The Contact Properties of Monolayer and Multilayer MoS2-Metal van der Waals Interfaces
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Model Structures
3.2. SBH of 1L MoS2-Metal-Close and -vdW Interfaces
3.3. FLP Strength of 1L MoS2-Metal-Close and -vdW Interfaces
3.4. SBH of Multilayer MoS2-Metal vdW Interfaces
3.5. FLP Strength of Multilayer MoS2-Metal vdW Interfaces
3.6. Layer-Dependent FLP Strength Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Duan, X.; Wang, C.; Pan, A.; Yu, R.; Duan, X. Two-dimensional transition metal dichalcogenides as atomically thin semiconductors: Opportunities and challenges. Chem. Soc. Rev. 2015, 44, 8859–8876. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.H.; Mehlich, K.; Busse, C.; Krasheninnikov, A.V. What governs the atomic structure of the interface between 2D transition metal dichalcogenides in lateral heterostructures? 2D Mater. 2024, 11, 015003. [Google Scholar] [CrossRef]
- Komsa, H.-P.; Kotakoski, J.; Kurasch, S.; Lehtinen, O.; Kaiser, U.; Krasheninnikov, A.V. Two-dimensional transition metal dichalcogenides under electron irradiation: Defect production and doping. Phys. Rev. Lett. 2012, 109, 035503. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, S.; Ma, Y.; Du, A.; Liao, T.; Gu, Y.; Kou, L. Distorted Janus transition metal dichalcogenides: Stable two-dimensional materials with sizable band gap and ultrahigh carrier mobility. J. Phys. Chem. C 2018, 122, 19153–19160. [Google Scholar] [CrossRef]
- Dou, K.P.; Hu, H.H.; Wang, X.H.; Wang, X.Y.; Jin, H.; Zhang, G.-P.; Shi, X.Q.; Kou, L. Asymmetrically flexoelectric gating effect of Janus transition-metal dichalcogenides and their sensor applications. J. Mater. Chem. C 2020, 8, 11457–11467. [Google Scholar] [CrossRef]
- Komsa, H.-P.; Krasheninnikov, A.V. Engineering the electronic properties of two-dimensional transition metal dichalcogenides by introducing mirror twin boundaries. Adv. Electron. Mater. 2017, 3, 1600468. [Google Scholar] [CrossRef]
- Ghorbani-Asl, M.; Kretschmer, S.; Spearot, D.E.; Krasheninnikov, A.V. Two-dimensional MoS2 under ion irradiation: From controlled defect production to electronic structure engineering. 2D Mater. 2017, 4, 025078. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, T.; Tan, X.; He, L.; Yin, K.; Wan, N.; Sun, L. In situ repair of 2D chalcogenides under electron beam irradiation. Adv. Mater. 2018, 30, 1705954. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Shen, X.; Krasheninnikov, A.V.; Sun, L.; Chen, Z. 1T phase as an efficient hole injection layer to TMDs transistors: A universal approach to achieve p-type contacts. 2D Mater. 2018, 5, 031012. [Google Scholar] [CrossRef]
- Allain, A.; Kang, J.; Banerjee, K.; Kis, A. Electrical contacts to two-dimensional semiconductors. Nat. Mater. 2015, 14, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gong, X.; Yu, Z.; Ma, L.; Sun, W.; Gao, S.; Köroğlu, Ç.; Wang, W.; Liu, L.; Li, T.; et al. Approaching the quantum limit in two-dimensional semiconductor contacts. Nature 2023, 613, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.-C.; Su, C.; Lin, Y.; Chou, A.-S.; Cheng, C.-C.; Park, J.-H.; Chiu, M.-H.; Lu, A.-Y.; Tang, H.-L.; Tavakoli, M.M.; et al. Ultralow contact resistance between semimetal and monolayer semiconductors. Nature 2021, 593, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Hu, X.; Sun, L. Electric field induced Schottky to Ohmic contact transition in Fe3GeTe2/TMDs contacts. ACS Appl. Electron. Mater. 2023, 5, 3071–3077. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Li, Q.; Quhe, R.; Yang, C.; Guo, Y.; Zhang, X.; Pan, Y.; Li, J.; Zhang, H.; et al. Schottky barrier heights in two-dimensional field-effect transistors: From theory to experiment. Rep. Prog. Phys. 2021, 84, 056501. [Google Scholar] [CrossRef]
- Kim, C.; Moon, I.; Lee, D.; Choi, M.S.; Ahmed, F.; Nam, S.; Cho, Y.; Shin, H.-J.; Park, S.; Yoo, W.J. Fermi level pinning at electrical metal contacts of monolayer molybdenum dichalcogenides. ACS Nano 2017, 11, 1588–1596. [Google Scholar] [CrossRef]
- Quhe, R.; Peng, X.; Pan, Y.; Ye, M.; Wang, Y.; Zhang, H.; Feng, S.; Zhang, Q.; Shi, J.; Yang, J.; et al. Can a black phosphorus Schottky barrier transistor be good enough? ACS Appl. Mater. Interfaces 2017, 9, 3959–3966. [Google Scholar] [CrossRef]
- Kang, J.; Liu, W.; Sarkar, D.; Jena, D.; Banerjee, K. Computational study of metal contacts to monolayer transition metal dichalcogenide semiconductors. Phys. Rev. X 2014, 4, 031005. [Google Scholar] [CrossRef]
- Kaushik, N.; Nipane, A.; Basheer, F.; Dubey, S.; Grover, S.; Deshmukh, M.M.; Lodha, S. Schottky barrier heights for Au and Pd contacts to MoS2. Appl. Phys. Lett. 2014, 105, 113505. [Google Scholar] [CrossRef]
- Bardeen, J. Surface states and rectification at a metal semiconductor contact. Phys. Rev. 1947, 71, 717–727. [Google Scholar] [CrossRef]
- Hasegawa, H.; Sawada, T. On the electrical properties of compound semiconductor interfaces in metal/insulator/semiconductor structures and the possible origin of interface states. Thin Solid Films 1983, 103, 119–140. [Google Scholar] [CrossRef]
- Tung, R.T. The physics and chemistry of the Schottky barrier height. Appl. Phys. Rev. 2014, 1, 011304. [Google Scholar] [CrossRef]
- Taghinejad, M.; Xia, C.; Hrton, M.; Lee, K.-T.; Kim, A.S.; Li, Q.; Guzelturk, B.; Kalousek, R.; Xu, F.; Cai, W.; et al. Determining hot-carrier transport dynamics from terahertz emission. Science 2023, 382, 299–305. [Google Scholar] [CrossRef]
- Kappera, R.; Voiry, D.; Yalcin, S.E.; Branch, B.; Gupta, G.; Mohite, A.D.; Chhowalla, M. Phase-engineered low-resistance contacts for ultrathin MoS2 transistors. Nat. Mater. 2014, 13, 1128–1134. [Google Scholar] [CrossRef]
- Yang, Z.; Kim, C.; Lee, K.Y.; Lee, M.; Appalakondaiah, S.; Ra, C.H.; Watanabe, K.; Taniguchi, T.; Cho, K.; Hwang, E.; et al. A Fermi-level-pinning-free 1D electrical contact at the intrinsic 2D MoS2-metal junction. Adv. Mater. 2019, 31, 1808231. [Google Scholar] [CrossRef] [PubMed]
- Pande, G.; Siao, J.-Y.; Chen, W.-L.; Lee, C.-J.; Sankar, R.; Chang, Y.-M.; Chen, C.-D.; Chang, W.-H.; Chou, F.-C.; Lin, M.-T. Ultralow Schottky barriers in hexagonal boron nitride-encapsulated monolayer WSe2 tunnel field-effect transistors. ACS Appl. Mater. Interfaces 2020, 12, 18667–18673. [Google Scholar] [CrossRef]
- Chuang, S.; Battaglia, C.; Azcatl, A.; McDonnell, S.; Kang, J.S.; Yin, X.; Tosun, M.; Kapadia, R.; Fang, H.; Wallace, R.M.; et al. MoS2 p-type transistors and diodes enabled by high work function MoOx contacts. Nano Lett. 2014, 14, 1337–1342. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, X.; Wang, Y.; Lu, C.; Krasheninnikov, A.V.; Chen, Z.; Sun, L. Suppressed Fermi level pinning and wide-range tunable Schottky barrier in CrX3 (X= I, Br)/2D metal contacts. J. Phys. Chem. Lett. 2023, 14, 2807–2815. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Gao, L.; Yu, H.; Liu, X.; Du, J.; Xiao, J.; Liu, Y.; Gu, L.; Liao, Q.; et al. Near-ideal van der Waals rectifiers based on all-two-dimensional Schottky junctions. Nat. Commun. 2021, 12, s41467. [Google Scholar] [CrossRef]
- McDonnell, S.; Azcatl, A.; Addou, R.; Gong, C.; Battaglia, C.; Chuang, S.; Cho, K.; Javey, A.; Wallace, R.M. Hole contacts on transition metal dichalcogenides: Interface chemistry and band alignments. ACS Nano 2014, 8, 6265–6272. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wu, R.; Chen, Y.; Huangfu, Y.; Liu, L.; Li, W.; Lu, D.; Tao, Q.; Song, W.; Li, W.; et al. Wafer-scale and universal van der Waals metal semiconductor contact. Nat. Commun. 2023, 14, 1014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Duan, X. Van der Waals integration before and beyond two-dimensional materials. Nature 2019, 567, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kong, L.; Li, Q.; He, C.; Ren, L.; Tao, Q.; Yang, X.; Lin, J.; Zhao, B.; Li, Z.; et al. Transferred van der Waals metal electrodes for sub-1-nm MoS2 vertical transistors. Nat. Electron. 2021, 4, 342–347. [Google Scholar] [CrossRef]
- Kwon, G.; Choi, Y.-H.; Lee, H.; Kim, H.-S.; Jeong, J.; Jeong, K.; Baik, M.; Kwon, H.; Ahn, J.; Lee, E.; et al. Interaction- and defect-free van der Waals contacts between metals and two-dimensional semiconductors. Nat. Electron. 2022, 5, 241–247. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, J.C.; Wu, R.J.; Martinez, J.; Song, X.; Yang, J.; Zhao, F.; Mkhoyan, A.; Jeong, H.Y.; Chhowalla, M. Van der Waals contacts between three-dimensional metals and two-dimensional semiconductors. Nature 2019, 568, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Taghinejad, H.; Rehn, D.A.; Muccianti, C.; Eftekhar, A.A.; Tian, M.; Fan, T.; Zhang, X.; Meng, Y.; Chen, Y.; Nguyen, T.-V.; et al. Defect-mediated alloying of monolayer transition-metal dichalcogenides. ACS Nano 2018, 12, 12795–12804. [Google Scholar] [CrossRef] [PubMed]
- Sfuncia, G.; Nicotra, G.; Giannazzo, F.; Pécz, B.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. 2D graphitic-like gallium nitride and other structural selectivity in confinement at the graphene/SiC interface. Cryst. Eng. Comm. 2023, 25, 5810–5817. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Zhu, E.; Liao, L.; Lee, S.J.; Ding, M.; Shakir, I.; Gambin, V.; Huang, Y.; Duan, X. Approaching the Schottky-Mott limit in van der Waals metal-semiconductor junctions. Nature 2018, 557, 696–700. [Google Scholar] [CrossRef]
- Zhong, M.; Xia, Q.; Pan, L.; Liu, Y.; Chen, Y.; Deng, H.-X.; Li, J.; Wei, Z. Thickness-dependent carrier transport characteristics of a new 2D elemental semiconductor: Black arsenic. Adv. Funct. Mater. 2018, 28, 1802581. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, H.; Zhu, M.; Shang, J.; Liu, D.; Cui, X.; Shen, D.; Kou, L.; Mao, D.; Zheng, J.; et al. Electrostatic functionalization and passivation of water-exfoliated few-layer black phosphorus by poly dimethyldiallyl ammonium chloride and its ultrafast laser application. ACS Appl. Mater. Interfaces 2018, 10, 9679–9687. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Frauenheim, T.; Chen, C. Nanoscale multilayer transition-metal dichalcogenide heterostructures: Band gap modulation by interfacial strain and spontaneous polarization. J. Phys. Chem. Lett. 2013, 4, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lee, G.-H.; Kim, Y.D.; Arefe, G.; Huang, P.Y.; Lee, C.-H.; Chenet, D.A.; Zhang, X.; Wang, L.; Ye, F.; et al. Multi-terminal transport measurements of MoS2 using a van der Waals heterostructure device platform. Nat. Nanotechnol. 2015, 10, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, J.-Y.; Yu, Y.-J.; Lee, C.-H.; Cui, X.; Honed, J.; Lee, G.-H. Thickness-dependent Schottky barrier height of MoS2 field-effect transistors. Nanoscale 2017, 9, 6151–6157. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E. Projector augmented-wave method. Phys Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Qiu, H.; Xu, T.; Wang, Z.; Ren, W.; Nan, H.; Ni, Z.; Chen, Q.; Yuan, S.; Miao, F.; Song, F.; et al. Hopping transport through defect-induced localized states in molybdenum disulphide. Nat. Commun. 2013, 4, 2642. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.X.; Quhe, R.; Zhong, H.; Cong, L.; Ye, M.; Ni, Z.; Song, Z.; Yang, J.; Shi, J.; et al. Does p-type Ohmic contact exist in WSe2-metal interfaces? Nanoscale 2016, 8, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, M.; Weng, M.; Li, J.; Zhang, X.; Zhang, H.; Guo, Y.; Pan, Y.; Xiao, L.; Liu, J.; et al. Electrical contacts in monolayer arsenene devices. ACS Appl. Mater. Interfaces 2017, 9, 29273–29284. [Google Scholar] [CrossRef] [PubMed]
- Batsanov, S.S. Van der Waals radii of elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Ma, F. Performance upper limit of sub-10 nm Monolayer MoS2 transistors with MoS2-Mo electrodes. J. Phys. Chem. C 2022, 126, 12100–12112. [Google Scholar] [CrossRef]
- Fang, Q.; Zhao, X.; Huang, Y.; Xu, K.; Min, T.; Ma, F. Junction-configuration-dependent interfacial electronic states of a monolayer MoS2/metal contact. J. Mater. Chem. C 2019, 7, 3607–3616. [Google Scholar] [CrossRef]
- Lim, J.Y.; Pezeshki, A.; Oh, S.; Kim, J.S.; Lee, Y.T.; Yu, S.; Hwang, D.K.; Lee, G.-H.; Choi, H.J.; Im, S. Homogeneous 2D MoTe2 p-n Junctions and CMOS Inverters formed by Atomic-Layer-Deposition-Induced Doping. Adv. Mater. 2017, 29, 1701798. [Google Scholar] [CrossRef]
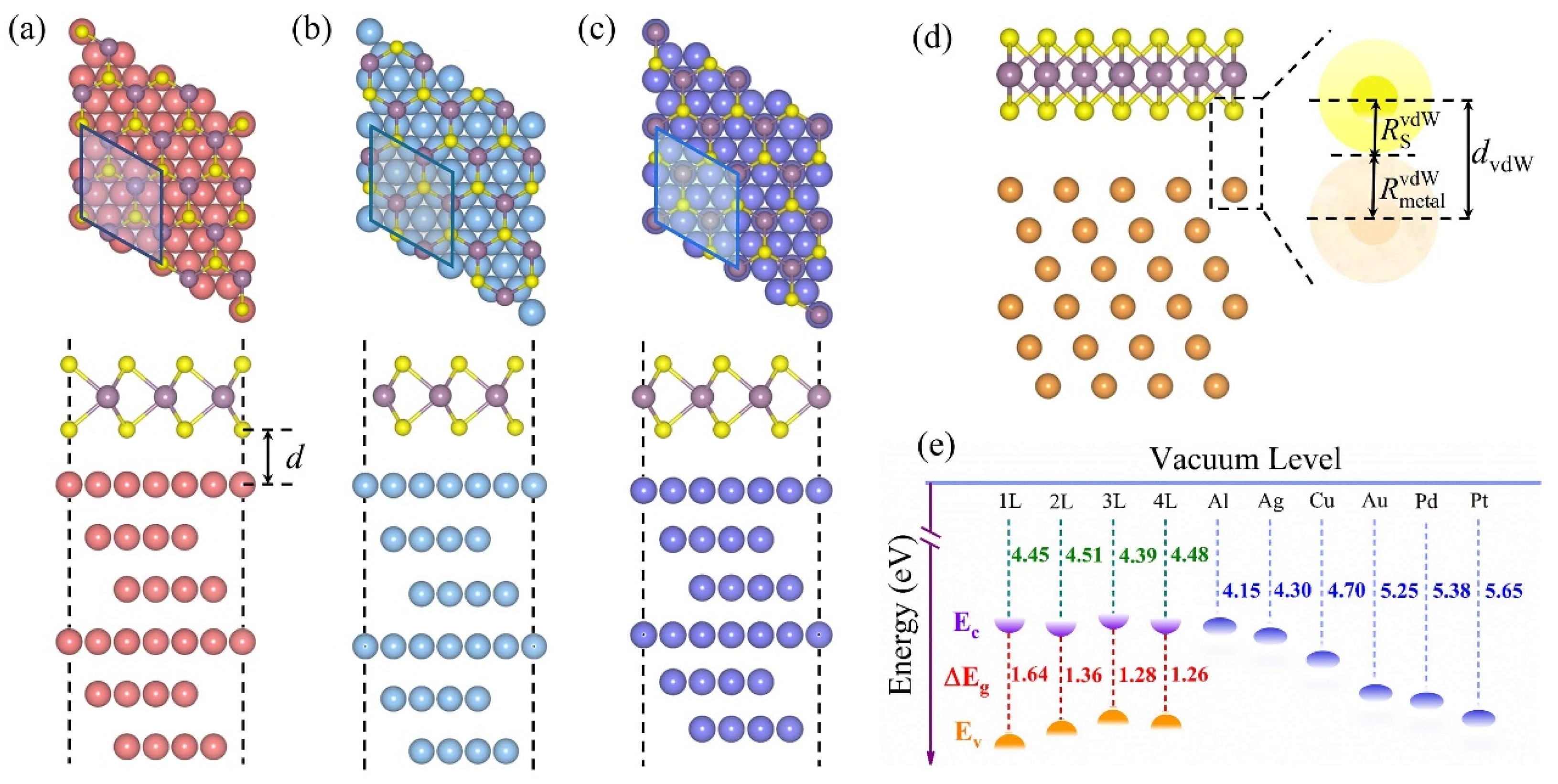
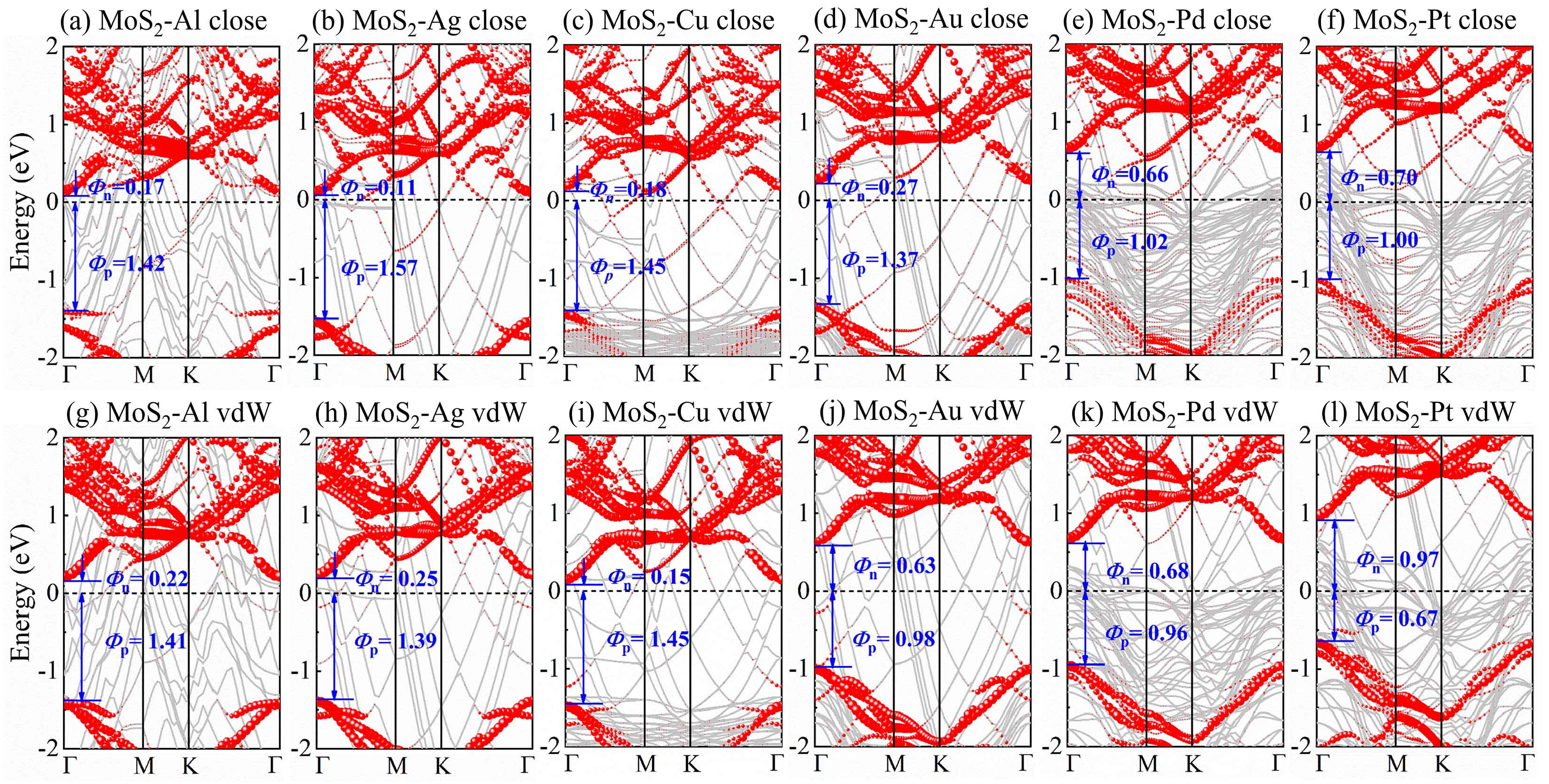


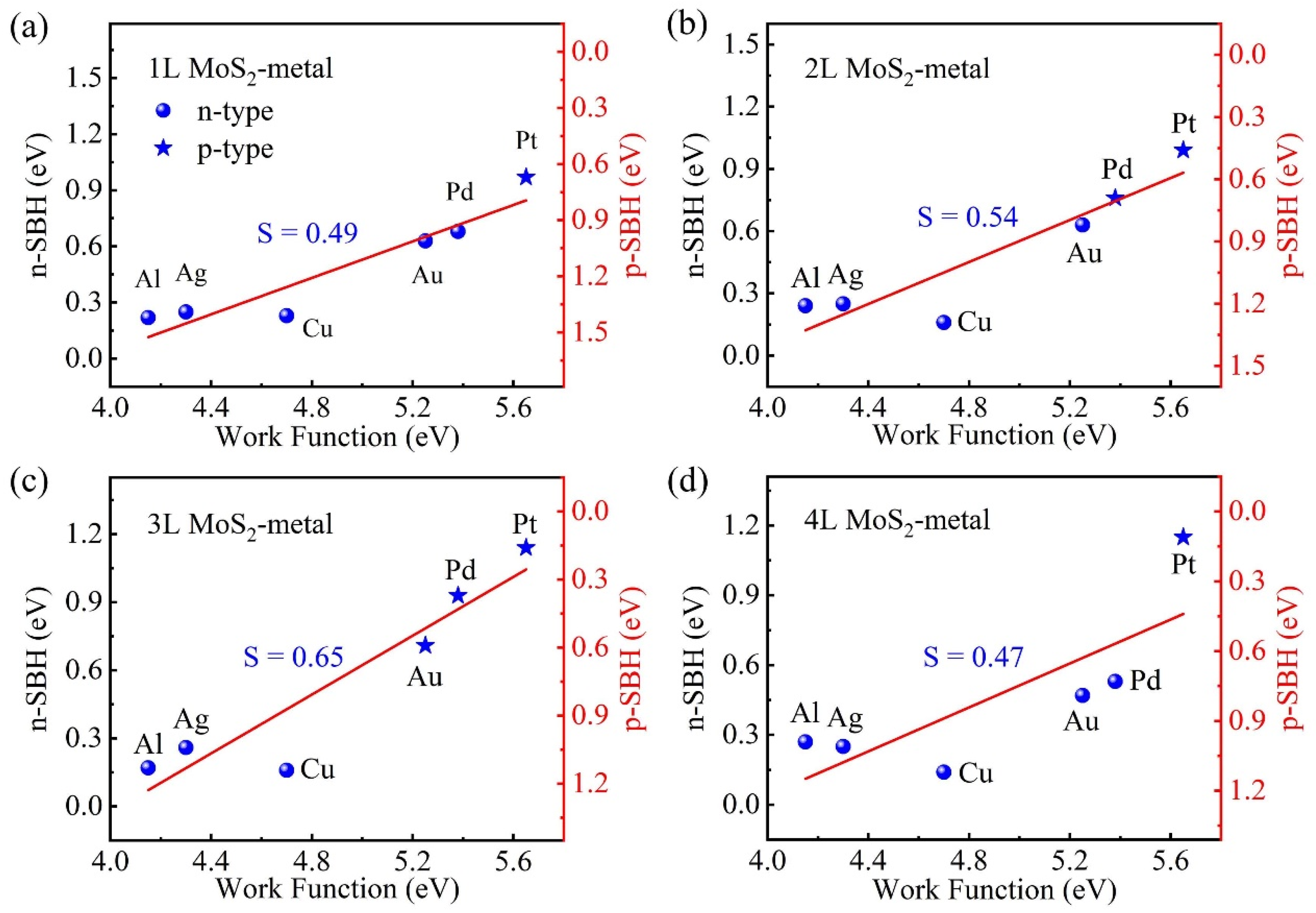
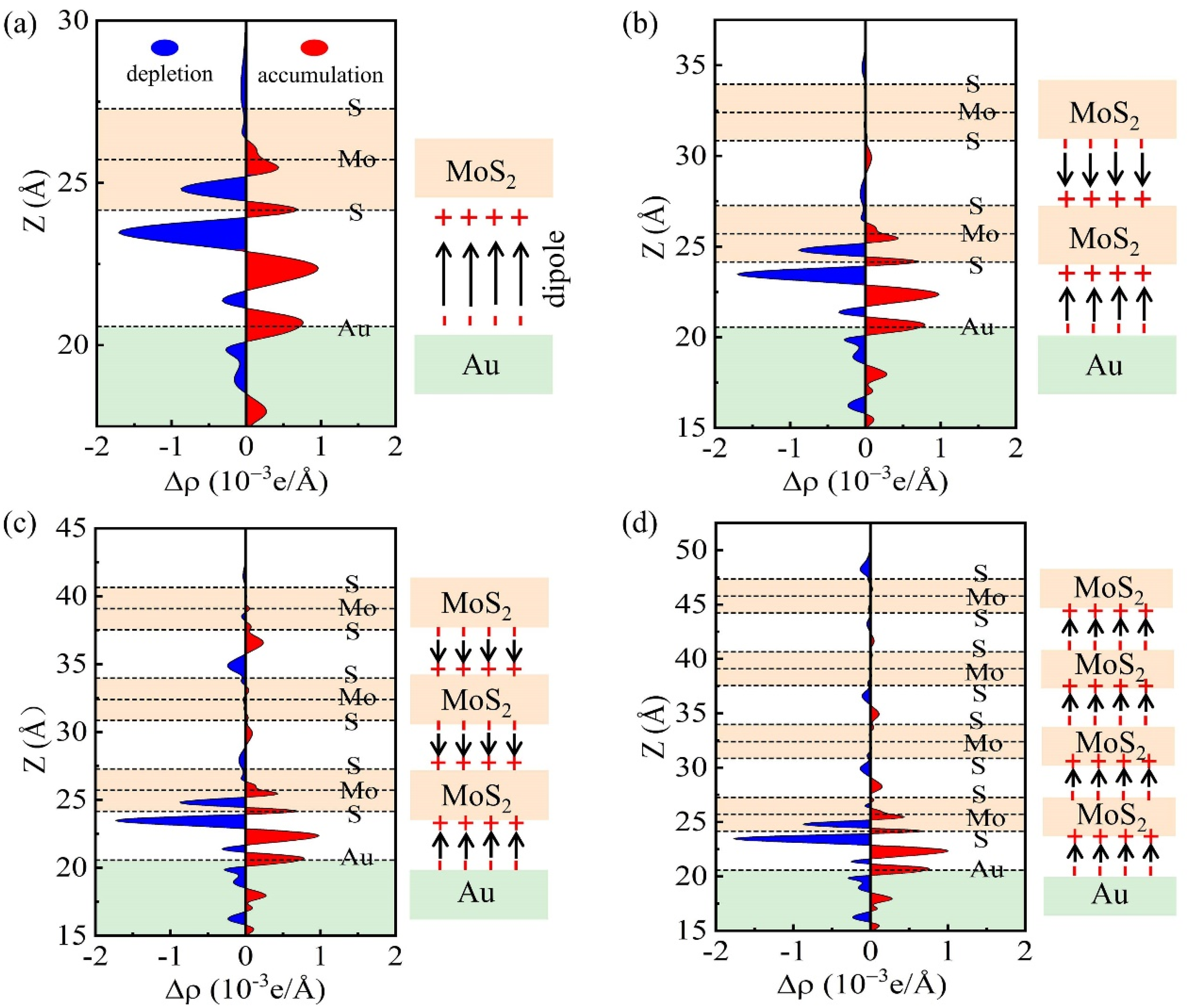
| Metal | WM (eV) | δ (%) | MoS2-Metal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| d (Å) | dvdW (Å) | Eb (eV) | SBH (eV) | ||||||||
| 1L | 1L | 2L vdW | 3L vdW | 4L vdW | |||||||
| Close | vdW | Close | vdW | ||||||||
| Al | 4.15 | 3.35 | 2.59 | 3.48 | −0.42 | −0.29 | 0.17 n | 0.22 n | 0.24 n | 0.17 n | 0.27 n |
| Ag | 4.30 | 4.57 | 2.64 | 3.50 | −0.59 | −0.37 | 0.11 n | 0.25 n | 0.25 n | 0.26 n | 0.25 n |
| Cu | 4.70 | 6.94 | 2.22 | 3.46 | −0.89 | −0.39 | 0.18 n | 0.15 n | 0.16 n | 0.16 n | 0.14 n |
| Au | 5.25 | 4.39 | 2.81 | 3.59 | −0.50 | −0.37 | 0.27 n | 0.63 n | 0.63 n | 0.59 p | 0.47 n |
| Pd | 5.38 | 0.42 | 2.26 | 3.59 | −0.98 | −0.40 | 0.66 n | 0.68 n | 0.64 p | 0.37 p | 0.53 n |
| Pt | 5.65 | 0.45 | 2.32 | 3.60 | −0.81 | −0.43 | 0.70 n | 0.67 p | 0.41 p | 0.16 p | 0.11 p |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, X.; Hu, X.; Xu, T.; Sun, L. The Contact Properties of Monolayer and Multilayer MoS2-Metal van der Waals Interfaces. Nanomaterials 2024, 14, 1075. https://doi.org/10.3390/nano14131075
Pei X, Hu X, Xu T, Sun L. The Contact Properties of Monolayer and Multilayer MoS2-Metal van der Waals Interfaces. Nanomaterials. 2024; 14(13):1075. https://doi.org/10.3390/nano14131075
Chicago/Turabian StylePei, Xin, Xiaohui Hu, Tao Xu, and Litao Sun. 2024. "The Contact Properties of Monolayer and Multilayer MoS2-Metal van der Waals Interfaces" Nanomaterials 14, no. 13: 1075. https://doi.org/10.3390/nano14131075
APA StylePei, X., Hu, X., Xu, T., & Sun, L. (2024). The Contact Properties of Monolayer and Multilayer MoS2-Metal van der Waals Interfaces. Nanomaterials, 14(13), 1075. https://doi.org/10.3390/nano14131075









