Enhancement of Photodetector Characteristics by Zn-Porphyrin-Passivated MAPbBr3 Single Crystals
Abstract
:1. Introduction
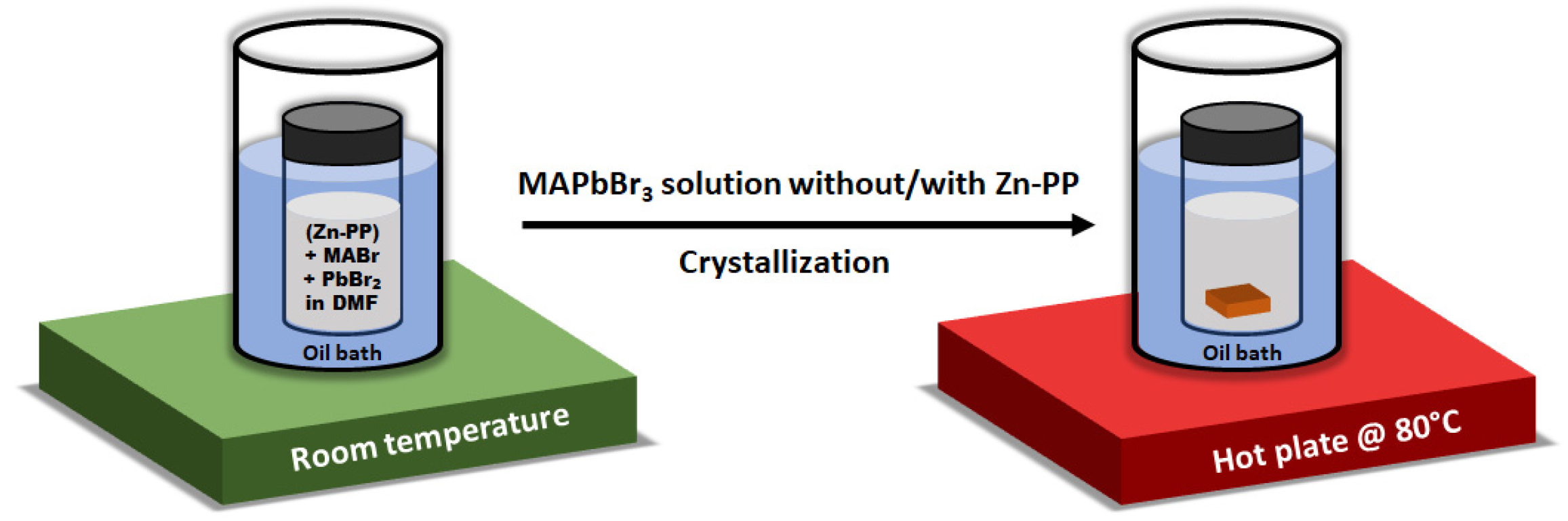
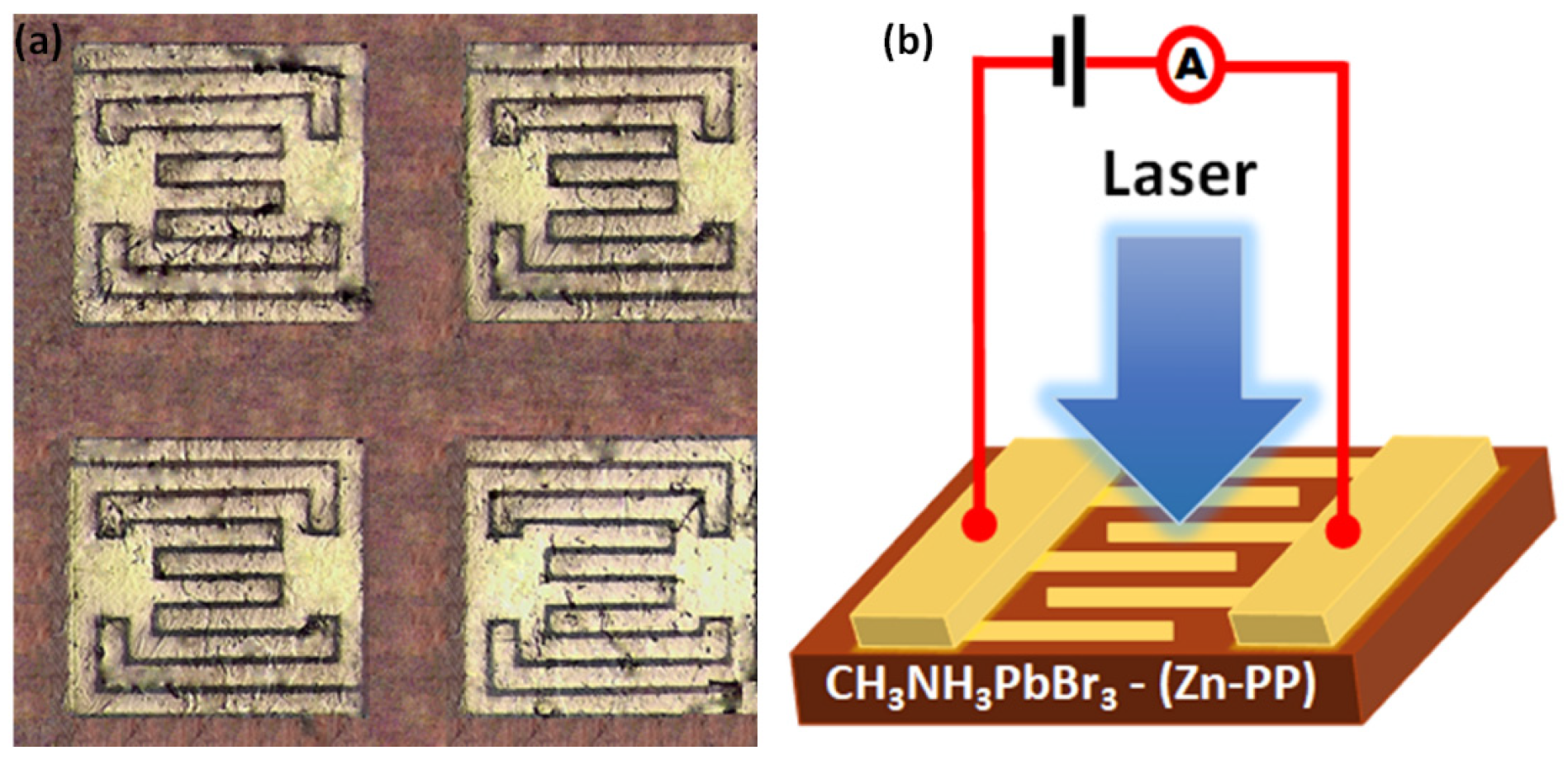
2. Materials and Methods
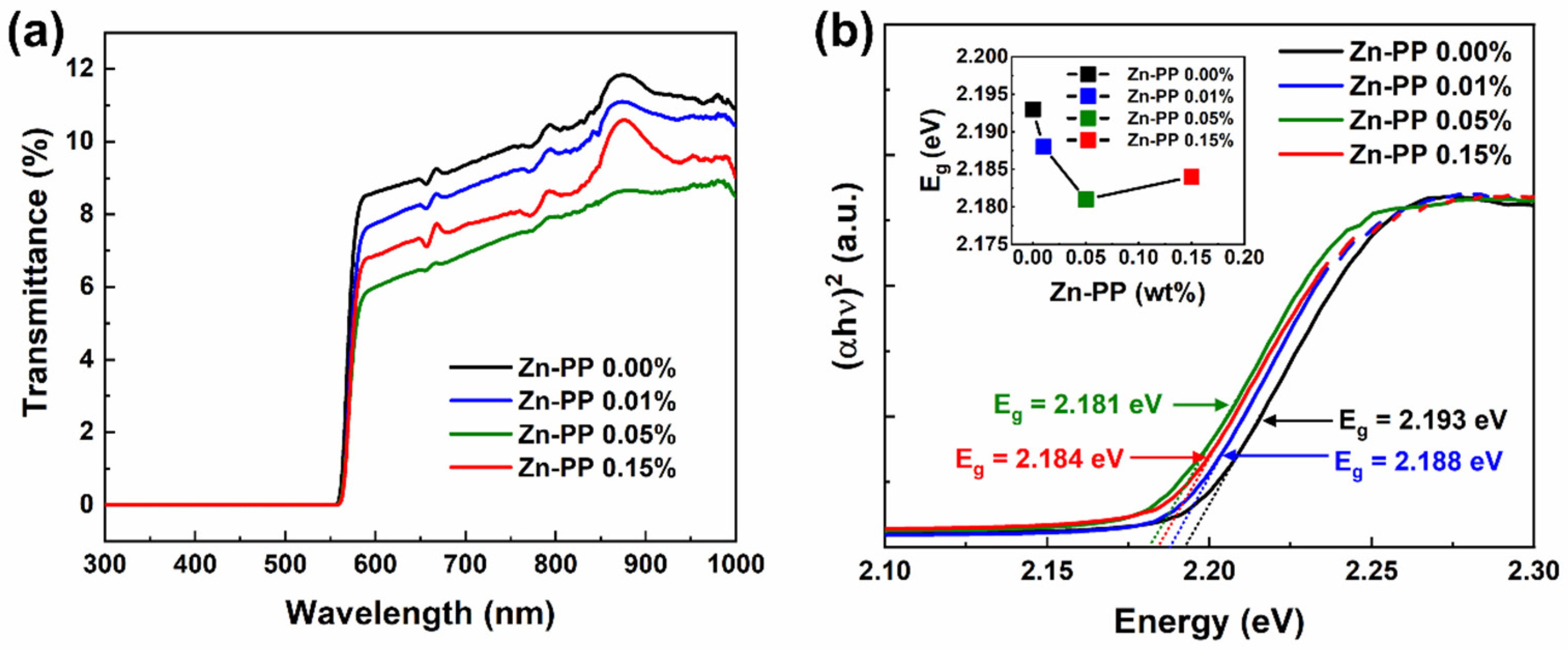
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tong, Y.; Najar, A.; Wang, L.; Liu, L.; Du, M.; Yang, J.; Li, J.; Wang, K.; Liu, S. Wide-Bandgap Organic–Inorganic Lead Halide Perovskite Solar Cells. Adv. Sci. 2022, 9, 2105085. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ma, S.; Xue, X.; Li, T.; Sha, S.; Ren, X.; Zhang, J.; Lu, H.; Ma, J.; Guo, S.; et al. Highly Efficient and Stable CsPbTh3 (Th = I, Br, Cl) Perovskite Solar Cells by Combinational Passivation Strategy. Adv. Sci. 2022, 9, 2105103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Najar, A.; Zhang, J.; Feng, J.; Cao, Y.; Li, Z.; Zhu, X.; Yang, D.; Liu, S.F. Effect of Solvent Residue in the Thin-Film Fabrication on Perovskite Solar Cell Performance. ACS Appl. Mater. Interfaces 2022, 14, 28729–28737. [Google Scholar] [CrossRef] [PubMed]
- Soopy, A.K.K.; Parida, B.; Aravindh, S.A.; SahulHameed, H.; Swain, B.S.; Saleh, N.i.; Taha, I.M.A.; Anjum, D.H.; Alberts, V.; Liu, S.; et al. Zn–Porphyrin Antisolvent Engineering-Enhanced Grain Boundary Passivation for High-Performance Perovskite Solar Cell. Sol. RRL 2024, 8, 2400054. [Google Scholar] [CrossRef]
- Soopy, A.K.K.; Parida, B.; Aravindh, S.A.; Al Ghaithi, A.O.; Qamhieh, N.; Amrane, N.; Benkraouda, M.; Liu, S.; Najar, A. Towards High Performance: Solution-Processed Perovskite Solar Cells with Cu-Doped CH3NH3PbI3. Nanomaterials 2024, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Li, Y.; Yang, Z.; Liu, S. Perovskite CH3NH3Pb(BrxI1−x)3 single crystals with controlled composition for fine-tuned bandgap towards optimized optoelectronic applications. J. Mater. Chem. C 2016, 4, 9172–9178. [Google Scholar] [CrossRef]
- Park, S.; Chang, W.J.; Lee, C.W.; Park, S.; Ahn, H.-Y.; Nam, K.T. Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution. Nat. Energy 2016, 2, 16185. [Google Scholar] [CrossRef]
- Zhang, X.; Chu, D.; Jia, B.; Zhao, Z.; Pi, J.; Yang, Z.; Li, Y.; Hao, J.; Shi, R.; Dong, X.; et al. Heterointerface Design of Perovskite Single Crystals for High-Performance X-Ray Imaging. Adv. Mater. 2024, 36, 2305513. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, Z.; Wang, P.; Wu, F.; Liu, L.; Najar, A.; Liu, Y.; Yang, Z.; Liu, S. Cs3Cu2I5 Nanocrystals with Near-Unity Photoluminescence Quantum Yield for Stable and High-Spatial-Resolution X-ray Imaging. ACS Appl. Nano Mater. 2023, 6, 11472–11480. [Google Scholar] [CrossRef]
- Chen, M.; Dong, X.; Chu, D.; Jia, B.; Zhang, X.; Zhao, Z.; Hao, J.; Zhang, Y.; Feng, J.; Ren, X.; et al. Interlayer-Spacing Engineering of Lead-Free Perovskite Single Crystal for High-Performance X-ray Imaging. Adv. Mater. 2023, 35, 2211977. [Google Scholar] [CrossRef]
- Lozhkina, O.A.; Yudin, V.I.; Murashkina, A.A.; Shilovskikh, V.V.; Davydov, V.G.; Kevorkyants, R.; Emeline, A.V.; Kapitonov, Y.V.; Bahnemann, D.W. Low Inhomogeneous Broadening of Excitonic Resonance in MAPbBr3 Single Crystals. J. Phys. Chem. Lett. 2018, 9, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Liu, Y.; Sun, Y.; Yuan, D.; Liu, X.; Lu, W.; Liu, G.; Xia, H.; Tao, X. Bulk crystal growth of hybrid perovskite material CH3NH3PbI3. CrystEngComm 2015, 17, 665–670. [Google Scholar] [CrossRef]
- Ding, J.; Yan, Q. Progress in organic-inorganic hybrid halide perovskite single crystal: Growth techniques and applications. Sci. China Mater. 2017, 60, 1063–1078. [Google Scholar] [CrossRef]
- Su, J.; Chen, D.P.; Lin, C.T. Growth of large CH3NH3PbX3 (X = I, Br) single crystals in solution. J. Cryst. Growth 2015, 422, 75–79. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhang, B.; Dong, J.; Jie, W.; Xu, Y. Charge Transport Behavior in Solution-Grown Methylammonium Lead Tribromide Perovskite Single Crystal Using α Particles. J. Phys. Chem. C 2018, 122, 14355–14361. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xu, Z.; Ye, H.; Li, Q.; Hu, M.; Yang, Z.; Liu, S. Two-dimensional (PEA)2PbBr4 perovskite single crystals for a high performance UV-detector. J. Mater. Chem. C 2019, 7, 1584–1591. [Google Scholar] [CrossRef]
- Cho, Y.; Jung, H.R.; Kim, Y.S.; Kim, Y.; Park, J.; Yoon, S.; Lee, Y.; Cheon, M.; Jeong, S.-Y.; Jo, W. High speed growth of MAPbBr3 single crystals via low-temperature inverting solubility: Enhancement of mobility and trap density for photodetector applications. Nanoscale 2021, 13, 8275–8282. [Google Scholar] [CrossRef]
- Maculan, G.; Sheikh, A.D.; Abdelhady, A.L.; Saidaminov, M.I.; Haque, M.A.; Murali, B.; Alarousu, E.; Mohammed, O.F.; Wu, T.; Bakr, O.M. CH3NH3PbCl3 Single Crystals: Inverse Temperature Crystallization and Visible-Blind UV-Photodetector. J. Phys. Chem. Lett. 2015, 6, 3781–3786. [Google Scholar] [CrossRef]
- Kim, T.; Chu, Y.H.; Lee, J.; Cho, S.H.; Kim, S.; Bang, K.; Lee, H.; Lim, C.; Lee, Y.S. Confined Growth of High-quality Single-Crystal MAPbBr3 by Inverse Temperature Crystallization for Photovoltaic Applications. Electron. Mater. Lett. 2021, 17, 347–354. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Abdelhady, A.L.; Murali, B.; Alarousu, E.; Burlakov, V.M.; Peng, W.; Dursun, I.; Wang, L.; He, Y.; Maculan, G.; et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 2015, 6, 7586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, Q.; Xiang, W.; Du, Y.; Wang, Z.; Liu, Y.; Liu, L.; Yang, T.; Wu, H.; Nie, T.; et al. Tailored Cysteine-Derived Molecular Structures toward Efficient and Stable Inorganic Perovskite Solar Cells. Adv. Mater. 2023, 35, 2301140. [Google Scholar] [CrossRef] [PubMed]
- Gkini, K.; Balis, N.; Papadakis, M.; Verykios, A.; Skoulikidou, M.-C.; Drivas, C.; Kennou, S.; Golomb, M.; Walsh, A.; Coutsolelos, A.G.; et al. Manganese Porphyrin Interface Engineering in Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 7353–7363. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, L.; Mu, X.; Chen, B.; Xiong, Q.; Wang, W.D.; Ding, J.; Gao, P.; Wu, Y.; Cao, J. Grain Boundary Engineering with Self-Assembled Porphyrin Supramolecules for Highly Efficient Large-Area Perovskite Photovoltaics. J. Am. Chem. Soc. 2021, 143, 18989–18996. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, C.; Lin, P.; Hu, H.; Meng, Q.; Xu, L.; Wang, P.; Wu, X.; Cui, C. Homologous bromides passivation of CH3NH3PbBr3 single crystals for photodetectors with improved properties. J. Alloys Compd. 2023, 935, 168132. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yang, Z.; Feng, J.; Xu, Z.; Li, Q.; Hu, M.; Ye, H.; Zhang, X.; Liu, M.; et al. Low-temperature-gradient crystallization for multi-inch high-quality perovskite single crystals for record performance photodetectors. Mater. Today 2019, 22, 67–75. [Google Scholar] [CrossRef]
- Shen, H.; Nan, R.; Jian, Z.; Li, X. Defect step controlled growth of perovskite MAPbBr3 single crystal. J. Mater. Sci. 2019, 54, 11596–11603. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Yuan, S.; Wang, J.; Zhan, Y.; Zheng, L. An optical dynamic study of MAPbBr3 single crystals passivated with MAPbCl3/I3-MAPbBr3 heterojunctions. Phys. Chem. Chem. Phys. 2017, 19, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-H.; Li, L.-C.; Shellaiah, M.; Wen Sun, K. Structural and Photophysical Properties of Methylammonium Lead Tribromide (MAPbBr3) Single Crystals. Sci. Rep. 2017, 7, 13643. [Google Scholar] [CrossRef]
- Konda, S.R.; Lin, Y.; Rajan, R.A.; Yu, W.; Li, W. Measurement of Optical Properties of CH3NH3PbX3 (X = Br, I) Single Crystals Using Terahertz Time-Domain Spectroscopy. Materials 2023, 16, 610. [Google Scholar] [CrossRef]
- Liu, H.; Wei, X.; Zhang, Z.; Lei, X.; Xu, W.; Luo, L.; Zeng, H.; Lu, R.; Liu, J. Microconcave MAPbBr3 Single Crystal for High-Performance Photodetector. J. Phys. Chem. Lett. 2019, 10, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Huang, Z.; Li, C.; Li, M.; Song, Y. A general printing approach for scalable growth of perovskite single-crystal films. Sci. Adv. 2018, 4, eaat2390. [Google Scholar] [CrossRef] [PubMed]
- Grancini, G.; Srimath Kandada, A.R.; Frost, J.M.; Barker, A.J.; De Bastiani, M.; Gandini, M.; Marras, S.; Lanzani, G.; Walsh, A.; Petrozza, A. Role of microstructure in the electron–hole interaction of hybrid lead halide perovskites. Nat. Photonics 2015, 9, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, L.; Du, C. Polarization-Sensitive Light Sensors Based on a Bulk Perovskite MAPbBr3 Single Crystal. Materials 2021, 14, 1238. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-x.; Su, X.-q.; Wang, J.; Gao, D.-w.; Pan, Y.; Wang, L. Modulation of photoluminescence intensity by surface defects of MAPbBr3 crystals. Opt. Mater. 2023, 138, 113561. [Google Scholar] [CrossRef]
- D’Innocenzo, V.; Srimath Kandada, A.R.; De Bastiani, M.; Gandini, M.; Petrozza, A. Tuning the Light Emission Properties by Band Gap Engineering in Hybrid Lead Halide Perovskite. J. Am. Chem. Soc. 2014, 136, 17730–17733. [Google Scholar] [CrossRef] [PubMed]
- Grancini, G.; D’Innocenzo, V.; Dohner, E.R.; Martino, N.; Srimath Kandada, A.R.; Mosconi, E.; De Angelis, F.; Karunadasa, H.I.; Hoke, E.T.; Petrozza, A. CH3NH3PbI3 perovskite single crystals: Surface photophysics and their interaction with the environment. Chem. Sci. 2015, 6, 7305–7310. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-Q.; Zhang, T.-Y.; Chen, L.; Guo, N.; Wang, Y.; Liu, G.-K.; Wang, J.-R.; Zhou, J.-Z.; Yan, J.-W.; Zhao, Y.-X.; et al. Organic–inorganic interactions of single crystalline organolead halide perovskites studied by Raman spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 18112–18118. [Google Scholar] [CrossRef]
- Hills-Kimball, K.; Nagaoka, Y.; Cao, C.; Chaykovsky, E.; Chen, O. Synthesis of formamidinium lead halide perovskite nanocrystals through solid–liquid–solid cation exchange. J. Mater. Chem. C 2017, 5, 5680–5684. [Google Scholar] [CrossRef]
- Yamanaka, T.; Masumori, K.; Ishikawa, R.; Ueno, K.; Shirai, H. Role of Isopropyl Alcohol Solvent in the Synthesis of Organic–Inorganic Halide CH(NH2)2PbIxBr3–x Perovskite Thin Films by a Two-Step Method. J. Phys. Chem. C 2016, 120, 25371–25377. [Google Scholar] [CrossRef]
- Williams, A.E.; Holliman, P.J.; Carnie, M.J.; Davies, M.L.; Worsley, D.A.; Watson, T.M. Perovskite processing for photovoltaics: A spectro-thermal evaluation. J. Mater. Chem. A 2014, 2, 19338–19346. [Google Scholar] [CrossRef]
- Guo, X.; McCleese, C.; Kolodziej, C.; Samia, A.C.S.; Zhao, Y.; Burda, C. Identification and characterization of the intermediate phase in hybrid organic–inorganic MAPbI3 perovskite. Dalton Trans. 2016, 45, 3806–3813. [Google Scholar] [CrossRef]
- Glaser, T.; Müller, C.; Sendner, M.; Krekeler, C.; Semonin, O.E.; Hull, T.D.; Yaffe, O.; Owen, J.S.; Kowalsky, W.; Pucci, A.; et al. Infrared Spectroscopic Study of Vibrational Modes in Methylammonium Lead Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Syed, A.; Akhtar, F.H.; Shevate, R.; Singh, S.; Peinemann, K.-V.; Baran, D.; Wu, T. Giant Humidity Effect on Hybrid Halide Perovskite Microstripes: Reversibility and Sensing Mechanism. ACS Appl. Mater. Interfaces 2019, 11, 29821–29829. [Google Scholar] [CrossRef]
- Attique, S.; Ali, N.; Khatoon, R.; Ali, S.; Abbas, A.; Yu, Y.; Hou, J.; Cao, B.; Wu, H.; Yang, S. Aqueous phase fabrication and conversion of Pb(OH)Br into a CH3NH3PbBr3 perovskite and its application in resistive memory switching devices. Green Chem. 2020, 22, 3608–3614. [Google Scholar] [CrossRef]
- Craven, C.W.; Reissmann, K.R.; Chinn, H.I. Infrared Absorption Spectra of Porphyrins. Anal. Chem. 1952, 24, 1214–1215. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Wang, A.; Wu, L.; Wang, Q. Facile synthesis and photocatalytic activity of a novel titanium dioxide nanocomposite coupled with zinc porphyrin. Nanomater. Nanotechnol. 2016, 6, 1847980416669487. [Google Scholar] [CrossRef]
- Słota, R.; Broda, M.A.; Dyrda, G.; Ejsmont, K.; Mele, G. Structural and Molecular Characterization of meso-Substituted Zinc Porphyrins: A DFT Supported Study. Molecules 2011, 16, 9957–9971. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Yang, Z.; Yang, D.; Ren, X.; Xu, H.; Yang, Z.; Liu, S. 20-mm-Large Single-Crystalline Formamidinium-Perovskite Wafer for Mass Production of Integrated Photodetectors. Adv. Opt. Mater. 2016, 4, 1829–1837. [Google Scholar] [CrossRef]
- Ueda, T.; An, Z.; Hirakawa, K.; Komiyama, S. Charge-sensitive infrared phototransistors: Characterization by an all-cryogenic spectrometer. J. Appl. Phys. 2008, 103, 093109. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yang, Z.; Yang, D.; Ren, X.; Pang, L.; Liu, S. Thinness- and Shape-Controlled Growth for Ultrathin Single-Crystalline Perovskite Wafers for Mass Production of Superior Photoelectronic Devices. Adv. Mater. 2016, 28, 9204–9209. [Google Scholar] [CrossRef] [PubMed]
- Shafa, M.; Wu, D.; Chen, X.; Alvi, N.u.H.; Pan, Y.; Najar, A. Flexible infrared photodetector based on indium antimonide nanowire arrays. Nanotechnology 2021, 32, 27LT01. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Ye, S.; Peng, X.; Sun, Z.; Lian, J.; Liu, L.; Qu, J.; Song, J. Self-powered photodetectors based on CsxDMA1-xPbI3 perovskite films with high detectivity and stability. Nano Energy 2020, 71, 104611. [Google Scholar] [CrossRef]
- Sun, R.; Tian, Q.; Li, M.; Wang, H.; Chang, J.; Xu, W.; Li, Z.; Pan, Y.; Wang, F.; Qin, T. Over 24% Efficient Poly(vinylidene fluoride) (PVDF)-Coordinated Perovskite Solar Cells with a Photovoltage up to 1.22 V. Adv. Funct. Mater. 2023, 33, 2210071. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Du, P.; Wang, E.; Gong, X.; Heeger, A.J. Ultrasensitive solution-processed broad-band photodetectors using CH3NH3PbI3 perovskite hybrids and PbS quantum dots as light harvesters. Nanoscale 2015, 7, 16460–16469. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhao, K.; Yang, Z.; Feng, J.; Zhang, X.; Wang, K.; Meng, L.; Ye, H.; Liu, M.; et al. A 1300 mm2 Ultrahigh-Performance Digital Imaging Assembly using High-Quality Perovskite Single Crystals. Adv. Mater. 2018, 30, 1707314. [Google Scholar] [CrossRef] [PubMed]
- Murali, B.; Saidaminov, M.I.; Abdelhady, A.L.; Peng, W.; Liu, J.; Pan, J.; Bakr, O.M.; Mohammed, O.F. Robust and air-stable sandwiched organo-lead halide perovskites for photodetector applications. J. Mater. Chem. C 2016, 4, 2545–2552. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Adinolfi, V.; Comin, R.; Abdelhady, A.L.; Peng, W.; Dursun, I.; Yuan, M.; Hoogland, S.; Sargent, E.H.; Bakr, O.M. Planar-integrated single-crystalline perovskite photodetectors. Nat. Commun. 2015, 6, 8724. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Haque, M.A.; Savoie, M.; Abdelhady, A.L.; Cho, N.; Dursun, I.; Buttner, U.; Alarousu, E.; Wu, T.; Bakr, O.M. Perovskite Photodetectors Operating in Both Narrowband and Broadband Regimes. Adv. Mater. 2016, 28, 8144–8149. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yang, Z.; Liu, S. High-quality perovskite MAPbI3 single crystals for broad-spectrum and rapid response integrate photodetector. J. Energy Chem. 2018, 27, 722–727. [Google Scholar] [CrossRef]
- Shaikh, P.A.; Shi, D.; Retamal, J.R.D.; Sheikh, A.D.; Haque, M.A.; Kang, C.-F.; He, J.-H.; Bakr, O.M.; Wu, T. Schottky junctions on perovskite single crystals: Light-modulated dielectric constant and self-biased photodetection. J. Mater. Chem. C 2016, 4, 8304–8312. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Liang, L.; Bao, J.; Li, S.; Yang, W.; Xie, Y. High-Performance Flexible Broadband Photodetector Based on Organolead Halide Perovskite. Adv. Funct. Mater. 2014, 24, 7373–7380. [Google Scholar] [CrossRef]
- Xia, H.-R.; Li, J.; Sun, W.-T.; Peng, L.-M. Organohalide lead perovskite based photodetectors with much enhanced performance. Chem. Commun. 2014, 50, 13695–13697. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wang, Y.; Li, M.; Huang, L.; Yang, J.; Li, G.; Zhang, X.; Xiao, K.; Sun, W. Improved optoelectronic performance from the internal secondary excitation of MAPbCl3-MAPbBr3 single crystal photodetectors. Ceram. Int. 2023, 49, 518–527. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Zhang, W.; Li, F.; Wang, Z.; Wang, X.; Shao, Y.; Shao, J. Surface Passivation of MAPbBr3 Perovskite Single Crystals to Suppress Ion Migration and Enhance Photoelectronic Performance. ACS Appl. Mater. Interfaces 2022, 14, 10917–10926. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yang, X.; Dong, D.; Li, B.; Yang, D.; Yuan, S.; Qiao, K.; Cheng, Y.-B.; Tang, J.; Song, H. Flexible and Semitransparent Organolead Triiodide Perovskite Network Photodetector Arrays with High Stability. Nano Lett. 2015, 15, 7963–7969. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, V.; Mahapatra, A.; Nawrocki, J.; Chavan, R.D.; Yadav, P.; Prochowicz, D. Metal-Doped MAPbBr3 Single Crystal p-n Junction Photodiode for Self-Powered Photodetection. Adv. Opt. Mater. 2024, 12, 2302032. [Google Scholar] [CrossRef]
- Rong, S.; Xiao, Y.; Jiang, J.; Zeng, Q.; Li, Y. Strongly Enhanced Photoluminescence and Photoconductivity in Erbium-Doped MAPbBr3 Single Crystals. J. Phys. Chem. C 2020, 124, 8992–8998. [Google Scholar] [CrossRef]


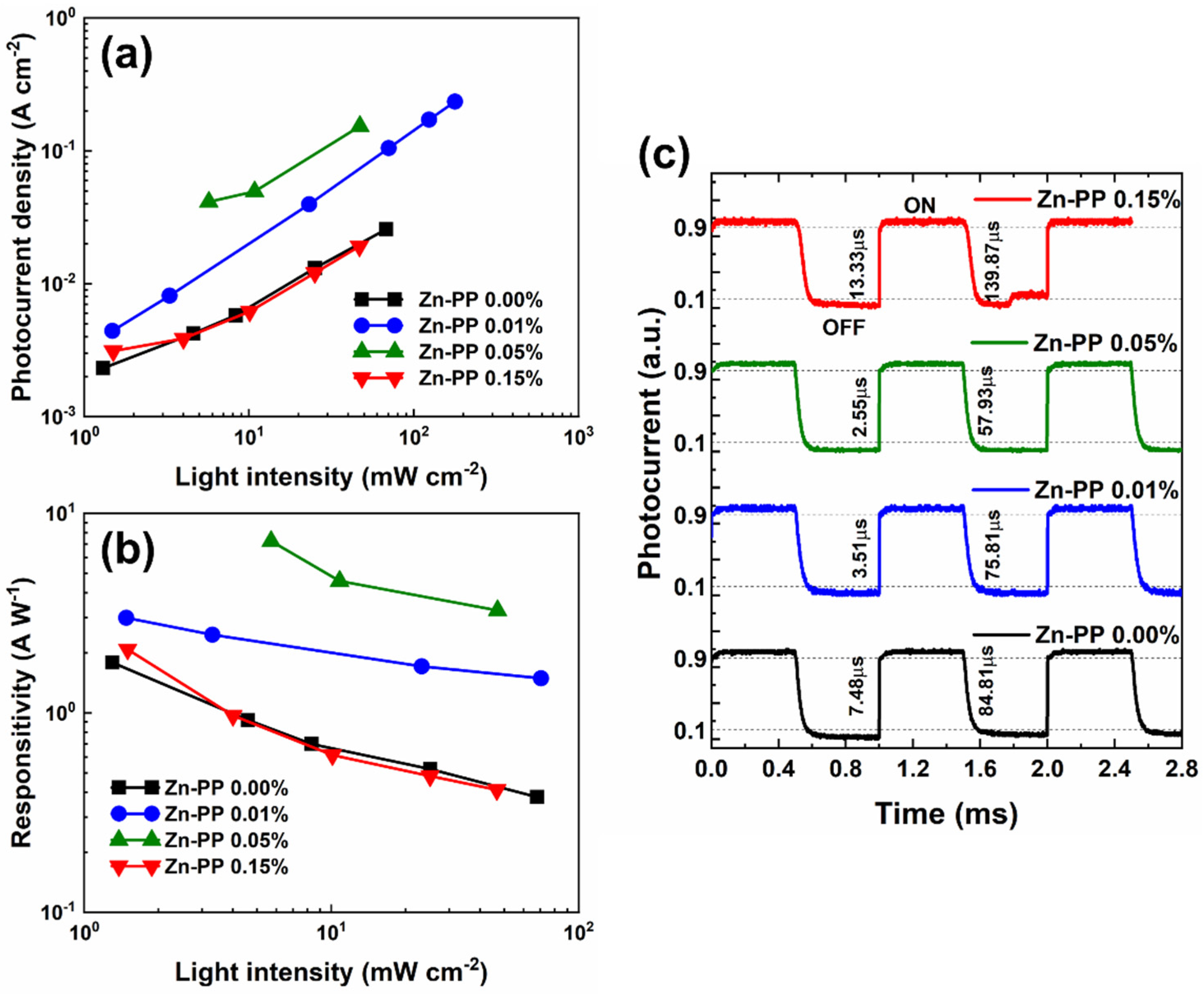
| Device | Responsivity (AW−1) | EQE (%) | Detectivity (Jones) | NEP (WHz1/2) |
|---|---|---|---|---|
| Zn-PP 0.00% | 1.05 | 320 | 8.08 × 1011 | 1.24 × 10−12 |
| Zn-PP-0.01% | 2.22 | 679 | 4.24 × 1012 | 2.36 × 10−13 |
| Zn-PP-0.05% | 5.16 | 1581 | 4.76 × 1012 | 2.10 × 10−13 |
| Zn-PP-0.15% | 4.01 | 1227 | 1.33 × 1012 | 7.54 × 10−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soopy, A.K.K.; Liu, S.; Najar, A. Enhancement of Photodetector Characteristics by Zn-Porphyrin-Passivated MAPbBr3 Single Crystals. Nanomaterials 2024, 14, 1068. https://doi.org/10.3390/nano14131068
Soopy AKK, Liu S, Najar A. Enhancement of Photodetector Characteristics by Zn-Porphyrin-Passivated MAPbBr3 Single Crystals. Nanomaterials. 2024; 14(13):1068. https://doi.org/10.3390/nano14131068
Chicago/Turabian StyleSoopy, Abdul Kareem Kalathil, Shengzhong (Frank) Liu, and Adel Najar. 2024. "Enhancement of Photodetector Characteristics by Zn-Porphyrin-Passivated MAPbBr3 Single Crystals" Nanomaterials 14, no. 13: 1068. https://doi.org/10.3390/nano14131068





