disulfide. Chem. Eur. J. 2020, 26, 6535–6544. [Google Scholar] [CrossRef]
- Liu, L.Z.; Wang, Z.L.; Zhang, J.F.; Ruzimuradov, O.; Dai, K.; Low, J. Tunable Interfacial Charge Transfer in a 2D-2D Composite for Efficient Visible-Light-Driven CO2 Conversion. Adv. Mater. 2023, 35, 2300643. [Google Scholar] [CrossRef]
- Liao, Y.L.; Zhu, Y.J.; Zou, R.Y.; Yu, Q.; Tang, Z.H. Defect engineering of Fe-N doped crumpled graphene for improved ORR performance. Prog. Nat. Sci. Mater. Int. 2024, 34, 147–154. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chiao, Y.C.; Hsu, P.C. Rapid Microwave-Assisted Synthesis of ZnIn2S4 Nanosheets for Highly Efficient Photocatalytic Hydrogen Production. Nanomaterials 2023, 13, 1957. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Hou, J.G.; Cao, S.Y.; Li, Z.W.; Zhang, Y.T.; Wu, Y.Z.; Zhang, B.; Zhai, P.L.; Sun, L.C. Defect Engineering of Photocatalysts for Solar Energy Conversion. Solar RRL 2020, 4, 2070045. [Google Scholar] [CrossRef]
- Yu, Z.H.; Pan, Y.M.; Shen, Y.T.; Wang, Z.L.; Ong, Z.Y.; Xu, T.; Xin, R.; Pan, L.J.; Wang, B.G.; Sun, L.T.; et al. Towards intrinsic charge transport in monolayer molybdenum disulfide by defect and interface engineering. Nat. Commun. 2014, 5, 5290. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Chen, X.Z.; Sanchis-Gual, R.; Veciana, A.; Franco, C.; Kim, D.; Surin, I.; Pérez-Ramfrez, J.; Mattera, M.; et al. Tuning oxygen vacancies in Bi4Ti3O12 nanosheets to boost piezo-photocatalytic activity. Nano Energy 2023, 108, 108202. [Google Scholar] [CrossRef]
- Liu, B.Y.; Wang, X.; Zhang, Y.J.; Xu, L.C.; Wang, T.S.; Xiao, X.; Wang, S.C.; Wang, L.Z.; Huang, W. A BiVO4 Photoanode with a VOx Layer Bearing Oxygen Vacancies Offers Improved Charge Transfer and Oxygen Evolution Kinetics in Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2023, 62, e202217346. [Google Scholar] [CrossRef]
- Li, J.; Zhan, G.M.; Yu, Y.; Zhang, L.Z. Superior visible light hydrogen evolution of Janus bilayer junctions via atomic-level charge flow steering. Nat. Commun. 2016, 7, 11480. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, Y.; Rauwel, E.; Nagpal, K.; Haddad, R.; Estephan, E.; Boissière, C.; Rauwel, P. Revealing the Dependency of Dye Adsorption and Photocatalytic Activity of ZnO Nanoparticles on Their Morphology and Defect States. Nanomaterials 2023, 13, 1998. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.J.; Wang, C.; Gan, L.Y.; Chen, X.J.; Zhang, P.; Wang, Y.; Li, H.; Wang, L.H.; Zhou, X.Y.; et al. Unraveling the dual defect sites in graphite carbon nitride for ultra-high photocatalytic H2O2 evolution. Energy Environ. Sci. 2022, 15, 830–842. [Google Scholar] [CrossRef]
- Shen, Y.; Ren, C.J.; Zheng, L.R.; Xu, X.Y.; Long, R.; Zhang, W.Q.; Yang, Y.; Zhang, Y.C.; Yao, Y.F.; Chi, H.Q.; et al. Room-temperature photosynthesis of propane from CO2 with Cu single atoms on vacancy-rich TiO2. Nat. Commun. 2023, 14, 1117. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Q.; Xu, C.G.; Wang, W.T.; Chen, L.; Li, X.; Wu, Y.T. Oxygen Vacancy Mediated Band-Gap Engineering via B-Doping for Enhancing Z-Scheme A-TiO2/R-TiO2 Heterojunction Photocatalytic Performance. Nanomaterials 2023, 13, 794. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Song, H.Q.; Wu, H.; Zhang, P.K.; Tang, Z.Y.; Lu, S.Y. Defects Enhance the Electrocatalytic Hydrogen Evolution Properties of MoS2-based Materials. Chem. Asian J. 2020, 15, 3123–3134. [Google Scholar] [CrossRef]
- Jiang, W.B.; Loh, H.Y.; Low, B.Q.L.; Zhu, H.J.; Low, J.X.; Heng, J.Z.X.; Tang, K.Y.; Li, Z.B.; Loh, X.J.; Ye, E.Y.; et al. Role of oxygen vacancy in metal oxides for photocatalytic CO2 reduction. Appl. Catal. B Environ. 2023, 321, 122079. [Google Scholar] [CrossRef]
- Alyami, M. Ultra-Violet-Assisted Scalable Method to Fabricate Oxygen-Vacancy-Rich Titanium-Dioxide Semiconductor Film for Water Decontamination under Natural Sunlight Irradiation. Nanomaterials 2023, 13, 703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Suenaga, K.; Wang, Z.Y.; Shi, Z.J.; Okunishi, E.; Iijima, S. Identification of active atomic defects in a monolayered tungsten disulphide nanoribbon. Nat. Commun. 2011, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Tang, M.T.; Wu, S.D.; Geng, J.; Han, Z.J.; Chan, K.R.; Gao, P.Q.; Li, H.J. Rational design of stable sulfur vacancies in molybdenum disulfide for hydrogen evolution. Catalysis 2020, 382, 320–328. [Google Scholar] [CrossRef]
- Liu, G.; Robertson, A.W.; Li, M.M.-J.; Kuo, W.C.H.; Darby, M.T.; Muhieddine, M.H.; Lin, Y.-C.; Suenaga, K.; Stamatakis, M.; Warner, J.H.; et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 2017, 9, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.W.; Si, H.N.; Zhang, Q.H.; Wu, J.; Gao, L.; Wei, X.F.; Sun, Y.; Liao, Q.L.; Zhang, Z.; et al. Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2. Am. Chem. Soc. 2020, 142, 4298–4308. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.Y.; Yan, B.; Xia, C.X.; Yang, G.W. Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 248, 193–201. [Google Scholar] [CrossRef]
- Fang, Z.B.; Weng, S.X.; Ye, X.X.; Feng, W.H.; Zheng, Z.Y.; Lu, M.L.; Lin, S.; Fu, X.Z.; Liu, P. Defect Engineering and Phase Junction Architecture of Wide-Bandgap ZnS for Conflicting Visible Light Activity in Photocatalytic H2 Evolution. ACS Appl. Mater. Interfaces 2015, 7, 13915–13924. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Xu, X.X.; An, K.L.; Wang, Y.; Shi, F.N. Coordination Polymer Derived NiS@g-C3N4 Composite Photocatalyst for Sulfur Vacancy and Photothermal Effect Synergistic Enhanced H2 Production. ACS Sustain. Chem. Eng. 2018, 6, 11869–11876. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Xie, Y.; Liu, L.J.; Jiao, J.L. Sulfur vacancy induced high performance for photocatalytic H2 production over 1T@2H phase MoS2 nanolayers. Catal. Sci. Technol. 2017, 7, 5635–5643. [Google Scholar] [CrossRef]
- Ma, Y.W.; Hai, G.T.; Atinafu, D.G.; Dong, W.J.; Li, R.J.; Hou, C.M.; Wang, G.J. Carbon inserted defect-rich MoS2−x nanosheets@CdS nanospheres for efficient photocatalytic hydrogen evolution under visible light irradiation. Colloid Interface Sci. 2020, 569, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.B.; Han, L.L.; Li, Y.; He, W.J.; Liu, C.C.; Zhang, J.; Chen, C.; Liu, H.; Xin, H.L. Optimizing electron density of nickel sulfide electrocatalysts through sulfur vacancy engineering for alkaline hydrogen evolution. J. Mater. Chem. A 2020, 8, 18207–18214. [Google Scholar] [CrossRef]
- Wang, L.S.; Xu, X.X.; Wang, Y.; Wang, X.J.; Shi, F.N. Sulfur vacancy-rich CdS loaded on filter paper-derived 3D nitrogen-doped mesoporous carbon carrier for photocatalytic VOC removal. Inorg. Chem. Front. 2018, 5, 1470–1476. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, X.; Xuan, X.X.; Liu, N.; Zhou, J.H. Development of an efficient catalyst with controlled sulfur vacancies and high pyridine nitrogen content for the photoelectrochemical reduction of CO2 into methanol. Sci. Total Environ. 2020, 702, 134981. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Hu, C.Y.; Guo, S.H.; Zhang, L.B.; Wang, M.J.; Peng, J.H.; Xu, L.; Wang, J. Sulfur-Vacancy-Enriched MoS2 Nanosheets Based Heterostructures for Near-Infrared Optoelectronic NO2 Sensing. ACS Appl. Nano Mater. 2020, 3, 665–673. [Google Scholar] [CrossRef]
- Sun, B.T.; Liang, Z.Q.; Qian, Y.Y.; Xu, X.S.; Han, Y.; Tian, J. Sulfur Vacancy-Rich O-Doped 1T-MoS2 Nanosheets for Exceptional Photocatalytic Nitrogen Fixation over CdS. ACS Appl. Mater. Interfaces 2020, 12, 7257–7269. [Google Scholar] [CrossRef]
- Hu, S.Z.; Li, Y.M.; Li, F.Y.; Fan, Z.P.; Ma, H.F.; Li, W.; Kang, X.X. Construction of g-C3N4/Zn0.11Sn0.12Cd0.88S1.12 Hybrid Heterojunction Catalyst with Outstanding Nitrogen Photofixation Performance Induced by Sulfur Vacancies. ACS Sustain. Chem. Eng. 2016, 4, 2269–2278. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Y.M.; Yu, Y.F.; Du, Y.H.; Zhang, B. Engineering Sulfur Defects, Atomic Thickness, and Porous Structures into Cobalt Sulfide Nanosheets for Efficient Electrocatalytic Alkaline Hydrogen Evolution. ACS Catal. 2018, 8, 8077–8083. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Jeong, H.Y.; Lee, M.H.; Shin, H.S.; Lee, J.S. Efficient Hydrogen Evolution Reaction Catalysis in Alkaline Media by All-in-One MoS2 with Multifunctional Active Sites. Adv. Mater. 2018, 30, 1707105. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Hu, Z.X.; Probert, M.; Li, K.; Lv, D.H.; Yang, X.A.; Gu, L.; Mao, N.N.; Feng, Q.L.; Xie, L.M.; et al. Exploring atomic defects in molybdenum disulphide monolayers. Nat. Commun. 2015, 6, 6293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zou, X.L.; Najmaei, S.; Liu, Z.; Shi, Y.M.; Kong, J.; Lou, J.; Ajayan, P.M.; Yakobson, B.I.; Idrobo, J.C. Intrinsic Structural Defects in Monolayer Molybdenum Disulfide. Nano Lett. 2013, 13, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; He, J.F.; Liu, Q.H.; Yao, T.; Chen, L.; Yan, W.S.; Hu, F.C.; Jiang, Y.; Zhao, Y.D.; Hu, T.D.; et al. Vacancy-Induced Ferromagnetism of MoS2 Nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Yin, J.; An, L.; Lu, M.; Sun, K.; Zhao, Y.Q.; Gao, D.Q.; Cheng, F.Y.; Xi, P.X. FeS2/CoS2 Interface Nanosheets as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Small 2018, 14, 1801070. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.T.; Jiao, Q.Z.; Li, Q.; Shi, Q.; Dai, Z.; Zhao, Y.; Li, H.S.; Feng, C.H.; Zhou, W.; Feng, T.Y. NiCo2S4 spheres grown on N,S co-doped rGO with high sulfur vacancies as superior oxygen bifunctional electrocatalysts. Electrochim. Acta 2020, 331, 135356. [Google Scholar] [CrossRef]
- Tsai, C.; Li, H.; Park, S.; Park, J.; Han, H.S.; Nørskov, J.K.; Zheng, X.L.; Abild-Pedersen, F. Electrochemical generation of sulfur vacancies in the basal plane of MoS2 for hydrogen evolution. Nat. Commun. 2017, 8, 15113. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Wang, C.H.; Lu, Y.; Wu, X.; Mamba, B.B.; Kuvarega, A.T.; Kefeni, K.K.; Gui, J.Z.; Liu, D. Ultrathin NiFeS Nanomeshes with Sulfur Vacancy for Electrocatalytic Hydrogen Evolution. ChemElectroChem 2020, 7, 2199–2204. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Li, H.; Lu, J.; Feng, Y.H.; Meng, F.Y.; Ma, C.C.; Yan, Y.S.; Meng, M.J. Synergy between van der waals heterojunction and vacancy in ZnIn2S4/g-C3N4 2D/2D photocatalysts for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 277, 119254. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Yu, Z.B.; Jiang, R.H.; Huang, J.; Hou, Y.P.; Zhou, Q.Y.; Zhu, S.Y.; Huang, X.C.; Zheng, F.; Luo, Z. Optimization of the overall water-splitting performance of N, S co-doped carbon-supported NiCoMnSx-10 at high current densities by the introduction of sulfur defects and oxygen vacancies. CrystEngComm 2020, 22, 6239–6248. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Xu, X.X.; Mei, M.L.; Shi, F.N. Coordination Polymer Derived Sulfur Vacancies Rich CdS Composite Photocatalyst with Nitrogen Doped Carbon as Matrix for H2 Production. ACS Sustain. Chem. Eng. 2018, 6, 854–861. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.B.; Li, Z.J.; Lou, Z.Z.; Wang, Z.Y.; Dai, Y.; Whangbo, M.H. Synthesis and characterization of ZnS with controlled amount of S vacancies for photocatalytic H2 production under visible light. Sci. Rep. 2015, 5, 8544. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Shi, T.L.; Wei, C.H.; Zeng, H.; Hu, X.G.; Yan, B. A 2D-2D heterojunction Bi2WO6/WS2−x as a broad-spectrum bactericide: Sulfur vacancies mediate the interface interactions between biology and nanomaterials. Biomaterials 2020, 243, 119937. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Chen, D.; Qin, L.S.; Liang, J.H.; Huang, Y.X. Hydrogenated ZnIn2S4 microspheres: Boosting photocatalytic hydrogen evolution by sulfur vacancy engineering and mechanism insight. Phys. Chem. Chem. Phys. 2019, 21, 25484–25494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, J.; Liu, R. Defect engineering of zeolite imidazole framework derived ZnS nanosheets towards enhanced visible light driven photocatalytic hydrogen production. Appl. Catal. B Environ. 2020, 278, 119265. [Google Scholar] [CrossRef]
- Lee, J.; Ham, S.; Choi, D.; Jang, D.J. Facile fabrication of porous ZnS nanostructures with a controlled amount of S vacancies for enhanced photocatalytic performances. Nanoscale 2018, 10, 14254–14263. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Yu, Z.B.; Jiang, R.H.; Hou, Y.P.; Huang, J.; Zhu, H.X.; Yang, F.; Li, M.J.; Li, F.Y.; Sun, Q.Q. A novel, noble-metal-free core-shell structure Ni-P@C cocatalyst modified sulfur vacancy-rich ZnIn2S4 2D ultrathin sheets for visible light-driven photocatalytic hydrogen evolution. J. Alloys Compd. 2021, 855, 157333. [Google Scholar] [CrossRef]
- Wu, H.Y.; Li, X.; Cheng, Y.; Xiao, Y.H.; Wu, Q.P.; Lin, H.; Xu, J.; Wang, Y.S. The synergistic role of double vacancies within AgGaS2 nanocrystals in carrier separation and transfer for efficient photocatalytic hydrogen evolution. Catal. Sci. Technol. 2019, 9, 5838–5844. [Google Scholar] [CrossRef]
- Fodor, S.; Baia, L.; Hernadi, K.; Pap, Z. Controlled synthesis of visible light active CuxS photocatalyst: The effect of heat treatment on their adsorption capacity and photoactivity. Materials 2020, 13, 3665. [Google Scholar] [CrossRef]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.H.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jorgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Norskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, H.B.; Zeng, X.Y.; Xu, J.Y.; Yu, X.T.; Liu, H.X.; Cao, H.L.; Lü, J.; Cao, R. CdZnS nanorods with rich sulphur vacancies for highly efficient photocatalytic hydrogen production. Chem. Commun. 2020, 56, 7765–7768. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Wang, Y.; Dong, X.L.; Zheng, N.; Ma, H.C.; Zhang, X.F. Indium sulfide nanotubes with sulfur vacancies as an efficient photocatalyst for nitrogen fixation. RSC Adv. 2019, 9, 21646–21652. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Z.; Chen, X.; Li, Q.; Zhao, Y.F.; Mao, W. Effect of sulfur vacancies on the nitrogen photofixation performance of ternary metal sulfide photocatalysts. Catal. Sci. Technol. 2016, 6, 5884–5890. [Google Scholar] [CrossRef]
- Li, Z.W.; Hou, J.G.; Zhang, B.; Cao, S.Y.; Wu, Y.Z.; Gao, Z.M.; Nie, X.W.; Sun, L.C. Two-dimensional Janus heterostructures for superior Z-scheme photocatalytic water splitting. Nano Energy 2019, 59, 537–544. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhao, Z.; Zhang, W.W.; Zhang, G.Q.; Qu, D.; Miao, X.; Sun, S.R.; Sun, Z.C. Surface Defects Enhanced Visible Light Photocatalytic H2 Production for Zn-Cd-S Solid Solution. Small 2016, 12, 793–801. [Google Scholar] [CrossRef]

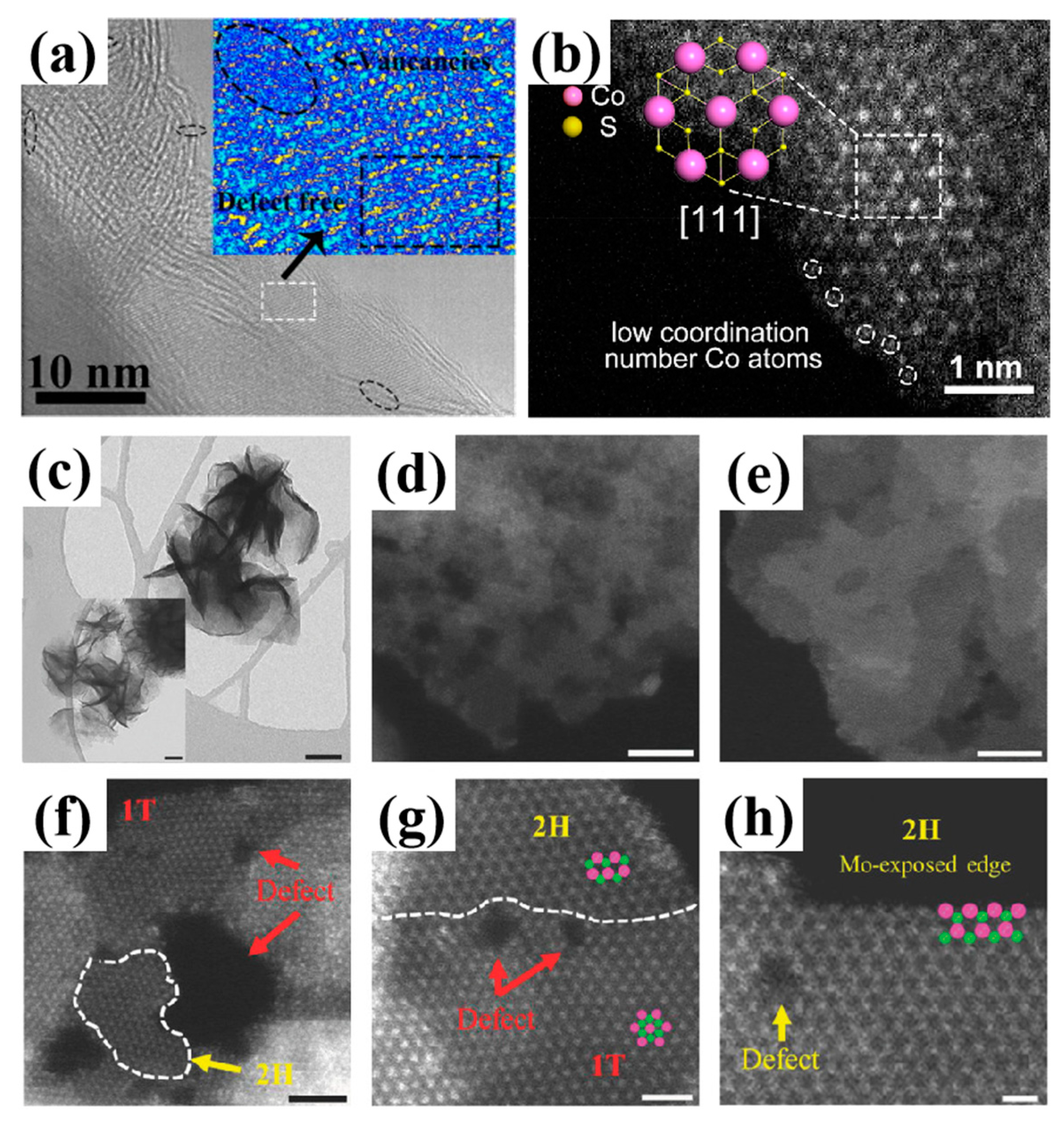


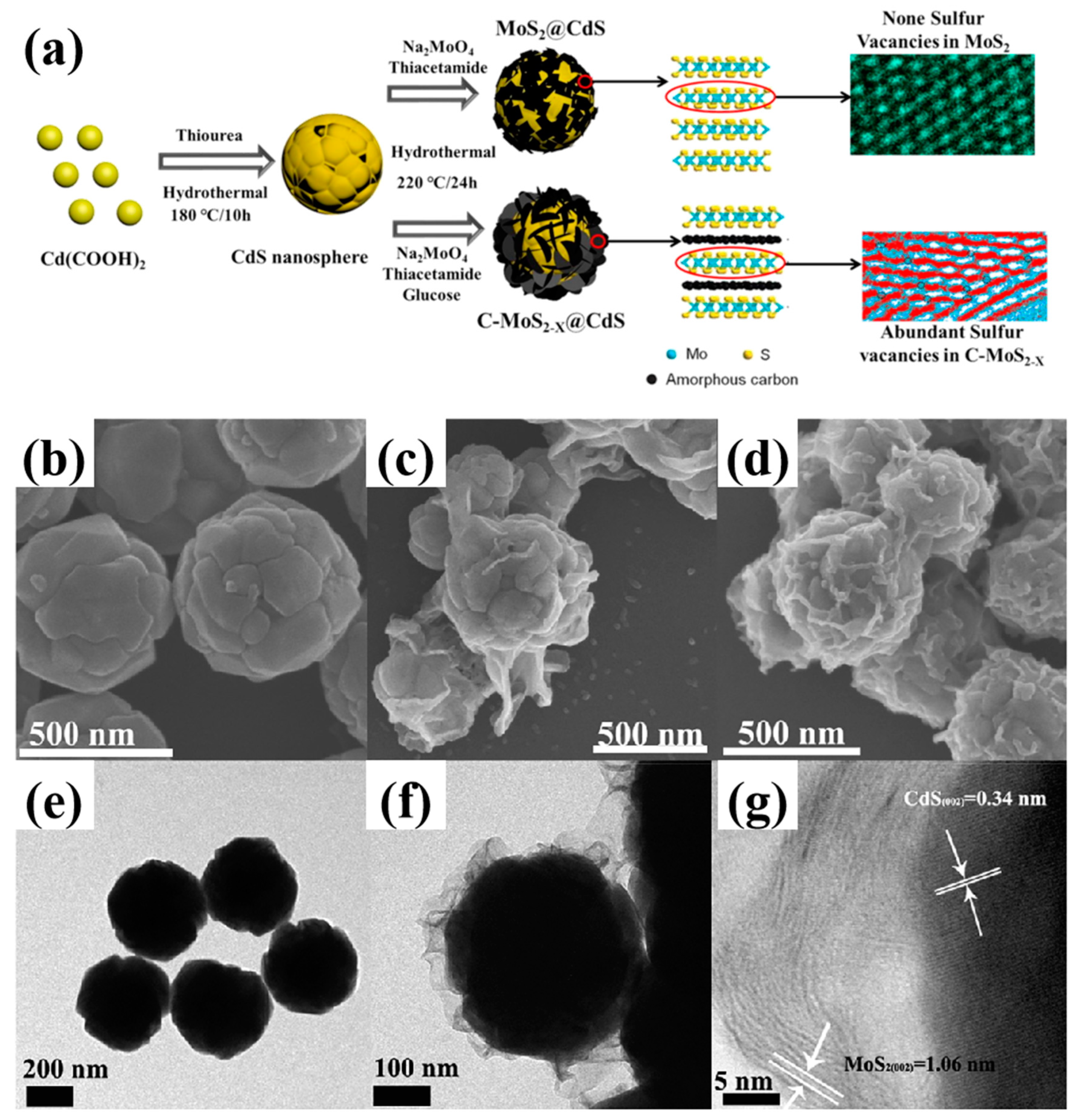

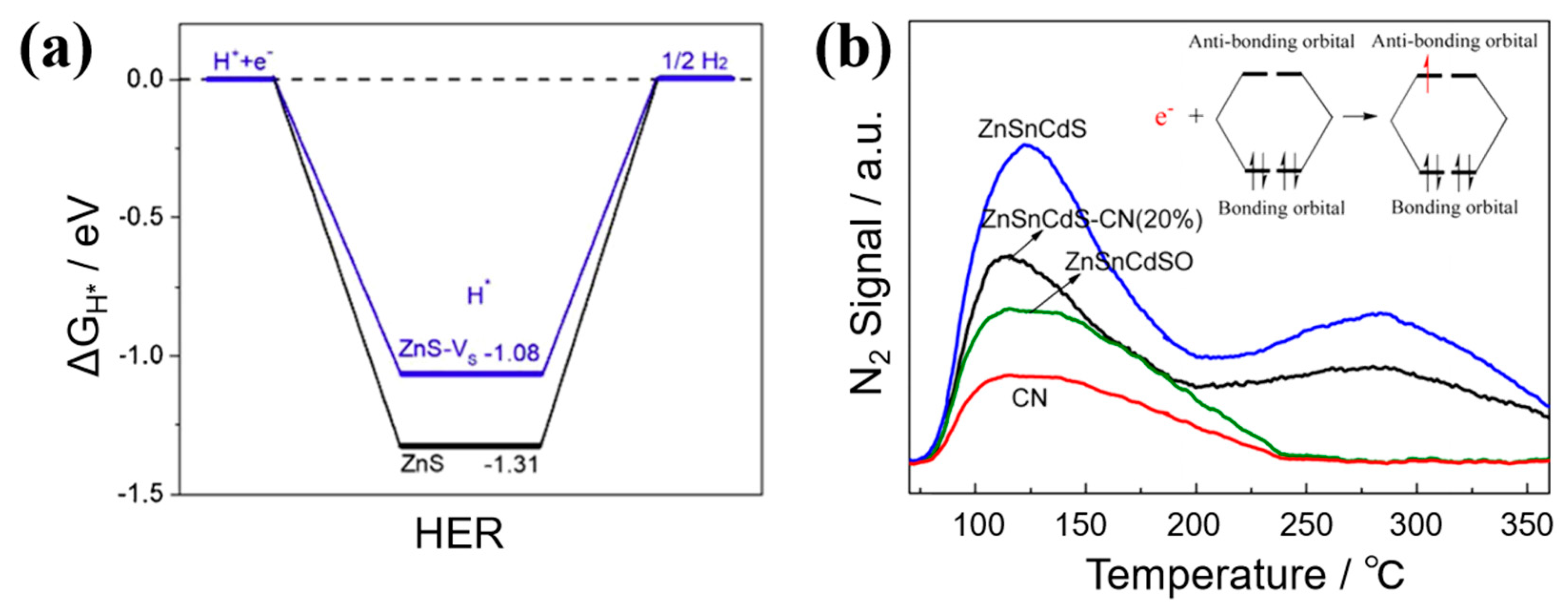
| Catalysts | Synthesis Methods | Characte- Rization Methods | Role of Defects | Tunable Properties | Refs. |
|---|---|---|---|---|---|
| ZnIn2S4 | Exfoliation using n-butyllithium/ Hydrothermal processes | False-color HRTEM XPS EPR HAADF- STEM | Blue-shifted absorption edge Enhanced light absorption Efficient charge separation | Photocatalytic hydrogen evolution | [19,40] |
| ZnS | Hydrothermal processes | XPS ESR | New energy levels Efficient charge separation Active sites | Photocatalytic hydrogen evolution | [41] |
| NiS@g-C3N4 | Calcination of coordination polymer | XPS ESR | Hydrophilicity with enhanced water adsorption Efficient charge separation | Photocatalytic hydrogen evolution | [42] |
| 1T@2H MoS2 | Hydrothermal processes | XPS EPR | H2O activation sites Efficient charge separation | Photocatalytic hydrogen evolution | [43] |
| MoS2−X@CdS | Hydrothermal processes | False-color STEM XPS EPR | Electronic reservoir Active sites | Photocatalytic hydrogen evolution | [44] |
| Zn0.5Cd0.5S1−x | Co-precipitation-hydrothermal strategy | XPS | Mid-gap impurity level Electron trapping site | Photocatalytic hydrogen evolution | [45] |
| CdS@3D-NPC | Calcination of coordination polymer | XPS EPR | Electron carriers VOC traps | Photocatalytic VOC removal | [46] |
| CdS/NCP | Thermal treatment | STEM XPS | CO2 adsorption sites Active sites Efficient charge separation | Photoelectrochemical reduction CO2 | [47] |
| SnS2 | Hydrothermal processes | ICP-AES XPS EPR HAADF-STEM | Efficient charge separation Cr(VI) adsorption sites Enhanced light harvesting | Photocatalytic reduction Cr(VI) | [48] |
| 1T-MoS2@CdS | Hydrothermal processes | XPS ESR | Enhanced light absorption Improved electron separation Active edge sites | Photocatalytic Nitrogen Fixation | [49] |
| g-C3N4/ZnSnCdS | Hydrothermal processes | XPS | Active sites Improved electron separation | Photocatalytic Nitrogen Fixation | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Li, Y.; Ren, Q.; Zhang, X.; Fan, X.; Liu, X.; Fan, J.; Shen, S.; Tang, Z.; Xue, Y. Unveiling the Role of Sulfur Vacancies in Enhanced Photocatalytic Activity of Hybrids Photocatalysts. Nanomaterials 2024, 14, 1009. https://doi.org/10.3390/nano14121009
Ren Z, Li Y, Ren Q, Zhang X, Fan X, Liu X, Fan J, Shen S, Tang Z, Xue Y. Unveiling the Role of Sulfur Vacancies in Enhanced Photocatalytic Activity of Hybrids Photocatalysts. Nanomaterials. 2024; 14(12):1009. https://doi.org/10.3390/nano14121009
Chicago/Turabian StyleRen, Zhenxing, Yang Li, Qiuyu Ren, Xiaojie Zhang, Xiaofan Fan, Xinjuan Liu, Jinchen Fan, Shuling Shen, Zhihong Tang, and Yuhua Xue. 2024. "Unveiling the Role of Sulfur Vacancies in Enhanced Photocatalytic Activity of Hybrids Photocatalysts" Nanomaterials 14, no. 12: 1009. https://doi.org/10.3390/nano14121009





