Evaluating Fluorinated-Aniline Units with Functionalized Spiro[Fluorene-9,9′-Xanthene] as Hole-Transporting Materials in Perovskite Solar Cells and Light-Emitting Diodes
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Fabrication of PSCs
2.3. Fabrication of PeLEDs
3. Results and Discussion
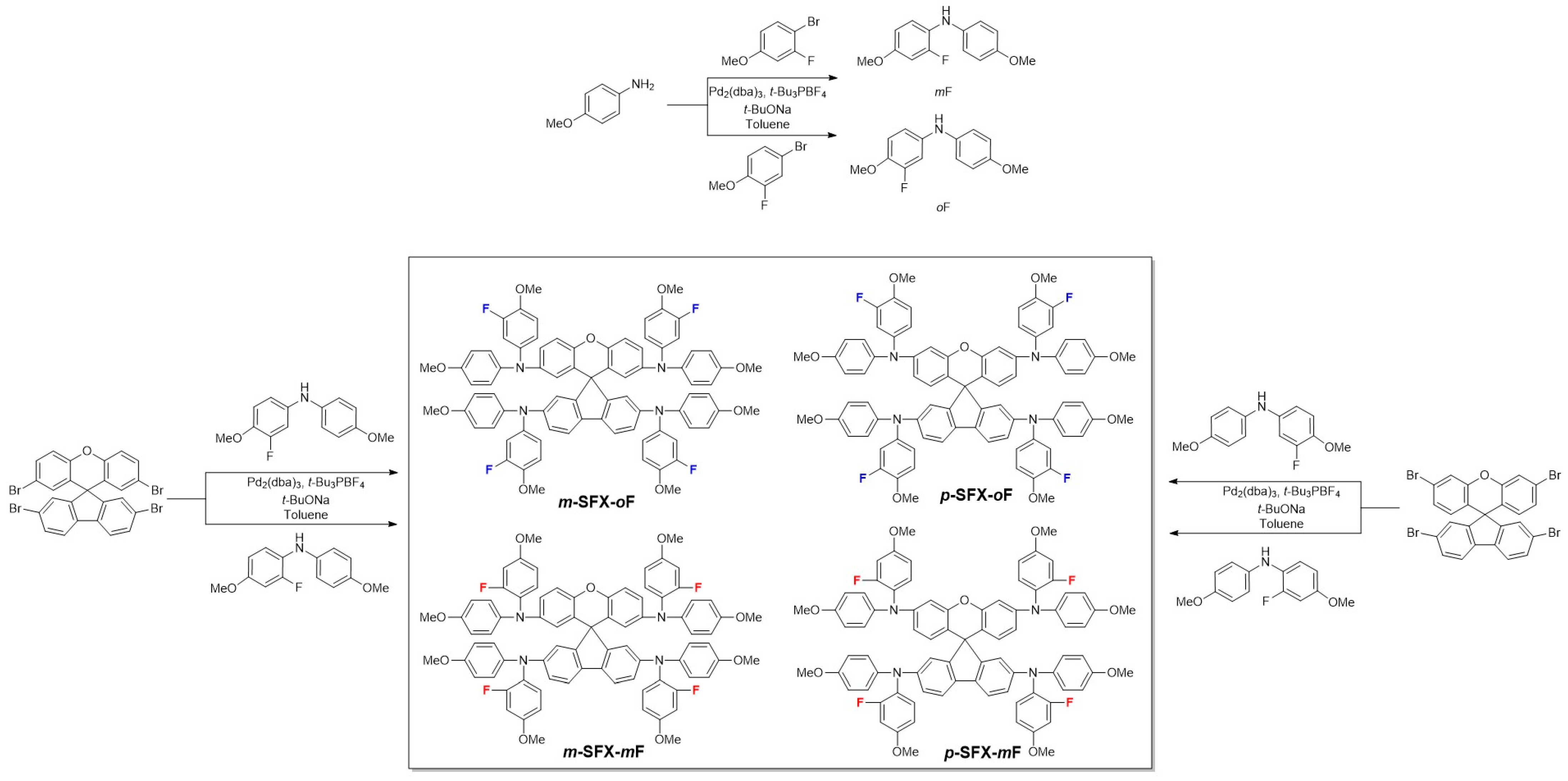
3.1. Synthesis, Theoretical Calculation, Photophysical and Electrochemical Properties
3.2. PSC Performance
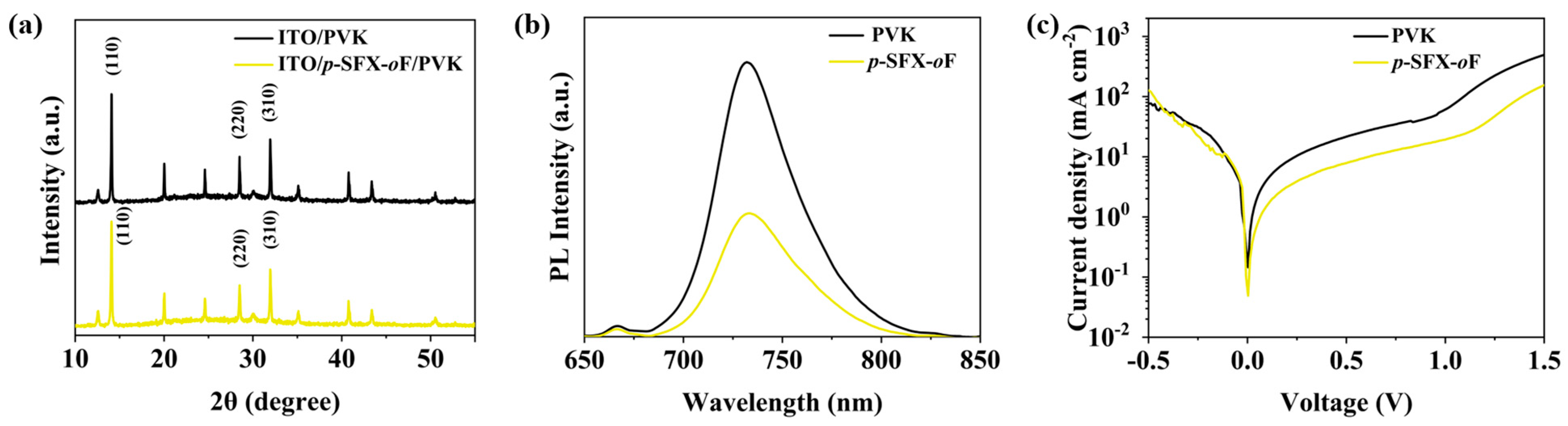
3.3. PeLED Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stoumpos, C.C.; Kanatzidis, M.G. Halide Perovskites: Poor Man’s High-Performance Semiconductors. Adv. Mater. 2016, 28, 5778–5793. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, R.; Chen, H.; Liu, Y.; Zhang, S.; Fang, T.; Guo, R.; Zhang, J.; Shan, Q.; Jin, Y.; et al. A Demulsification–Crystallization Model for High-Quality Perovskite Nanocrystals. Adv. Mater. 2023, 35, 2206969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Jiang, Q.; Wang, P.; Yin, Z.; Zhang, X.; Tan, H.; Yang, Y.; Wei, M.; Sutherland, B.R.; et al. Ultra-bright and highly efficient inorganic based perovskite light-emitting diodes. Nat. Commun. 2017, 8, 15640. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, Q.; Kang, S.; Ouyang, T.; Chen, Q.; Liu, X.; Xia, Z.; Yang, Z.; Zhang, Q.; Qiu, J.; et al. Three-dimensional laser-assisted patterning of blue-emissive metal halide perovskite nanocrystals inside a glass with switchable photoluminescence. ACS Nano 2020, 14, 3150–3158. [Google Scholar] [CrossRef]
- Konidakis, I.; Maksudov, T.; Serpetzoglou, E.; Kakavelakis, G.; Kymakis, E.; Stratakis, E. Improved charge carrier dynamics of CH3NH3PbI3 perovskite films synthesized by means of laser-assisted crystallization. ACS Appl. Energy Mater. 2018, 1, 5101–5111. [Google Scholar] [CrossRef]
- Pham, H.; Hu, H.; Feron, K.; Manzhos, S.; Wang, H.; Lam, Y.; Sona, P. Thienylvinylenethienyl and Naphthalene Core Substituted with Triphenylamines—Highly Efficient Hole Transporting Materials and Their Comparative Study for Inverted Perovskite Solar Cell. Sol. RRL 2017, 1, 1700105. [Google Scholar] [CrossRef]
- Haque, F.; Yi, H.; Lim, J.; Duan, L.; Pham, H.; Sonar, P.; Uddin, A. Small molecular material as an interfacial layer in hybrid inverted structure perovskite solar cells. Mater. Sci. Semicond. Process. 2020, 108, 104908. [Google Scholar] [CrossRef]
- Rombach, F.M.; Haque, S.A.; Macdonald, T.J. Lessons learned from spiro-OMeTAD and PTAA in perovskite solar cells. Energy Environ. Sci. 2021, 14, 5161–5190. [Google Scholar] [CrossRef]
- Saragi, T.P.I.; Spehr, T.; Siebert, A.; Fuhrmann-Lieker, T.; Salbeck, J. Spiro Compounds for Organic Optoelectronics. Chem. Rev. 2007, 107, 1011–1065. [Google Scholar] [CrossRef]
- Luo, X.; Boschloo, G.; Kloo, L.; Sun, L.; Xu, B. Spiro[fluorene-9,9′-xanthene]-Based Hole-Transporting Materials for Photovoltaics: Molecular Design, Structure–Property Relationship, and Applications. Acc. Mater. Res. 2024, 5, 220–235. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Ren, B.-Y.; Sun, Y.-G.; Xie, L.-H.; Huang, W. Research Progress of Hole Transport Materials Based on Spiro Aromatic-Skeleton in Perovskite Solar Cells. Acta Chim. Sin. 2021, 79, 1181–1196. [Google Scholar] [CrossRef]
- Li, C.-S.; Ren, B.-Y.; Sun, Y.-G. Low-cost and stable SFX-based semiconductor materials in organic optoelectronics. Resour. Chem. Mater. 2023, 2, 100–109. [Google Scholar] [CrossRef]
- Bach, U.; Lupo, D.; Comte, P.; Moser, J.E.; Weissörtel, F.; Salbeck, J.; Spreitzer, H.; Grätzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 1998, 395, 583–585. [Google Scholar] [CrossRef]
- Kirkpatrick, J.; Nelson, J. Theoretical study of the transfer integral and density of states in spiro-linked triphenylamine derivatives. J. Chem. Phys. 2005, 123, 084703. [Google Scholar] [CrossRef]
- Agarwala, P.; Kabra, D. A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): Evolution and molecular engineering. J. Mater. Chem. A 2017, 5, 1348–1373. [Google Scholar] [CrossRef]
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J.; et al. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3-V voltage loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef]
- Du, G.; Yang, L.; Zhang, J. Light Soaking Induced Halide Doping of Evaporated Spiro-OMeTAD in Perovskite Solar Cells. Laser Photonics Rev. 2023, 17, 2200475. [Google Scholar] [CrossRef]
- Liu, T.; Chen, K.; Hu, Q.; Zhu, R.; Gong, Q. Inverted Perovskite Solar Cells: Progresses and Perspectives. Adv. Energy Mater. 2016, 6, 1600457. [Google Scholar] [CrossRef]
- Khan, D.; Liu, X.; Qu, G.; Nath, A.R.; Xie, P.; Xu, Z.-X. Nexuses Between the Chemical Design and Performance of Small Molecule Dopant-Free Hole Transporting Materials in Perovskite Solar Cells. Small 2023, 19, 2205926. [Google Scholar] [CrossRef]
- Du, P.; Wang, L.; Li, J.; Luo, J.; Ma, Y.; Tang, J.; Zhai, T. Thermal Evaporation for Halide Perovskite Optoelectronics: Fundamentals, Progress, and Outlook. Adv. Opt. Mater. 2022, 10, 2101770. [Google Scholar] [CrossRef]
- Liu, K.; Yao, Y.; Wang, J.; Zhu, L.; Sun, M.; Ren, B.; Xie, L.; Luo, Y.; Meng, Q.; Zhan, X. Spiro[fluorene-9,9′-xanthene]-based hole transporting materials for efficient perovskite solar cells with enhanced stability. Mater. Chem. Front. 2017, 1, 100–110. [Google Scholar] [CrossRef]
- Gao, K.; Xu, B.; Hong, C.; Shi, X.; Liu, H.; Li, X.; Xie, L.; Jen, A.K.Y. Di-Spiro-Based Hole-Transporting Materials for Highly Efficient Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1800809. [Google Scholar] [CrossRef]
- Yan, L.; Qiu, S.; Yu, B.; Huang, J.; Qiu, J.; Zhang, C.; Guo, F.; Ynag, Y.; Mai, Y. Synergistic Passivation of Perovskite Absorber Films for Efficient Four-Terminal Perovskite/Silicon Tandem Solar Cells. Adv. Energy Sustain. Res. 2022, 3, 2100199. [Google Scholar] [CrossRef]
- Lee, K.-M.; Huang, Y.-S.; Chiu, W.-H.; Huang, Y.-K.; Chen, G.; Adugna, G.B.; Li, S.-R.; Lin, F.-J.; Lu, S.-I.; Hsieh, H.-C.; et al. Fluorinated Pentafulvalene-Fused Hole-Transporting Material Enhances the Performance of Perovskite Solar Cells with Efficiency Exceeding 23%. Adv. Funct. Mater. 2023, 33, 2306367. [Google Scholar] [CrossRef]
- Wu, J.; Hu, M.; Zhang, L.; Song, G.; Li, Y.; Tan, W.; Tian, Y.; Xu, B. Fluorinated Cross-linkable and Dopant-free hole transporting materials for efficient and stable perovskite solar cells. Chem. Eng. J. 2021, 422, 130124. [Google Scholar] [CrossRef]
- Li, Z.; Yun, Y.; Huang, H.; Ding, Z.; Li, X.; Zhao, B.; Huang, W. Fluorine substitution position effects on spiro(fluorene-9,9′-xanthene) cored hole transporting materials for high-performance planar perovskite solar cells. J. Energy Chem. 2021, 57, 341–350. [Google Scholar] [CrossRef]
- Liu, K.; Dai, S.; Meng, F.; Shi, J.; Li, Y.; Wu, J.; Meng, Q.; Zhan, X. Fluorinated fused nonacyclic interfacial materials for efficient and stable perovskite solar cells. J. Mater. Chem. A 2017, 5, 21414–21421. [Google Scholar] [CrossRef]
- Philippe, B.; Jacobsson, T.J.; Correa-Baena, J.-P.; Jena, N.K.; Banerjee, A.; Chakraborty, S.; Cappel, U.B.; Ahuja, R.; Hagfeldt, A.; Odelius, M.; et al. Valence Level Character in a Mixed Perovskite Material and Determination of the Valence Band Maximum from Photoelectron Spectroscopy: Variation with Photon Energy. J. Phys. Chem. C 2017, 121, 26655–26666. [Google Scholar] [CrossRef]
- Liu, X.; Ding, B.; Han, M.; Yang, Z.; Chen, J.; Shi, P.; Xue, X.; Ghadari, R.; Zhang, X.; Wang, R.; et al. Extending the π-Conjugated System in Spiro-Type Hole Transport Material Enhances the Efficiency and Stability of Perovskite Solar Modules. Angew. Chem. Int. Ed. 2023, 62, e202304350. [Google Scholar] [CrossRef]
- Jiang, Z.; Du, T.; Lin, C.-T.; Macdonald, T.J.; Chen, J.; Chin, Y.-C.; Xu, W.; Ding, B.; Kim, J.-S.; Durrant, J.R.; et al. Deciphering the Role of Hole Transport Layer HOMO Level on the Open Circuit Voltage of Perovskite Solar Cells. Adv. Mater. Interfaces 2023, 10, 2201737. [Google Scholar] [CrossRef]
- Tvingstedt, K.; Gil-Escrig, L.; Momblona, C.; Rieder, P.; Kiermasch, D.; Sessolo, M.; Baumann, A.; Bolink, H.J.; Dyakonov, V. Removing Leakage and Surface Recombination in Planar Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 424–430. [Google Scholar] [CrossRef]
- Tress, W.; Marinova, N.; Ingans, O.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Graetzel, M. Predicting the Open-Circuit Voltage of CH3NH3PbI3 Perovskite Solar Cells Using Electroluminescence and Photovoltaic Quantum Efficiency Spectra: The Role of Radiative and Non-Radiative Recombination. Adv. Energy Mater. 2015, 5, 1400812. [Google Scholar] [CrossRef]
- Yu, J.C.; Badgujar, S.; Jung, E.D.; Singh, V.K.; Kim, D.W.; Gierschner, J.; Lee, E.; Kim, Y.S.; Cho, S.; Kwon, M.S. Highly Efficient and Stable Inverted Perovskite Solar Cell Obtained via Treatment by Semiconducting Chemical Additive. Adv. Mater. 2019, 31, 1805554. [Google Scholar] [CrossRef]
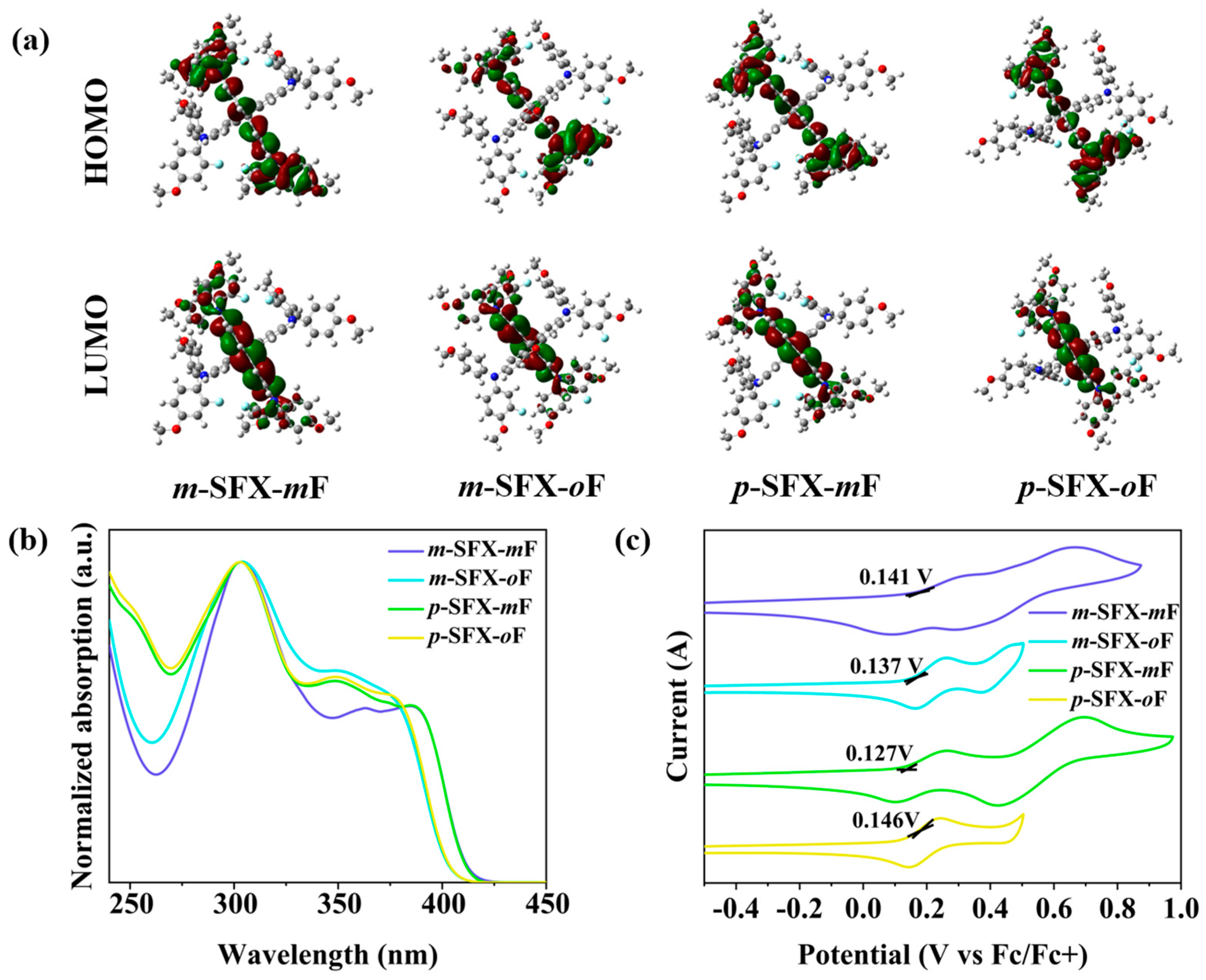


| Compound | λabs a [nm] | λPL b [nm] | Eg c [eV] | EHOMO d [eV] | Td [°C] |
|---|---|---|---|---|---|
| m-SFX-mF | 303 ± 1 | 423 ± 1 | 2.99 | −5.24 | 395 |
| m-SFX-oF | 304 ± 1 | 413 ± 1 | 3.06 | −5.24 | 398 |
| p-SFX-mF | 302 ± 1 | 423 ± 1 | 2.99 | −5.23 | 403 |
| p-SFX-oF | 303 ± 1 | 413 ± 1 | 3.05 | −5.25 | 425 |
| HTMs | Voc [V] | Jsc [mA·cm−2] | FF [%] | PCE [%] | |
|---|---|---|---|---|---|
| Average | Maximum | ||||
| m-SFX-mF | 1.07 ± 0.01 | 17.72 ± 0.76 | 63.01 ± 9.56 | 11.68 | 13.82 |
| m-SFX-oF | 1.14 ± 0.01 | 17.52 ± 1.21 | 63.64 ± 8.40 | 12.70 | 15.27 |
| p-SFX-mF | 1.03 ± 0.03 | 17.64 ± 0.46 | 70.09 ± 4.65 | 12.59 | 13.52 |
| p-SFX-oF | 1.13 ± 0.01 | 17.99 ± 0.62 | 72.63 ± 1.72 | 14.29 | 15.21 |
| Device | V a [V] | CE b [cd·A−1] | PE b [lm·W−1] | EQE b [%] | Lmax [cd·m−2] | CIE c |
|---|---|---|---|---|---|---|
| m-SFX-mF | 3.3, 3.8 | 4.94, 2.79 | 4.69, 1.82 | 3.15, 1.69 | 5182 | (0.05, 0.53) |
| m-SFX-oF | 3.3, 4.0 | 3.81, 2.19 | 3.63, 1.29 | 2.45, 1.30 | 3444 | (0.05, 0.55) |
| p-SFX-mF | 3.0, 4.7 | 4.87, 2.25 | 5.09, 1.27 | 3.16, 1.31 | 3152 | (0.05, 0.55) |
| p-SFX-oF | 3.0, 4.1 | 4.24, 2.37 | 4.44, 1.45 | 2.71, 1.43 | 4377 | (0.05, 0.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Sun, L.; Liu, Q.-L.; Ren, B.-Y.; Guo, R.-D.; Wang, L.; Sun, Y.-G.; Wang, Y.-S. Evaluating Fluorinated-Aniline Units with Functionalized Spiro[Fluorene-9,9′-Xanthene] as Hole-Transporting Materials in Perovskite Solar Cells and Light-Emitting Diodes. Nanomaterials 2024, 14, 1044. https://doi.org/10.3390/nano14121044
Liu K, Sun L, Liu Q-L, Ren B-Y, Guo R-D, Wang L, Sun Y-G, Wang Y-S. Evaluating Fluorinated-Aniline Units with Functionalized Spiro[Fluorene-9,9′-Xanthene] as Hole-Transporting Materials in Perovskite Solar Cells and Light-Emitting Diodes. Nanomaterials. 2024; 14(12):1044. https://doi.org/10.3390/nano14121044
Chicago/Turabian StyleLiu, Kuo, Liang Sun, Qing-Lin Liu, Bao-Yi Ren, Run-Da Guo, Lei Wang, Ya-Guang Sun, and You-Sheng Wang. 2024. "Evaluating Fluorinated-Aniline Units with Functionalized Spiro[Fluorene-9,9′-Xanthene] as Hole-Transporting Materials in Perovskite Solar Cells and Light-Emitting Diodes" Nanomaterials 14, no. 12: 1044. https://doi.org/10.3390/nano14121044
APA StyleLiu, K., Sun, L., Liu, Q.-L., Ren, B.-Y., Guo, R.-D., Wang, L., Sun, Y.-G., & Wang, Y.-S. (2024). Evaluating Fluorinated-Aniline Units with Functionalized Spiro[Fluorene-9,9′-Xanthene] as Hole-Transporting Materials in Perovskite Solar Cells and Light-Emitting Diodes. Nanomaterials, 14(12), 1044. https://doi.org/10.3390/nano14121044







