Systematic Review of Solubility, Thickening Properties and Mechanisms of Thickener for Supercritical Carbon Dioxide
Abstract
:1. Introduction
2. Characterization Parameters of Solubility and Thickening Properties
2.1. Solubility Properties
2.2. Thickening Properties
3. Supercritical CO2 Thickeners
3.1. Surfactants
3.2. Hydrocarbon Polymers
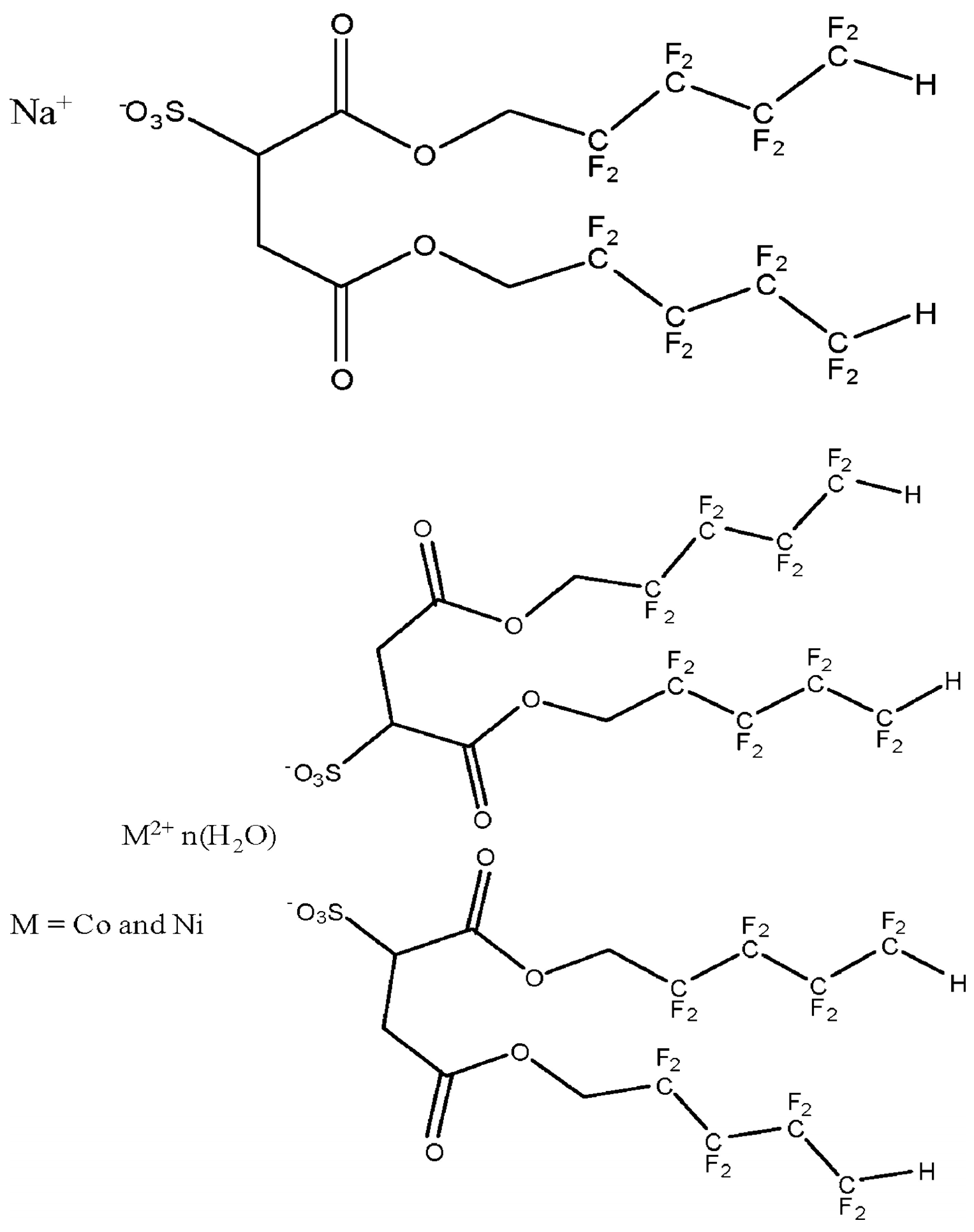
3.3. Fluorinated Polymers
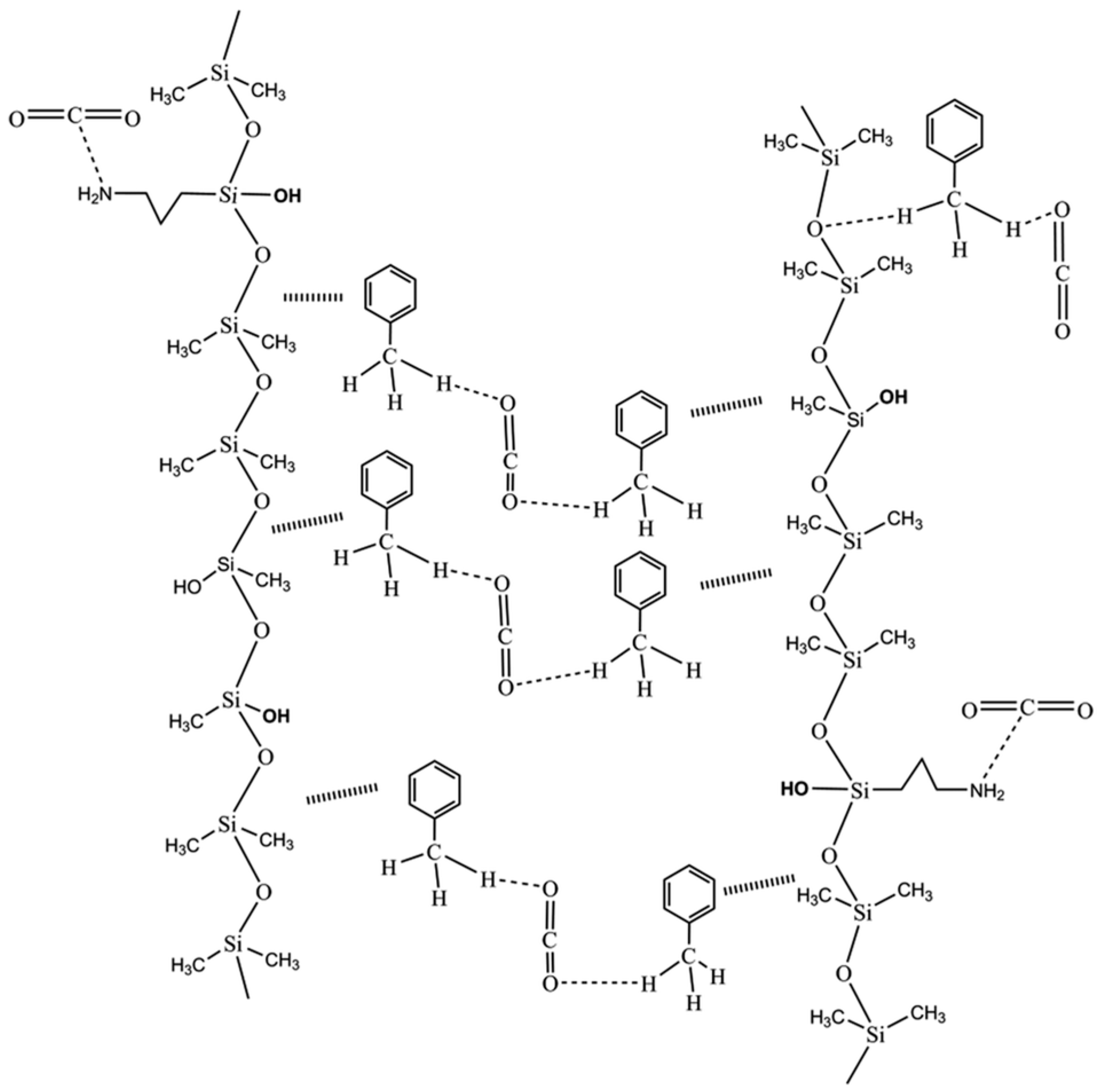
3.4. Silicone Polymer
4. Thickening Mechanism
5. Thickening Supercritical CO2 in Porous Media
5.1. The Flow of CO2 in Porous Media
5.2. Adsorption in Porous Media
6. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muhammad, A.; Nurudeen, Y.; Nilanjan, P.; Alireza, K.; Stefan, I.; Hussein, H. Influence of pressure, temperature and organic surface concentration on hydrogen wettability of caprock; implications for hydrogen geo–storage. Energy Rep. 2021, 7, 5988–5996. [Google Scholar] [CrossRef]
- Muhammad, A.; Nurudeen, Y.; Nilanjan, P.; Alireza, K.; Stefan, I.; Hussein, H. Influence of organic molecules on wetting characteristics of mica/H2/brine systems: Implications for hydrogen structural trapping capacities. J. Colloid Interface Sci. 2022, 608, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Sherif, F.; Ahmed, E.-T.; Hesham, A.; Youssef, E.; Abdulmohsin, I. Increasing Oil Recovery from Unconventional Shale Reservoirs Using Cyclic Carbon Dioxide Injection. In Proceedings of the SPE Europec, EAGE Conference and Exhibition, Virtual, 1–3 December 2020. [Google Scholar] [CrossRef]
- Sean, S.; Patricia, C.; Barbara, K.; Sittichai, N. Characterizing Pore–Scale Geochemical Alterations in Eagle Ford and Barnett Shale from Exposure to Hydraulic Fracturing Fluid and CO2/H2O. Energy Fuels 2020, 35, 583–598. [Google Scholar] [CrossRef]
- Zhang, Q.; Zuo, L.; Wu, C.; Sun, C.; Zhu, X. Effects of crude oil characteristics on foaming and defoaming behavior at separator during CO2 flooding. Colloids Surf. A Physicochem. Eng. Asp. 2020, 608, 125562. [Google Scholar] [CrossRef]
- Aiguo, S.; Jinbo, L.; Yuehui, S.; Fuchang, S.; Zhengliang, W. Synthesis of the Copolymer of Vinylacete–Methylsilsesquioxane as a Potential Carbon Dioxide Thickener. Polym. Mater. Sci. Eng. 2011, 27, 157–159. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Zhang, B.; Gan, B.; Li, G. Experimental Study on Supercritical CO2 Fracturing. Nat. Gas Explor. Dev. 2016, 39, 58–63+14. (In Chinese) [Google Scholar]
- Etienne, G.; Thierry, T.; Jean-Daniel, M.; Mathias, D. Structure–Property Relationships in CO2–philic (Co)polymers: Phase Behavior, Self–Assembly, and Stabilization of Water/CO2 Emulsions. Chem. Rev. 2016, 116, 4125–4169. [Google Scholar] [CrossRef] [PubMed]
- XueSong, X.; Guo, Y.; Zhang, J.; Sun, N.; Shen, G.; Chang, X.; Yu, W.; Tang, Z.; Chen, W.; Wei, W.; et al. Fracturing with Carbon Dioxide: From Microscopic Mechanism to Reservoir Application. Joule 2019, 3, 1913–1926. [Google Scholar] [CrossRef]
- Liu, W. Research advance in supercritical CO2 thickeners. Fault-Block Oil Gas Field 2019, 19, 658–661. (In Chinese) [Google Scholar] [CrossRef]
- Whorton, L.P.; Brownscombe, E.R.; Dyes, A.B. Method for producing oil by means of carbon dioxide. US. Patent US2623596A, 30 December 1952. [Google Scholar]
- Birgit, C.G.; Ewers, U.; Fritz, H.F. Hydraulic fracturing: A toxicological threat for groundwater and drinking–water? Environ. Earth Sci. 2013, 70, 3875–3893. [Google Scholar] [CrossRef]
- Min, L.; Mehdi, S.; Peyman, M. Impact of mineralogical heterogeneity on reactive transport modelling. Comput. Geosci. 2017, 104, 12–19. [Google Scholar] [CrossRef]
- Zhang, Y. Study on the Influencing Factors of Viscous Fingering of CO2 in Oil Reservoirs; China University of Petroleum: Beijing, China, 2010. [Google Scholar]
- Shen, Z.; Wang, H.; Li, G. Numerical simulation of the cutting–carrying ability of supercritical carbon dioxide drilling at horizontal section. Pet. Explor. Dev. 2011, 38, 233–236. (In Chinese) [Google Scholar] [CrossRef]
- Li, Q.; Chen, M.; Jin, Y.; Wang, M.; Jiang, H. Application of New Fracturing Technologies in Shale Gas Development. Spec. Oil Gas Reserv. 2012, 19, 1–7+141. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Zhu, B.; Sepehrnoori, K.; Shi, L.; Zheng, Y.; Shi, X. Key problems and solutions in supercritical CO2 fracturing technology. Front. Energy 2019, 13, 667–672. [Google Scholar] [CrossRef]
- Pal, N.; Zhang, X.; Ali, M.; Mandal, A.; Hoteit, H. Carbon dioxide thickening: A review of technological aspects, advances and challenges for oilfield application. Fuel 2022, 315, 122947. [Google Scholar] [CrossRef]
- Xie, W.; Chen, S.; Wang, M.; Yu, Z.; Wang, H. Progress and Prospects of Supercritical CO2 Application in the Exploitation of Shale Gas Reservoirs. Energy Fuels 2021, 35, 18370–18384. [Google Scholar] [CrossRef]
- LI, Q.; WANG, Y.; LI, Q.; WANG, F.; YUAN, L.; BAI, H. Thickening Performance and Thickening Mechanism of a Viscosifier for CO2 Fracturing Fluid. Drill. Fluid Complet. Fluid 2019, 36, 102–108. (In Chinese) [Google Scholar] [CrossRef]
- Xue, W.; Wenge, C.; Qiuyuan, Y.; Hongyun, N.; Qian, L.; Yun, L.; Ming, G.; Ming, X.; An, X.; Sijin, L.; et al. Preliminary investigation on cytotoxicity of fluorinated polymer nanoparticles. J. Environ. Sci. China 2018, 69, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, W.; Ye, L.; Ren, P. Progress in Research on Pollution Status and Hazards of Perfluorinated Organic Compounds (Pfcs) in Surface Water. Water Pollut. Control 2015, 33, 43–47. (In Chinese) [Google Scholar] [CrossRef]
- Salar, A.; Mohamed, H.A.; Abbas, F. Improvement in CO2 geo–sequestration in saline aquifers by viscosification: From molecular scale to core scale. Int. J. Greenh. Gas Control 2023, 125, 103888. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, N.; Wu, Q.; Wang, S.; Zhang, P.; Zhao, J. Research progress on the solubility of supercritical CO2. Refin. Chem. Ind. 2011, 22, 1–5+83. (In Chinese) [Google Scholar] [CrossRef]
- Gong, H.; Zhang, H.; Xu, L.; Li, Y.; Dong, M. Effects of cosolvent on dissolution behaviors of PVAc in supercritical CO2: A molecular dynamics study. Chem. Eng. Sci. 2019, 206, 22–30. [Google Scholar] [CrossRef]
- Robert, M.E.; Eric, J.B.; Hamilton, A. Novel CO2–Thickeners for Improved Mobility Control; UNT Digital Library: Denton, TX, USA, 2001. [Google Scholar] [CrossRef]
- Huang, Z. Preparation of CO2 Thickening Agent and Evaluation of Its Fracturing Performance; SouthWest Petroleum University: Chengdu, China, 2017. [Google Scholar]
- Poovathinthodiyil, R.; Yutaka, I.; Scott, L.W. Polar Attributes of Supercritical Carbon Dioxide. Acc. Chem. Res. 2005, 38, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Romain, D.; Delmas, G. Thermodynamic properties of polyolefin solutions at high temperature: 2. Lower critical solubility temperatures for polybutene–1, polypentene–1 and poly(4–methylpentene–1) in hydrocarbon solvents and determination of the polymer–solvent interaction parameter for PB1 and one ethylene–propylene copolymer. Polymer 1981, 22, 1190–1198. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Liang, L.; Zeng, Y. Achieving solubility alteration with functionalized polydimethylsiloxane for improving the viscosity of supercritical CO2 fracturing fluids. RSC Adv. 2021, 11, 17197–17205. [Google Scholar] [CrossRef]
- Hu, D.; Sun, S.; Yuan, P.-Q.; Zhao, L.; Liu, T. Exploration of CO2–Philicity of Poly(vinyl acetate–co–alkyl vinyl ether) through Molecular Modeling and Dissolution Behavior Measurement. J. Phys. Chem. B 2015, 119, 12490–12501. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Shi, J.; Cao, X.; Yuan, S. Molecular dynamics simulation of thickening mechanism of supercritical CO2 thickener. Chem. Phys. Lett. 2018, 706, 658–664. [Google Scholar] [CrossRef]
- Sui, S.; Xiao, P.; CUI, M. Effect of Modified Silicone CO2 Thickener on Fluid Rheology and Oil Displacement Efficiency. Oilfield Chem. 2023, 40, 229–234. (In Chinese) [Google Scholar] [CrossRef]
- Li, S.; Wang, J. Recent Developments of the Surfactants Using in Supercritical Carbon Dioxide. Chem. World 2007, 496–499+510. (In Chinese) [Google Scholar] [CrossRef]
- Liu, J.; Li, G.L.; Han, B. Research on the Aggregation and Microenvironment of Fluorine-Free and Silicon-Free Nonionic Surfactants in Supercritical Carbon Dioxide; Shandong University: Jinan, China, 2022. [Google Scholar]
- Du, M. Investigation on Supercritical Carbon Dioxide Fracturing Fluid System; China University of Petroleum (East China): Qingdao, China, 2016. [Google Scholar]
- Masanobu, S.; Yuuki, S.; Sajad, K.; Shirin, A.; Tretya, A.; Azmi, M.; Robert, M.E.; Sarah, E.R.; Christopher, H.; Julian, E. Thickening supercritical CO2 at high temperatures with rod-like reverse micelles. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133302. [Google Scholar] [CrossRef]
- Zhai, H.; Zhang, J.; Dong, J.; Wang, J.; Zhang, F.; Xiaofeng, L. Solubility Evaluation of Supercritical Carbon Dioxide Thickener. Oilfield Chem. 2021, 38, 422–426. (In Chinese) [Google Scholar] [CrossRef]
- Enick, M.R. A Literature Review of Attempts to Increase the Viscosity of Dense Carbon Dioxide. 1998. Available online: https://www.semanticscholar.org/paper/A-Literature-Review-of-Attempts-to-Increase-the-of-Enick/899dccfc1e36981b94973f48d5bf62064ae56e14 (accessed on 11 March 2024).
- Joel, F.; Naiping, H. The molecular basis of CO2 interaction with polymers containing fluorinated groups: Computational chemistry of model compounds and molecular simulation of poly bis(2,2,2–trifluoroethoxy)phosphazene. Polymer 2003, 44, 4363–4372. [Google Scholar] [CrossRef]
- Zhang, Y. Design, Synthesis and Properties of Polyethers as Carbon Dioxide Thickening Agent; Jilin University: Changchun City, China, 2017. [Google Scholar]
- Chao, S.; Zhihua, H.; Eric, J.B.; Robert, M.E.; Sun-Young, K.; Dennis, P.C. Semi-Fluorinated Trialkyltin Fluorides and Fluorinated Telechelic Ionomers as Viscosity–Enhancing Agents for Carbon Dioxide. Ind. Eng. Chem. Res. 2001, 40, 908–913. [Google Scholar] [CrossRef]
- Robert, M.E.; Eric, J.B.; Chen, S.; Eddy, K. Formation of fluoroether polyurethanes in CO2. In Proceedings of the 4th International Symposium on Supercritical Fluids (ISSF 97), Sendai, Japan, 11–14 May 1998. [Google Scholar]
- Kieran, T.; Dazun, X.; Robert, M.E.; Julian, E.; Martin, J.H.; Kevin, J.M.; Sarah, E.R.; Richard, K.H.; David, C.S. Rod-Like Micelles Thicken CO2. Langmuir 2009, 26, 83–88. [Google Scholar] [CrossRef]
- Hoefling, T.A.; Newman, D.A.; Robert, M.E.; Eric, J.B. Effect of structure on the cloud-point curves of silicone-based amphiphiles in supercritical carbon dioxide. J. Supercrit. Fluids 1993, 6, 165–171. [Google Scholar] [CrossRef]
- Stephen, C.; Robert, M.E.; Sarah, E.R.; Richard, K.H.; Julian, E. Amphiphiles for supercritical CO2. Biochimie 2012, 94, 94–100. [Google Scholar] [CrossRef]
- Xin, F.; Vijay, P.; Michael, C.M.; Yan, W.; Juncheng, L.; Robert, M.E.; Andrew, D.H.; Christopher, B.R.; Johnson, J.K.; Eric, J.B. Oxygenated Hydrocarbon Ionic Surfactants Exhibit CO2 Solubility. J. Am. Chem. Soc. 2005, 127, 11754–11762. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, C.; Xu, J.; Duan, Y. Application and research of liquid carbon dioxide based gel fracturing fluid in tight gas reservoir. In Proceedings of the 2016 Natural Gas Academic Annual Conference, Yinchuan, China, 28 September 2016. [Google Scholar]
- Heller, J.P.; Dandge, D.K.; Roger, J.C.; Donaruma, L.G. Direct Thickeners for Mobility Control of CO2 Floods. Soc. Pet. Eng. J. 1985, 25, 679–686. [Google Scholar] [CrossRef]
- Traian, S.; Thomas, S.; Eric, J.B. Non-fluorous polymers with very high solubility in supercritical CO2 down to low pressures. Nature 2000, 405, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; McHugh, M.; Xu, J.; Belardi, J.; Kilic, S.; Mesiano, A.; Bane, S.; Karnikas, C.; Beckman, E.; Enick, R. CO2–solubility of oligomers and polymers that contain the carbonyl group. Polymer 2003, 44, 1491–1498. [Google Scholar] [CrossRef]
- Shen, A.; Liu, J.; She, Y.; Shu, F.; Wang, Z. Design and Synthesis of Styrene Vinyl-acetate Copolymer as Potential CO2 Thickener. J. Oil Gas Technol. 2011, 33, 131–134+168. (In Chinese) [Google Scholar]
- Zhang, J. Carbon Dioxide Thickening Using Poly(Vinyl Acetate) and Amphiphilic Surfactant; East China University of Science and Technology: Shanghai, China, 2017. [Google Scholar]
- Goicochea, A.G.; Abbas, F. CO2 Viscosification by Functional Molecules from Mesoscale Simulations. J. Phys. Chem. C 2019, 123, 29461–29467. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, J.; Ma, Z.; Zhang, X.; Fan, H.; Bittencourt, C. Evaluation of CO2–philicity and thickening capability of multichain poly(ether–carbonate) with assistance of molecular simulations. J. Appl. Polym. Sci. 2020, 138, 49700. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H.; Zha, Y.; Yu, J.; Zhang, J.; Ge, Y.; Sun, B.; Zhang, Y.; Gao, C. Experimental and microscopic investigations of the performance of copolymer thickeners in supercritical CO2. Chem. Eng. Sci. 2020, 226, 115857. [Google Scholar] [CrossRef]
- Kazuya, K.; Abbas, F. Branching in molecular structure enhancement of solubility in CO2. PNAS Nexus 2023, 2, pgad393. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Kantzas, A.; Firoozabadi, A. Spatiotemporal X-ray Imaging of Neat and Viscosified CO2 in Displacement of Brine-Saturated Porous Media. SPE J. 2024, 1–16. [Google Scholar] [CrossRef]
- Joseph, M.D.; Zihibin, G.; Elsbernd, C.S. Synthesis of Fluoropolymers in Supercritical Carbon Dioxide. Science 1992, 257, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shi, C.; Xu, J.; Kilic, S.; Enick, R.M.; Beckman, E.J. Enhancement of the Viscosity of Carbon Dioxide Using Styrene/Fluoroacrylate Copolymers. Macromolecules 2000, 33, 5437–5442. [Google Scholar] [CrossRef]
- Robert, M.E.; Eric, J.B.; Ali Vaziri, Y.; Val, J.K.; Hans, S.; Jon, L.H. Phase behavior of CO2–perfluoropolyether oil mixtures and CO2–perfluoropolyether chelating agent mixtures. J. Supercrit. Fluids 1998, 13, 121–126. [Google Scholar] [CrossRef]
- Baojiang, S.; Wenchao, S.; Haige, W.; Ying, L.; Haiming, F.; Hao, L.; Xiuping, C. Molecular simulation aided design of copolymer thickeners for supercritical CO2 as non-aqueous fracturing fluid. J. CO2 Util. 2018, 28, 107–116. [Google Scholar] [CrossRef]
- Sevgi, K.; Robert, M.E.; Eric, J.B. Fluoroacrylate–aromatic acrylate copolymers for viscosity enhancement of carbon dioxide. J. Supercrit. Fluids 2019, 146, 38–46. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Q.; Wang, F.; Li, Y.; Tang, L. Synthesis and performance evaluation of supercritical CO2 thickener for fracturing. Fault-Block Oil Gas Field 2018, 25, 541–544. (In Chinese) [Google Scholar]
- Jae-Heum, B.; Irani, C.A. A Laboratory Investigation of Viscosified CO2 Process. SPE Adv. Technol. Ser. 1993, 1, 166–171. [Google Scholar] [CrossRef]
- Jae-Heum, B. Viscosified CO2 Process: Chemical Transport and Other Issues. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Gao, M.; Wang, T.; Dai, C.; Wang, X.; Guan, B.; Liu, P. Formulation and performance evaluation of polymer-thickened supercritical CO2 fracturing fluid. J. Pet. Sci. Eng. 2021, 201, 108474. [Google Scholar] [CrossRef]
- Fink, R.; Hancu, D.; Valentine, R.; Beckman, E.J. Toward the Development of “CO2–philic” Hydrocarbons. 1. Use of Side–Chain Functionalization to Lower the Miscibility Pressure of Polydimethylsiloxanes in CO2. J. Phys. Chem. B 1999, 103, 6441–6444. [Google Scholar] [CrossRef]
- Sevgi, K.; Yang, W.; Johnson, J.K.; Eric, J.B.; Robert, M.E. Influence of tert–amine groups on the solubility of polymers in CO2. Polymer 2009, 50, 2436–2444. [Google Scholar] [CrossRef]
- Michael, J.O.B.; Robert, J.P.; Mark, D.D.; Jason, J.L.; Aman, D.; Eric, J.B.; Robert, M.E. Anthraquinone Siloxanes as Thickening Agents for Supercritical CO2. Energy Fuels 2016, 30, 5990–5998. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Q.; Foster, G.; Lei, C. Study on the optimization of silicone copolymer synthesis and the evaluation of its thickening performance. RSC Adv. 2018, 8, 8770–8778. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Dong, W.; Li, Q.; Wang, F.; Bai, H.; Zhang, R.; Owusu, A.B. Effect of different factors on the yield of epoxy-terminated polydimethylsiloxane and evaluation of CO2 thickening. RSC Adv. 2018, 8, 39787–39796. [Google Scholar] [CrossRef] [PubMed]
- Masanobu, S.; Shotaro, O.; Craig, J.; Atsushi, Y.; Azmi, M.; Frédéric, G.; Robert, M.E.; Sarah, E.R.; Adam, C.; Christopher, H.; et al. Anisotropic reversed micelles with fluorocarbon–hydrocarbon hybrid surfactants in supercritical CO2. Colloids Surf. B Biointerfaces 2018, 168, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hoefling, T.A.; David, S.; Reid, M.D.; Eric, J.B.; Robert, M.E. The incorporation of a fluorinated ether functionality into a polymer or surfactant to enhance CO2–solubility. J. Supercrit. Fluids 1992, 5, 237–241. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, L.; Zhao, C.; Zhang, Y.; Song, Y. A review of research on the dispersion process and CO2 enhanced natural gas recovery in depleted gas reservoir. J. Pet. Sci. Eng. 2021, 208, 109682. [Google Scholar] [CrossRef]
- Miri, R. Effects of CO2–Brine–Rock Interactions on CO2 Injectivity–Implications for CCS; University of Oslo: Oslo, Norway, 2015. [Google Scholar]
- Lenormand, R.; Éric, T.; Zarcone, C. Numerical models and experiments on immiscible displacements in porous media. J. Fluid Mech. 1988, 189, 165–187. [Google Scholar] [CrossRef]
- Li, Y. The Numerical Simulation Study on CO2–Water Two Phase Flow in Porous Media Based on CFD; Dalian University of Technology: Dalian, China, 2015. [Google Scholar]
- Li, Q.; Wang, Y.; Owusu, A.B. A modified Ester-branched thickener for rheology and wettability during CO2 fracturing for improved fracturing property. Environ. Sci. Pollut. Res. 2019, 26, 20787–20797. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Q.; Liang, S.; Zhao, S. Systematic Review of Solubility, Thickening Properties and Mechanisms of Thickener for Supercritical Carbon Dioxide. Nanomaterials 2024, 14, 996. https://doi.org/10.3390/nano14120996
Wang X, Zhang Q, Liang S, Zhao S. Systematic Review of Solubility, Thickening Properties and Mechanisms of Thickener for Supercritical Carbon Dioxide. Nanomaterials. 2024; 14(12):996. https://doi.org/10.3390/nano14120996
Chicago/Turabian StyleWang, Xiaohui, Qihong Zhang, Shiwei Liang, and Songqing Zhao. 2024. "Systematic Review of Solubility, Thickening Properties and Mechanisms of Thickener for Supercritical Carbon Dioxide" Nanomaterials 14, no. 12: 996. https://doi.org/10.3390/nano14120996





