Emerging Trends in Nanomedicine: Carbon-Based Nanomaterials for Healthcare
Abstract
:1. Introduction
2. Carbon Quantum Dots in Biological Applications
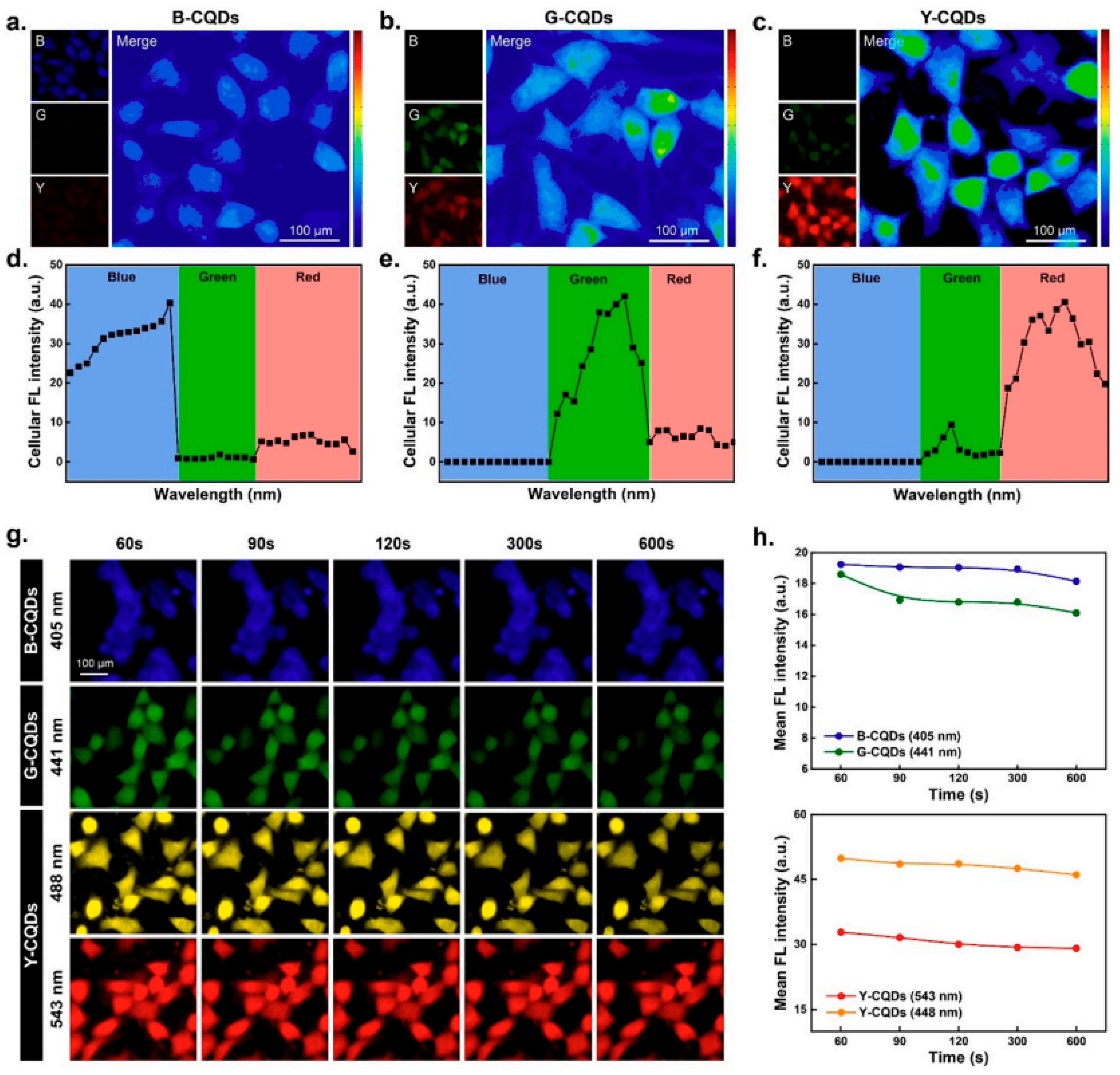
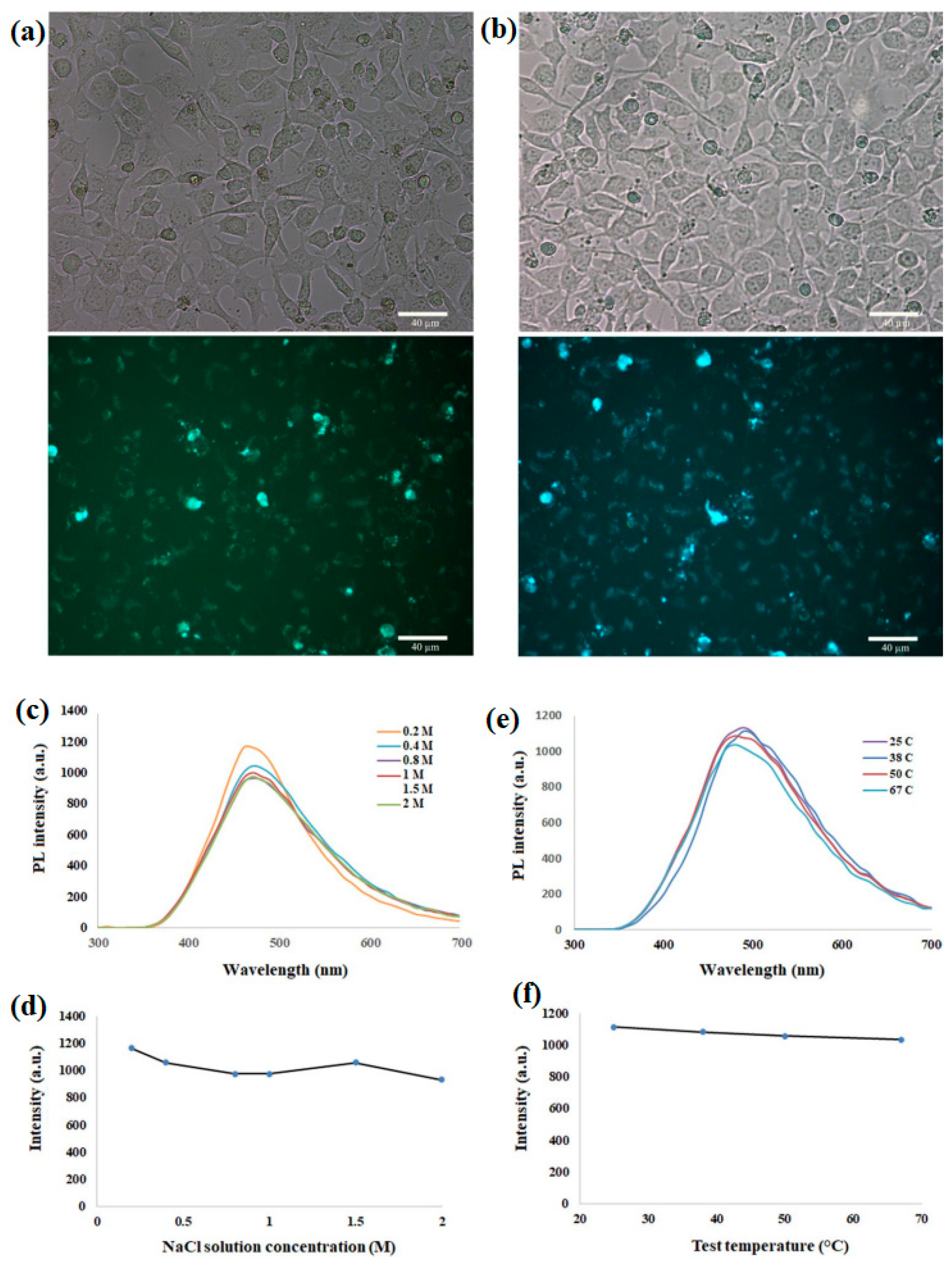
2.1. Imaging and Bioimaging
2.2. Drug Delivery
2.3. Bio-Sensing and Diagnostics
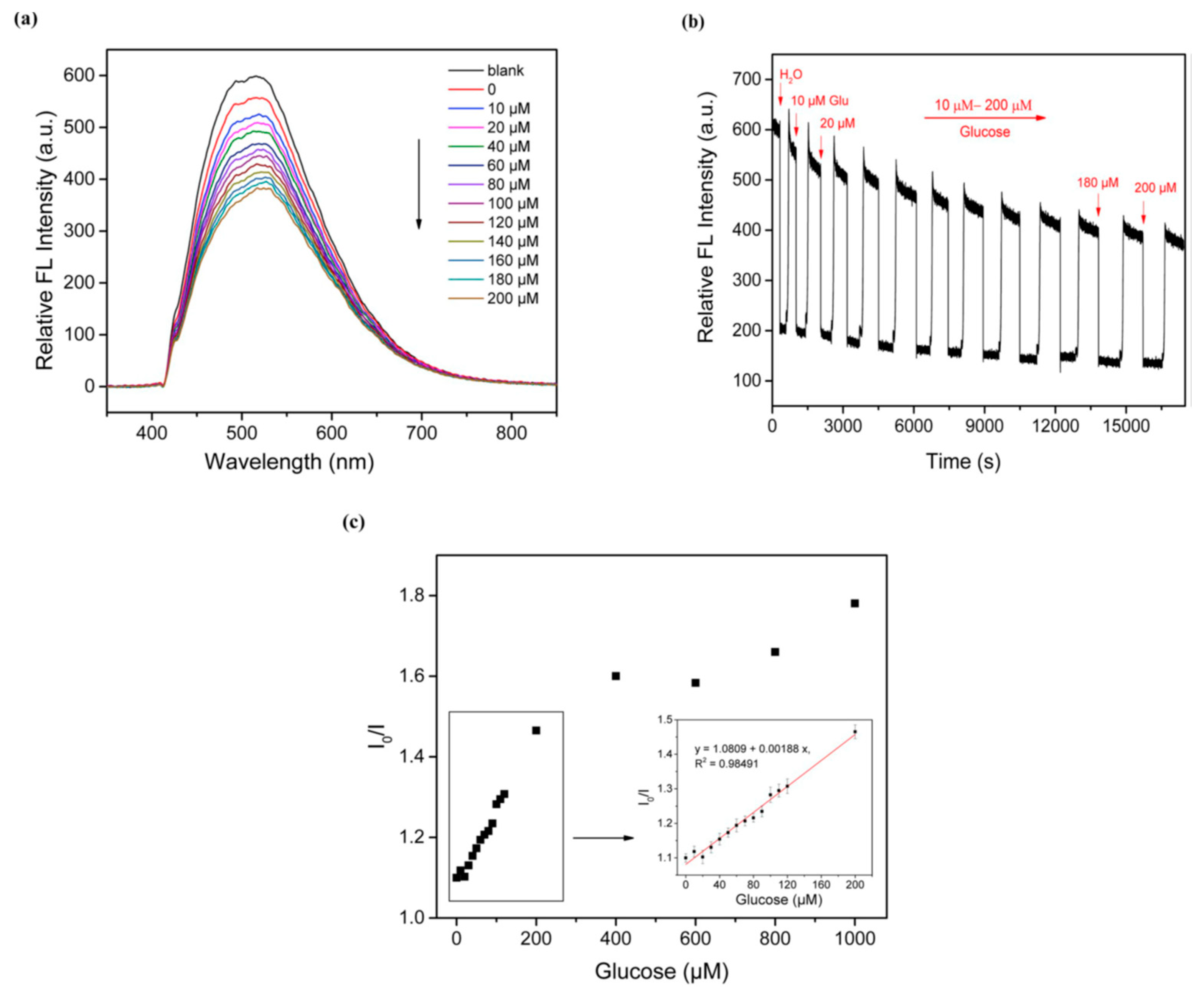
2.3.1. Glucose Biosensors
2.3.2. Biomolecule Sensors
2.3.3. DNA Sensors
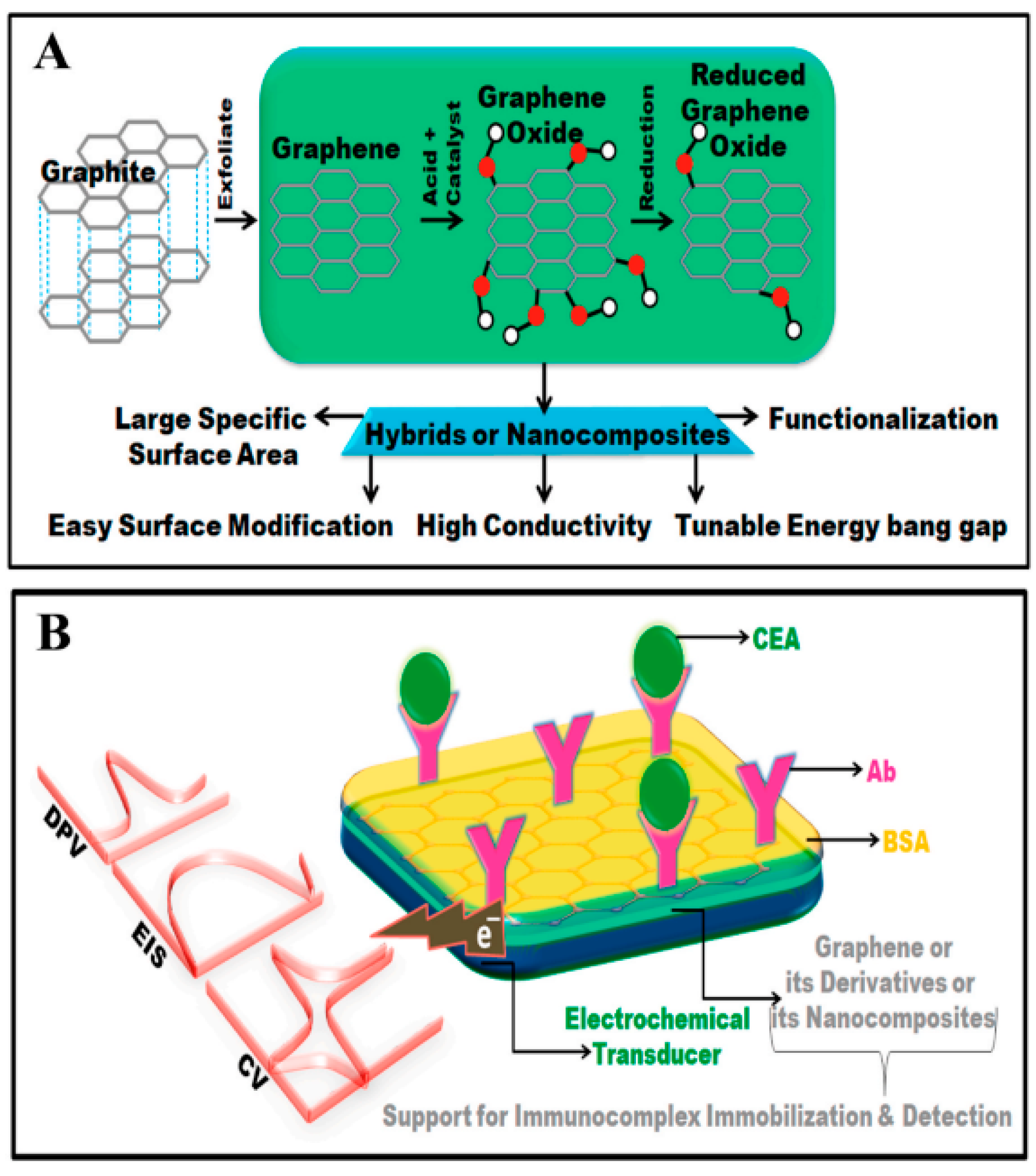
2.3.4. Cancer Biomarker Detection
2.4. Photodynamic Therapy (PDT)
2.4.1. Photodynamic Therapy for Cancer
2.4.2. Photodynamic Therapy for Microbial Infections
2.4.3. Targeted PDT Using CQDs
2.4.4. Imaging and Monitoring during PDT
2.5. Bioimaging-Guided Therapy
2.5.1. Bioimaging Applications
2.5.2. Targeted Drug Delivery
2.5.3. Theranostic Applications
2.5.4. Improved Diagnostics and Treatments
2.6. Carbon Quantum Dots in Future Prospective Biological Applications Which Is Not Yet Done Research
2.6.1. Theranostics
2.6.2. Immunomodulation
2.6.3. Neural Interfaces and Neuroprotection
2.6.4. Bioelectronic Medicine
2.6.5. Bioimaging beyond Visible Light
2.6.6. Drug-Resistant Pathogen Treatment
2.7. Limitations of Carbon Quantum Dots in Biological Applications
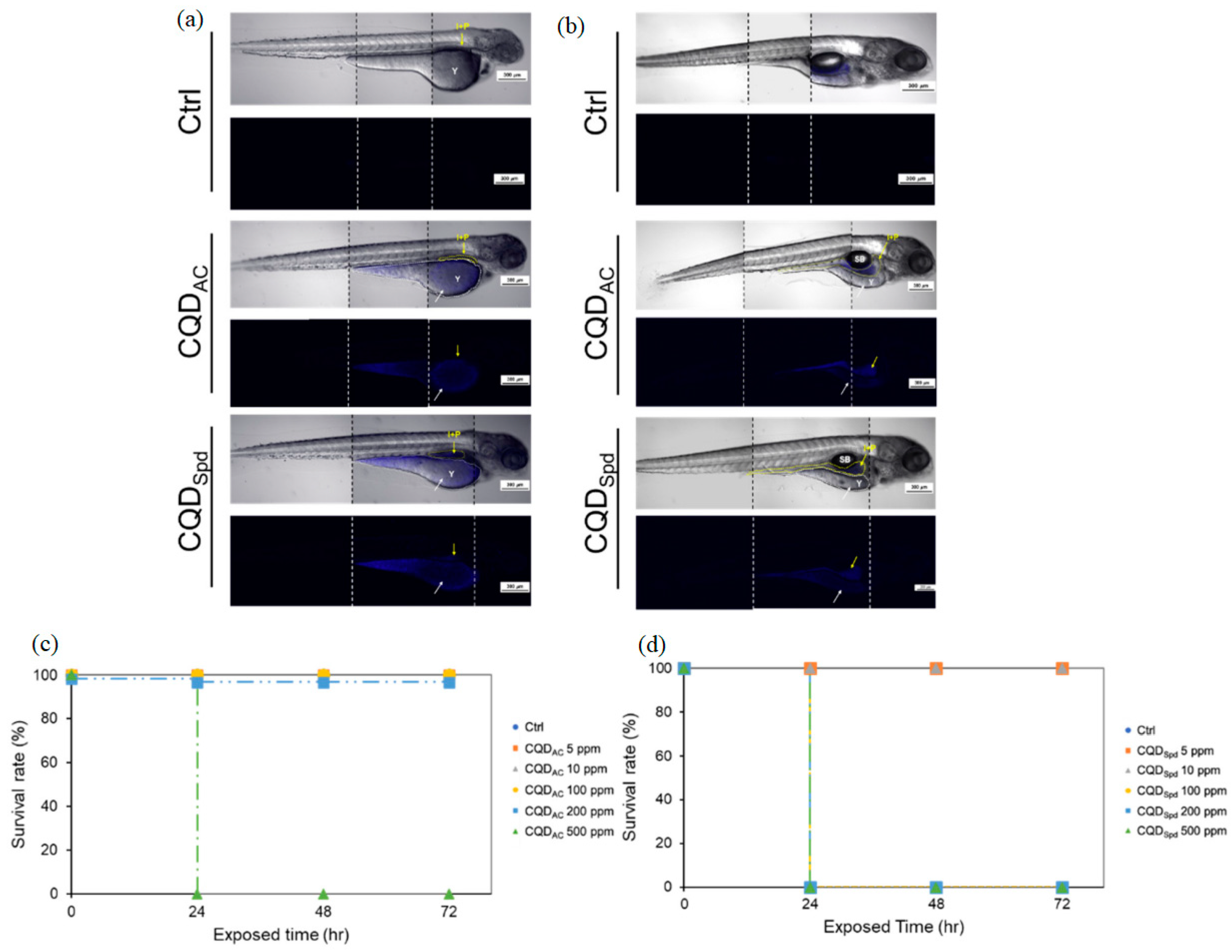
2.7.1. Size and Stability
2.7.2. Biocompatibility and Toxicity
2.7.3. Long-Term Effects
2.7.4. Biodistribution and Clearance
2.7.5. Synthesis and Scalability
2.7.6. Specificity in Targeting
2.7.7. Regulatory Challenges
2.7.8. Cost Effective
2.7.9. Photobleaching
3. Carbon 2D Nanosheets in Biological Applications
3.1. Graphene in Biosensing and Bioimaging
3.1.1. DNA Sensing
3.1.2. Protein Detection
3.1.3. Disease Marker Detection
3.1.4. Fluorescence Imaging and Photoacoustic Imaging
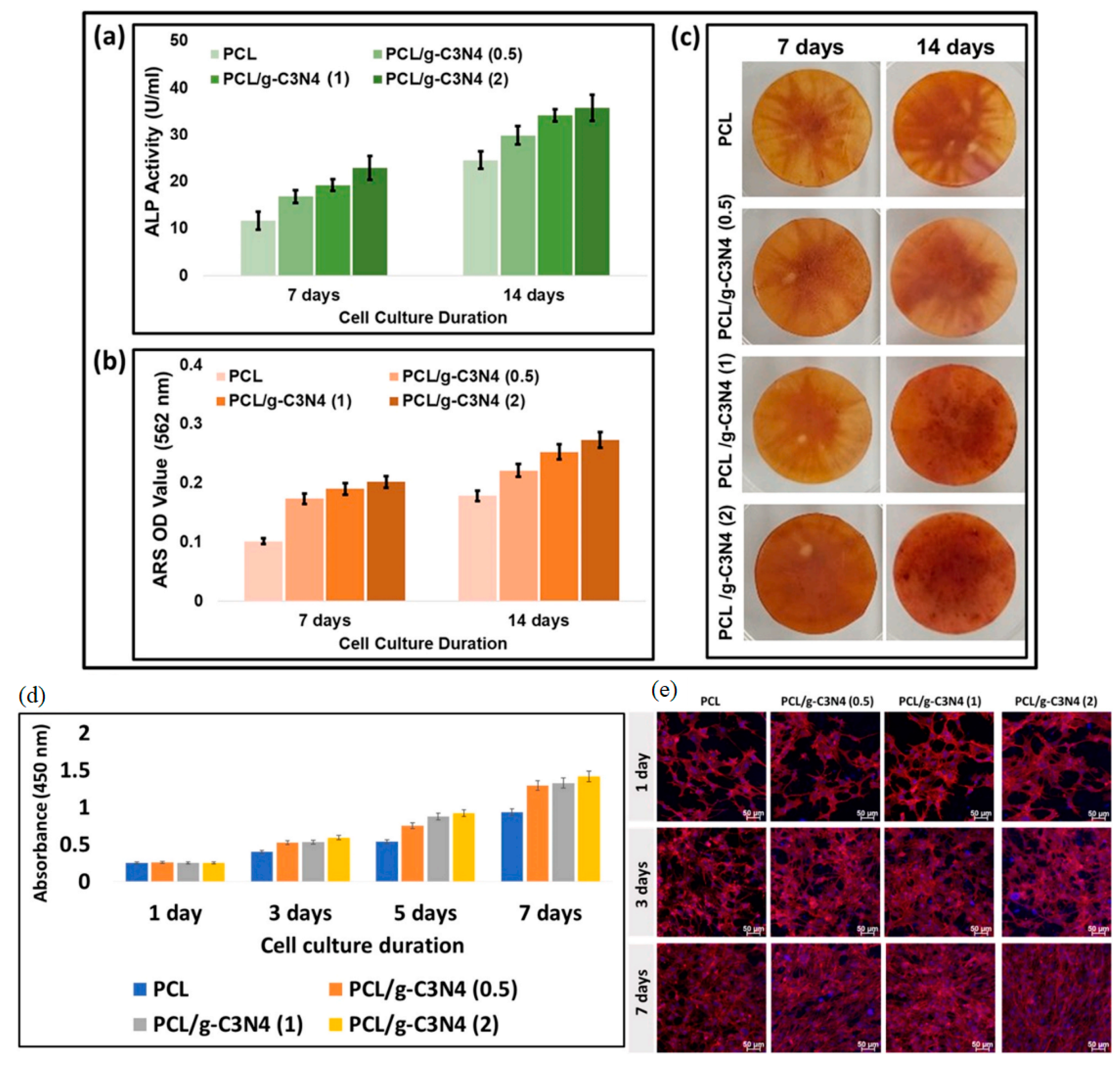
3.2. Graphdiyne in Tissue Engineering and Biomedical Devices
3.2.1. Graphdiyne Scaffolds for Tissue Engineering
3.2.2. Cell Adhesion and Proliferation
3.2.3. Controlled Drug Release
3.2.4. Biomedical Devices
4. Carbon 3D Nanosheets in Biological Applications
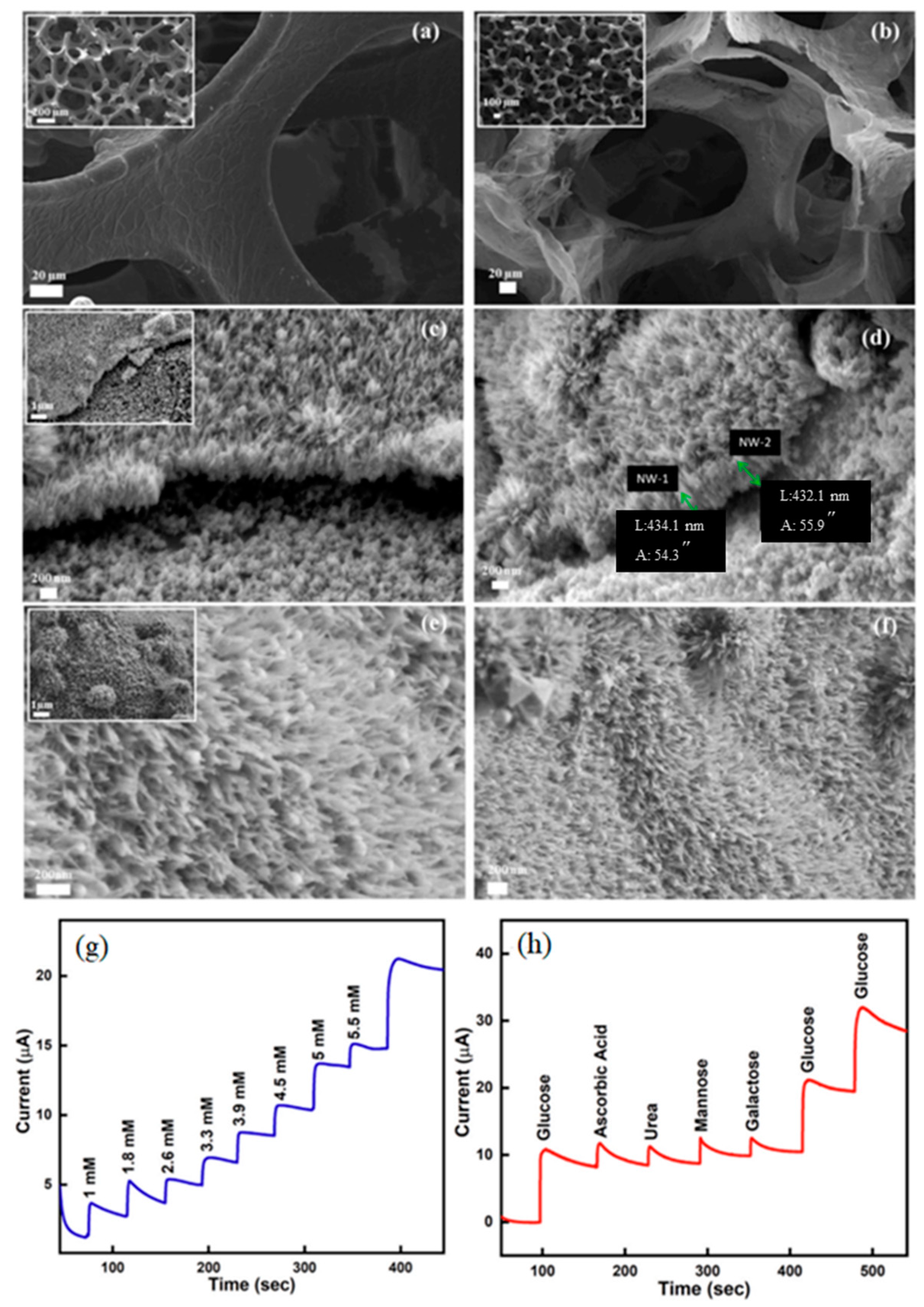
4.1. D Graphene Foams
4.1.1. Biosensing Applications
4.1.2. Drug Delivery Systems
4.1.3. Tissue Engineering
4.1.4. Targeted Drug Delivery
4.2. Vertically Aligned Carbon Nanosheets
5. Biofuel Cells
5.1. Biosensors
5.2. Electrochemical Reactions in Bioelectrochemical Devices
5.3. Enzyme Immobilization
6. Carbon Nanosheet Aerogels
6.1. Tissue Engineering Scaffolds
6.2. Lightweight Structural Materials in Biomedical Devices
6.3. Drug Delivery
6.4. Regenerative Medicine
7. 3D Graphdiyne Nanosheets
7.1. Controlled Release of Gases in Biomedical Applications
7.2. Gas Separation Membranes
7.3. Biomedical Gas Sensors
7.4. Drug Delivery and Controlled Gas Release
8. Carbon Nanosheet Composites
8.1. Tissue Engineering Scaffolds
8.2. Enhanced Electrical Conductivity in Bioelectrochemical Devices
9. Carbon Nanotubes and their Derivatives in Biological Applications
9.1. Drug Delivery
9.2. Bioimaging
9.3. Biosensing
9.4. Tissue Engineering
9.5. Biocompatibility and Toxicity
10. Clinical and Practical Aspects
10.1. Clinical Aspects
10.1.1. Targeting Strategies
10.1.2. Large-Scale Production
10.1.3. Clinical Imaging
10.1.4. Drug Delivery Challenges
10.1.5. Personalized Medicine
10.1.6. Tissue Engineering
10.2. Practical Aspects
Biocompatibility and Toxicity
11. Challenges and Future Prospects
11.1. Challenges
11.1.1. Safety and Toxicity
11.1.2. Standardization and Reproducibility
11.1.3. Biocompatibility and Immunogenicity
11.2. Future Prospects
11.2.1. Advanced Drug Delivery Systems
11.2.2. Diagnostic and Imaging Technologies
11.2.3. Tissue Engineering and Regenerative Medicine
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Teradal, N.L.; Jelinek, R. Carbon Nanomaterials in Biological Studies and Biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, W.; Gao, X.; Liu, X.; Zhang, Z.; Xing, B. Nanoparticles-Mediated Ion Channels Manipulation: From Their Membrane Interactions to Bioapplications. Adv. Drug Deliv. Rev. 2023, 195, 114763. [Google Scholar] [CrossRef] [PubMed]
- Sabui, P.; Mallick, S.; Singh, K.R.; Natarajan, A.; Verma, R.; Singh, J.; Singh, R.P. Potentialities of Fluorescent Carbon Nanomaterials as Sensor for Food Analysis. Luminescence 2022, 38, 1047–1063. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.-H.; Qi, M.-Y.; Zhang, F.; Tang, Z.-R.; Xu, Y.-J. Surface/Interface Engineering of Carbon-Based Materials for Constructing Multidimensional Functional Hybrids. Sol. RRL 2020, 4, 1900577. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Ghafori Gorab, M.; Masoud Hashemi, S.; Javanshir, S.; Ahangari Cohan, R.; et al. Applications of Carbon-Based Conductive Nanomaterials in Biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, B.; Cao, L.; Deng, Y.; Su, J. Tailored Mesoporous Inorganic Biomaterials: Assembly, Functionalization, and Drug Delivery Engineering. Adv. Mater. 2021, 33, 2005215. [Google Scholar] [CrossRef]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of Nanoparticles in Biomedical Imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N.; Joo, S.W. PH-Responsive Biocompatible Fluorescent Core-Shell Nanogel for Intracellular Imaging and Control Drug Release. Part. Part. Syst. Charact. 2021, 38, 2100110. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Synthesis of a Highly Fluorescence Nitrogen-Doped Carbon Quantum Dots Bioimaging Probe and Its in vivo Clearance and Printing Applications. RSC Adv. 2016, 6, 18134–18140. [Google Scholar] [CrossRef]
- Erdem, Ö.; Derin, E.; Zeibi Shirejini, S.; Sagdic, K.; Yilmaz, E.G.; Yildiz, S.; Akceoglu, G.A.; Inci, F. Carbon-Based Nanomaterials and Sensing Tools for Wearable Health Monitoring Devices. Adv. Mater. Technol. 2022, 7, 2100572. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N. Rapid Detection of Bacteria by Carbon Quantum Dots. J. Biomed. Nanotechnol. 2011, 7, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.K.; Lee, Y.R.; Parvin, N. Red Phosphorus Decorated Graphene Oxide Nanosheets: Label-Free DNA Detection. Biomater. Sci. 2020, 8, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Tondnevis, F.; Keshvari, H.; Mohandesi, J.A. Fabrication, Characterization, and in vitro Evaluation of Electrospun Polyurethane-gelatin-carbon Nanotube Scaffolds for Cardiovascular Tissue Engineering Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2276–2293. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; He, J.; Li, D. Electrohydrodynamic 3D Printing of Layer-Specifically Oriented, Multiscale Conductive Scaffolds for Cardiac Tissue Engineering. Nanoscale 2019, 11, 15195–15205. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of ATP–Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Peng, Q.; Ruttkay-Nedecky, B.; Milnerowicz, H.; Kizek, R. Carbon Nanomaterials for Targeted Cancer Therapy Drugs: A Critical Review. Chem. Rec. 2019, 19, 502–522. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2022, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Manimegalai, S.; Vickram, S.; Deena, S.R.; Rohini, K.; Thanigaivel, S.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Carbon-Based Nanomaterial Intervention and Efficient Removal of Various Contaminants from Effluents—A Review. Chemosphere 2023, 312, 137319. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Nie, G. Multifunctional Biomolecule Nanostructures for Cancer Therapy. Nat. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of Nucleic Acids. Nat. Rev. Methods Prim. 2022, 2, 24. [Google Scholar] [CrossRef]
- Han, X.; Alu, A.; Liu, H.; Shi, Y.; Wei, X.; Cai, L.; Wei, Y. Biomaterial-Assisted Biotherapy: A Brief Review of Biomaterials Used in Drug Delivery, Vaccine Development, Gene Therapy, and Stem Cell Therapy. Bioact. Mater. 2022, 17, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Mandal, T.K. Dually Emissive P,N-Co-Doped Carbon Dots for Fluorescent and Photoacoustic Tissue Imaging in Living Mice. Microchim. Acta 2017, 184, 1117–1125. [Google Scholar] [CrossRef]
- Hsu, J.C.; Tang, Z.; Eremina, O.E.; Sofias, A.M.; Lammers, T.; Lovell, J.F.; Zavaleta, C.; Cai, W.; Cormode, D.P. Nanomaterial-Based Contrast Agents. Nat. Rev. Methods Prim. 2023, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Refaat, A.; Yap, M.L.; Pietersz, G.; Walsh, A.P.G.; Zeller, J.; del Rosal, B.; Wang, X.; Peter, K. In Vivo Fluorescence Imaging: Success in Preclinical Imaging Paves the Way for Clinical Applications. J. Nanobiotechnol. 2022, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, Y.; Zhang, N.; Qu, S.; Zeng, W.; Li, R.; Dai, Z. Nanotechnology for Enhancing Medical Imaging. In Nanomedicine; Springer Nature: Singapore, 2023; pp. 99–156. [Google Scholar]
- Masoudi Asil, S.; Guerrero, E.D.; Bugarini, G.; Cayme, J.; De Avila, N.; Garcia, J.; Hernandez, A.; Mecado, J.; Madero, Y.; Moncayo, F.; et al. Theranostic Applications of Multifunctional Carbon Nanomaterials. View 2023, 4, 20220056. [Google Scholar] [CrossRef] [PubMed]
- van Moolenbroek, G.T.; Patiño, T.; Llop, J.; Sánchez, S. Engineering Intelligent Nanosystems for Enhanced Medical Imaging. Adv. Intell. Syst. 2020, 2, 2000087. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N.; Joo, S.W.; Roy, P. Risk Assessment of Cosmetics Using Triclosan on Future Generation’s Germ Cell Maturation via Lactating Mother Rats. Int. J. Environ. Res. Public Health 2020, 17, 1143. [Google Scholar] [CrossRef] [PubMed]
- Gergeroglu, H.; Yildirim, S.; Ebeoglugil, M.F. Nano-Carbons in Biosensor Applications: An Overview of Carbon Nanotubes (CNTs) and Fullerenes (C60). SN Appl. Sci. 2020, 2, 603. [Google Scholar] [CrossRef]
- Jeykumari, D.R.S.; Narayanan, S.S. Fabrication of Bienzyme Nanobiocomposite Electrode Using Functionalized Carbon Nanotubes for Biosensing Applications. Biosens. Bioelectron. 2008, 23, 1686–1693. [Google Scholar] [CrossRef]
- Chary, P.S.; Bhawale, R.; Vasave, R.; Rajana, N.; Singh, P.K.; Madan, J.; Singh, S.B.; Mehra, N.K. A Review on Emerging Role of Multifunctional Carbon Nanotubes as an Armament in Cancer Therapy, Imaging and Biosensing. J. Drug Deliv. Sci. Technol. 2023, 85, 104588. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, T.; Cao, Y.; Sun, J.; Zhou, Q.; Chen, H.; Guo, S.; Wang, Y.; Zhen, Y.; Liang, X.-J.; et al. Temperature-Sensitive Lipid-Coated Carbon Nanotubes for Synergistic Photothermal Therapy and Gene Therapy. ACS Nano 2021, 15, 6517–6529. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Obeid, M.A.; Amawi, H.A.; Rezigue, M.M.; Hamzat, Y.; Satija, S.; Tambuwala, M.M. Correction to: Application of Nanomaterials in the Diagnosis and Treatment of Genetic Disorders. In Applications of Nanomaterials in Human Health; Springer: Singapore, 2020; p. C1. [Google Scholar]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon Nanotubes: Evaluation of Toxicity at Biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Dalla Colletta, A.; Pelin, M.; Sosa, S.; Fusco, L.; Prato, M.; Tubaro, A. CARBON-BASED Nanomaterials and SKIN: An Overview. Carbon 2022, 196, 683–698. [Google Scholar] [CrossRef]
- Kundu, S.; Ghosh, M.; Sarkar, N. State of the Art and Perspectives on the Biofunctionalization of Fluorescent Metal Nanoclusters and Carbon Quantum Dots for Targeted Imaging and Drug Delivery. Langmuir 2021, 37, 9281–9301. [Google Scholar] [CrossRef]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaidya, S.S.; Yadav, M.D. Carbon Nanotubes in Biomedical Applications: Current Status, Promises, and Challenges. Carbon Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef]
- Manikandan, V.; Lee, N.Y. Green Synthesis of Carbon Quantum Dots and Their Environmental Applications. Environ. Res. 2022, 212, 113283. [Google Scholar] [CrossRef]
- Soumya, K.; More, N.; Choppadandi, M.; Aishwarya, D.A.; Singh, G.; Kapusetti, G. A Comprehensive Review on Carbon Quantum Dots as an Effective Photosensitizer and Drug Delivery System for Cancer Treatment. Biomed. Technol. 2023, 4, 11–20. [Google Scholar] [CrossRef]
- Latha, B.D.; Soumya, K.; More, N.; Mounika, C.; Guduru, A.T.; Singh, G.; Kapusetti, G. Fluorescent Carbon Quantum Dots for Effective Tumor Diagnosis: A Comprehensive Review. Biomed. Eng. Adv. 2023, 5, 100072. [Google Scholar] [CrossRef]
- John, V.L.; Nair, Y.; Vinod, T.P. Doping and Surface Modification of Carbon Quantum Dots for Enhanced Functionalities and Related Applications. Part. Part. Syst. Charact. 2021, 38, 2100170. [Google Scholar] [CrossRef]
- Al-Hetty, H.R.A.K.; Jalil, A.T.; Al-Tamimi, J.H.Z.; Shakier, H.G.; Kandeel, M.; Saleh, M.M.; Naderifar, M. Engineering and Surface Modification of Carbon Quantum Dots for Cancer Bioimaging. Inorg. Chem. Commun. 2023, 149, 110433. [Google Scholar] [CrossRef]
- Jana, P.; Dev, A. Carbon Quantum Dots: A Promising Nanocarrier for Bioimaging and Drug Delivery in Cancer. Mater. Today Commun. 2022, 32, 104068. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K.; Nagajyothi, P.C.; Mohan Reddy, P.; Ramesh Reddy, N.; Joo, S.-W. Highly Fluorescent Doped Fe3O4@C Nanoparticles Cross the Blood–Brain Barrier: Help in Brain Imaging and Blocking the Life Cycle of Mosquitoes. J. Clust. Sci. 2021, 32, 1761–1767. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.; Roy, P. Soluble Nanoparticles Versatile Tools for Plant Tissue Imaging. J. Bionanosci. 2013, 7, 256–259. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Deb Dutta, S.; Lim, K.-T.; Kim, J. Wet Chemistry-Based Processing of Tunable Polychromatic Carbon Quantum Dots for Multicolor Bioimaging and Enhanced NIR-Triggered Photothermal Bactericidal Efficacy. Appl. Surf. Sci. 2022, 597, 153630. [Google Scholar] [CrossRef]
- Kargozar, S.; Hoseini, S.J.; Milan, P.B.; Hooshmand, S.; Kim, H.; Mozafari, M. Quantum Dots: A Review from Concept to Clinic. Biotechnol. J. 2020, 15, 2000117. [Google Scholar] [CrossRef] [PubMed]
- Soldado, A.; Barrio, L.C.; Díaz-Gonzalez, M.; de la Escosura-Muñiz, A.; Costa-Fernandez, J.M. Advances in Quantum Dots as Diagnostic Tools. Adv. Clin. Chem. 2022, 107, 1–40. [Google Scholar]
- Mandal, T.K.; Parvin, N.; Roy, P. Polyelectrolyte Carbon Quantum-Dots: New Player as a Noninvasive Imaging Probe in Drosophila Dots. J. Nanosci. Nanotechnol. 2013, 13, 6499–6505. [Google Scholar]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural Carbon-Based Quantum Dots and Their Applications in Drug Delivery: A Review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef] [PubMed]
- Brindhadevi, K.; Al Garalleh, H.; Alalawi, A.; Al-Sarayreh, E.; Pugazhendhi, A. Carbon Nanomaterials: Types, Synthesis Strategies and Their Application as Drug Delivery System for Cancer Therapy. Biochem. Eng. J. 2023, 192, 108828. [Google Scholar] [CrossRef]
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, M.A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting Cancer Cells with Nanotherapeutics and Nanodiagnostics: Current Status and Future Perspectives. Semin. Cancer Biol. 2021, 69, 52–68. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Rajabzadeh-Khosroshahi, M.; Samadi, A.; Behzadmehr, R.; Rahdar, A.; Ferreira, L.F.R. Properties and Application of Carbon Quantum Dots (CQDs) in Biosensors for Disease Detection: A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2023, 80, 104156. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Bhandari, B.; Yang, C. Recent Development of Carbon Quantum Dots: Biological Toxicity, Antibacterial Properties and Application in Foods. Food Rev. Int. 2022, 38, 1513–1532. [Google Scholar] [CrossRef]
- Thulasinathan, B.D.S.; Murugan, S.; Panda, S.K.; Veerapandian, M.; Manickam, P. DNA-Functionalized Carbon Quantum Dots for Electrochemical Detection of Pyocyanin: A Quorum Sensing Molecule in Pseudomonas Aeruginosa. Biosens. Bioelectron. 2023, 227, 115156. [Google Scholar] [CrossRef]
- Chu, H.-W.; Unnikrishnan, B.; Anand, A.; Lin, Y.-W.; Huang, C.-C. Carbon Quantum Dots for the Detection of Antibiotics and Pesticides. J. Food Drug Anal. 2020, 28, 540–558. [Google Scholar] [CrossRef]
- Feng, H.; Qian, Z. Functional Carbon Quantum Dots: A Versatile Platform for Chemosensing and Biosensing. Chem. Rec. 2018, 18, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Diao, H.; Chang, H.; Wang, H.; Li, T.; Wei, W. Green Synthesis of Carbon Dots from Rose-Heart Radish and Application for Fe3+ Detection and Cell Imaging. Sens. Actuators B Chem. 2017, 241, 190–198. [Google Scholar] [CrossRef]
- Yu, S.; Ding, L.; Lin, H.; Wu, W.; Huang, J. A Novel Optical Fiber Glucose Biosensor Based on Carbon Quantum Dots-Glucose Oxidase/Cellulose Acetate Complex Sensitive Film. Biosens. Bioelectron. 2019, 146, 111760. [Google Scholar] [CrossRef]
- Eddin, F.B.K.; Fen, Y.W.; Fauzi, N.I.M.; Daniyal, W.M.E.M.M.; Omar, N.A.S.; Anuar, M.F.; Hashim, H.S.; Sadrolhosseini, A.R.; Abdullah, H. Direct and Sensitive Detection of Dopamine Using Carbon Quantum Dots Based Refractive Index Surface Plasmon Resonance Sensor. Nanomaterials 2022, 12, 1799. [Google Scholar] [CrossRef]
- Zhu, Z.; Niu, H.; Li, R.; Yang, Z.; Wang, J.; Li, X.; Pan, P.; Liu, J.; Zhou, B. One-Pot Hydrothermal Synthesis of Fluorescent Carbon Quantum Dots with Tunable Emission Color for Application in Electroluminescence Detection of Dopamine. Biosens. Bioelectron. X 2022, 10, 100141. [Google Scholar] [CrossRef]
- Shamsipur, M.; Nasirian, V.; Mansouri, K.; Barati, A.; Veisi-Raygani, A.; Kashanian, S. A Highly Sensitive Quantum Dots-DNA Nanobiosensor Based on Fluorescence Resonance Energy Transfer for Rapid Detection of Nanomolar Amounts of Human Papillomavirus 18. J. Pharm. Biomed. Anal. 2017, 136, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef]
- Li, N.-L.; Jia, L.-P.; Ma, R.-N.; Jia, W.-L.; Lu, Y.-Y.; Shi, S.-S.; Wang, H.-S. A Novel Sandwiched Electrochemiluminescence Immunosensor for the Detection of Carcinoembryonic Antigen Based on Carbon Quantum Dots and Signal Amplification. Biosens. Bioelectron. 2017, 89, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Zhang, X.; Liu, S.; Pei, Q.; Zheng, M.; Xie, Z. Porphyrin-Based Carbon Dots for Photodynamic Therapy of Hepatoma. Adv. Healthc. Mater. 2017, 6, 1600924. [Google Scholar] [CrossRef]
- Li, X.; Vinothini, K.; Ramesh, T.; Rajan, M.; Ramu, A. Combined Photodynamic-Chemotherapy Investigation of Cancer Cells Using Carbon Quantum Dot-Based Drug Carrier System. Drug Deliv. 2020, 27, 791–804. [Google Scholar] [CrossRef]
- Hao, X.; Huang, L.; Zhao, C.; Chen, S.; Lin, W.; Lin, Y.; Zhang, L.; Sun, A.; Miao, C.; Lin, X.; et al. Antibacterial Activity of Positively Charged Carbon Quantum Dots without Detectable Resistance for Wound Healing with Mixed Bacteria Infection. Mater. Sci. Eng. C 2021, 123, 111971. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Pang, Q.; Mai, K.; He, X.; Xu, L.; Zhou, F.; Liu, Y. Silver Nanoparticle@carbon Quantum Dot Composite as an Antibacterial Agent. RSC Adv. 2022, 12, 9621–9627. [Google Scholar] [CrossRef]
- Nicholas, D.; Fowley, C.; McHale, A.P.; Kamila, S.; Sheng, J.; Atchison, J.; Callan, J.F. A Folic Acid Labelled Carbon Quantum Dot-Protoporphryin IX Conjugate for Use in Folate Receptor Targeted Two-Photon Excited Photodynamic Therapy; Parak, W.J., Osinski, M., Liang, X.-J., Eds.; SPIE: Bellingham, WA, USA, 2015; p. 933813. [Google Scholar]
- Zhang, L.; Lin, Z.; Yu, Y.-X.; Jiang, B.-P.; Shen, X.-C. Multifunctional Hyaluronic Acid-Derived Carbon Dots for Self-Targeted Imaging-Guided Photodynamic Therapy. J. Mater. Chem. B 2018, 6, 6534–6543. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J.; Jiang, K.; Tang, Z.; Wang, Y.; Li, Z.; Liu, C.; Wu, A.; Lin, H. Ce6-Modified Carbon Dots for Multimodal-Imaging-Guided and Single-NIR-Laser-Triggered Photothermal/Photodynamic Synergistic Cancer Therapy by Reduced Irradiation Power. ACS Appl. Mater. Interfaces 2019, 11, 5791–5803. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Li, S.; Su, W.; Wu, H.; Yuan, T.; Yuan, C.; Liu, J.; Deng, G.; Gao, X.; Chen, Z.; Bao, Y.; et al. Targeted Tumour Theranostics in Mice via Carbon Quantum Dots Structurally Mimicking Large Amino Acids. Nat. Biomed. Eng. 2020, 4, 704–716. [Google Scholar] [CrossRef]
- Das, R.K.; Panda, S.; Bhol, C.S.; Bhutia, S.K.; Mohapatra, S. N-Doped Carbon Quantum Dot (NCQD)-Deposited Carbon Capsules for Synergistic Fluorescence Imaging and Photothermal Therapy of Oral Cancer. Langmuir 2019, 35, 15320–15329. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. Gadolinium-Doped Fluorescent Carbon Quantum Dots as MRI Contrast Agents and Fluorescent Probes. Sci. Rep. 2022, 12, 17681. [Google Scholar] [CrossRef]
- Jeong, Y.; Jin, M.; Kim, K.S.; Na, K. Biocompatible Carbonized Iodine-Doped Dots for Contrast-Enhanced CT Imaging. Biomater. Res. 2022, 26, 27. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Chen, C.; Quan, B.; Zeng, Z.; Xu, J.; Chen, Z.; Wang, L. Tunable Photoacoustic and Fluorescence Imaging of Nitrogen-Doped Carbon Quantum Dots. Appl. Mater. Today 2023, 30, 101706. [Google Scholar] [CrossRef]
- Mahani, M.; Pourrahmani-Sarbanani, M.; Yoosefian, M.; Divsar, F.; Mousavi, S.M.; Nomani, A. Doxorubicin Delivery to Breast Cancer Cells with Transferrin-Targeted Carbon Quantum Dots: An in Vitro and in Silico Study. J. Drug Deliv. Sci. Technol. 2021, 62, 102342. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, Y.; Tang, Y.; Zhu, S.; Liu, X.; Yin, L.; Liu, Q.; Yu, Z.; Xu, Q.; Luo, D.; et al. Bi-Doped Carbon Quantum Dots Functionalized Liposomes with Fluorescence Visualization Imaging for Tumor Diagnosis and Treatment. Chin. Chem. Lett. 2023, 35, 108689. [Google Scholar] [CrossRef]
- Qin, X.; Liu, J.; Zhang, Q.; Chen, W.; Zhong, X.; He, J. Synthesis of Yellow-Fluorescent Carbon Nano-Dots by Microplasma for Imaging and Photocatalytic Inactivation of Cancer Cells. Nanoscale Res. Lett. 2021, 16, 14. [Google Scholar] [CrossRef]
- Biswal, M.R.; Bhatia, S. Carbon Dot Nanoparticles: Exploring the Potential Use for Gene Delivery in Ophthalmic Diseases. Nanomaterials 2021, 11, 935. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kaur, H.; Sharma, A.; Singh, J.; Alajangi, H.K.; Kumar, S.; Singla, N.; Kaur, I.P.; Barnwal, R.P. Carbon Based Nanodots in Early Diagnosis of Cancer. Front. Chem. 2021, 9, 669169. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sun, L.; Xue, S.; Qu, D.; An, L.; Wang, X.; Sun, Z. Recent Advances of Carbon Dots as New Antimicrobial Agents. SmartMat 2022, 3, 226–248. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, C.; Andrews, G.; Wang, J.; Ge, Y. Recent Advances in Carbon Quantum Dots for Virus Detection, as Well as Inhibition and Treatment of Viral Infection. Nano Converg. 2022, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Barve, K.; Singh, U.; Yadav, P.; Bhatia, D. Carbon-Based Designer and Programmable Fluorescent Quantum Dots for Targeted Biological and Biomedical Applications. Mater. Chem. Front. 2023, 7, 1781–1802. [Google Scholar] [CrossRef]
- Azam, N.; Najabat Ali, M.; Javaid Khan, T. Carbon Quantum Dots for Biomedical Applications: Review and Analysis. Front. Mater. 2021, 8, 700403. [Google Scholar] [CrossRef]
- Lesani, P.; Mohamad Hadi, A.H.; Lu, Z.; Palomba, S.; New, E.J.; Zreiqat, H. Design Principles and Biological Applications of Red-Emissive Two-Photon Carbon Dots. Commun. Mater. 2021, 2, 108. [Google Scholar] [CrossRef]
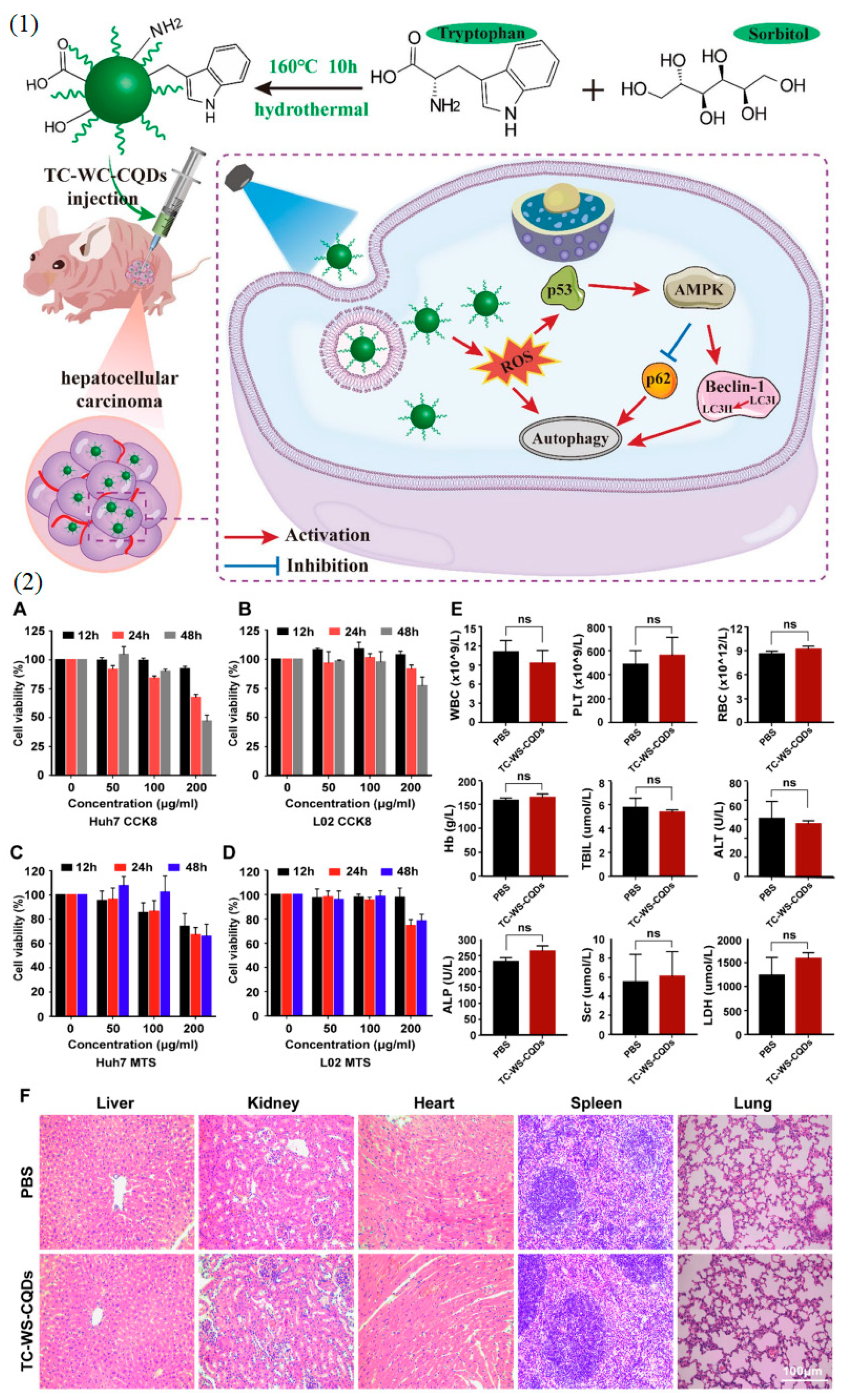
- Wang, Y.; Chen, J.; Tian, J.; Wang, G.; Luo, W.; Huang, Z.; Huang, Y.; Li, N.; Guo, M.; Fan, X. Tryptophan-Sorbitol Based Carbon Quantum Dots for Theranostics against Hepatocellular Carcinoma. J. Nanobiotechnol. 2022, 20, 78. [Google Scholar] [CrossRef]
- Truskewycz, A.; Yin, H.; Halberg, N.; Lai, D.T.H.; Ball, A.S.; Truong, V.K.; Rybicka, A.M.; Cole, I. Carbon Dot Therapeutic Platforms: Administration, Distribution, Metabolism, Excretion, Toxicity, and Therapeutic Potential. Small 2022, 18, 2106342. [Google Scholar] [CrossRef]
- Henriquez, G.; Ahlawat, J.; Fairman, R.; Narayan, M. Citric Acid-Derived Carbon Quantum Dots Attenuate Paraquat-Induced Neuronal Compromise In Vitro and In Vivo. ACS Chem. Neurosci. 2022, 13, 2399–2409. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Chen, L.; Yan, M.; Ge, L.; Ge, S.; Yu, J. A Disposable Electrochemiluminescence Device for Ultrasensitive Monitoring of K562 Leukemia Cells Based on Aptamers and ZnO@carbon Quantum Dots. Biosens. Bioelectron. 2013, 49, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kenry; Duan, Y.; Liu, B. Recent Advances of Optical Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, 1802394. [Google Scholar] [CrossRef] [PubMed]
- Serra-Burriel, M.; Keys, M.; Campillo-Artero, C.; Agodi, A.; Barchitta, M.; Gikas, A.; Palos, C.; López-Casasnovas, G. Impact of Multi-Drug Resistant Bacteria on Economic and Clinical Outcomes of Healthcare-Associated Infections in Adults: Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0227139. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Nanoparticles as Therapeutic Options for Treating Multidrug-Resistant Bacteria: Research Progress, Challenges, and Prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Jiang, S.; Park, S.-J.; Meng, L.-Y. A Review: Recent Advances in Preparations and Applications of Heteroatom-Doped Carbon Quantum Dots. Dalt. Trans. 2020, 49, 6915–6938. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-Y.; Chen, Y.-J.; Kang, C.-H.; Lin, H.-Y.; Huang, C.-C.; Hsu, P.-H.; Lin, H.-J. Toxic or Not Toxic, That Is the Carbon Quantum Dot’s Question: A Comprehensive Evaluation with Zebrafish Embryo, Eleutheroembryo, and Adult Models. Polymers 2021, 13, 1598. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, K.; Hu, Y.; Tang, M. A Review of Pulmonary Toxicity of Different Types of Quantum Dots in Environmental and Biological Systems. Chem. Biol. Interact. 2022, 368, 110247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sultan, D.; Liu, Y. Biodistribution, Excretion, and Toxicity of Nanoparticles. In Theranostic Bionanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–53. [Google Scholar]
- Yang, H.-L.; Bai, L.-F.; Geng, Z.-R.; Chen, H.; Xu, L.-T.; Xie, Y.-C.; Wang, D.-J.; Gu, H.-W.; Wang, X.-M. Carbon Quantum Dots: Preparation, Optical Properties, and Biomedical Applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, Properties and Biomedical Applications of Carbon-Based Quantum Dots: An Updated Review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Shin, G.H.; Kim, J.T. Carbon Dots: Opportunities and Challenges in Cancer Therapy. Pharmaceutics 2023, 15, 1019. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Cheng, L.-W.; Ma, Y.-Y.; Kong, J.-L.; Xiong, H.-M. Luminescent Carbon Quantum Dots and Their Application in Cell Imaging. New J. Chem. 2013, 37, 2515. [Google Scholar] [CrossRef]
- Longo, A.V.; Sciortino, A.; Cannas, M.; Messina, F. UV Photobleaching of Carbon Nanodots Investigated by in Situ Optical Methods. Phys. Chem. Chem. Phys. 2020, 22, 13398–13407. [Google Scholar] [CrossRef]
- Desmond, L.J.; Phan, A.N.; Gentile, P. Critical Overview on the Green Synthesis of Carbon Quantum Dots and Their Application for Cancer Therapy. Environ. Sci. Nano 2021, 8, 848–862. [Google Scholar] [CrossRef]
- Arshad, F.; Nabi, F.; Iqbal, S.; Khan, R.H. Applications of Graphene-Based Electrochemical and Optical Biosensors in Early Detection of Cancer Biomarkers. Colloids Surf. B Biointerfaces 2022, 212, 112356. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Ahsan, W.; Mangla, B.; Javed, S.; Hassan, M.Z.; Asmari, M.; Al Bratty, M.; Najmi, A. Graphene-Based Biosensors for Disease Theranostics: Development, Applications, and Recent Advancements. Nanotechnol. Rev. 2021, 11, 96–116. [Google Scholar] [CrossRef]
- Hunsur Ravikumar, C.; Ira Gowda, M.; Balakrishna, R.G. An “OFF–ON” Quantum Dot–Graphene Oxide Bioprobe for Sensitive Detection of Micrococcal Nuclease of Staphylococcus Aureus. Analyst 2019, 144, 3999–4005. [Google Scholar] [CrossRef]
- Maturi, M.; Locatelli, E.; Monaco, I.; Comes Franchini, M. Current Concepts in Nanostructured Contrast Media Development for in vivo Photoacoustic Imaging. Biomater. Sci. 2019, 7, 1746–1775. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.D.; Paschoalino, W.J.; Damasceno, J.P.V.; Kubota, L.T. Structure, Properties, and Electrochemical Sensing Applications of Graphene-Based Materials. ChemElectroChem 2020, 7, 4508–4525. [Google Scholar] [CrossRef]
- Simoska, O.; Stevenson, K.J. Electrochemical Sensors for Rapid Diagnosis of Pathogens in Real Time. Analyst 2019, 144, 6461–6478. [Google Scholar] [CrossRef]
- Karki, N.; Tiwari, H.; Tewari, C.; Rana, A.; Pandey, N.; Basak, S.; Sahoo, N.G. Functionalized Graphene Oxide as a Vehicle for Targeted Drug Delivery and Bioimaging Applications. J. Mater. Chem. B 2020, 8, 8116–8148. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Punetha, V.D.; Pathak, R.; Punetha, M. Two-Dimensional Carbon Nanomaterial-Based Biosensors: Micromachines for Advancing the Medical Diagnosis. In Recent Advances in Graphene Nanophotonics; Springer Nature: Cham, Switzerland, 2023; pp. 181–225. [Google Scholar]
- Tripathi, A.; Bonilla-Cruz, J. Review on Healthcare Biosensing Nanomaterials. ACS Appl. Nano Mater. 2023, 6, 5042–5074. [Google Scholar] [CrossRef]
- Kilic, T.; Kaplan, M.; Demiroglu, S.; Erdem, A.; Ozsoz, M. Label-Free Electrochemical Detection of MicroRNA-122 in Real Samples by Graphene Modified Disposable Electrodes. J. Electrochem. Soc. 2016, 163, B227–B233. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K.; Joo, S.-W. Architecture of dual emissive three-dimensional nanostructure composites containing graphitic 2D sheets and iron oxide nanoparticles: Detection of short single-stranded DNA sequences. Biomater. Sci. 2023, 11, 3851–3859. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Nguyen, E.P.; Merkoçi, A.; Guo, Z. 2-Dimensional Materials-Based Electrical/Optical Platforms for Smart on-off Diagnostics Applications. 2D Mater. 2020, 7, 032001. [Google Scholar] [CrossRef]
- Parvin, N.; Jin, Q.; Wei, Y.; Yu, R.; Huang, L.; Zhang, Y.; Wang, L.; Gao, M.; Zhao, H.; Hu, W.; et al. Few-Layer Graphdiyne Nanosheets Applied for Multiplexed Real-Time DNA Detection. Adv. Mater. 2017, 29, 1606755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Li, C.; Han, J.; Huang, W.; Zhou, J.; Yang, Y. Hydrophilic Antifouling 3D Porous MXene/Holey Graphene Nanocomposites for Electrochemical Determination of Dopamine. Microchem. J. 2022, 181, 107713. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Z.; Xiao, B.; Deng, M.; Wang, Z.; Li, Z.; Zhang, H.; Zhang, L.; Qian, J.; Li, J. Aptamer-Based Solution-Gated Graphene Transistors for Highly Sensitive and Real-Time Detection of Thrombin Molecules. Anal. Chem. 2021, 93, 13673–13679. [Google Scholar] [CrossRef] [PubMed]
- Manasa, G.; Mascarenhas, R.J.; Malode, S.J.; Shetti, N.P. Graphene-Based Electrochemical Immunosensors for Early Detection of Oncomarker Carcinoembryonic Antigen. Biosens. Bioelectron. X 2022, 11, 100189. [Google Scholar] [CrossRef]
- Kalyani, T.; Sangili, A.; Kotal, H.; Kaushik, A.; Chaudhury, K.; Jana, S.K. Ultra-Sensitive Label-Free Detection of Haptoglobin Using Au-RGO Decorated Electrochemical Sensing Platform: Towards Endometriosis Diagnostic Application. Biosens. Bioelectron. X 2023, 14, 100353. [Google Scholar] [CrossRef]
- Toumia, Y.; Domenici, F.; Orlanducci, S.; Mura, F.; Grishenkov, D.; Trochet, P.; Lacerenza, S.; Bordi, F.; Paradossi, G. Graphene Meets Microbubbles: A Superior Contrast Agent for Photoacoustic Imaging. ACS Appl. Mater. Interfaces 2016, 8, 16465–16475. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Wu, F.-G. A Graphene Oxide-Based Switch-on Fluorescent Probe for Glutathione Detection and Cancer Diagnosis. J. Colloid Interface Sci. 2018, 530, 511–520. [Google Scholar] [CrossRef]
- Jun, S.W.; Manivasagan, P.; Kwon, J.; Nguyen, V.T.; Mondal, S.; Ly, C.D.; Lee, J.; Kang, Y.-H.; Kim, C.-S.; Oh, J. Folic Acid–Conjugated Chitosan-Functionalized Graphene Oxide for Highly Efficient Photoacoustic Imaging-Guided Tumor-Targeted Photothermal Therapy. Int. J. Biol. Macromol. 2020, 155, 961–971. [Google Scholar] [CrossRef]
- Xie, C.; Wang, N.; Li, X.; Xu, G.; Huang, C. Research on the Preparation of Graphdiyne and Its Derivatives. Chem. A Eur. J. 2020, 26, 569–583. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Iqbal, M.; Mahmood, A.; Mahmood, N.; Shi, Z.; Yin, J.; Qing, D.; Ma, C.; Zhang, H. Recent Development in Graphdiyne and Its Derivative Materials for Novel Biomedical Applications. J. Mater. Chem. B 2021, 9, 9461–9484. [Google Scholar] [CrossRef]
- Yu, W.; Song, G.; Lv, F.; Huang, Y.; Bai, H.; Wang, S. Recent Advances in Graphdiyne Materials for Biomedical Applications. Nano Today 2022, 46, 101616. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Huang, X.; Xu, R.; He, Y.; Deng, F.; Chen, G. The Influences of PEG-functionalized Graphdiyne on Cell Growth and Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, N.; Li, X.; Wang, X.; Zhan, L.; Yuan, W.-E.; Song, J.; Ouyang, Y. Graphdiyne-Loaded Polycaprolactone Nanofiber Scaffold for Peripheral Nerve Regeneration. J. Colloid Interface Sci. 2023, 646, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, X.; Wu, Y.; Jie, J.; Wang, Z.; Chen, G.-Q.; Sun, J.; Wu, L.-P. Amphiphilic and Fatigue-Resistant Organohydrogels for Small-Diameter Vascular Grafts. Sci. Adv. 2022, 8, eabn5360. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; He, N.; Yuan, W.-E.; Qian, Y.; Ouyang, Y. Graphdiyne-Related Materials in Biomedical Applications and Their Potential in Peripheral Nerve Tissue Engineering. Cyborg Bionic Syst. 2022, 2022, 9892526. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Shi, F.; Liu, X.; Tang, Z.; Wang, Y.; Huang, Y.; Hou, H.; Xie, X.; Zhi, C. An Intrinsically Stretchable and Compressible Supercapacitor Containing a Polyacrylamide Hydrogel Electrolyte. Angew. Chem. Int. Ed. 2017, 56, 9141–9145. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Guo, M.; Liu, J.; Liu, J.; Zhou, H.; Li, J.; Wang, L.; Liu, H.; Li, Y.; Zhao, Y.; et al. Graphdiyne Nanosheet-Based Drug Delivery Platform for Photothermal/Chemotherapy Combination Treatment of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 8436–8442. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Hu, X.; Yu, L.; Fan, X.; Huang, C.; Li, Y. Graphdiyne-Based Flexible Respiration Sensors for Monitoring Human Health. Nano Today 2021, 39, 101214. [Google Scholar] [CrossRef]
- Güneş, F.; Aykaç, A.; Erol, M.; Erdem, Ç.; Hano, H.; Uzunbayir, B.; Şen, M.; Erdem, A. Synthesis of Hierarchical Hetero-Composite of Graphene Foam/α-Fe2O3 Nanowires and Its Application on Glucose Biosensors. J. Alloys Compd. 2022, 895, 162688. [Google Scholar] [CrossRef]
- Ezzati, N.; Mahjoub, A.R.; Shokrollahi, S.; Amiri, A.; Abolhosseini Shahrnoy, A. Novel Biocompatible Amino Acids-Functionalized Three-Dimensional Graphene Foams: As the Attractive and Promising Cisplatin Carriers for Sustained Release Goals. Int. J. Pharm. 2020, 589, 119857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhong, H.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.; Luo, T. Functional Nanostructure-Loaded Three-Dimensional Graphene Foam as a Non-Enzymatic Electrochemical Sensor for Reagentless Glucose Detection. RSC Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Baheiraei, N.; Mohseni, M.; Razavi, M.; Ghaderi, A.; Azizi, B.; Rabiee, N.; Karimi, M. Three-Dimensional Graphene Foam as a Conductive Scaffold for Cardiac Tissue Engineering. J. Biomater. Appl. 2019, 34, 74–85. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Mohammadi, Z.; Khavani, M.; Sanati, A.; Shariati, L.; Seyedhosseini Ghaheh, H.; Bidram, E.; Zarrabi, A. Fluorescence Anisotropy Cytosensing of Folate Receptor Positive Tumor Cells Using 3D Polyurethane-GO-Foams Modified with Folic Acid: Molecular Dynamics and in Vitro Studies. Microchim. Acta 2023, 190, 44. [Google Scholar] [CrossRef]
- Cui, H.-F.; Zhang, K.; Zhang, Y.-F.; Sun, Y.-L.; Wang, J.; Zhang, W.-D.; Luong, J.H.T. Immobilization of Glucose Oxidase into a Nanoporous TiO2 Film Layered on Metallophthalocyanine Modified Vertically-Aligned Carbon Nanotubes for Efficient Direct Electron Transfer. Biosens. Bioelectron. 2013, 46, 113–118. [Google Scholar] [CrossRef]
- Amade, R.; Moreno, H.A.; Hussain, S.; Vila-Costa, M.; Bertran, E. Vertically Aligned Carbon Nanotubes as Anode and Air-Cathode in Single Chamber Microbial Fuel Cells. Appl. Phys. Lett. 2016, 109, 163904. [Google Scholar] [CrossRef]
- TermehYousefi, A.; Bagheri, S.; Kadri, N.A.; Mahmood, M.R.; Ikeda, S. Constant Glucose Biosensor Based on Vertically Aligned Carbon Nanotube Composites. Int. J. Electrochem. Sci. 2015, 10, 4183–4192. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Li, C. Enhanced Performance of Microbial Fuel Cells with a Bacteria/Shape-Controllable Aligned Carbon Nanofibers Hybrid Biofilm. Int. J. Hydrogen Energy 2023, 48, 1107–1119. [Google Scholar] [CrossRef]
- Jiang, C.; Zheng, L.; Liu, Y. Aligned Carbon Nanotube Films for Immobilization of Glucose Oxidase and Its Application in Glucose Biosensor. Aust. J. Chem. 2021, 75, 89–93. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Dolatabadi, J.E.-N.; Hasanzadeh, M.; Soleymani, J. Carbon-Based Aerogels for Biomedical Sensing: Advances toward Designing the Ideal Sensor. Adv. Colloid Interface Sci. 2021, 298, 102550. [Google Scholar] [CrossRef]
- Rodriguez Sala, M.; Lynch, K.J.; Chandrasekaran, S.; Skalli, O.; Worsley, M.; Sabri, F. PC-12 Cells Adhesion and Differentiation on Carbon Aerogel Scaffolds. MRS Commun. 2018, 8, 1426–1432. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Zhang, S.; Lai, X.; Zeng, X. Superhydrophobic MXene@carboxylated Carbon Nanotubes/Carboxymethyl Chitosan Aerogel for Piezoresistive Pressure Sensor. Chem. Eng. J. 2021, 425, 130462. [Google Scholar] [CrossRef]
- Lim, M.B.; Ganas, A.S.; Hanson, J.L.; Zhou, X.; Hellner, B.; Manandhar, S.; Gariepy, R.E.; Baneyx, F.; Wilbur, D.S.; Pauzauskie, P.J. Crystalline Loading of Lipophilic Coenzyme Q10 Pharmaceuticals within Conjugated Carbon Aerogel Derivatives. Carbon 2020, 164, 451–458. [Google Scholar] [CrossRef]
- Atya, A.M.N.; Tevlek, A.; Almemar, M.; Gökcen, D.; Aydin, H.M. Fabrication and Characterization of Carbon Aerogel/Poly(Glycerol-Sebacate) Patches for Cardiac Tissue Engineering. Biomed. Mater. 2021, 16, 065027. [Google Scholar] [CrossRef] [PubMed]
- Tabandeh, Z.; Reisi-Vanani, A. Manipulation of the CO2 Capture Capability of Graphdiyne Using Transition Metal Decoration and Charge Injection: A DFT-D2 Study. Fuel 2023, 333, 126295. [Google Scholar] [CrossRef]
- Min, H.; Qi, Y.; Zhang, Y.; Han, X.; Cheng, K.; Liu, Y.; Liu, H.; Hu, J.; Nie, G.; Li, Y. A Graphdiyne Oxide-Based Iron Sponge with Photothermally Enhanced Tumor-Specific Fenton Chemistry. Adv. Mater. 2020, 32, 2000038. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, P.; Naeij, H.R. A New Approach to Separate Hydrogen from Carbon Dioxide Using Graphdiyne-like Membrane. Sci. Rep. 2020, 10, 13549. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yu, J.; Cao, C.; Song, W. Graphdiyne: A Highly Sensitive Material for Ppb-Level NO2 Gas Sensing at Room Temperature. Chem. Res. Chin. Univ. 2021, 37, 1317–1322. [Google Scholar] [CrossRef]
- Tabandeh, Z.; Reisi-Vanani, A. Investigation of the Adsorption Behavior of Two Anticancer Drugs on the Pristine and BN-Doped Graphdiyne Nanosheet: A DFT-D3 Perception. Diam. Relat. Mater. 2021, 119, 108564. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon Based Nanomaterials for Tissue Engineering of Bone: Building New Bone on Small Black Scaffolds: A Review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Awasthi, G.P.; Kaliannagounder, V.K.; Park, J.; Maharjan, B.; Shin, M.; Yu, C.; Park, C.H.; Kim, C.S. Assembly of Porous Graphitic Carbon Nitride Nanosheets into Electrospun Polycaprolactone Nanofibers for Bone Tissue Engineering. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126584. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-Inspired Electroactive Chitosan/Graphene Oxide Composite Hydrogel with Rapid Self-Healing and Recovery Behavior for Tissue Engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Mahalingam, S.; Ayyaru, S.; Ahn, Y.-H. Enhanced Cathode Performance of Fe2O3, Boron Nitride-Doped RGO Nanosheets for Microbial Fuel Cell Applications. Sustain. Energy Fuels 2020, 4, 1454–1468. [Google Scholar] [CrossRef]
- Muthusankar, E.; Ragupathy, D. Graphene/Poly(Aniline-Co-Diphenylamine) Nanohybrid for Ultrasensitive Electrochemical Glucose Sensor. Nano-Struct. Nano-Objects 2019, 20, 100390. [Google Scholar] [CrossRef]
- Gifani, M.; Eddins, D.J.; Kosuge, H.; Zhang, Y.; Paluri, S.L.A.; Larson, T.; Leeper, N.; Herzenberg, L.A.; Gambhir, S.S.; McConnell, M.V.; et al. Ultraselective Carbon Nanotubes for Photoacoustic Imaging of Inflamed Atherosclerotic Plaques. Adv. Funct. Mater. 2021, 31, 2101005. [Google Scholar] [CrossRef]
- Dai, B.; Zhou, R.; Ping, J.; Ying, Y.; Xie, L. Recent Advances in Carbon Nanotube-Based Biosensors for Biomolecular Detection. TrAC Trends Anal. Chem. 2022, 154, 116658. [Google Scholar] [CrossRef]
- Xia, Y.; Li, S.; Nie, C.; Zhang, J.; Zhou, S.; Yang, H.; Li, M.; Li, W.; Cheng, C.; Haag, R. A Multivalent Polyanion-Dispersed Carbon Nanotube toward Highly Bioactive Nanostructured Fibrous Stem Cell Scaffolds. Appl. Mater. Today 2019, 16, 518–528. [Google Scholar] [CrossRef]
- Yan, H.; Xue, Z.; Xie, J.; Dong, Y.; Ma, Z.; Sun, X.; Kebebe Borga, D.; Liu, Z.; Li, J. Toxicity of Carbon Nanotubes as Anti-Tumor Drug Carriers. Int. J. Nanomed. 2019, 14, 10179–10194. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical Applications of Carbon Nanomaterials: Drug and Gene Delivery Potentials. J. Cell. Physiol. 2019, 234, 298–319. [Google Scholar] [CrossRef] [PubMed]
- Rashed, A.O.; Merenda, A.; Kondo, T.; Lima, M.; Razal, J.; Kong, L.; Huynh, C.; Dumée, L.F. Carbon Nanotube Membranes—Strategies and Challenges towards Scalable Manufacturing and Practical Separation Applications. Sep. Purif. Technol. 2021, 257, 117929. [Google Scholar] [CrossRef]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A. Legal and Practical Challenges in Classifying Nanomaterials According to Regulatory Definitions. Nat. Nanotechnol. 2019, 14, 208–216. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lin, J.-F.; Zeng, Z.; Ma, X.; Liang, L.; Li, Y.; Zhao, Z.; Mei, Z.; Yang, H.; Li, Q.; et al. Engineering van Der Waals Contacts by Interlayer Dipoles. Nano Lett. 2024, 24, 4408–4414. [Google Scholar] [CrossRef]
- Li, X.; Deng, N.; Wei, Y.; Qiao, Y.; Yang, Y.; Yang, F.; Zhong, G.; Chen, B.; Tian, H.; Ren, T.-L. Roll-to-Roll Graphene Films for Non-Disposable Electrocardiogram Electrodes. J. Phys. D Appl. Phys. 2021, 54, 364003. [Google Scholar] [CrossRef]
- Matsunaga, Y.T.; Morimoto, Y.; Takeuchi, S. Molding Cell Beads for Rapid Construction of Macroscopic 3D Tissue Architecture. Adv. Mater. 2011, 23, H90–H94. [Google Scholar] [CrossRef]
- Zhang, C.; Mcadams, D.A.; Grunlan, J.C. Nano/Micro-Manufacturing of Bioinspired Materials: A Review of Methods to Mimic Natural Structures. Adv. Mater. 2016, 28, 6292–6321. [Google Scholar] [CrossRef]
- Williams, A.G.; Moore, E.; Thomas, A.; Johnson, J.A. Graphene-Based Materials in Dental Applications: Antibacterial, Biocompatible, and Bone Regenerative Properties. Int. J. Biomater. 2023, 2023, 8803283. [Google Scholar] [CrossRef] [PubMed]
- Krishna Saraswat, S.; Ahmed Mustafa, M.; Kamil Ghadir, G.; Kaur, M.; Guamán Lozada, D.F.; Hasen Shuhata Alubiady, M.; Muzahem Al-Ani, A.; Alshahrani, M.Y.; Kadhem Abid, M.; Salih Jumaa, S.; et al. Carbon Quantum Dots: A Comprehensive Review of Green Synthesis, Characterization and Investigation Their Applications in Bioimaging. Inorg. Chem. Commun. 2024, 162, 112279. [Google Scholar] [CrossRef]
- Demirel, E.; Yuksel Durmaz, Y. PEGylated Reduced Graphene Oxide as Nanoplatform for Targeted Gene and Drug Delivery. Eur. Polym. J. 2023, 186, 111841. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Q.; Mei, T.; Zheng, M.; Kumar, R.R.; Yu, B.; Wu, C.; Zhang, H.; An, F. Nanosized Modification Strategies for Improving the Antitumor Efficacy of MEK Inhibitors. Curr. Drug Targets 2020, 21, 228–251. [Google Scholar] [CrossRef] [PubMed]
- Raslan, A.; Saenz del Burgo, L.; Ciriza, J.; Pedraz, J.L. Graphene Oxide and Reduced Graphene Oxide-Based Scaffolds in Regenerative Medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef]
- Xia, Y.; Gao, W.; Gao, C. A Review on Graphene-Based Electromagnetic Functional Materials: Electromagnetic Wave Shielding and Absorption. Adv. Funct. Mater. 2022, 32, 2204591. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of Oxidative Stress in Nanoparticles Toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Kumar, A.; Arya, V.; Rohela, A.; Verma, R.; Nepovimova, E.; Krejcar, O.; Kumar, D.; Thakur, N.; Kuca, K. Phytoantioxidant Functionalized Nanoparticles: A Green Approach to Combat Nanoparticle-Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 3155962. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The Chemistry and Promising Applications of Graphene and Porous Graphene Materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, X.; Hong, H.; Chen, J.; Zhao, Q.; Wu, F. Computational Nanotoxicology Models for Environmental Risk Assessment of Engineered Nanomaterials. Nanomaterials 2024, 14, 155. [Google Scholar] [CrossRef]
- Kundu, A.; Sharma, S.; Pathania, A.R. Engineered Nanomaterials and Human Exposure: An Overview. Mater. Today Proc. 2023, 72, 1216–1223. [Google Scholar] [CrossRef]
- Lai, D.; Chen, Z.; Han, Z.; Wu, Z.-S.; Chen, X. Progress and Roadmap for Graphene Films in Electromagnetic Interference Shielding. Resour. Chem. Mater. 2023, 2, 11–38. [Google Scholar] [CrossRef]
- Döscher, H.; Schmaltz, T.; Neef, C.; Thielmann, A.; Reiss, T. Graphene Roadmap Briefs (No. 2): Industrialization Status and Prospects 2020. 2D Mater. 2021, 8, 022005. [Google Scholar] [CrossRef]
- Laganà, P.; Visalli, G.; Facciolà, A.; Ciarello, M.P.; Laganà, A.; Iannazzo, D.; Di Pietro, A. Is the Antibacterial Activity of Multi-Walled Carbon Nanotubes (MWCNTs) Related to Antibiotic Resistance? An Assessment in Clinical Isolates. Int. J. Environ. Res. Public Health 2021, 18, 9310. [Google Scholar] [CrossRef] [PubMed]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Holban, A.M.; Nicoara, A.I.; Alexandrescu, E.; Somoghi, R.; Ganciarov, M.; Vasilievici, G.; et al. Hybrid Materials Based on Multi-Walled Carbon Nanotubes and Nanoparticles with Antimicrobial Properties. Nanomaterials 2021, 11, 1415. [Google Scholar] [CrossRef]
- Xiao, W.; Li, B.; Yan, J.; Wang, L.; Huang, X.; Gao, J. Three Dimensional Graphene Composites: Preparation, Morphology and Their Multi-Functional Applications. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107335. [Google Scholar] [CrossRef]
- Devi, N.; Kumar, R.; Singh, S.; Singh, R.K. Recent Development of Graphene-Based Composite for Multifunctional Applications: Energy, Environmental and Biomedical Sciences. Crit. Rev. Solid State Mater. Sci. 2024, 49, 72–140. [Google Scholar] [CrossRef]
- Jin, C.R.; Kim, J.Y.; Kim, D.H.; Jeon, M.S.; Choi, Y.-E. In Vivo Monitoring of Intracellular Metabolite in a Microalgal Cell Using an Aptamer/Graphene Oxide Nanosheet Complex. ACS Appl. Bio Mater. 2021, 4, 5080–5089. [Google Scholar] [CrossRef]
- Chergui, S.; Rhili, K.; Poorahong, S.; Siaj, M. Graphene Oxide Membrane Immobilized Aptamer as a Highly Selective Hormone Removal. Membranes 2020, 10, 229. [Google Scholar] [CrossRef]
- Han, X.; Guo, Z. Graphene and Its Derivative Composite Materials with Special Wettability: Potential Application in Oil-Water Separation. Carbon 2021, 172, 647–681. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Emerging Trends in Nanomedicine: Carbon-Based Nanomaterials for Healthcare. Nanomaterials 2024, 14, 1085. https://doi.org/10.3390/nano14131085
Parvin N, Kumar V, Joo SW, Mandal TK. Emerging Trends in Nanomedicine: Carbon-Based Nanomaterials for Healthcare. Nanomaterials. 2024; 14(13):1085. https://doi.org/10.3390/nano14131085
Chicago/Turabian StyleParvin, Nargish, Vineet Kumar, Sang Woo Joo, and Tapas Kumar Mandal. 2024. "Emerging Trends in Nanomedicine: Carbon-Based Nanomaterials for Healthcare" Nanomaterials 14, no. 13: 1085. https://doi.org/10.3390/nano14131085






