Distinguishing the Charge Trapping Centers in CaF2-Based 2D Material MOSFETs
Abstract
1. Introduction
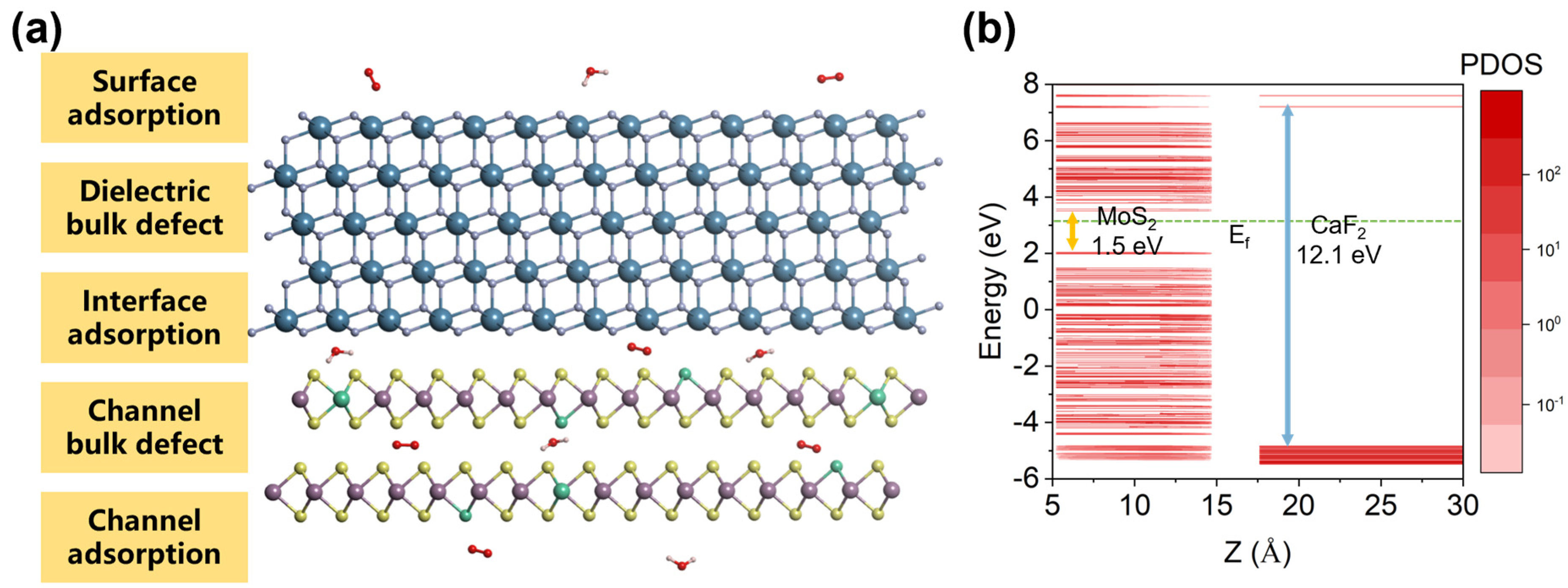
2. Materials and Methods
3. Results
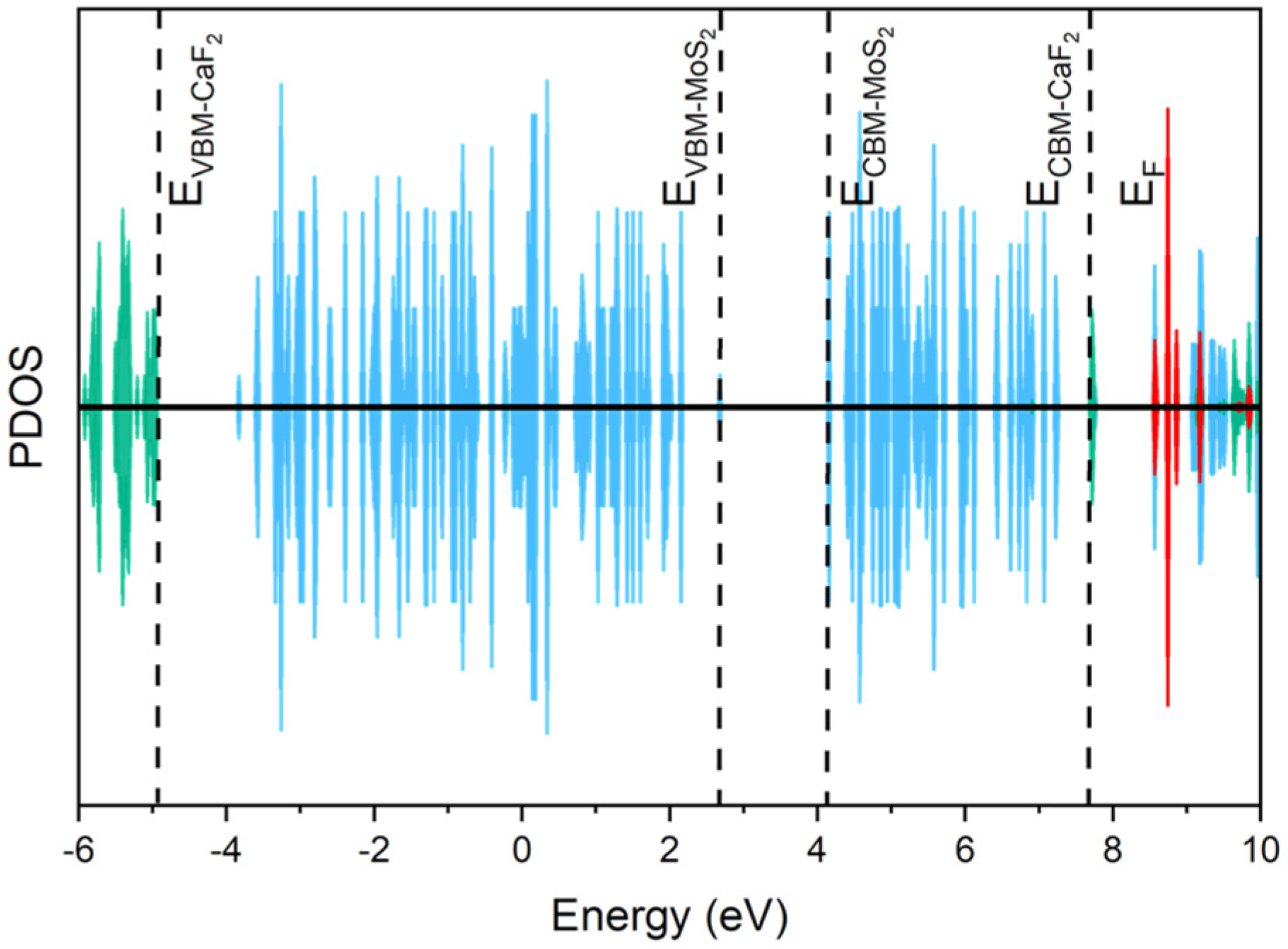
3.1. The Charge-Trapping Centers in CaF2-MoS2
3.1.1. The Charge-Trapping Centers in CaF2
3.1.2. The Charge-Trapping Centers in the Channel
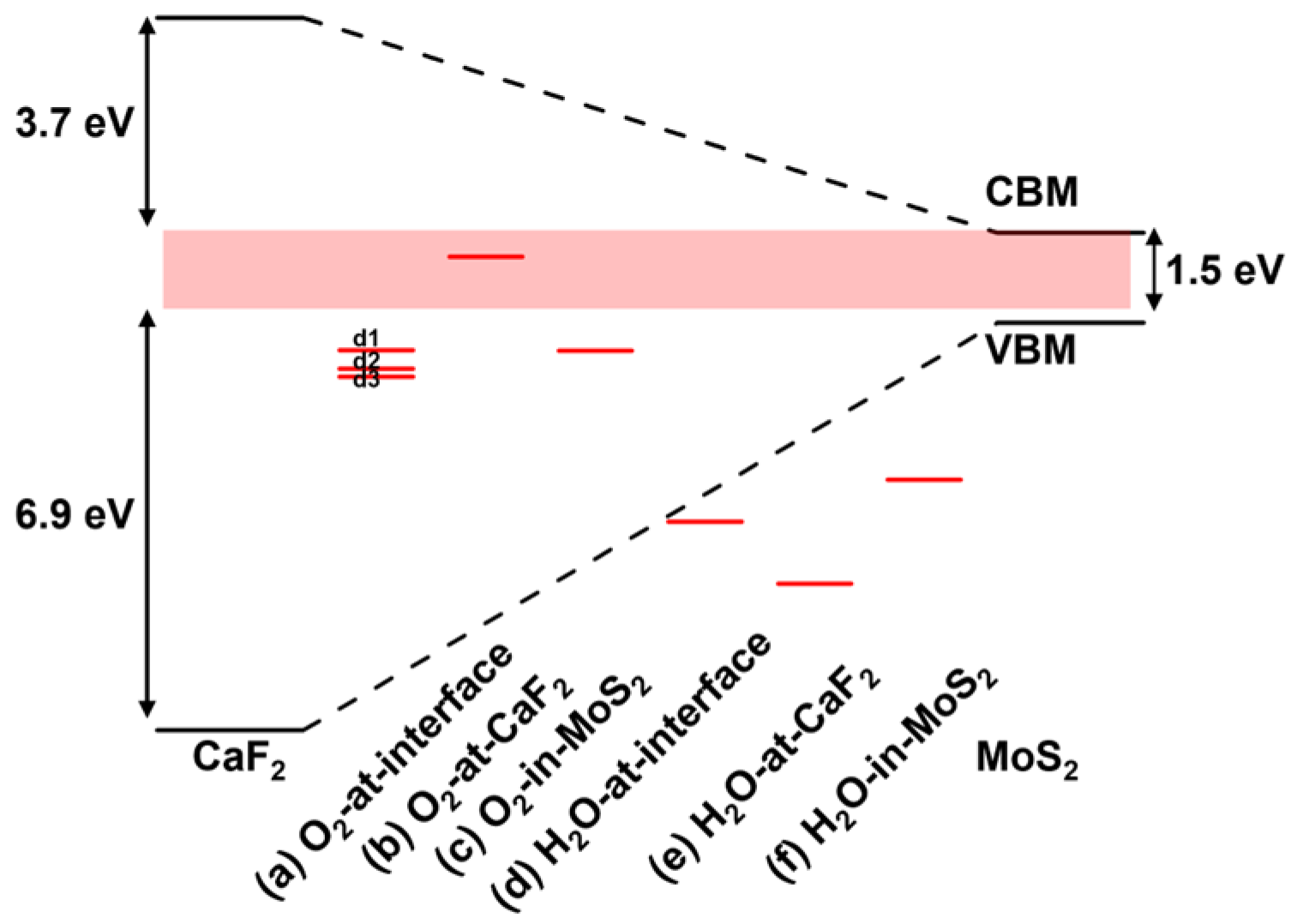
3.1.3. The Charge-Trapping Centers in the Interface and Surface
3.2. The Charge-Trapping Centers in CaF2-MoSi2N4
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-Dimensional Gas of Massless Dirac Fermions in Graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Benka, S.G. Two-Dimensional Atomic Crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-Layer MoS2 Transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black Phosphorus Field-Effect Transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D Materials and van Der Waals Heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Balbuena, J.; Tutor De Ureta, P.; Rivera Ruiz, E.; Mellor Pita, S. Enfermedad de Vogt-Koyanagi-Harada. Med. Clin. 2016, 146, 93–94. [Google Scholar] [CrossRef]
- Wse, L.; Allain, A.; Kis, A. Terms of Use Electron and Hole Mobilities in Single-layer WSe2. ACS Nano 2014, 8, 7180–7185. [Google Scholar]
- Pan, Y.; Jia, K.; Huang, K.; Wu, Z.; Bai, G.; Yu, J.; Zhang, Z.; Zhang, Q.; Yin, H. Near-Ideal Subthreshold Swing MoS 2 Back-Gate Transistors with an Optimized Ultrathin HfO2 Dielectric Layer. Nanotechnology 2019, 30, 095202. [Google Scholar] [CrossRef] [PubMed]
- Bolshakov, P.; Zhao, P.; Azcatl, A.; Hurley, P.K.; Wallace, R.M.; Young, C.D. Improvement in Top-Gate MoS2 Transistor Performance Due to High Quality Backside Al2O3 Layer. Appl. Phys. Lett. 2017, 111, 032110. [Google Scholar] [CrossRef]
- Lee, C.; Rathi, S.; Khan, M.A.; Lim, D.; Kim, Y.; Yun, S.J.; Youn, D.H.; Watanabe, K.; Taniguchi, T.; Kim, G.H. Comparison of Trapped Charges and Hysteresis Behavior in HBN Encapsulated Single MoS2 Flake Based Field Effect Transistors on SiO2 and HBN Substrates. Nanotechnology 2018, 29, 335202. [Google Scholar] [CrossRef] [PubMed]
- Cassabois, G.; Valvin, P.; Gil, B. Hexagonal Boron Nitride Is an Indirect Bandgap Semiconductor. Nat. Photonics 2016, 10, 262–266. [Google Scholar] [CrossRef]
- Geick, R.; Perry, C.H.; Rupprecht, G. Normal Modes in Hexagonal Boron Nitride. Phys. Rev. 1966, 146, 543–547. [Google Scholar] [CrossRef]
- Illarionov, Y.Y.; Vexler, M.I.; Fedorov, V.V.; Suturin, S.M.; Sokolov, N.S. Electrical and Optical Characterization of Au/CaF2/p-Si(111) Tunnel-Injection Diodes. J. Appl. Phys. 2014, 115, 223706. [Google Scholar] [CrossRef]
- Usiskin, R.; Sigle, W.; Kelsch, M.; van Aken, P.A.; Maier, J. Ion Transport in Nanocrystalline CaF2 Films. J. Appl. Phys. 2021, 130, 105301. [Google Scholar] [CrossRef]
- Tyaginov, S.E.; Illarionov, Y.Y.; Vexler, M.I.; Bina, M.; Cervenka, J.; Franco, J.; Kaczer, B.; Grasser, T. Modeling of Deep-Submicron Silicon-Based MISFETs with Calcium Fluoride Dielectric. J. Comput. Electron. 2014, 13, 733–738. [Google Scholar] [CrossRef]
- Illarionov, Y.Y.; Banshchikov, A.G.; Polyushkin, D.K.; Wachter, S.; Knobloch, T.; Thesberg, M.; Mennel, L.; Paur, M.; Stöger-Pollach, M.; Steiger-Thirsfeld, A.; et al. Ultrathin Calcium Fluoride Insulators for Two-Dimensional Field-Effect Transistors. Nat. Electron. 2019, 2, 230–235. [Google Scholar] [CrossRef]
- Foster, A.S.; Trevethan, T.; Shluger, A.L. Structure and Diffusion of Intrinsic Defects, Adsorbed Hydrogen, and Water Molecules at the Surface of Alkali-Earth Fluorides Calculated Using Density Functional Theory. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 80, 115421. [Google Scholar] [CrossRef]
- Song, H.; Zhou, F.; Yan, S.; Su, X.; Wu, H.; Wu, Q.; Gao, Y.; Chen, R.; Chen, T.; Yao, J.; et al. Enhanced Transport and Optoelectronic Properties of van Der Waals Materials on CaF2 Films. Nano Lett. 2023, 23, 4983–4990. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Banshchikov, A.G.; Illarionov, Y.Y.; Frammelsberger, W.; Knobloch, T.; Hui, F.; Sokolov, N.S.; Grasser, T.; Lanza, M. Dielectric Properties of Ultrathin CaF2 Ionic Crystals. Adv. Mater. 2020, 32, e2002525. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, H.; Cui, W. High-k Monolayer CaF2 as the Gate Dielectric for Two-Dimensional SiC-Based Field-Effect Transistors. ACS Appl. Electron. Mater. 2023, 5, 5082–5092. [Google Scholar] [CrossRef]
- Vishwanath, S.; Liu, X.; Rouvimov, S.; Mende, P.C.; Azcatl, A.; McDonnell, S.; Wallace, R.M.; Feenstra, R.M.; Furdyna, J.K.; Jena, D.; et al. Comprehensive Structural and Optical Characterization of MBE Grown MoSe2 on Graphite, CaF2 and Graphene. 2D Mater. 2015, 2, 024007. [Google Scholar] [CrossRef]
- Wen, C.; Lanza, M. Calcium Fluoride as High-k Dielectric for 2D Electronics. Appl. Phys. Rev. 2021, 8, 021307. [Google Scholar] [CrossRef]
- Grasser, T.; Kaczer, B.; Goes, W.; Reisinger, H.; Aichinger, T.; Hehenberger, P.; Wagner, P.J.; Schanovsky, F.; Franco, J.; Toledano Luque, M.; et al. The Paradigm Shift in Understanding the Bias Temperature Instability: From Reaction-Diffusion to Switching Oxide Traps. IEEE Trans. Electron Devices 2011, 58, 3652–3666. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, X.; Shu, J.; Liu, B.; Yin, J.; Guan, C.; Han, Y.; Gao, S.; Chen, Q. Charge Trapping at the MoS2-SiO2 Interface and Its Effects on the Characteristics of MoS2 Metal-Oxide-Semiconductor Field Effect Transistors. Appl. Phys. Lett. 2015, 106, 103109. [Google Scholar] [CrossRef]
- Illarionov, Y.Y.; Waltl, M.; Bartolomeo, A.D.; Genovese, L.; Illarionov, Y.Y.; Rzepa, G.; Waltl, M.; Knobloch, T.; Grill, A. The Role of Charge Trapping in MoS2/SiO2 and MoS2/HBN Field-Effect Transistors. 2D Mater. 2016, 3, 035004. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Genovese, L.; Giubileo, F.; Iemmo, L.; Luongo, G.; Foller, T.; Schleberger, M. Hysteresis in the Transfer Characteristics of MoS2 Transistors. 2D Mater. 2018, 5, 015014. [Google Scholar] [CrossRef]
- Late, D.J.; Liu, B.; Matte, H.S.S.R.; Dravid, V.P.; Rao, C.N.R. Hysteresis in Single-Layer MoS2 Field Effect Transistors. ACS Nano 2012, 6, 5635–5641. [Google Scholar] [CrossRef]
- Park, Y.; Baac, H.W.; Heo, J.; Yoo, G. Thermally Activated Trap Charges Responsible for Hysteresis in Multilayer MoS2 Field-Effect Transistors. Appl. Phys. Lett. 2016, 108, 083102. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.Y.; Zeng, L.; Chen, J.; Wang, R.; Wang, L.W.; Wu, Y.; Jiang, X. Defects Induced Charge Trapping/Detrapping and Hysteresis Phenomenon in MoS2 Field-Effect Transistors: Mechanism Revealed by Anharmonic Marcus Charge Transfer Theory. ACS Appl. Mater. Interfaces 2022, 14, 2185–2193. [Google Scholar] [CrossRef]
- Xiong, T.; Yang, J.; Deng, H.-X.; Wei, Z.; Liu, Y.-Y. The Mechanism of Improving Germanium Metal–Oxide–Semiconductor Field-Effect Transistors’ Reliability by High-k Dielectric and Yttrium-Doping: From the View of Charge Trapping. J. Appl. Phys. 2022, 132, 174506. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, F.; Wang, R.; Luo, J.W.; Jiang, X.; Huang, R.; Li, S.S.; Wang, L.W. Characterizing the Charge Trapping across Crystalline and Amorphous Si/SiO2/HfO2 Stacks from First-Principle Calculations. Phys. Rev. Appl. 2019, 12, 064012. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zheng, F.; Jiang, X.; Luo, J.W.; Li, S.S.; Wang, L.W. Ab Initio Investigation of Charge Trapping Across the Crystalline- Si -Amorphous- SiO2 Interface. Phys. Rev. Appl. 2019, 11, 044058. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Jiang, X. Physics of Hole Trapping Process in High-k Gate Stacks: A Direct Simulation Formalism for the Whole Interface System Combining Density-Functional Theory and Marcus Theory. In Proceedings of the 2018 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 1–5 December 2018. [Google Scholar] [CrossRef]
- Illarionov, Y.Y.; Knobloch, T.; Uzlu, B.; Banshchikov, A.G.; Ivanov, I.A.; Sverdlov, V.; Otto, M.; Stoll, S.L.; Vexler, M.I.; Waltl, M.; et al. Variability and High Temperature Reliability of Graphene Field-Effect Transistors with Thin Epitaxial CaF2 Insulators. npj 2D Mater. Appl. 2024, 8, 1–10. [Google Scholar] [CrossRef]
- Durante, O.; Intonti, K.; Viscardi, L.; De Stefano, S.; Faella, E.; Kumar, A.; Pelella, A.; Romeo, F.; Giubileo, F.; Alghamdi, M.S.G.; et al. Subthreshold Current Suppression in ReS2 Nanosheet-Based Field-Effect Transistors at High Temperatures. ACS Appl. Nano Mater. 2023, 6, 21663–21670. [Google Scholar] [CrossRef] [PubMed]
- Ishibe, T.; Tomeda, A.; Komatsubara, Y.; Kitaura, R.; Uenuma, M.; Uraoka, Y.; Yamashita, Y.; Nakamura, Y. Carrier and Phonon Transport Control by Domain Engineering for High-Performance Transparent Thin Film Thermoelectric Generator. Appl. Phys. Lett. 2021, 118, 151601. [Google Scholar] [CrossRef]
- Xia, J.; Sohail, M.; Nazeeruddin, M.K. Efficient and Stable Perovskite Solar Cells by Tailoring of Interfaces. Adv. Mater. 2023, 35, e2211324. [Google Scholar] [CrossRef]
- Li, Z.; Baskurt, M.; Sahin, H.; Gao, S.; Kang, J. Electronic Properties of Intrinsic Vacancies in Single-Layer CaF2 and Its Heterostructure with Monolayer MoS2. J. Appl. Phys. 2021, 130, 055301. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, B.; Xu, L.; Yan, J.; Zhao, W.; Zhang, Z.; Zhang, Z.; Lu, J. Sub-5 Nm Monolayer MoS2 Transistors toward Low-Power Devices. ACS Appl. Electron. Mater. 2021, 3, 1560–1571. [Google Scholar] [CrossRef]
- Hua, Q.; Gao, G.; Jiang, C.; Yu, J.; Sun, J.; Zhang, T.; Gao, B.; Cheng, W.; Liang, R.; Qian, H.; et al. Atomic Threshold-Switching Enabled MoS2 Transistors towards Ultralow-Power Electronics. Nat. Commun. 2020, 11, 6207. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, N.; Wang, Q.; Ouyang, C.; He, C.; Zhang, L.; Du, L.; Yang, W.; Yang, R.; Shi, D.; et al. Optoelectronic Synapses Based on MoS2 Transistors for Accurate Image Recognition. Adv. Mater. Interfaces 2022, 9, 2201558. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Kumar, A.; Durante, O.; Sessa, A.; Faella, E.; Viscardi, L.; Intonti, K.; Giubileo, F.; Martucciello, N.; Romano, P.; et al. Temperature-Dependent Photoconductivity in Two-Dimensional MoS2 Transistors. Mater. Today Nano 2023, 24, 100382. [Google Scholar] [CrossRef]
- Hong, Y.L.; Liu, Z.; Wang, L.; Zhou, T.; Ma, W.; Xu, C.; Feng, S.; Chen, L.; Chen, M.L.; Sun, D.M.; et al. Chemical Vapor Deposition of Layered Two-Dimensional MoSi2N4 Materials. Science 2020, 369, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Jiang, Z.Y.; Zheng, J.M.; Lin, Y.M.; Du, A. Theoretical Study of a Novel WSi2N4/MoSi2N4 Heterostructure with Ultrafast Carrier Transport. J. Phys. Chem. C 2022, 126, 11380–11388. [Google Scholar] [CrossRef]
- Mortazavi, B.; Javvaji, B.; Shojaei, F.; Rabczuk, T.; Shapeev, A.V.; Zhuang, X. Exceptional Piezoelectricity, High Thermal Conductivity and Stiffness and Promising Photocatalysis in Two-Dimensional MoSi2N4 Family Confirmed by First-Principles. Nano Energy 2021, 82, 105716. [Google Scholar] [CrossRef]
- Yang, J.-S.; Zhao, L.; LI, S.-Q.; Liu, H.; Wang, L.; Chen, M.; Gao, J.; Zhao, J. Accurate Electronic Properties and Non-Linear Optical Response of Two-Dimensional MA2Z4. Nanoscale 2021, 13, 5479–5488. [Google Scholar] [CrossRef]
- Jia, W.; Fu, J.; Cao, Z.; Wang, L.; Chi, X.; Gao, W.; Wang, L.W. Fast Plane Wave Density Functional Theory Molecular Dynamics Calculations on Multi-GPU Machines. J. Comput. Phys. 2013, 251, 102–115. [Google Scholar] [CrossRef]
- Jia, W.; Cao, Z.; Wang, L.; Fu, J.; Chi, X.; Gao, W.; Wang, L.W. The Analysis of a Plane Wave Pseudopotential Density Functional Theory Code on a GPU Machine. Comput. Phys. Commun. 2013, 184, 9–18. [Google Scholar] [CrossRef]
- Hamann, D.R. Optimized Norm-Conserving Vanderbilt Pseudopotentials. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 88, 085117. [Google Scholar] [CrossRef]
- Heyd, J.; Peralta, J.E.; Scuseria, G.E.; Martin, R.L. Energy Band Gaps and Lattice Parameters Evaluated with the Heyd-Scuseria-Ernzerhof Screened Hybrid Functional. J. Chem. Phys. 2005, 123, 174101. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.; Guo, Y.; Robertson, J. Electronic Properties of CaF2 bulk and Interfaces. J. Appl. Phys. 2022, 131, 215302. [Google Scholar] [CrossRef]
- Zhou, W.; Zou, X.; Najmaei, S.; Liu, Z.; Shi, Y.; Kong, J.; Lou, J.; Ajayan, P.M.; Yakobson, B.I.; Idrobo, J.C. Intrinsic Structural Defects in Monolayer Molybdenum Disulfide. Nano Lett. 2013, 13, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Falabretti, B. Band Offsets of High K Gate Oxides on III-V Semiconductors. J. Appl. Phys. 2006, 100, 014111. [Google Scholar] [CrossRef]
- Robertson, J. Band Offsets, Schottky Barrier Heights, and Their Effects on Electronic Devices. J. Vac. Sci. Technol. A Vacuum Surfaces Film. 2013, 31, 050821. [Google Scholar] [CrossRef]
- Robertson, J. Band Offsets of Wide-Band-Gap Oxides and Implications for Future Electronic Devices. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2000, 18, 1785. [Google Scholar] [CrossRef]

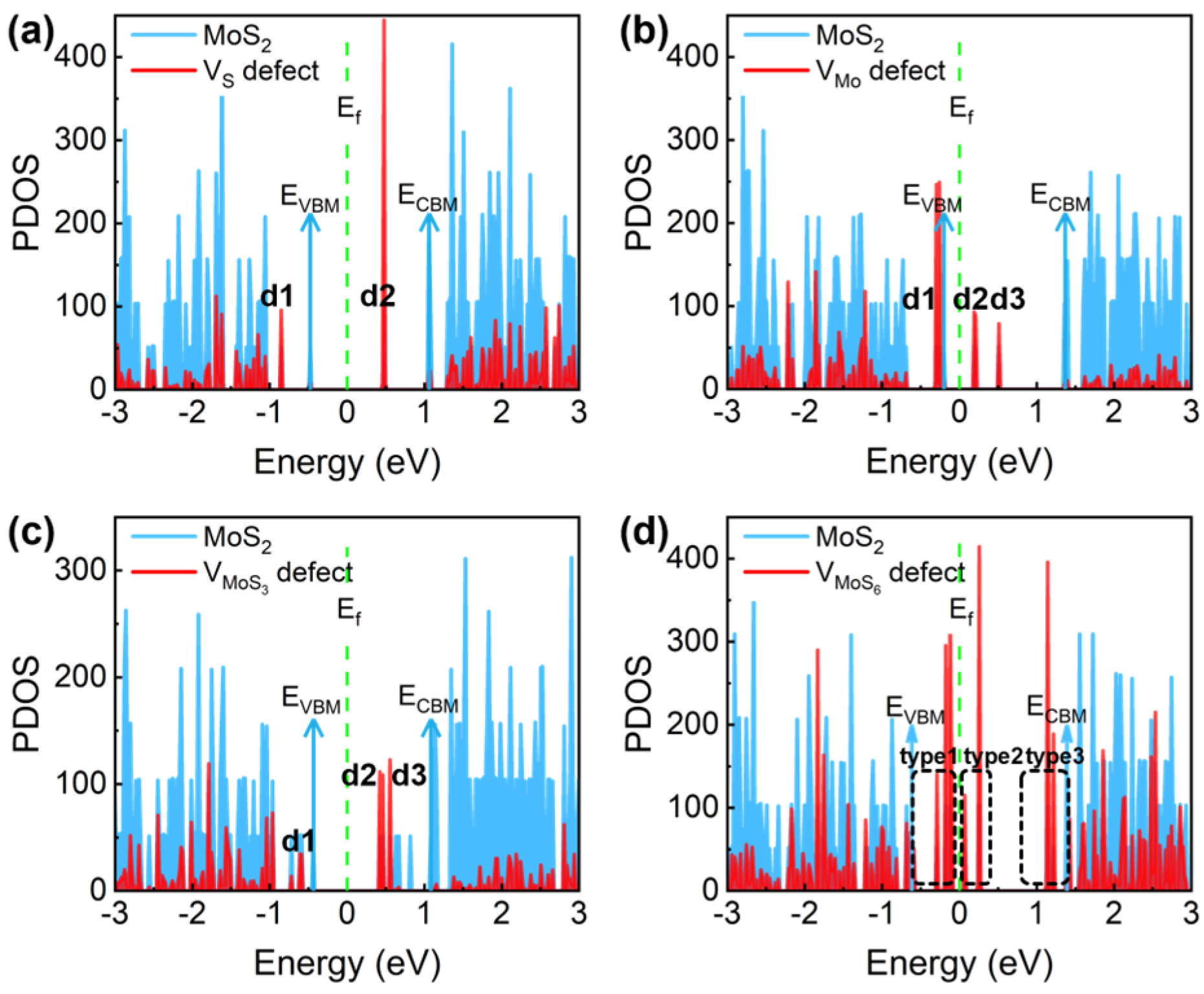
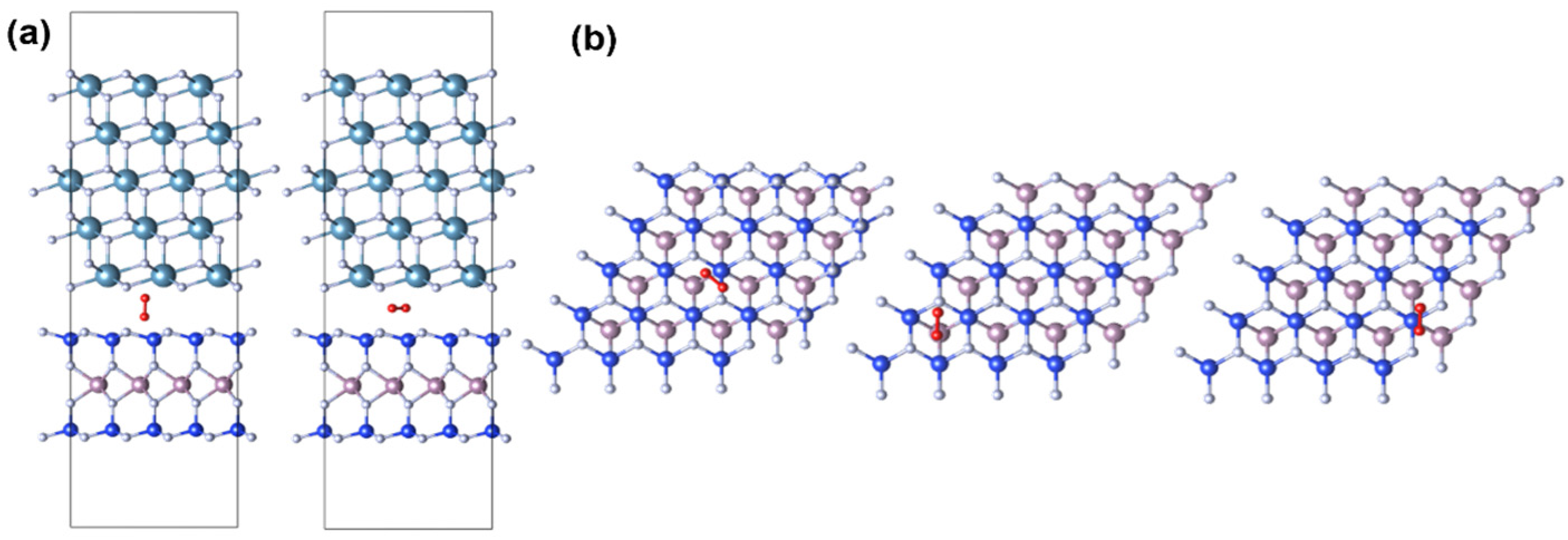

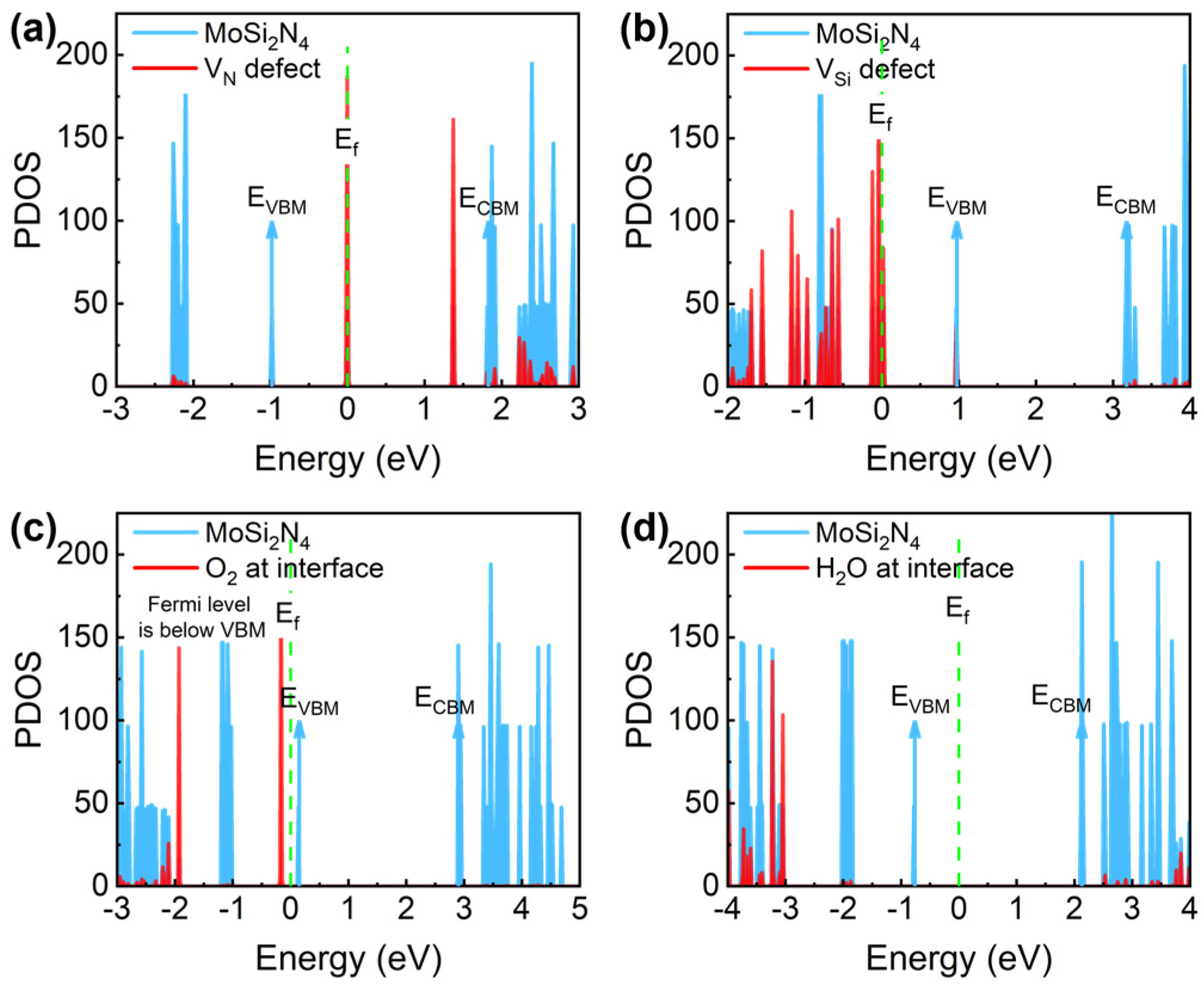
| Defect Types | Defect State | ΔE- VBM (eV) | ΔE- CBM (eV) | n-FET Importance | p-FET Importance | Fromation Energy (eV) | Overall Importance |
|---|---|---|---|---|---|---|---|
| VS | d1 | −0.38 | −1.91 | ✓ | ✗ | 2.91 | ✓ |
| d2 | 0.95 | −0.57 | ✓ | ✓ | |||
| VMo | d1 | −0.06 | −1.63 | ✓ | ✗ | 8.52 | ✓ |
| d2 | 0.40 | −1.17 | ✗ | ✓ | |||
| d3 | 0.71 | −0.86 | ✗ | ✓ | |||
| VMoS3 | d1 | −0.25 | −1.78 | ✓ | ✗ | 11.81 | ✓ |
| d2 | 0.89 | −0.64 | ✗ | ✓ | |||
| d3 | 0.99 | −0.53 | ✗ | ✓ | |||
| VMoS6 | type1 | <0.50 | >1.50 | ✓ | ✗ | 21.41 | ✗ |
| type2 | <1.00 | >1.00 | ✗ | ✗ | |||
| type3 | >1.75 | <0.25 | ✗ | ✓ | |||
| O2 at interface | d1 | −0.99 | −2.45 | ✓ | ✗ | 0.68 | ✓ |
| d2 | −0.55 | −2.00 | ✓ | ✗ | |||
| d3 | −0.85 | −2.31 | ✓ | ✗ | |||
| H2O at interface | −3.42 | −4.91 | ✗ | ✗ | 0.61 | ✗ | |
| O2 in MoS2 | −0.37 | −2.01 | ✓ | ✗ | 2.35 | ✓ | |
| O2 at surface | 1.11 | −0.41 | ✗ | ✓ | 2.25 | ✓ |
| Defect Types | Defect State | ΔE- VBM (eV) | ΔE- CBM (eV) | n-FET Importance | p-FET Importance | Fromation Energy (eV) | Overall Importance |
|---|---|---|---|---|---|---|---|
| VN | d1 | 0.98 | −1.83 | ✓ | ✗ | 5.97 | ✓ |
| d2 | 2.36 | −0.45 | ✗ | ✓ | |||
| VSi | d1 | −1.01 | −3.23 | ✗ | ✗ | 11.15 | ✓ |
| d2 | 0.00 | −2.22 | ✓ | ✗ | |||
| O2 at interface | −0.32 | −3.07 | ✓ | ✗ | 0.19 | ✓ | |
| H2O at interface | −2.29 | −5.17 | ✗ | ✗ | 0.34 | ✗ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Xiong, T.; Gong, J.; Liu, Y.-Y. Distinguishing the Charge Trapping Centers in CaF2-Based 2D Material MOSFETs. Nanomaterials 2024, 14, 1038. https://doi.org/10.3390/nano14121038
Zhao Z, Xiong T, Gong J, Liu Y-Y. Distinguishing the Charge Trapping Centers in CaF2-Based 2D Material MOSFETs. Nanomaterials. 2024; 14(12):1038. https://doi.org/10.3390/nano14121038
Chicago/Turabian StyleZhao, Zhe, Tao Xiong, Jian Gong, and Yue-Yang Liu. 2024. "Distinguishing the Charge Trapping Centers in CaF2-Based 2D Material MOSFETs" Nanomaterials 14, no. 12: 1038. https://doi.org/10.3390/nano14121038
APA StyleZhao, Z., Xiong, T., Gong, J., & Liu, Y.-Y. (2024). Distinguishing the Charge Trapping Centers in CaF2-Based 2D Material MOSFETs. Nanomaterials, 14(12), 1038. https://doi.org/10.3390/nano14121038





