Effect of Calcination Temperature on the Microstructure, Composition and Properties of Agglomerated Nanometer CeO2-Y2O3-ZrO2 Powders for Plasma Spray–Physical Vapor Deposition (PS-PVD) and Coatings Thereof
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Agglomerated Powders
2.3. Agglomerated Powder Calcination
2.4. Coating Preparation
2.5. Characterization of Powders and Coatings
3. Results and Discussion
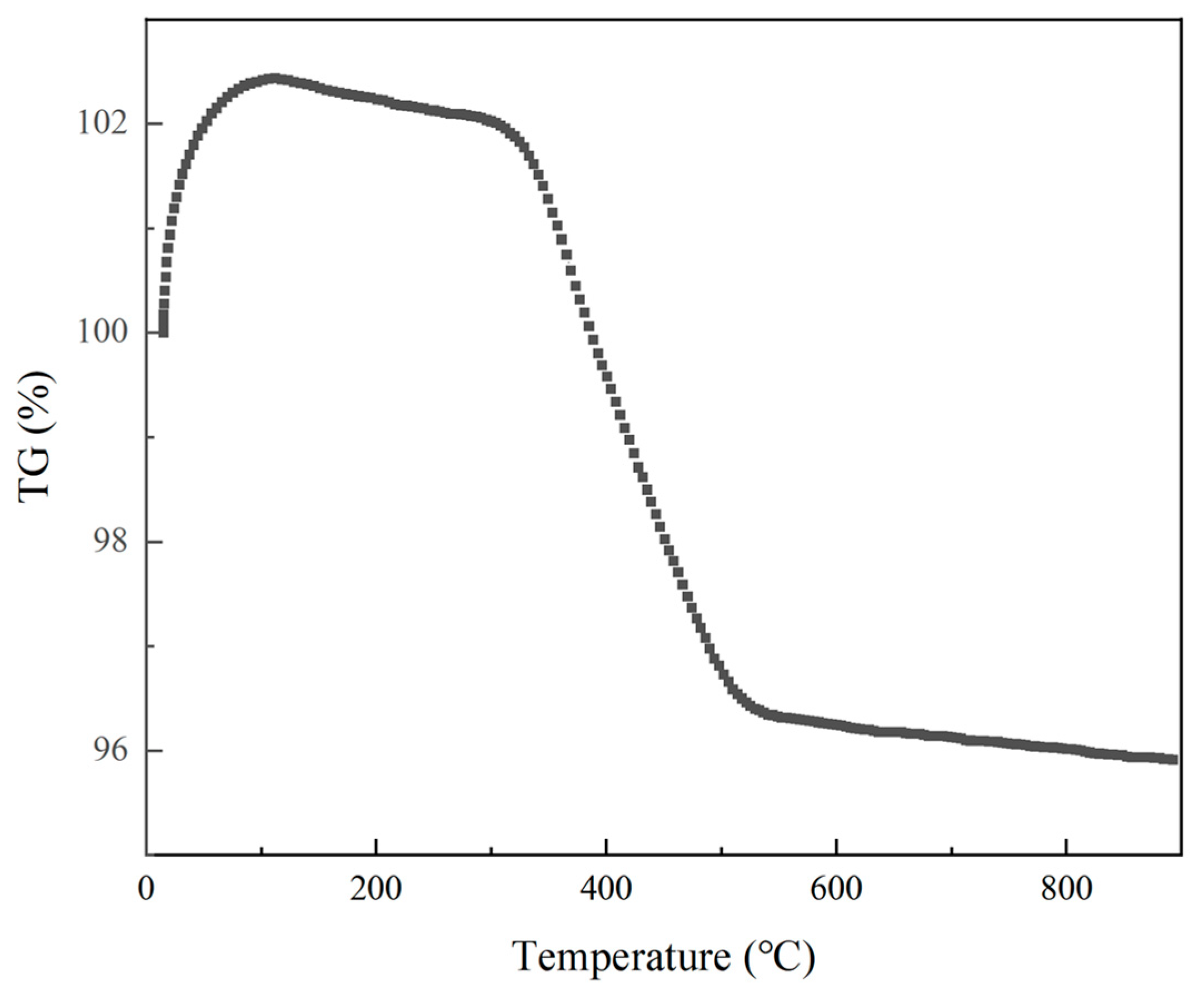
3.1. Effect of Calcination Temperature on the Microstructure of the Powders
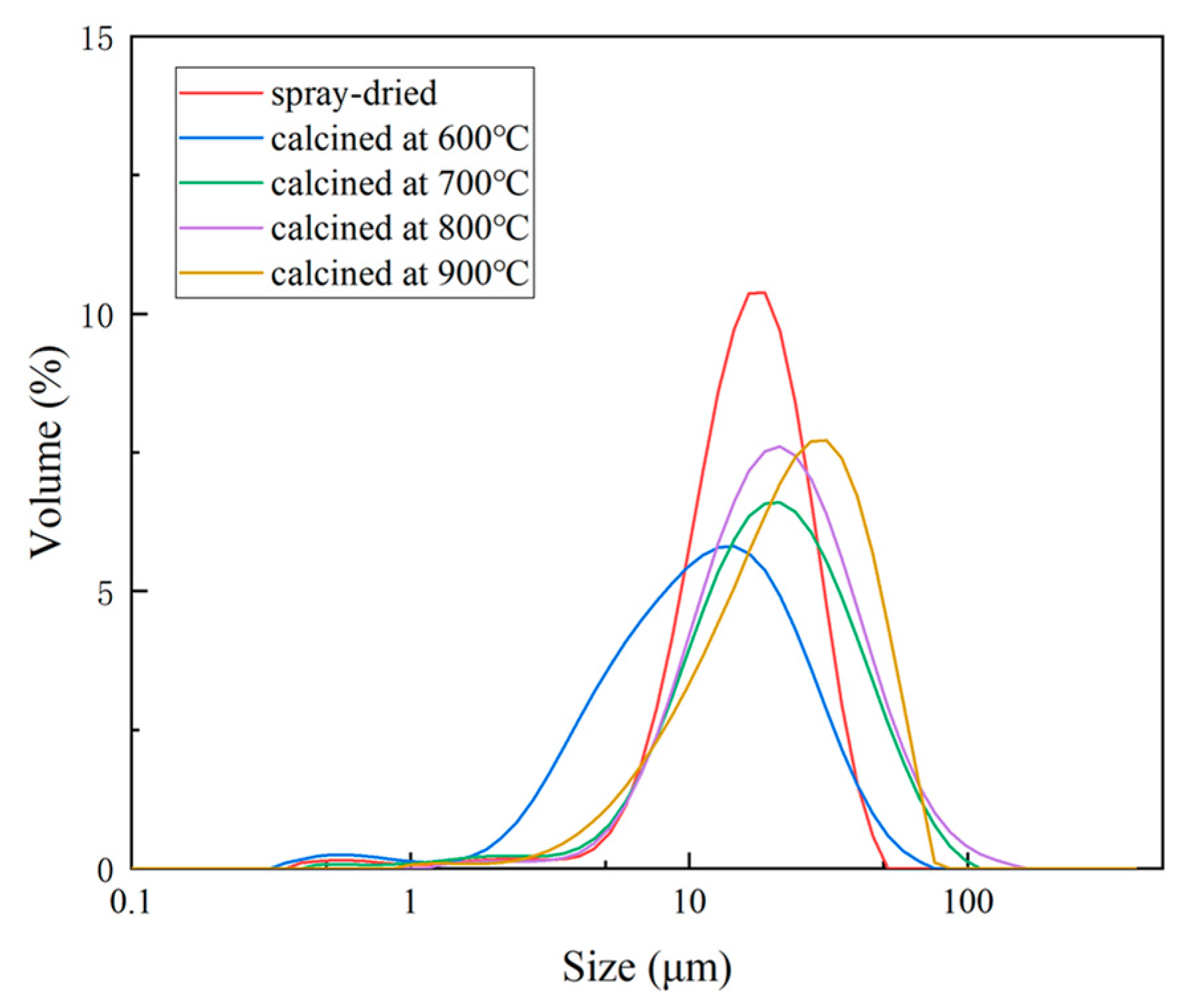
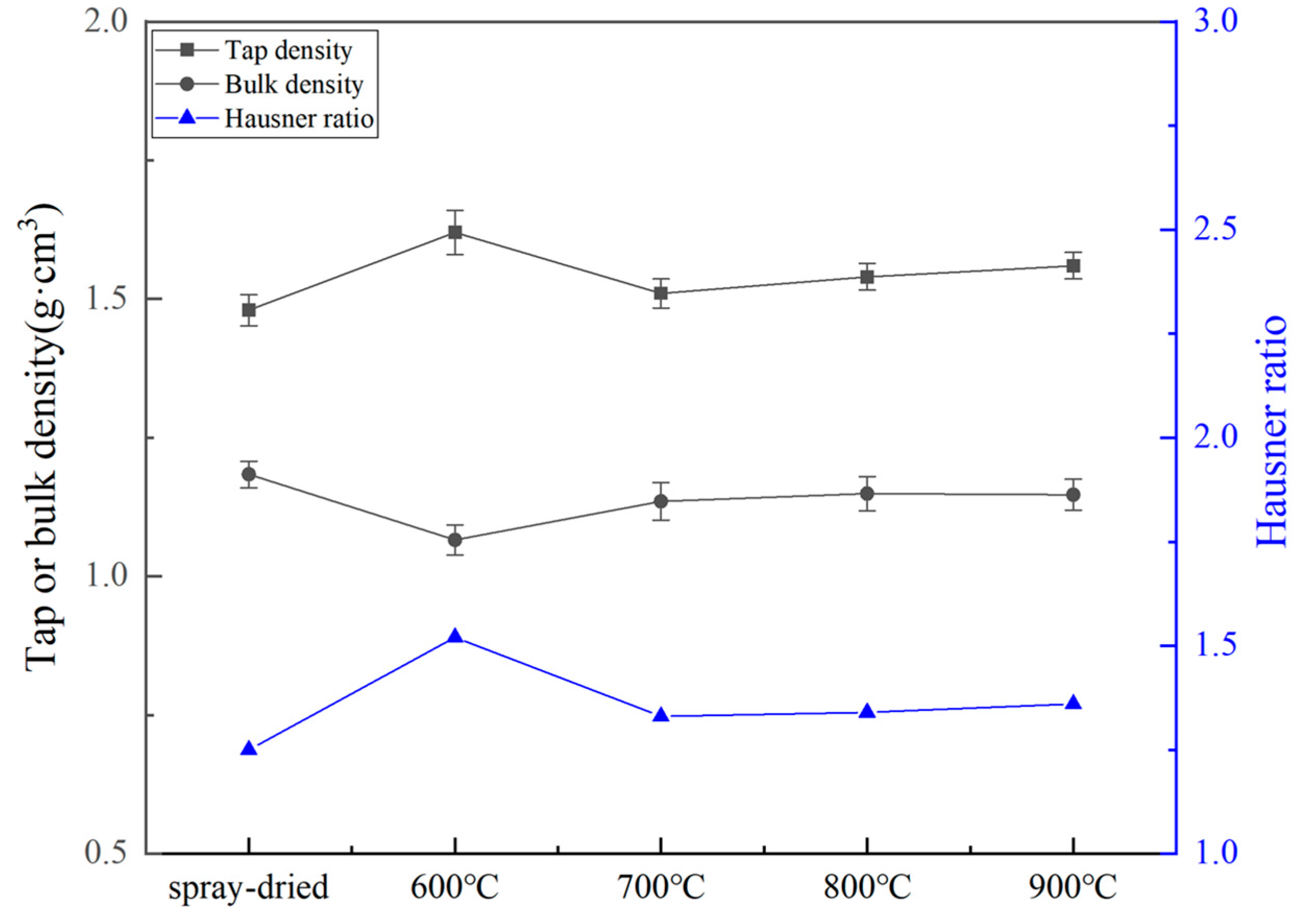
3.2. Influence of Calcination Temperature on Particle Size Distribution and Flowability
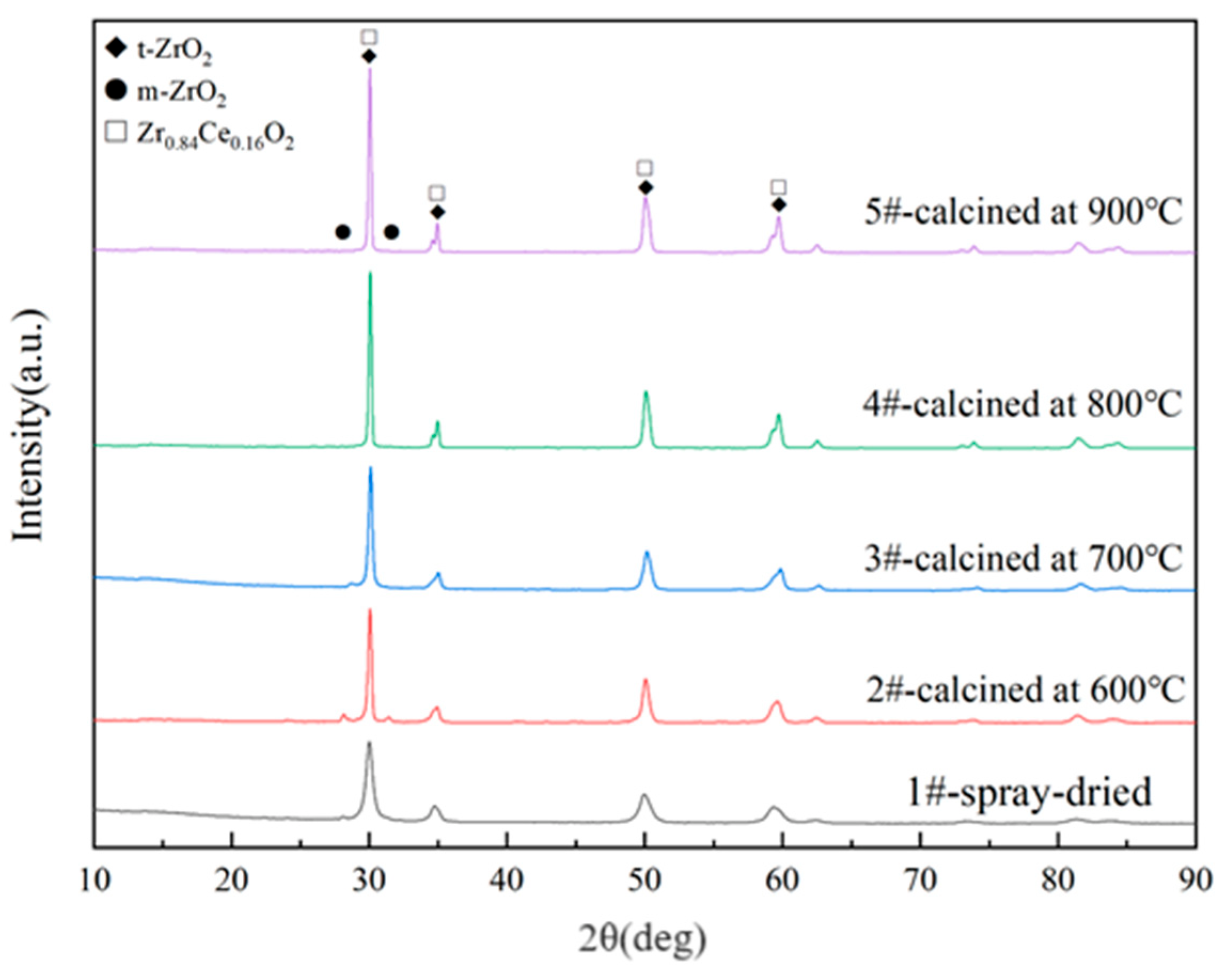
3.3. Effect of Calcination Temperature on Composition of the Powders
3.4. Effect of Powder Variation on the Microstructure, Composition, and Properties of the Prepared Coatings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, L.; Guo, H.; Wei, L.; Li, C.; Xu, H. Microstructure, Thermal Conductivity and Thermal Cycling Behavior of Thermal Barrier Coatings Prepared by Plasma Spray Physical Vapor Deposition. Surf. Coat. Technol. 2015, 276, 424–430. [Google Scholar] [CrossRef]
- Mao, J.; Liu, M.; Deng, Z.; Wen, K.; Deng, C.; Yang, K.; Chen, Z. Coating Deposition Regularity Depended on Orientation Difference in PS-PVD Plasma Jet. Chin. J. Aeronaut. 2019, 33, 3460–3468. [Google Scholar] [CrossRef]
- Zhao, C.; He, W.; He, J.; Wei, L.; Guo, H. Correlation of Feedstock Powder Characteristics with Microstructure, Composition, and Mechanical Properties of La2Ce2O7 Coatings Produced by Plasma Spray-physical Vapor Deposition. Coatings 2020, 10, 93. [Google Scholar] [CrossRef]
- Girolamo, D.; Caterina, B.; Leonardo, P.; Monica, S. Phase evolution and thermophysical properties of plasma sprayed thick zirconia coatings after annealing. Cream. Int. 2010, 36, 2273–2280. [Google Scholar] [CrossRef]
- Ma, B.; Li, Y.; Ke, S. Characterization of ceria–yttria stabilized zirconia plasma-sprayed coatings. Appl. Surf. Sci. 2009, 255, 7234–7237. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Kim, M.; Song, H.; Park, C. Microscopic observation of degradation behavior in yttria and ceria stabilized zirconia thermal barrier coatings under hot corrosion. Surf. Coat. Technol. 2005, 190, 357–365. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Choi, H.; Ahn, H.S. Phase transformation and bond coat oxidation behavior of plasma-sprayed zirconia thermal barrier coating. Surf. Coat. Technol. 2000, 124, 1–12. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, M.; Mao, J.; Deng, C.; Zhang, X. Stage growth of columnar 7YSZ coating prepared by plasma spray-physical vapor deposition. Vacuum 2017, 145, 39–46. [Google Scholar] [CrossRef]
- Renteria, A.; Saruhan, B.; Schulz, U.; Raetzer-Scheibe, H.-J.; Haug, J.; Wiedenmann, A. Effect of morphology on thermal conductivity of EB-PVD PYSZ TBCs. Surf. Coat. Technol. 2006, 201, 2611–2620. [Google Scholar] [CrossRef]
- HB-5476-1991; Test Method for Bonding Strength of Thermal Spray Coatings. Industry Standard-Aviation: Beijing, China, 1991.
- Ren, X.; Zhao, M.; Feng, J.; Pan, W. Phase transformation behavior in air plasma sprayed yttria stabilized zirconia coating. J. Alloys Compd. 2018, 750, 189–196. [Google Scholar] [CrossRef]
- Mercer, C.; Williams, J.R.; Clarke, D.R.; Evans, A.G. On a ferroelastic mechanism governing the toughness of metastable tetragonal-prime (t’) yttria-stabilized zirconia. Proc. R. Soc. A Math. Phys. Eng. Sci. 2007, 463, 1393–1408. [Google Scholar] [CrossRef]
- Smialek, J.; Miller, R. Revisiting the Birth of 7YSZ Thermal Barrier Coatings: Stephan Stecura. Coatings 2018, 8, 255. [Google Scholar] [CrossRef]
- Krogstad, J.; Kramer, S.; Lipkin, D.; Johnson, C.A.; Mitchell, D.R.; Cairney, J.M.; Levi, C.G. Phase Stability of t’-Zirconia-Based Thermal Barrier Coatings: Mechanistic Insights. J. Am. Ceram. Soc. 2011, 94, s168–s177. [Google Scholar] [CrossRef]
- Loganathan, A.; Gandhi, A. Effect of phase transformations on the fracture toughness of t’yttria stabilized zirconia. Mater. Sci. Eng. A 2012, 556, 927–935. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, X.; Xiao, P. The effects of temperature and composition on the thermal conductivities of [(ZrO2)1-x(CeO2)x]0.92(Y2O3)0.08 (0 ≤ x ≤ 1) solid solutions. Acta Mater. 2012, 60, 914–922. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, X.; Pan, W. Low Thermal Conductivity of SnO2-Doped Y2O3-Stabilized ZrO2: Effect of the Lattice Tetragonal Distortion. J. Am. Ceram. Soc. 2014, 98, 229–235. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, K.; Deng, C.; Liu, M.; Deng, Z.Q.; Deng, C.G.; Song, J.B. Gasdeposition mechanisms of 7YSZ coating based on plasma spray-physical vapor deposition. J. Eur. Ceram. Soc. 2016, 36, 697–703. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, X.; Zhou, K.; Liu, M.; Deng, C.; Mao, J.; Chen, Z. 7YSZ coating prepared by PS-PVD based on heterogeneous nucleation. Chin. J. Aeronaut. 2018, 31, 820–825. [Google Scholar] [CrossRef]






| Type | Product | CAS | Supplier |
|---|---|---|---|
| Dispersant | Polyacrylic acid (PAA) | 9003-01-4 | Tianjin Damao Chemical Reagent Factory, Tianjin, China |
| Binder | Polyvinylpyrrolidone (PVP) | 9003-39-8 | Institute of New Materials, Guangdong Academy of Sciences |
| Inter Temp./℃ | Outlet Temp./°C | Feeding Speed/r·min−1 | Pressure/Mpa |
|---|---|---|---|
| 250 | 120 | 35 | 0.2 |
| Sample | Calcination Temp./°C | Holding Time/h | Heating Rate/°C·min−1 |
|---|---|---|---|
| 1# | Spray-dried | / | / |
| 2# | 600 | 3 | 5 |
| 3# | 700 | 3 | 5 |
| 4# | 800 | 3 | 5 |
| 5# | 900 | 3 | 5 |
| Ar (L/min) | He (L/min) | Current (A) | Stand-off Distance (mm) |
|---|---|---|---|
| 30–40 | 55–65 | 2500–2600 | 900–1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Yang, W.; Zhan, Y.; Zhang, X.; Zhang, J. Effect of Calcination Temperature on the Microstructure, Composition and Properties of Agglomerated Nanometer CeO2-Y2O3-ZrO2 Powders for Plasma Spray–Physical Vapor Deposition (PS-PVD) and Coatings Thereof. Nanomaterials 2024, 14, 995. https://doi.org/10.3390/nano14120995
Hou Z, Yang W, Zhan Y, Zhang X, Zhang J. Effect of Calcination Temperature on the Microstructure, Composition and Properties of Agglomerated Nanometer CeO2-Y2O3-ZrO2 Powders for Plasma Spray–Physical Vapor Deposition (PS-PVD) and Coatings Thereof. Nanomaterials. 2024; 14(12):995. https://doi.org/10.3390/nano14120995
Chicago/Turabian StyleHou, Zhenning, Wenchao Yang, Yongzhong Zhan, Xiaofeng Zhang, and Jingqin Zhang. 2024. "Effect of Calcination Temperature on the Microstructure, Composition and Properties of Agglomerated Nanometer CeO2-Y2O3-ZrO2 Powders for Plasma Spray–Physical Vapor Deposition (PS-PVD) and Coatings Thereof" Nanomaterials 14, no. 12: 995. https://doi.org/10.3390/nano14120995
APA StyleHou, Z., Yang, W., Zhan, Y., Zhang, X., & Zhang, J. (2024). Effect of Calcination Temperature on the Microstructure, Composition and Properties of Agglomerated Nanometer CeO2-Y2O3-ZrO2 Powders for Plasma Spray–Physical Vapor Deposition (PS-PVD) and Coatings Thereof. Nanomaterials, 14(12), 995. https://doi.org/10.3390/nano14120995






