Degradation and Recondensation of Metal Oxide Nanoparticles in Laminar Premixed Flames
Abstract
1. Introduction
2. Materials and Methods
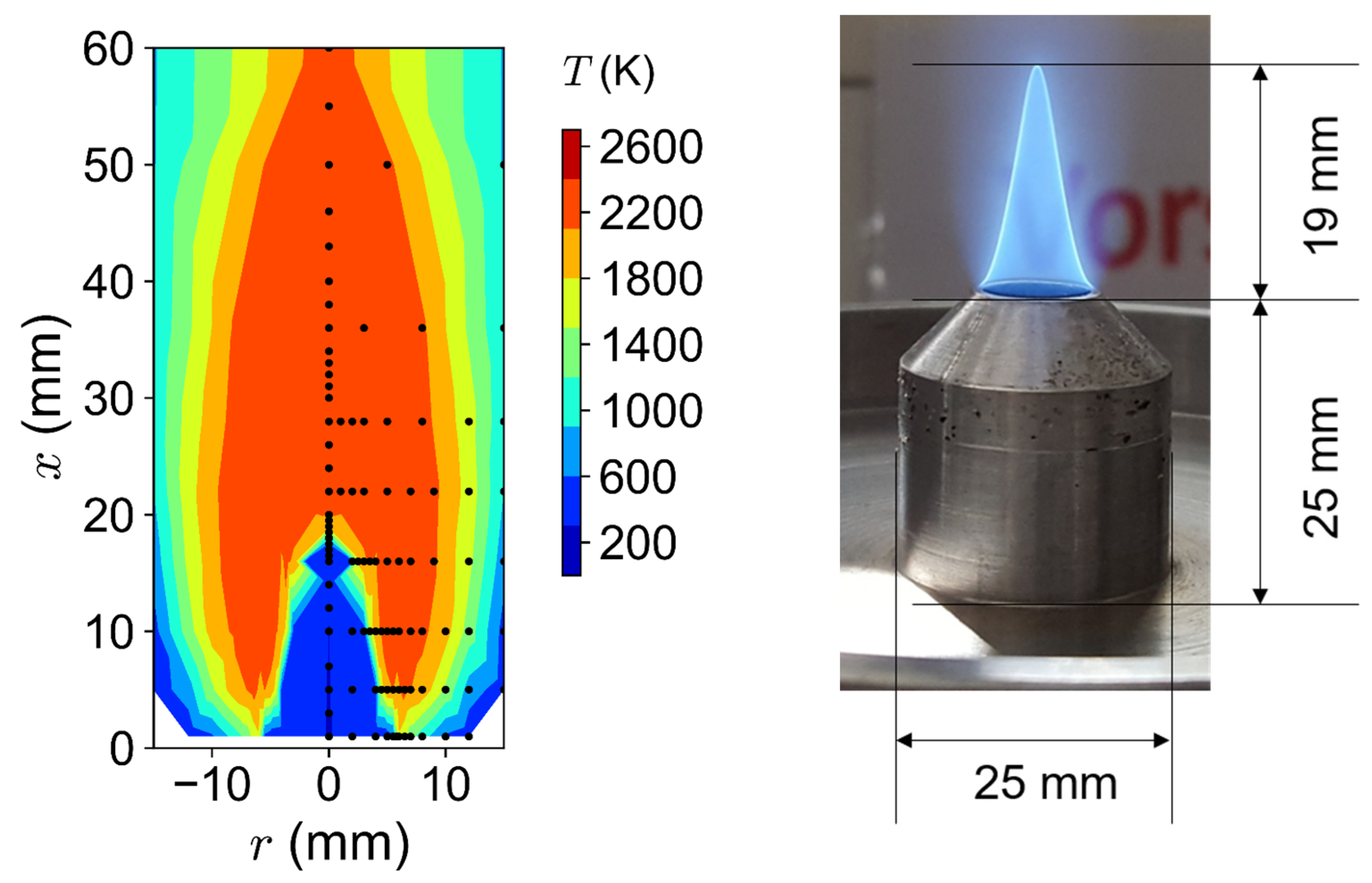
2.1. Laboratory Bunsen-Type Burner
2.2. Used Nanoparticles
2.3. Aerosol Sampling and Characterization
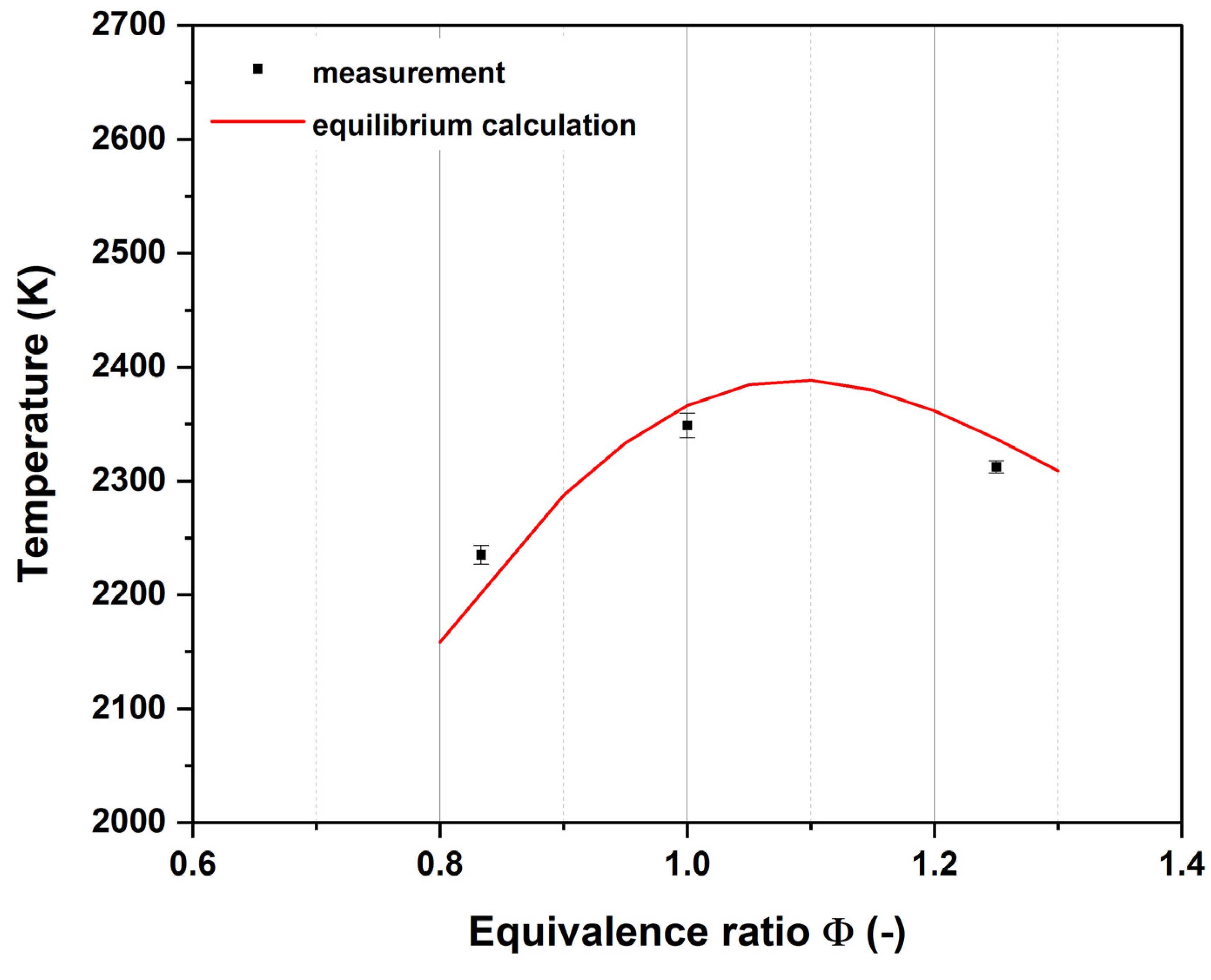
2.4. Equilibrium Temperature Calculations
2.5. Temperature Measurement via CARS
3. Results
3.1. Flame Temperatures
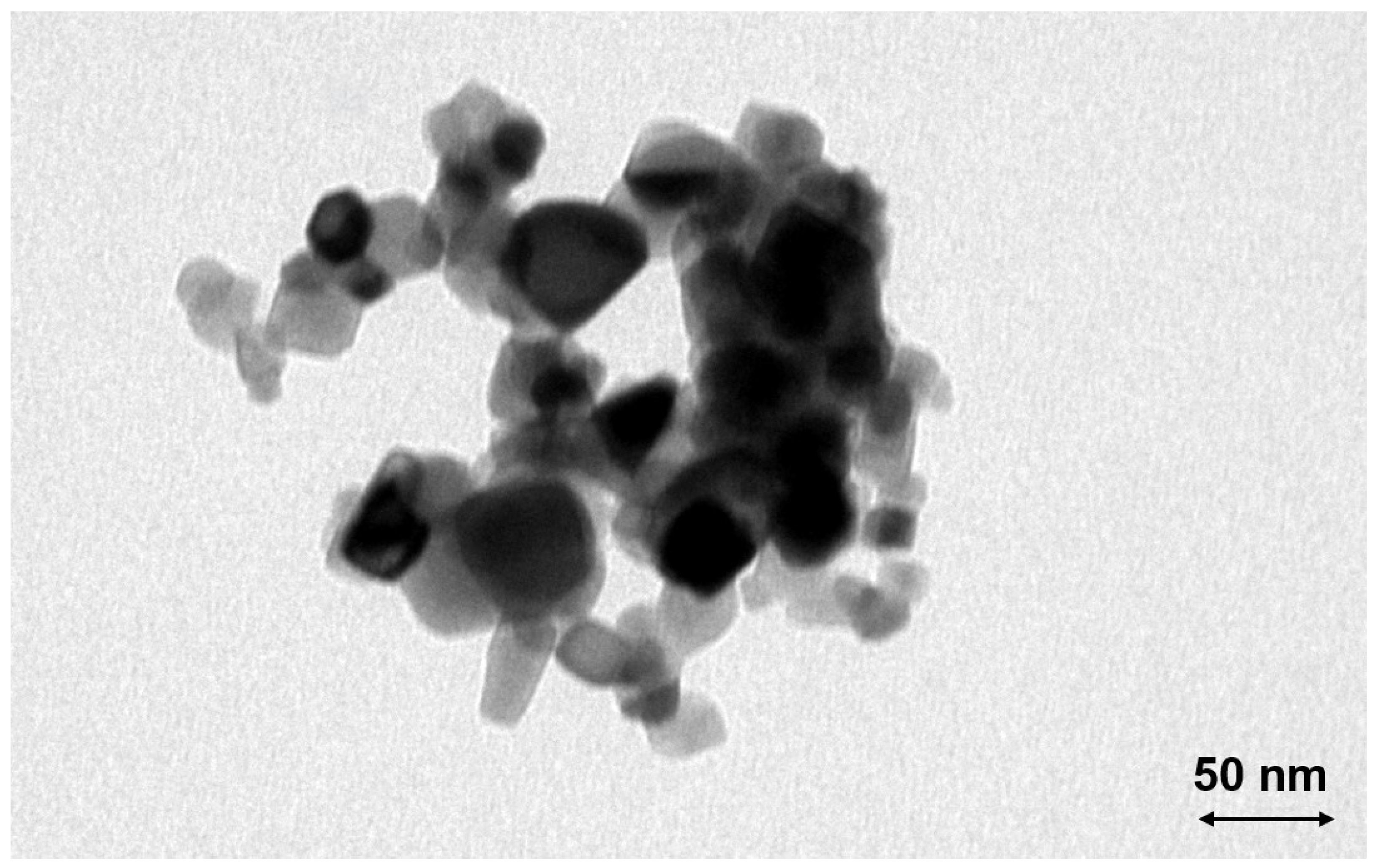
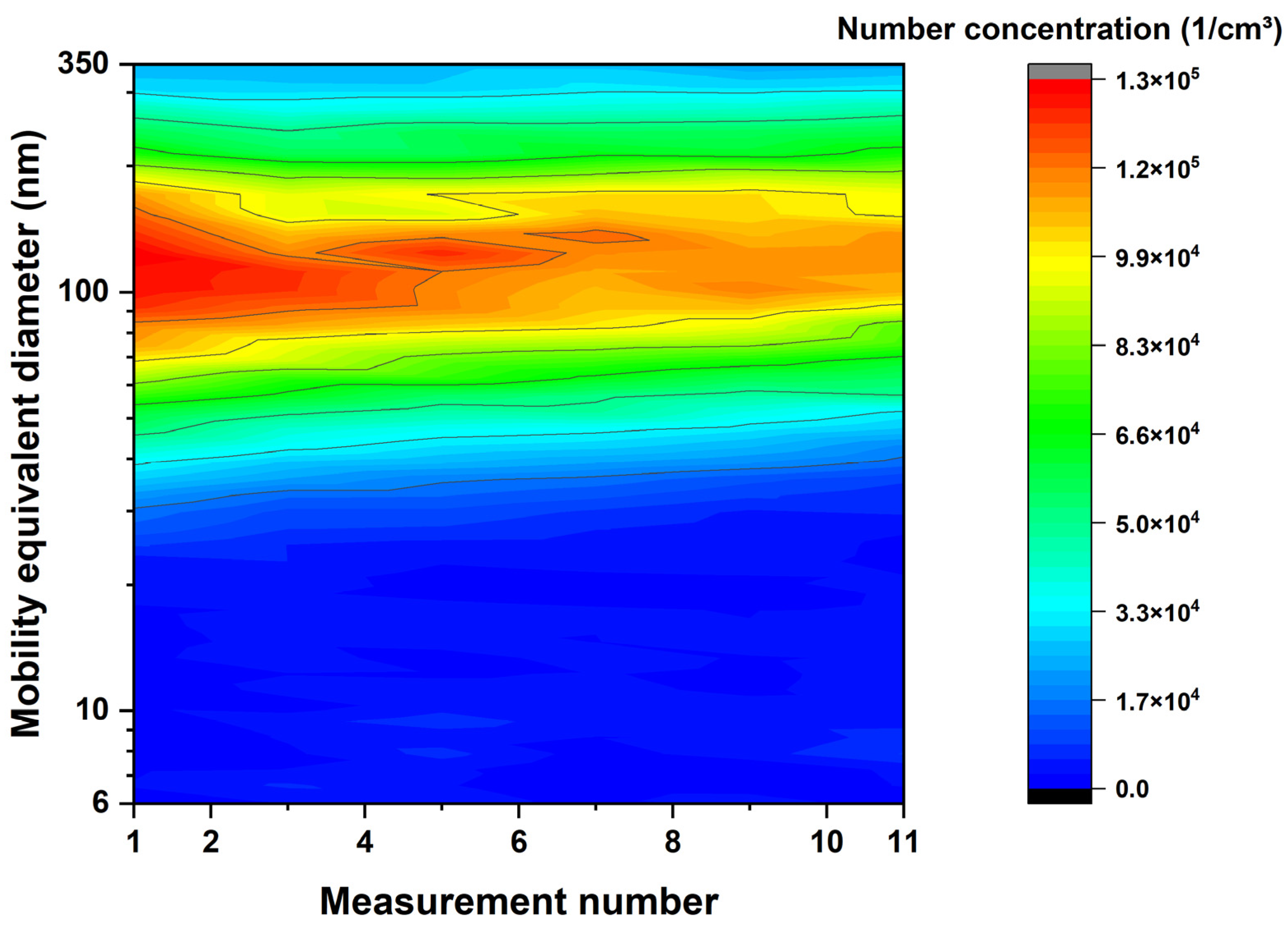
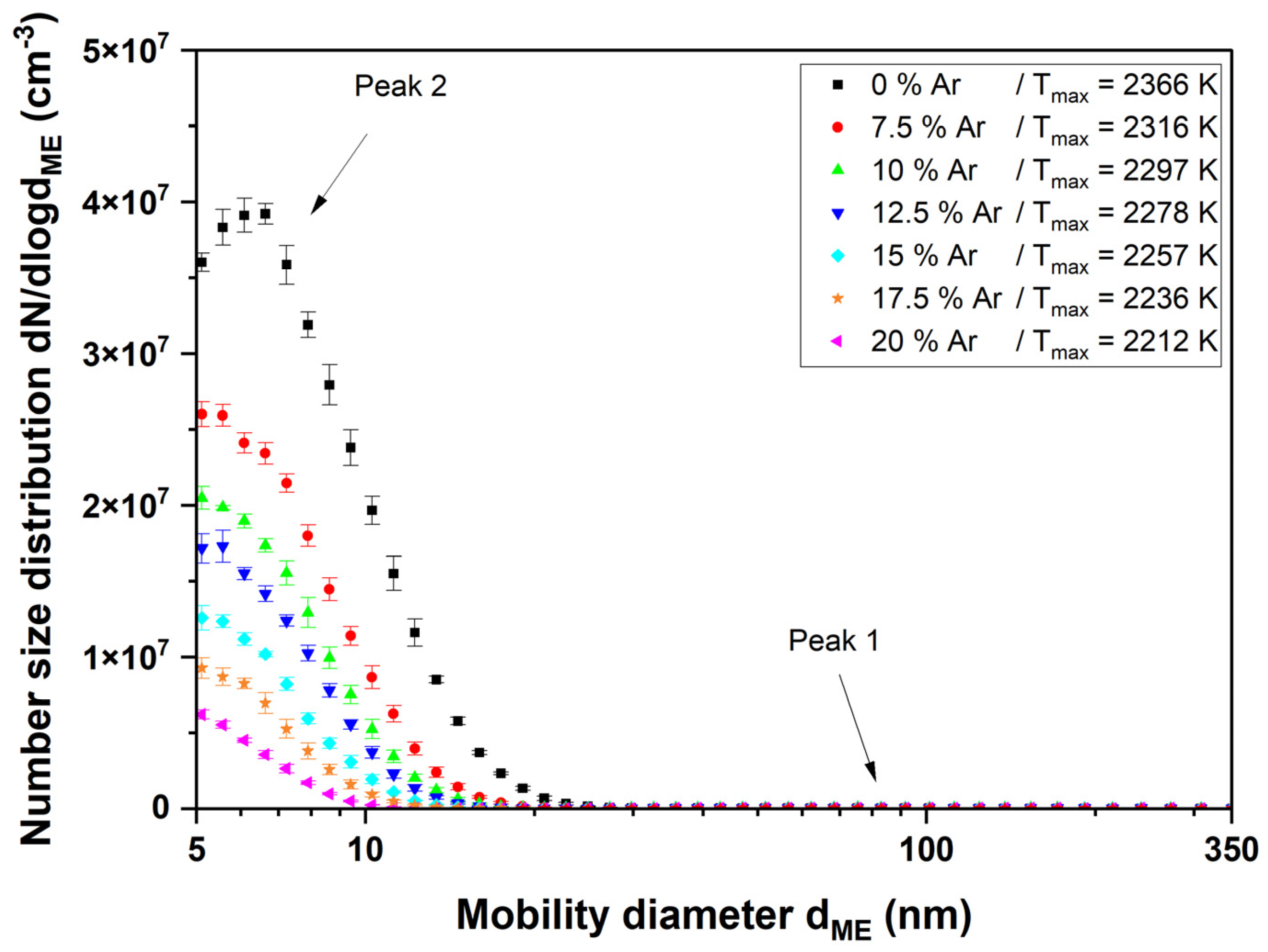
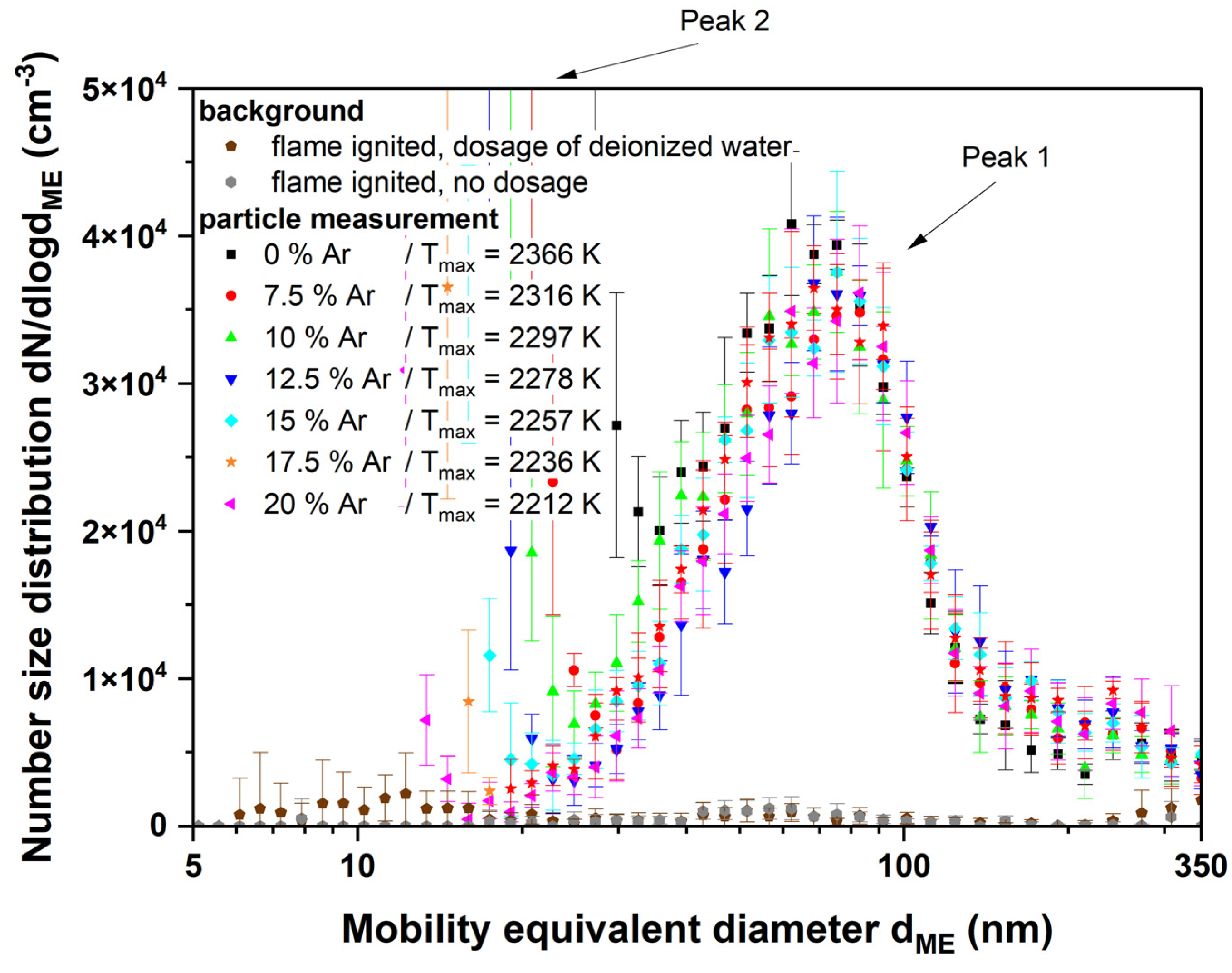
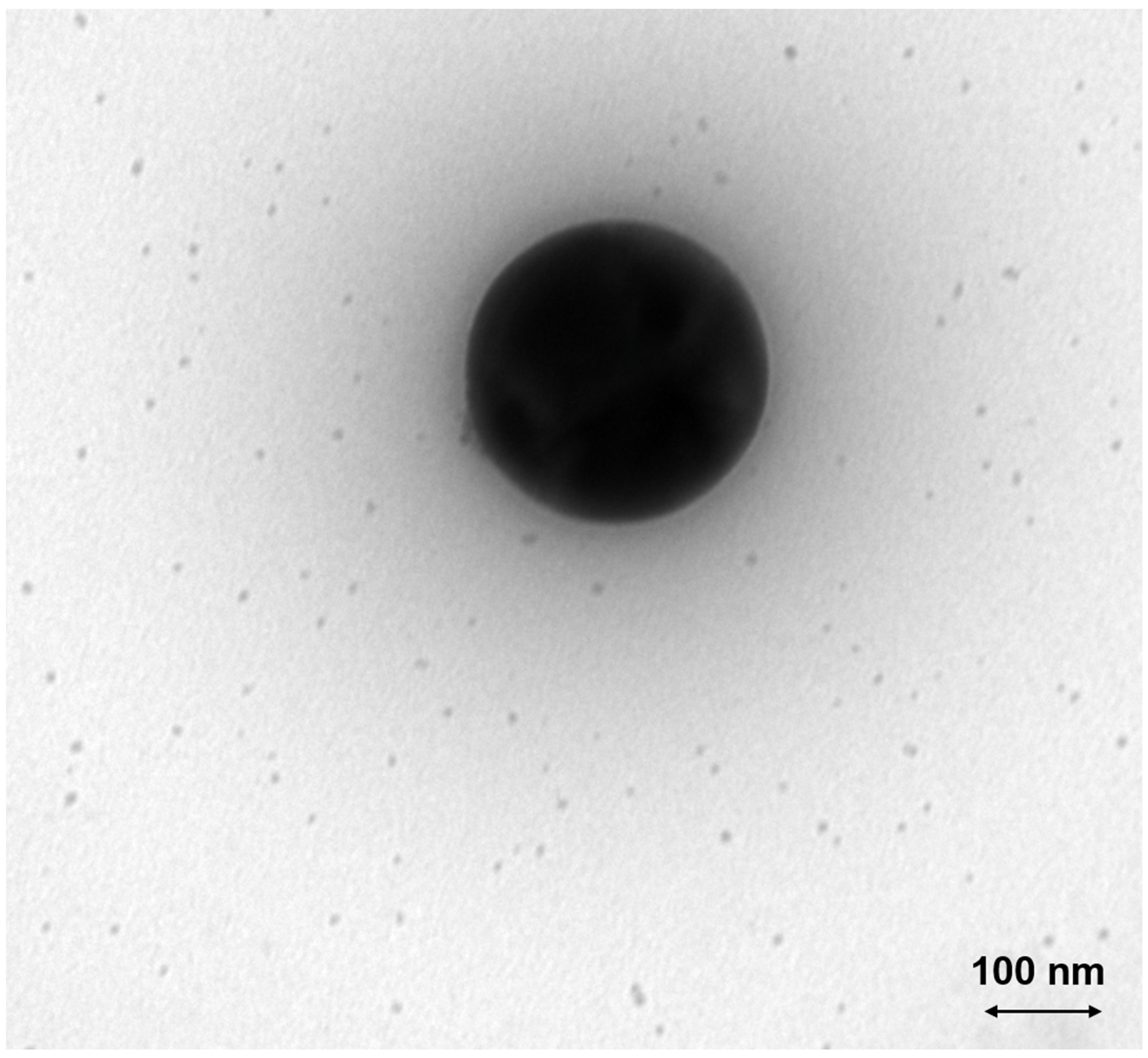
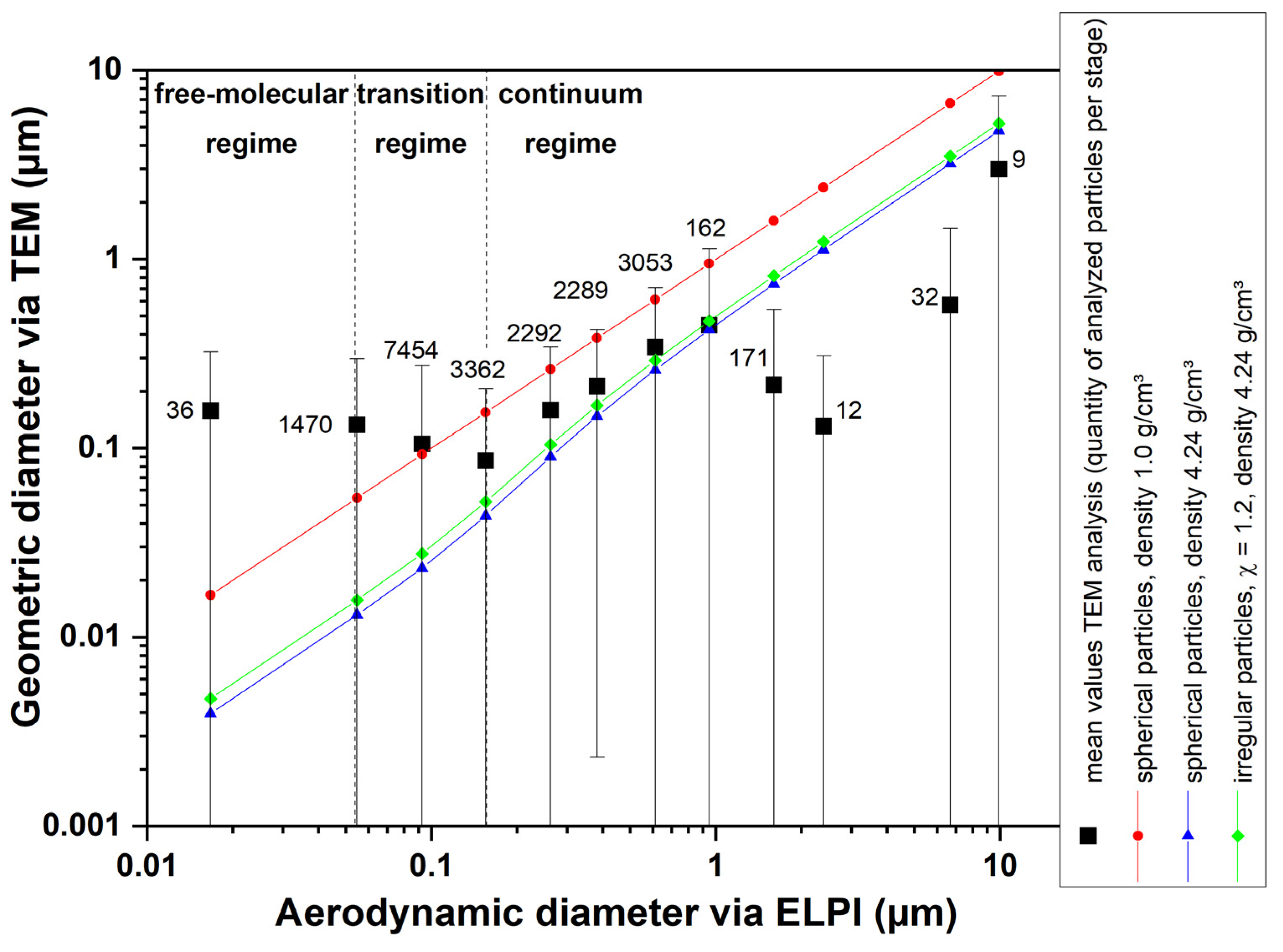
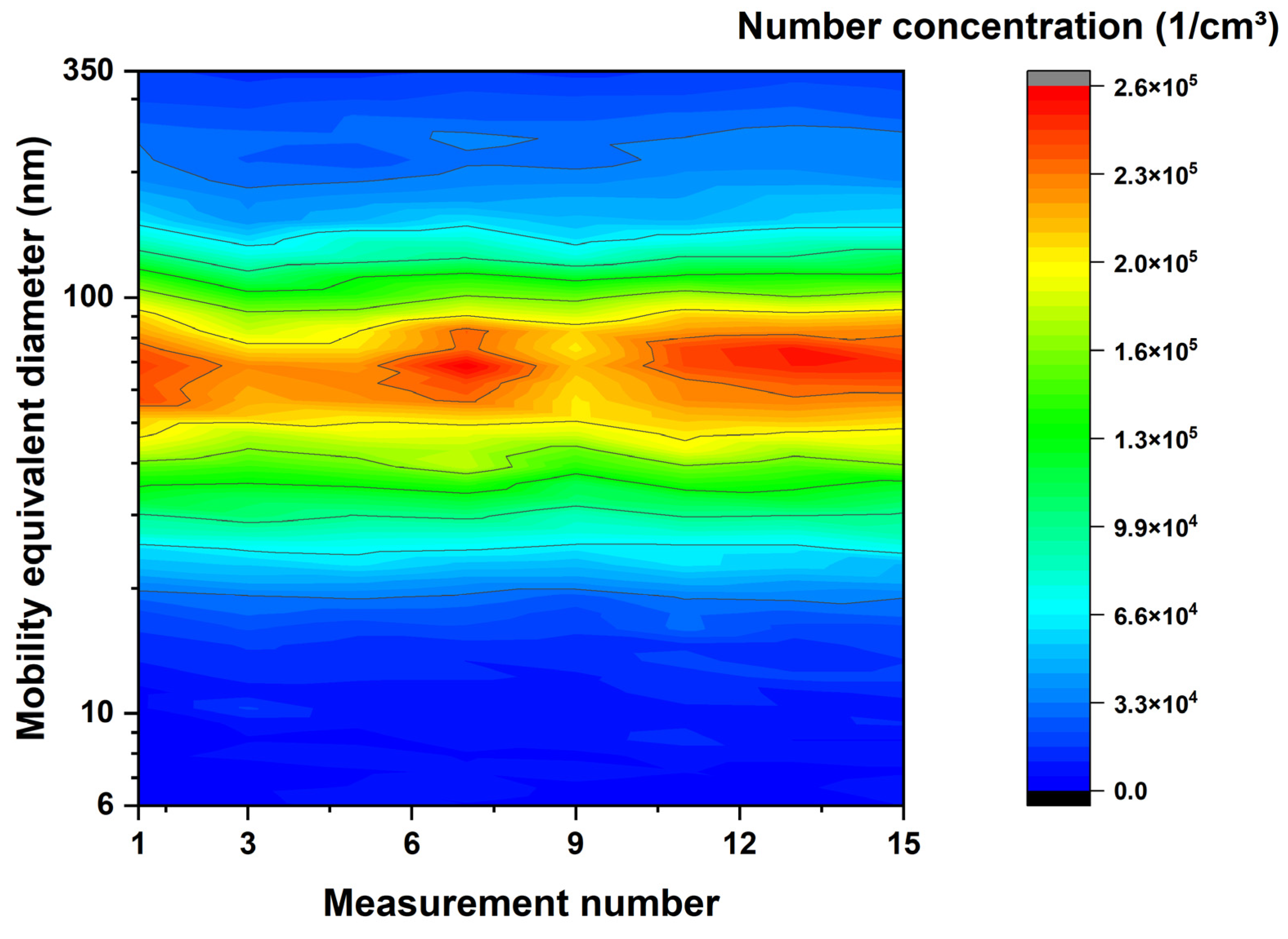
3.2. Aerosol and Particle Characterization
3.2.1. Titania
3.2.2. Ceria
4. Discussion
4.1. Flame Temperatures
4.2. Aerosol and Particle Characterization
4.2.1. Comparison of Different Equivalent Diameters
4.2.2. Titania
4.2.3. Ceria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Part, F.; Berge, N.; Baran, P.; Stringfellow, A.; Sun, W.; Bartelt-Hunt, S.; Mitrano, D.; Li, L.; Hennebert, P.; Quicker, P.; et al. A review of the fate of engineered nanomaterials in municipal solid waste streams. Waste Manag. 2018, 75, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Walser, T.; Limbach, L.K.; Brogioli, R.; Erismann, E.; Flamigni, L.; Hattendorf, B.; Juchli, M.; Krumeich, F.; Ludwig, C.; Prikopsky, K.; et al. Persistence of engineered nanoparticles in a municipal solid-waste incineration plant. Nat. Nanotechnol. 2012, 7, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Pavlicek, A.; Part, F.; Rose, G.; Praetorius, A.; Miernicki, M.; Gazsó, A.; Huber-Humer, M. A European nano-registry as a reliable database for quantitative risk assessment of nanomaterials? A comparison of national approaches. NanoImpact 2021, 21, 100276. [Google Scholar] [CrossRef]
- European Comission. Commission Staff Working Paper: Types and Uses of Nanomaterials, Including Safety Aspects, Accompanying the Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee on the Second Regulatory Review on Nanomaterials. 2012. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=SWD:2012:0288:FIN:EN:PDF (accessed on 4 November 2020).
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; Gleich, A.v.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef]
- Hendren, C.O.; Mesnard, X.; Dröge, J.; Wiesner, M.R. Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environ. Sci. Technol. 2011, 45, 2562–2569. [Google Scholar] [CrossRef]
- Holden, P.A.; Klaessig, F.; Turco, R.F.; Priester, J.H.; Rico, C.M.; Avila-Arias, H.; Mortimer, M.; Pacpaco, K.; Gardea-Torresdey, J.L. Evaluation of exposure concentrations used in assessing manufactured nanomaterial environmental hazards: Are they relevant? Environ. Sci. Technol. 2014, 48, 10541–10551. [Google Scholar] [CrossRef]
- Janković, N.Z.; Plata, D.L. Engineered nanomaterials in the context of global element cycles. Environ. Sci. Nano 2019, 6, 2697–2711. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Rauscher, H.; Rasmussen, K.; Sokull-Klüttgen, B. Regulatory Aspects of Nanomaterials in the EU. Chem. Ing. Tech. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Schwirn, K.; Völker, D. Nanomaterials in the Environment—Current State of Knowledge and Regulations on Chemical Safety: Recommendations of the German Environment Agency; German Environment Agency: Dessau-Roßlau, Germany, 2016. [Google Scholar]
- Hansen, S.F.; Mackevica, A.; Hull, M.S. Nano-enabled Consumer Products: Inventories, Release, and Exposures. In Nanotoxicology in Humans and the Environment, 1st ed.; Lead, J.R., Doak, S.H., Clift, M.J.D., Eds.; Springer International Publishing; Imprint Springer: Cham, Switzerland, 2021; pp. 85–127. ISBN 978-3-030-79807-9. [Google Scholar]
- Conversio Market & Strategy GmbH. Titandioxid in Kunststoffen: Kurzfassung der Ergebnisse aus der Analyse der Kunststoffverarbeitung, der Kunststoffabfallmengen und der Verwertung von Kunststoffabfällen in Deutschland 2017. 2019. Available online: https://www.bkv-gmbh.de/fileadmin/documents/Studien/Kurzfassung_Titandioxid_in_Kunststoffen_210219.pdf (accessed on 12 March 2019).
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef]
- Caballero-Guzman, A.; Nowack, B. A critical review of engineered nanomaterial release data: Are current data useful for material flow modeling? Environ. Pollut. 2016, 213, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Baumann, W.; Teuscher, N.; Hauser, M.; Gehrmann, J.; Paur, H.-R.; Stapf, D. Behaviour of engineered nanoparticles in a lab-scale flame and combustion chamber. Energy Procedia 2017, 120, 705–712. [Google Scholar] [CrossRef]
- Hölemann, K.; Daumann, B.; Baumann, W.; Teuscher, N. Untersuchungen zum Verhalten und zur Rückhaltung synthetischer Nanopartikel in einer Rückstandsverbrennungsanlage. In Energie aus Abfall; Gosten, A., Quicker, P., Thiel, S., Thomé-Kozmiensky, E., Eds.; TK: Neuruppin, Germany, 2019; pp. 681–692. ISBN 3944310454. [Google Scholar]
- Paur, H.-R.; Baumann, W.; Hauser, M.; Lang, I.; Teuscher, N.; Seifert, H.; Stapf, D. Thermal Stability and Material Balance of Nanomaterials in Waste Incineration. J. Phys. Conf. Ser. 2017, 838, 012012. [Google Scholar] [CrossRef]
- Oischinger, J.; Meiller, M.; Daschner, R.; Hornung, A.; Warnecke, R. Fate of nano titanium dioxide during combustion of engineered nanomaterial-containing waste in a municipal solid waste incineration plant. Waste Manag. Res. 2019, 37, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Widiyastuti, W.; Purwanto, A.; Wang, W.-N.; Iskandar, F.; Setyawan, H.; Okuyama, K. Nanoparticle formation through solid-fed flame synthesis: Experiment and modeling. AIChE J. 2009, 55, 885–895. [Google Scholar] [CrossRef]
- Widiyastuti, W.; Hidayat, D.; Purwanto, A.; Iskandar, F.; Okuyama, K. Particle dynamics simulation of nanoparticle formation in a flame reactor using a polydispersed submicron-sized solid precursor. Chem. Eng. J. 2010, 158, 362–367. [Google Scholar] [CrossRef]
- Buffat, P.; Borel, J.-P. Size effect on the melting temperature of gold particles. Phys. Rev. A 1976, 13, 2287–2298. [Google Scholar] [CrossRef]
- Xiong, S.; Qi, W.; Cheng, Y.; Huang, B.; Wang, M.; Li, Y. Universal relation for size dependent thermodynamic properties of metallic nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 10652–10660. [Google Scholar] [CrossRef]
- Nanda, K.K.; Kruis, F.E.; Fissan, H. Evaporation of free PbS nanoparticles: Evidence of the Kelvin effect. Phys. Rev. Lett. 2002, 89, 256103. [Google Scholar] [CrossRef]
- Shim, J.-H.; Lee, B.-J.; Cho, Y.W. Thermal stability of unsupported gold nanoparticle: A molecular dynamics study. Surf. Sci. 2002, 512, 262–268. [Google Scholar] [CrossRef]
- Wang, T.H.; Zhu, Y.F.; Jiang, Q. Size effect on evaporation temperature of nanocrystals. Mater. Chem. Phys. 2008, 111, 293–295. [Google Scholar] [CrossRef]
- Nanda, K.K. Bulk cohesive energy and surface tension from the size-dependent evaporation study of nanoparticles. Appl. Phys. Lett. 2005, 87, 21909. [Google Scholar] [CrossRef]
- Yang, C.C.; Mai, Y.-W. Thermodynamics at the nanoscale: A new approach to the investigation of unique physicochemical properties of nanomaterials. Mater. Sci. Eng. R Rep. 2014, 79, 1–40. [Google Scholar] [CrossRef]
- Evonik Industries AG. Aeroxide—Pyrogene Metalloxide: Technical Overview 13. Available online: https://products.evonik.com/assets/49/30/TO_13_AEROXIDE_Pyrogene_Metalloxide_DE_DE_244930.pdf (accessed on 7 September 2023).
- Keskinen, J.; Pietarinen, K.; Lehtimäki, M. Electrical low pressure impactor. J. Aerosol Sci. 1992, 23, 353–360. [Google Scholar] [CrossRef]
- ANSYS CHEMKIN R2; ANSYS, Inc.: San Diego, CA, USA, 2022.
- Eckbreth, A.C. Laser Diagnostics for Combustion Temperature and Species, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996; ISBN 9789056995324. [Google Scholar]
- Geigle, K.P.; Schneider-Kühnle, Y.; Tsurikov, M.S.; Hadef, R.; Lückerath, R.; Krüger, V.; Stricker, W.; Aigner, M. Investigation of laminar pressurized flames for soot model validation using SV-CARS and LII. Proc. Combust. Inst. 2005, 30, 1645–1653. [Google Scholar] [CrossRef]
- Geigle, K.P.; Köhler, M.; O’Loughlin, W.; Meier, W. Investigation of soot formation in pressurized swirl flames by laser measurements of temperature, flame structures and soot concentrations. Proc. Combust. Inst. 2015, 35, 3373–3380. [Google Scholar] [CrossRef]
- Parameswaran, T.; Snelling, D.R. The Calculation of Theoretical Coherent Anti-Stokes Raman Spectra; NRC Technical Report; TR-GD-013 NRC No. 29810; National Research Council: Ottawa, ON, Canada, 1988. [Google Scholar]
- Yin, Z. CARSpy: Synthesizing and Fitting Coherent Anti-Stokes Raman Spectra in Python. Available online: https://github.com/chuckedfromspace/carspy (accessed on 31 October 2023).
- Palmer, R.E. The CARSFT Computer Code Calculating Coherent Anti-Stokes Raman Spectra: User and Programmer Information; Sandia National Lab. (SNL-CA): Livermore, CA, USA, 1989. [Google Scholar]
- Ihalainen, M.; Lind, T.; Arffman, A.; Torvela, T.; Jokiniemi, J. Break-Up and Bounce of TiO2 Agglomerates by Impaction. Aerosol Sci. Technol. 2013, 48, 31–41. [Google Scholar] [CrossRef][Green Version]
- Ihalainen, M.; Lind, T.; Ruusunen, J.; Tiitta, P.; Lähde, A.; Torvela, T.; Jokiniemi, J. Experimental study on bounce of submicron agglomerates upon inertial impaction. Powder Technol. 2014, 268, 203–209. [Google Scholar] [CrossRef]
- Ristimäki, J.; Virtanen, A.; Marjamäki, M.; Rostedt, A.; Keskinen, J. On-line measurement of size distribution and effective density of submicron aerosol particles. J. Aerosol Sci. 2002, 33, 1541–1557. [Google Scholar] [CrossRef]




| Material | Hendren et al. [6] | Piccinno 1 et al. [9] | Piccinno 1 et al. [9] | EC [4] | Holden et al. [7] | Giese et al. [5] | Janković and Plata [8] |
|---|---|---|---|---|---|---|---|
| USA | Worldwide | Europe | Worldwide | Worldwide | Worldwide | Worldwide | |
| carbon black | - | - | - | 9,600,000 | >10,000,000 | - | - |
| SiO2 | - | 5500 (55–55,000) | 5500 (55–55,000) | 1,500,000 | >2,400,000 | 100,000–3,000,000 | ~1,000,000 |
| TiO2 | 7800–38,000 | 3000 (550–5500) | 550 (55–3000) | 10,000 | >30,000 | - | ~100,000 |
| CeO2 | 35–700 | 55 2 (5.5–550) | 55 2 (0.55–2800) | 10,000 | <10,000 | 1000–100,000 | ~1000 |
| CNTs | 55–1101 | 300 (55–550) | 550 (180–550) | Several hundreds | 250 | - | ~3300 |
| Argon Fraction | Equivalence Ratio Φ | Volume Flow, Argon | Volume Flow, Ethylene | Volume Flow, Air | Volume Flow, Atomizer |
|---|---|---|---|---|---|
| (%) | (-) | (L/min) | (L/min) | (L/min) | (L/min) |
| 0 | 0.83 | 0 | 0.60 | 9.24 | 1 |
| 0 | 1.25 | 0 | 0.87 | 8.97 | 1 |
| 0 | 1.0 | 0 | 0.71 | 9.13 | 1 |
| 7.5 | 1.0 | 0.81 | 0.66 | 8.37 | 1 |
| 10 | 1.0 | 1.08 | 0.64 | 8.12 | 1 |
| 12.5 | 1.0 | 1.35 | 0.62 | 7.86 | 1 |
| 15 | 1.0 | 1.63 | 0.60 | 7.61 | 1 |
| 17.5 | 1.0 | 1.90 | 0.58 | 7.36 | 1 |
| 20 | 1.0 | 2.17 | 0.57 | 7.10 | 1 |
| Argon Fraction | Teq | TCARS |
|---|---|---|
| (%) | (K) | (K) |
| 0 | 2366 | 2349 |
| 7.5 | 2316 | 2355 |
| 10 | 2297 | 2280 |
| 12.5 | 2278 | 2247 |
| 15 | 2257 | 2219 |
| 17.5 | 2236 | 2173 |
| 20 | 2212 | 2192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, N.; Baumann, W.; Hauser, M.; Yin, Z.; Geigle, K.P.; Stapf, D. Degradation and Recondensation of Metal Oxide Nanoparticles in Laminar Premixed Flames. Nanomaterials 2024, 14, 1047. https://doi.org/10.3390/nano14121047
May N, Baumann W, Hauser M, Yin Z, Geigle KP, Stapf D. Degradation and Recondensation of Metal Oxide Nanoparticles in Laminar Premixed Flames. Nanomaterials. 2024; 14(12):1047. https://doi.org/10.3390/nano14121047
Chicago/Turabian StyleMay, Nadine, Werner Baumann, Manuela Hauser, Zhiyao Yin, Klaus Peter Geigle, and Dieter Stapf. 2024. "Degradation and Recondensation of Metal Oxide Nanoparticles in Laminar Premixed Flames" Nanomaterials 14, no. 12: 1047. https://doi.org/10.3390/nano14121047
APA StyleMay, N., Baumann, W., Hauser, M., Yin, Z., Geigle, K. P., & Stapf, D. (2024). Degradation and Recondensation of Metal Oxide Nanoparticles in Laminar Premixed Flames. Nanomaterials, 14(12), 1047. https://doi.org/10.3390/nano14121047






