Investigation of Direct Electron Transfer of Glucose Oxidase on a Graphene-CNT Composite Surface: A Molecular Dynamics Study Based on Electrochemical Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. GOx/Carbon Nanocomposite Electrode Preparation
2.4. SEM/TEM Sample Preparation
2.5. CV Characteristics Measurements
2.6. Molecular Modeling: GOx, CNT, and Graphene
2.7. MD Simulations
2.8. Data Analysis for MD
2.9. Elastic Network Model and Normal-Mode Analysis of Enzyme
3. Results and Discussion
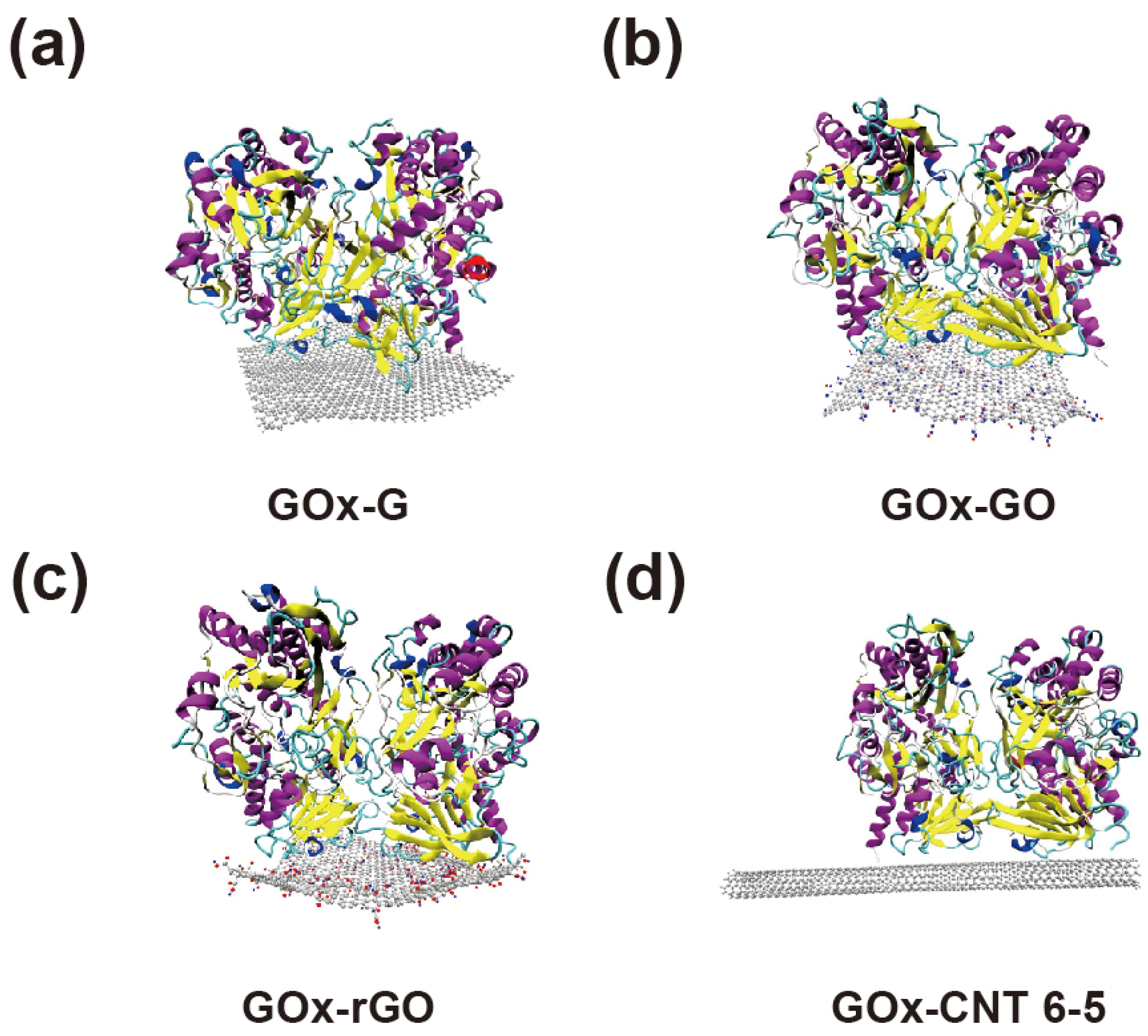
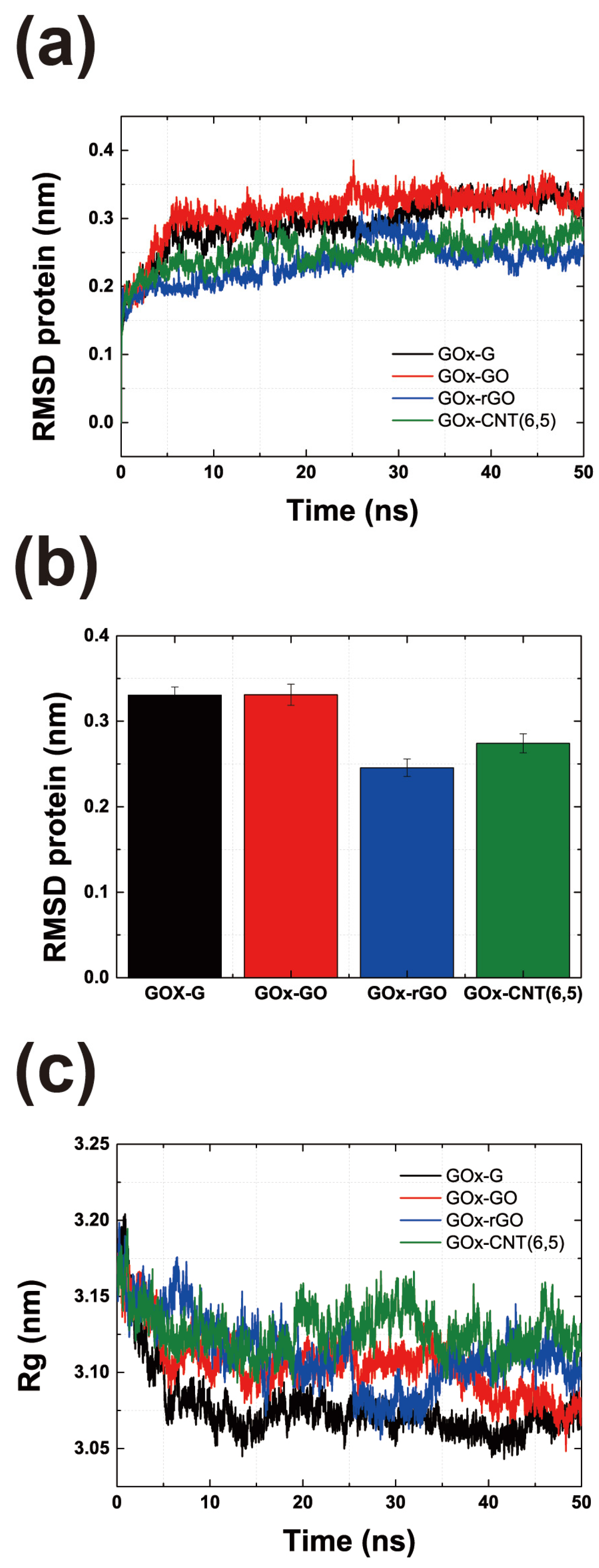
3.1. Atomics Scale Simulations for GOx-CNT/Graphene Interfaces
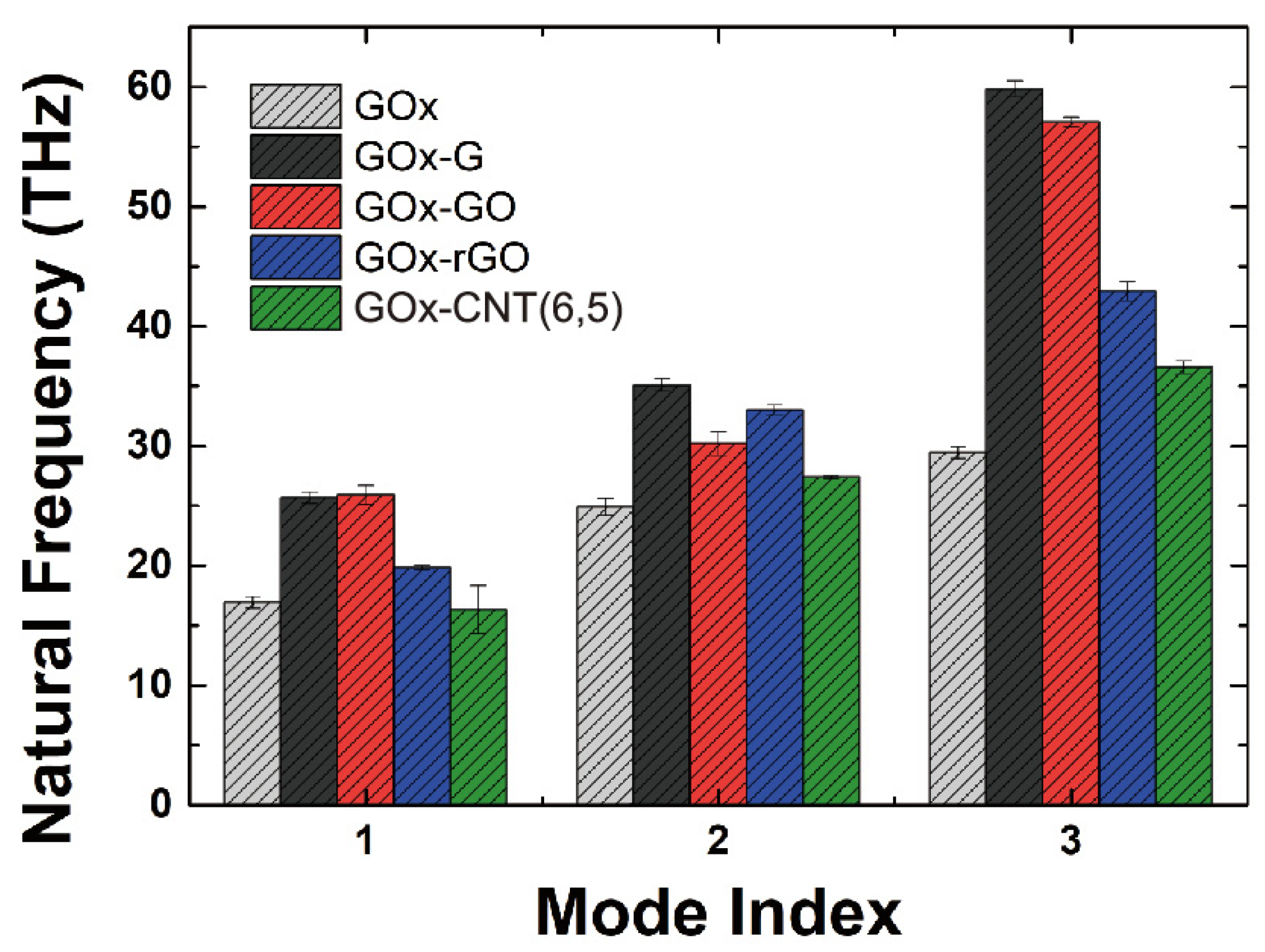
3.2. Morphology Analysis of Enzyme Interface Using Elastic Network Model
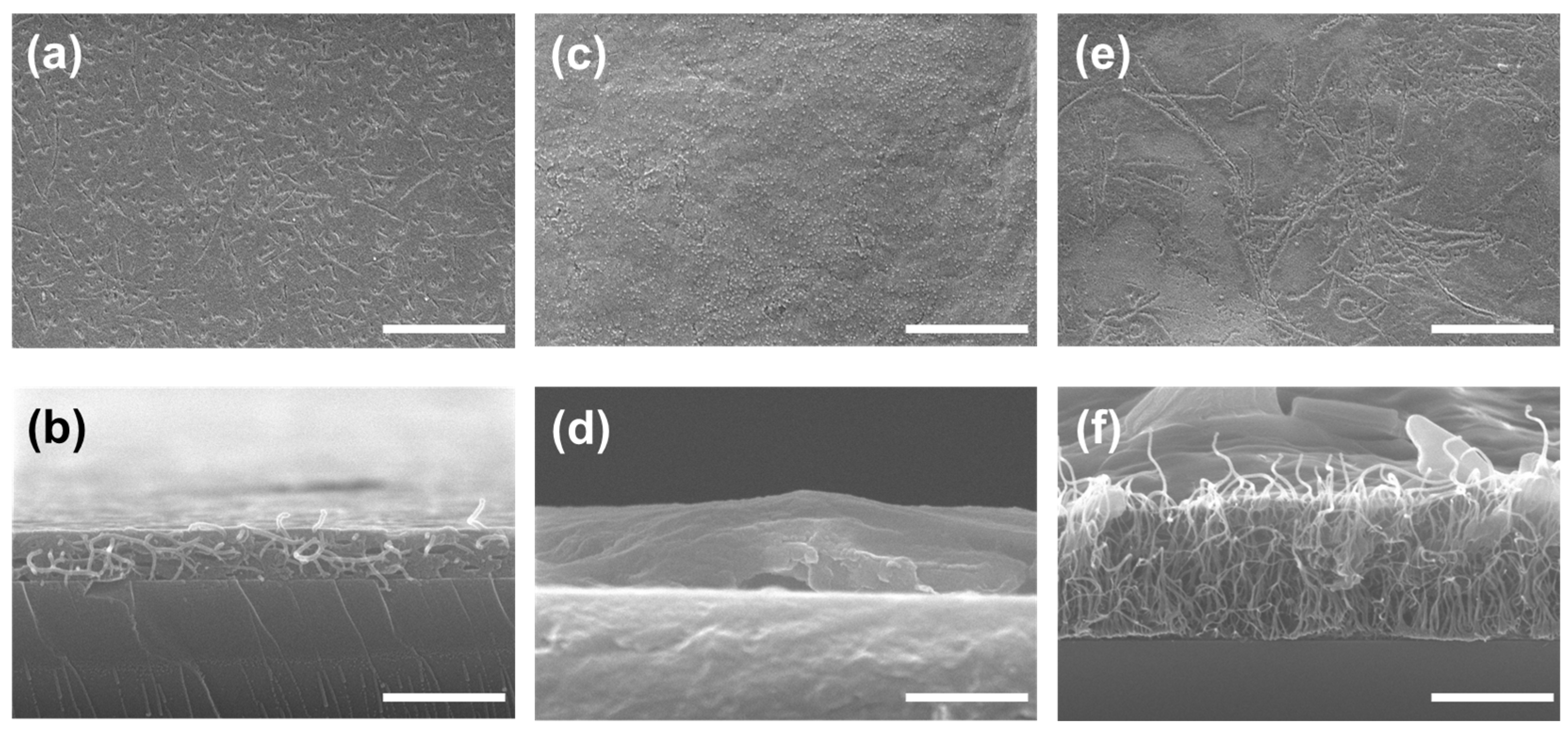
3.3. Confirmation of GOx-CNT/Graphene Structure
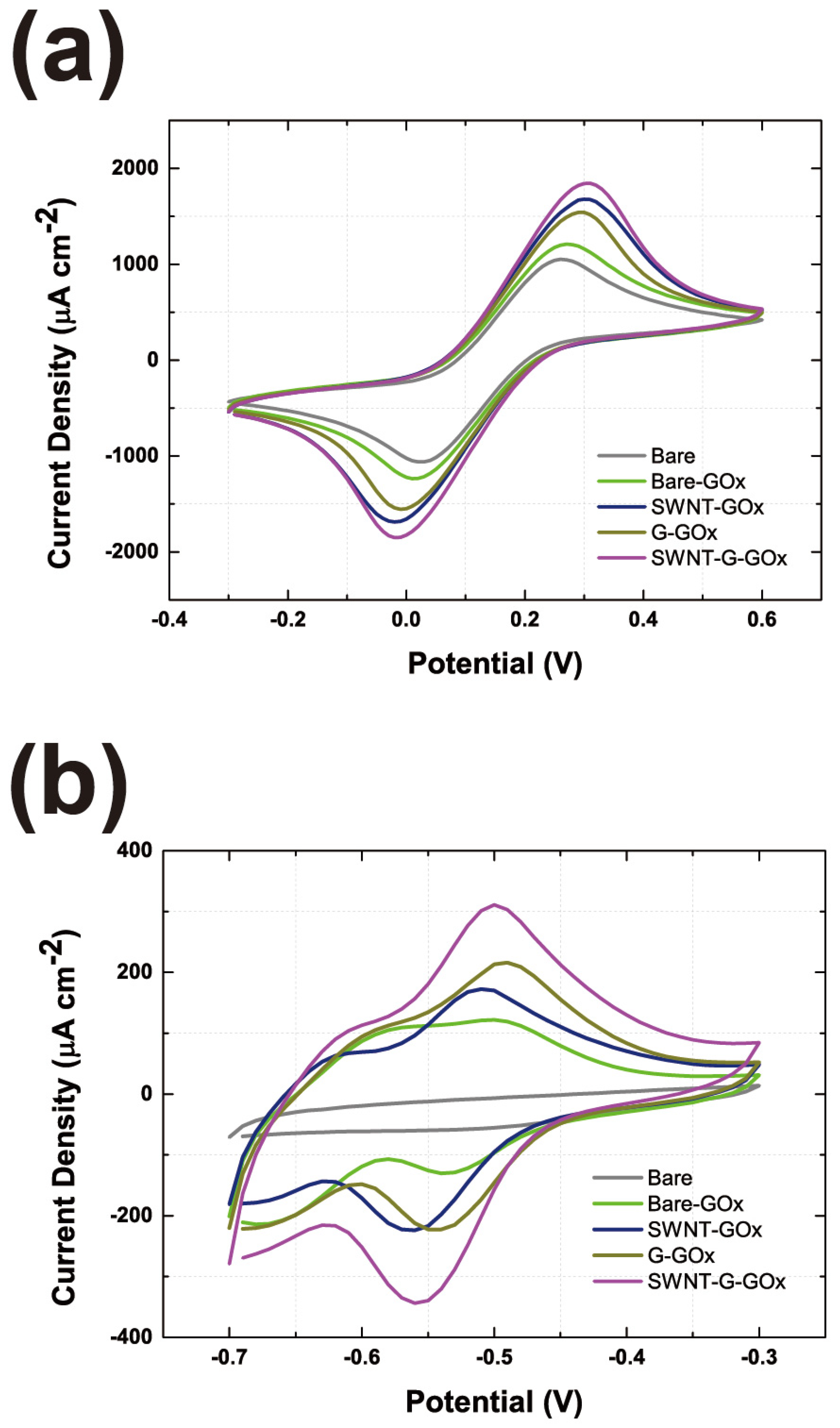
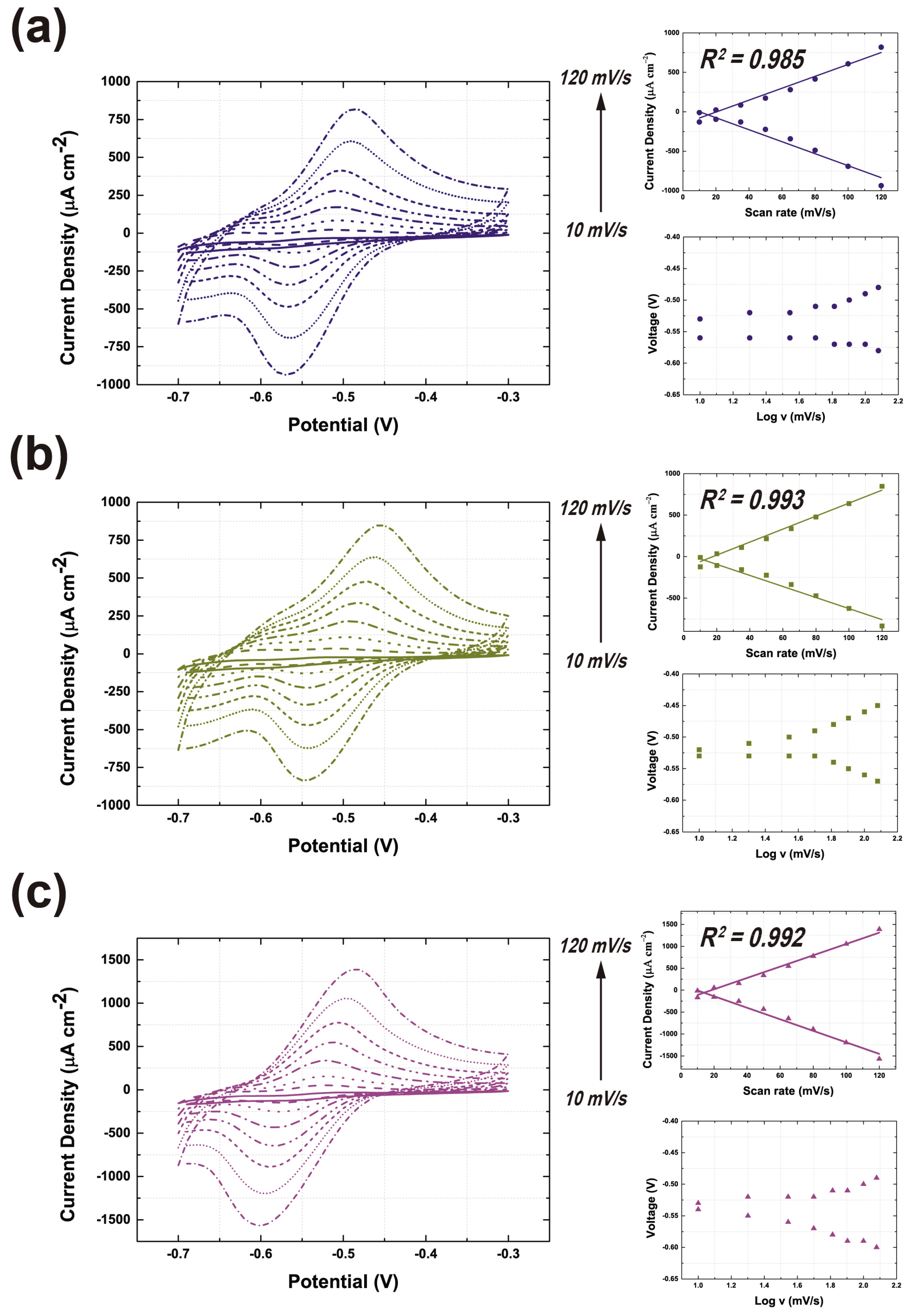
3.4. Evaluation of the GOx-CNT/Graphene Composite Electrode
4. Conclusions
- Biosensors for Glucose Monitoring: The insights gained from this study can be directly applied to the development of high-performance biosensors for glucose monitoring, which are crucial for diabetes management. The enhanced electron transfer rates and stability of the GOx-graphene-CNT composites make them ideal candidates for continuous glucose monitoring systems (CGMS). These systems require sensors that can provide accurate, real-time glucose measurements with high sensitivity and stability over extended periods. The superior electron transfer efficiency and structural stability of the GOx-graphene-CNT composite electrodes can lead to more reliable and longer-lasting sensors.
- Biofuel Cells: Enzyme-based biofuel cells (EBFCs) convert biochemical energy from glucose into electrical energy. The high electron transfer rates observed in the GOx-graphene-CNT composites can significantly improve the power output of these biofuel cells. The stability and efficient electron transfer of the immobilized GOx ensure sustained catalytic activity, which is essential for maintaining the performance of EBFCs over time. This can lead to the development of more efficient and cost-effective biofuel cells for powering small electronic devices and potential applications in medical implants and portable power sources.
- Environmental Monitoring: Beyond healthcare, the GOx-graphene-CNT composites can be utilized in environmental monitoring to detect glucose and other analytes in water and soil samples. The high sensitivity and specificity of the enzyme-based sensors can help monitor pollution levels and detect contaminants in the environment, contributing to better environmental management and protection.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GOx | Glucose oxidase |
| CNT | Carbon nanotube |
| MWNT | Multi-walled carbon nanotube |
| SWNT | Single-walled carbon nanotube |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| CVD | Chemical vapor deposition |
| FAD | Flavin adenine dinucleotide |
References
- Luong, J.H.; Glennon, J.D.; Gedanken, A.; Vashist, S.K. Achievement and assessment of direct electron transfer of glucose oxidase in electrochemical biosensing using carbon nanotubes, graphene, and their nanocomposites. Microchim. Acta 2017, 184, 369–388. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Graphene-based materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Navaee, A.; Salimi, A. Graphene-supported pyrene-functionalized amino-carbon nanotube: A novel hybrid architecture of laccase immobilization as effective bioelectrocatalyst for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 7623–7630. [Google Scholar] [CrossRef]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and electronic structure of graphene-oxide. Nano Lett. 2009, 9, 1058–1063. [Google Scholar] [CrossRef]
- Filip, J.; Tkac, J. Is graphene worth using in biofuel cells? Electrochim. Acta 2014, 136, 340–354. [Google Scholar] [CrossRef]
- Ivnitski, D.; Branch, B.; Atanassov, P.; Apblett, C. Glucose oxidase anode for biofuel cell based on direct electron transfer. Electrochem. Commun. 2006, 8, 1204–1210. [Google Scholar] [CrossRef]
- Cosnier, S.; Gross, A.J.; Le Goff, A.; Holzinger, M. Recent advances on enzymatic glucose/oxygen and hydrogen/oxygen biofuel cells: Achievements and limitations. J. Power Sources 2016, 325, 252–263. [Google Scholar] [CrossRef]
- Christwardana, M.; Kim, D.-H.; Chung, Y.; Kwon, Y. A hybrid biocatalyst consisting of silver nanoparticle and naphthalenethiol self-assembled monolayer prepared for anchoring glucose oxidase and its use for an enzymatic biofuel cell. Appl. Surf. Sci. 2018, 429, 180–186. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Carbon nanotube/enzyme biofuel cells. Electrochim. Acta 2012, 82, 179–190. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Degani, Y.; Heller, A. Direct electrical communication between chemically modified enzymes and metal electrodes. I. Electron transfer from glucose oxidase to metal electrodes via electron relays, bound covalently to the enzyme. J. Phys. Chem. 1987, 91, 1285–1289. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S.; Moon, J.M.; Bae, D.J.; Lim, S.C.; Lee, Y.S.; Lee, Y.H. Electrochemical properties of high-power supercapacitors using single-walled carbon nanotube electrodes. Adv. Funct. Mater. 2001, 11, 387–392. [Google Scholar] [CrossRef]
- Gonçales, V.R.; Colombo, R.N.; Minadeo, M.A.; Matsubara, E.Y.; Rosolen, J.M.; de Torresi, S.I.C. Three-dimensional graphene/carbon nanotubes hybrid composites for exploring interaction between glucose oxidase and carbon based electrodes. J. Electroanal. Chem. 2016, 775, 235–242. [Google Scholar] [CrossRef]
- Yoon, T.; Park, W.; Kim, Y.; Na, S. Electric field-mediated regulation of enzyme orientation for efficient electron transfer at the bioelectrode surface: A molecular dynamics study. Appl. Surf. Sci. 2023, 608, 155124. [Google Scholar] [CrossRef]
- Yoon, T.; Baek, I.; Lee, S.; Choi, H.; Yoon, S.; Lee, H.; Kim, S.U.; Na, S. Immobilization of laccase on a graphene interface: Direct electron transfer and molecular dynamics study. Appl. Surf. Sci. 2020, 521, 146378. [Google Scholar] [CrossRef]
- Baek, I.; Choi, H.; Yoon, S.; Na, S. Effects of the hydrophobicity of key residues on the characteristics and stability of glucose oxidase on a graphene surface. ACS Biomater. Sci. Eng. 2020, 6, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yang, C.; Ma, X.; Li, Q. Insights into the conformation changes of SARS-CoV-2 spike receptor-binding domain on graphene. Appl. Surf. Sci. 2022, 578, 151934. [Google Scholar] [CrossRef]
- Zhao, D.; Li, L.; Zhou, J. Simulation insight into the cytochrome c adsorption on graphene and graphene oxide surfaces. Appl. Surf. Sci. 2018, 428, 825–834. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Zhang, D.; Yang, J.; Zheng, M.; Li, Y. Chirality pure carbon nanotubes: Growth, sorting, and characterization. Chem. Rev. 2020, 120, 2693–2758. [Google Scholar] [CrossRef]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An automated force field topology builder (ATB) and repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Miller III, B.R.; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA. py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Brown, S.P.; Muchmore, S.W. High-throughput calculation of protein—Ligand binding affinities: Modification and adaptation of the MM-PBSA protocol to enterprise grid computing. J. Chem. Inf. Model. 2006, 46, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Amaro, R.E.; McCammon, J.A. MM-PBSA captures key role of intercalating water molecules at a protein—Protein interface. J. Chem. Theory Comput. 2009, 5, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, R.; Consortium, O.S.D.D.; Lynn, A. g_mmpbsa—A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Hinsen, K. Analysis of domain motions by approximate normal mode calculations. Proteins Struct. Funct. Bioinform. 1998, 33, 417–429. [Google Scholar] [CrossRef]
- Hinsen, K.; Thomas, A.; Field, M.J. Analysis of domain motions in large proteins. Proteins Struct. Funct. Bioinform. 1999, 34, 369–382. [Google Scholar] [CrossRef]
- Palanisamy, S.; Cheemalapati, S.; Chen, S.-M. Amperometric glucose biosensor based on glucose oxidase dispersed in multiwalled carbon nanotubes/graphene oxide hybrid biocomposite. Mater. Sci. Eng. C 2014, 34, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Devadas, B.; Chen, S.-M. Direct electrochemistry of glucose oxidase at electrochemically reduced graphene oxide-multiwalled carbon nanotubes hybrid material modified electrode for glucose biosensor. Biosens. Bioelectron. 2013, 41, 309–315. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Z.; He, S.; Zhang, B.; Li, X.; Yao, M. Direct electron transfer of glucose oxidase and biosensing for glucose based on PDDA-capped gold nanoparticle modified graphene/multi-walled carbon nanotubes electrode. Biosens. Bioelectron. 2014, 52, 147–152. [Google Scholar] [CrossRef]
- Ghoshdastider, U.; Wu, R.; Trzaskowski, B.; Mlynarczyk, K.; Miszta, P.; Gurusaran, M.; Viswanathan, S.; Renugopalakrishnan, V.; Filipek, S. Molecular effects of encapsulation of glucose oxidase dimer by graphene. RSC Adv. 2015, 5, 13570–13578. [Google Scholar] [CrossRef]
- Sumaryada, T.; Sandy Gunawan, M.; Perdana, S.; Arjo, S.; Maddu, A. A molecular interaction analysis reveals the possible roles of graphene oxide in a glucose biosensor. Biosensors 2019, 9, 18. [Google Scholar] [CrossRef]
- Tatke, S.S.; Loong, C.K.; D’Souza, N.; Schoephoerster, R.T.; Prabhakaran, M. Large scale motions in a biosensor protein glucose oxidase: A combined approach by QENS, normal mode analysis, and molecular dynamics studies. Biopolym. Orig. Res. Biomol. 2008, 89, 582–594. [Google Scholar] [CrossRef]
- Liu, J.; Xie, Y.; Peng, C.; Yu, G.; Zhou, J. Molecular understanding of laccase adsorption on charged self-assembled monolayers. J. Phys. Chem. B 2017, 121, 10610–10617. [Google Scholar] [CrossRef] [PubMed]
- Rusling, J.F.; Zhang, Z. Thin films on electrodes for direct protein electron transfer. In Handbook of Surfaces and Interfaces of Materials; Elsevier: Amsterdam, The Netherlands, 2001; pp. 33–71. [Google Scholar]
- Martins, M.V.; Pereira, A.R.; Luz, R.A.; Iost, R.M.; Crespilho, F.N. Evidence of short-range electron transfer of a redox enzyme on graphene oxide electrodes. Phys. Chem. Chem. Phys. 2014, 16, 17426–17436. [Google Scholar] [CrossRef]
- Roth, J.P.; Klinman, J.P. Catalysis of electron transfer during activation of O2 by the flavoprotein glucose oxidase. Proc. Natl. Acad. Sci. USA 2003, 100, 62–67. [Google Scholar] [CrossRef]
- Yoon, T.; Park, W.; Kim, Y.; Choi, H.; Chung, S.; Park, J.; Chang, H.J.; Na, S. Silk-based organic photoresists for extreme ultraviolet lithography: A multiscale in silico study. J. Mater. Chem. C 2023, 11, 4415–4425. [Google Scholar] [CrossRef]
- Park, W.; Yoon, T.; Chang, H.; You, J.; Na, S. An atomistic scale simulation study of structural properties in the silk-fibrohexamerin complex. Nanoscale 2024, 16, 821–832. [Google Scholar] [CrossRef]
- Xu, Z.; Paparcone, R.; Buehler, M.J. Alzheimer’s Aβ (1–40) amyloid fibrils feature size-dependent mechanical properties. Biophys. J. 2010, 98, 2053–2062. [Google Scholar] [CrossRef]
- Hu, Y.; Buehler, M.J. End-to-end protein normal mode frequency predictions using language and graph models and application to sonification. ACS Nano 2022, 16, 20656–20670. [Google Scholar] [CrossRef]
- Götze, J.P.; Bühl, M. Laccase redox potentials: pH dependence and mutants, a QM/MM study. J. Phys. Chem. B 2016, 120, 9265–9276. [Google Scholar] [CrossRef]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal structure of a laccase from the fungusTrametes versicolor at 1.90-Å resolution containing a full complement of coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef]
- Wohlfahrt, G.; Witt, S.; Hendle, J.; Schomburg, D.; Kalisz, H.M.; Hecht, H.-J. 1.8 and 1.9 Å resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr. Sect. D Biol. Crystallogr. 1999, 55, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.P.; Chen, Y.; Chen, P. Three-dimensional graphene-carbon nanotube hybrid for high-performance enzymatic biofuel cells. ACS Appl. Mater. Interfaces 2014, 6, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Filip, J.; Tkac, J. Effective bioelectrocatalysis of bilirubin oxidase on electrochemically reduced graphene oxide. Electrochem. Commun. 2014, 49, 70–74. [Google Scholar] [CrossRef]
- Filip, J.; Andicsová-Eckstein, A.; Vikartovská, A.; Tkac, J. Immobilization of bilirubin oxidase on graphene oxide flakes with different negative charge density for oxygen reduction. The effect of GO charge density on enzyme coverage, electron transfer rate and current density. Biosens. Bioelectron. 2017, 89, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Xu, H.; Zhang, Q.; Huang, Y.C.; Shi, C.; Chang, Y.C.; Xu, X.; Tang, J.; Gu, Y.; Pao, C.W. Identifying a universal activity descriptor and a unifying mechanism concept on perovskite oxides for green hydrogen production. Adv. Mater. 2023, 35, 2305074. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.-C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Degani, Y.; Heller, A. Direct electrical communication between chemically modified enzymes and metal electrodes. 2. Methods for bonding electron-transfer relays to glucose oxidase and D-amino-acid oxidase. J. Am. Chem. Soc. 1988, 110, 2615–2620. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemi-cal systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Allen, P.; Hickling, A. Electrochemistry of sulphur. Part 1—Overpotential in the discharge of the sulphide ion. Trans. Faraday Soc. 1957, 53, 1626–1635. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Fundamentals and applications. Electrochem. Methods 2001, 2, 482. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, T.; Park, W.; You, J.; Na, S. Investigation of Direct Electron Transfer of Glucose Oxidase on a Graphene-CNT Composite Surface: A Molecular Dynamics Study Based on Electrochemical Experiments. Nanomaterials 2024, 14, 1073. https://doi.org/10.3390/nano14131073
Yoon T, Park W, You J, Na S. Investigation of Direct Electron Transfer of Glucose Oxidase on a Graphene-CNT Composite Surface: A Molecular Dynamics Study Based on Electrochemical Experiments. Nanomaterials. 2024; 14(13):1073. https://doi.org/10.3390/nano14131073
Chicago/Turabian StyleYoon, Taeyoung, Wooboum Park, Juneseok You, and Sungsoo Na. 2024. "Investigation of Direct Electron Transfer of Glucose Oxidase on a Graphene-CNT Composite Surface: A Molecular Dynamics Study Based on Electrochemical Experiments" Nanomaterials 14, no. 13: 1073. https://doi.org/10.3390/nano14131073
APA StyleYoon, T., Park, W., You, J., & Na, S. (2024). Investigation of Direct Electron Transfer of Glucose Oxidase on a Graphene-CNT Composite Surface: A Molecular Dynamics Study Based on Electrochemical Experiments. Nanomaterials, 14(13), 1073. https://doi.org/10.3390/nano14131073






