Exploring Zinc-Doped Manganese Hexacyanoferrate as Cathode for Aqueous Zinc-Ion Batteries
Abstract
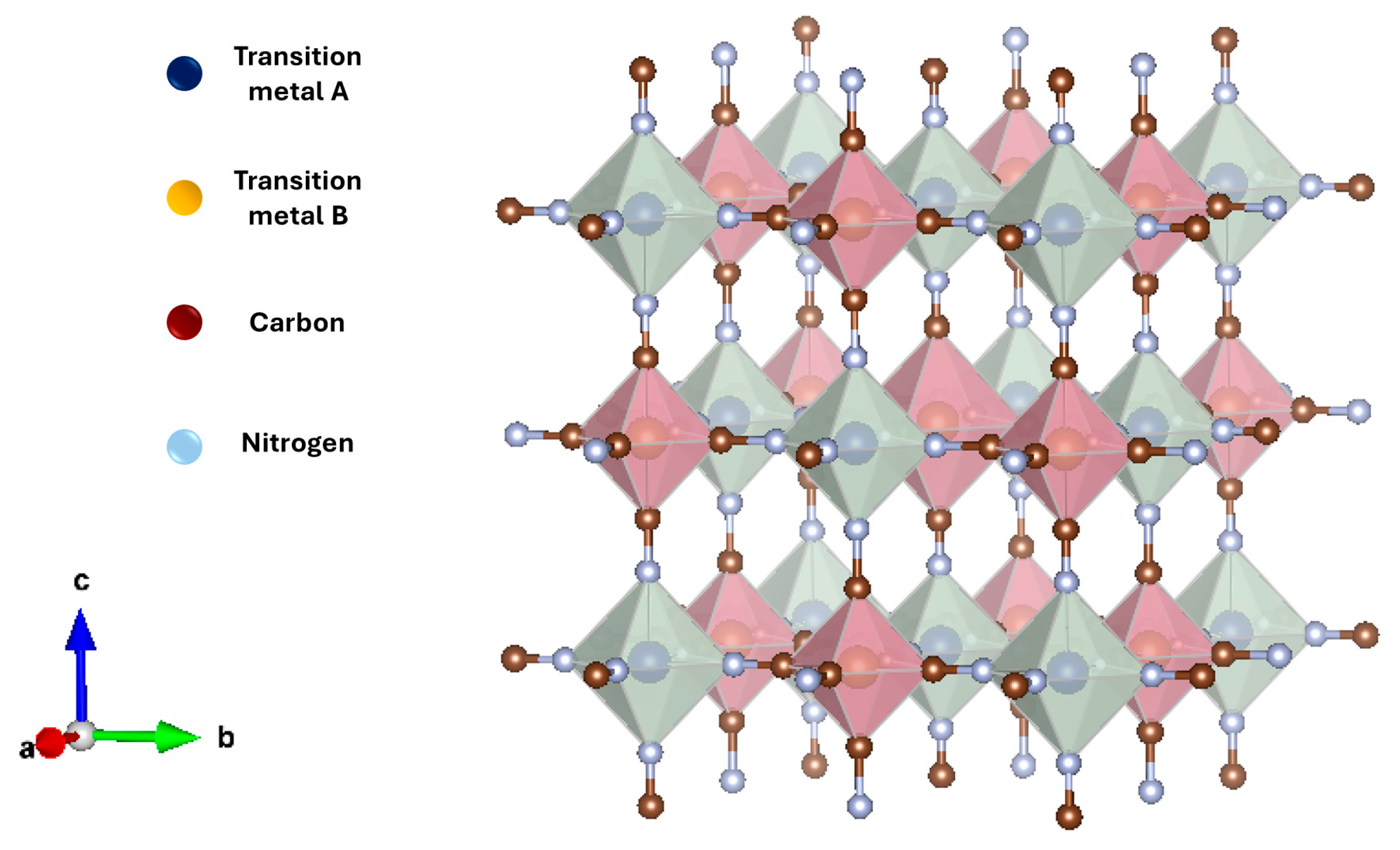
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the PBA Compounds
2.2. Physico-Chemical Characterization of the PBAs
2.3. Preparation of Electrodes
2.4. Electrochemical Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M.A. Renewable Energy and Energy Storage Systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Babuder, M.; Omahen, G.; Bregar, Z. New Age with Renewable Energy Sources. Elektrotechnik Informationstechnik 2013, 130, 241–246. [Google Scholar] [CrossRef]
- Tokar, D.; Foris, D.; Tokar, A.; Foris, T. Sustainable Development of Disadvantaged Regions by Renewable Energy Sources Integration. In Proceedings of the 2019 International Conference ESRD, Jelgava, Letonia, 8 May 2019; pp. 237–244. [Google Scholar]
- Goikolea, E.; Palomares, V.; Wang, S.; de Larramendi, I.R.; Guo, X.; Wang, G.; Rojo, T. Na-Ion Batteries—Approaching Old and New Challenges. Adv. Energy Mater. 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, D.; Lee, M.; Nam, K.W. Challenges and Possibilities for Aqueous Battery Systems. Commun. Mater. 2023, 4, 37. [Google Scholar] [CrossRef]
- Yi, J.; Xia, Y. Advanced Aqueous Batteries: Status and Challenges. MRS Energy Sustain. 2022, 9, 106–128. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Sun, S.; Wen, Y.; Huang, K. Electrode Materials for Practical Rechargeable Aqueous Zn-Ion Batteries: Challenges and Opportunities. ChemElectroChem 2020, 7, 2714–2734. [Google Scholar] [CrossRef]
- Hong, X.; Deng, C.; He, J.; Liang, B.; Wang, G.; Tu, Z. Existing Electrochemical Activation Mechanisms and Related Cathode Materials for Aqueous Zn Ion Batteries. Energy Convers. Manag. 2024, 299, 117906. [Google Scholar] [CrossRef]
- Bani Hashemi, A.; Kasiri, G.; La Mantia, F. The Effect of Polyethyleneimine as an Electrolyte Additive on Zinc Electrodeposition Mechanism in Aqueous Zinc-Ion Batteries. Electrochim. Acta 2017, 258, 703–708. [Google Scholar] [CrossRef]
- Higashi, S.; Lee, S.W.; Lee, J.S.; Takechi, K.; Cui, Y. Avoiding Short Circuits from Zinc Metal Dendrites in Anode by Backside-Plating Configuration. Nat. Commun. 2016, 7, 11801. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Open Challenges and Good Experimental Practices in the Research Field of Aqueous Zn-Ion Batteries. Nat. Commun. 2022, 13, 687. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fu, Y.; Dang, H.; Wang, D.; Ran, F. Optimization Strategies toward Advanced Aqueous Zinc-Ion Batteries: From Facing Key Issues to Viable Solutions. Nano Energy 2023, 116, 108858. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Wu, Y.; Fee, J.; Tobin, Z.; Shirazi-Amin, A.; Kerns, P.; Dissanayake, S.; Mirich, A.; Suib, S.L. Amorphous Manganese Oxides: An Approach for Reversible Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 1627–1633. [Google Scholar] [CrossRef]
- Dai, Q.; Li, L.; Hoang, T.K.A.; Tu, T.; Hu, B.; Jia, Y.; Zhang, M.; Song, L.; Trudeau, M.L. The Secondary Aqueous Zinc-Manganese Battery. J. Energy Storage 2022, 55, 105397. [Google Scholar] [CrossRef]
- Senguttuvan, P.; Han, S.D.; Kim, S.; Lipson, A.L.; Tepavcevic, S.; Fister, T.T.; Bloom, I.D.; Burrell, A.K.; Johnson, C.S. A High Power Rechargeable Nonaqueous Multivalent Zn/V2O5 Battery. Adv. Energy Mater. 2016, 6, 1600826. [Google Scholar] [CrossRef]
- Zhou, A.; Cheng, W.; Wang, W.; Zhao, Q.; Xie, J.; Zhang, W.; Gao, H.; Xue, L.; Li, J. Hexacyanoferrate-Type Prussian Blue Analogs: Principles and Advances Toward High-Performance Sodium and Potassium Ion Batteries. Adv. Energy Mater. 2021, 11, 1–35. [Google Scholar] [CrossRef]
- Paolella, A.; Faure, C.; Timoshevskii, V.; Marras, S.; Bertoni, G.; Guerfi, A.; Vijh, A.; Armand, M.; Zaghib, K. A Review on Hexacyanoferrate-Based Materials for Energy Storage and Smart Windows: Challenges and Perspectives. J. Mater. Chem. A Mater. 2017, 5, 18919–18932. [Google Scholar] [CrossRef]
- Li, W.J.; Han, C.; Cheng, G.; Chou, S.L.; Liu, H.K.; Dou, S.X. Chemical Properties, Structural Properties, and Energy Storage Applications of Prussian Blue Analogues. Small 2019, 15, 1900470. [Google Scholar] [CrossRef]
- Zeng, Y.; Lu, X.F.; Zhang, S.L.; Luan, D.; Li, S.; Lou, X.W. Construction of Co–Mn Prussian Blue Analog Hollow Spheres for Efficient Aqueous Zn-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 22189–22194. [Google Scholar] [CrossRef]
- Xu, C.; Yang, Z.; Zhang, X.; Xia, M.; Yan, H.; Li, J.; Yu, H.; Zhang, L.; Shu, J. Correction to: Prussian Blue Analogues in Aqueous Batteries and Desalination Batteries. Nanomicro Lett. 2021, 13, 187. [Google Scholar] [CrossRef]
- Wang, D.; Lv, H.; Hussain, T.; Yang, Q.; Liang, G.; Zhao, Y.; Ma, L.; Li, Q.; Li, H.; Dong, B.; et al. A Manganese Hexacyanoferrate Framework with Enlarged Ion Tunnels and Two-Species Redox Reaction for Aqueous Al-Ion Batteries. Nano Energy 2021, 84, 105945. [Google Scholar] [CrossRef]
- Cao, T.; Zhang, F.; Chen, M.; Shao, T.; Li, Z.; Xu, Q.; Cheng, D.; Liu, H.; Xia, Y. Cubic Manganese Potassium Hexacyanoferrate Regulated by Controlling of the Water and Defects as a High-Capacity and Stable Cathode Material for Rechargeable Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 26924–26935. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Maisuradze, M.; Sciacca, R.; Hasa, I.; Giorgetti, M. A Structural Perspective on Prussian Blue Analogues for Aqueous Zinc-Ion Batteries. Batter. Supercaps 2023, 6, e202300340. [Google Scholar] [CrossRef]
- Chen, J.; Liao, L.; Sun, B.; Song, X.; Wang, M.; Guo, B.; Ma, Z.; Yu, B.; Li, X. Manganese Hexacyanoferrate Anchoring MnO2 with Enhanced Stability for Aqueous Zinc-Ion Batteries. J. Alloys Compd. 2022, 903, 163833. [Google Scholar] [CrossRef]
- Dai, P.; Ma, W.; Zhou, Y.; Tang, Y.; Cao, X.; Wu, P.; Zhou, H. Superfast Phase Transformation Driven by Dual Chemical Equilibrium Enabling Enhanced Electrochemical Energy Storage. Adv. Funct. Mater. 2024, 2308357, 1–8. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, R.; Zhang, Q.; Ali, U.; Li, Y.; Hao, Y.; Jia, H.; Li, Y.; Zeng, G.; Sun, M.; et al. Ultrafine Manganese Hexacyanoferrate with Low Defects Regulated by Potassium Polyacrylate for High-Performance Aqueous Zn-Ion Batteries. J. Energy Storage 2023, 72, 108535. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Hong, H.; Yang, S.; Zhang, R.; Wang, X.; Jin, X.; Xiong, B.; Bai, S.; Zhi, C. Lean-Water Hydrogel Electrolyte for Zinc Ion Batteries. Nat. Commun. 2023, 14, 3890. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, Y.; Shen, Z.; Wen, Z.; Chen, S.; Hong, G. High-Voltage and Stable Manganese Hexacyanoferrate/Zinc Batteries Using Gel Electrolytes. ACS Appl. Mater. Interfaces 2023, 15, 29032–29041. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Yu, L.; Gao, P.; Liao, X.Z.; Wen, J.; He, Y.S.; Tan, G.; Ren, Y.; Ma, Z.F. Highly Crystalline Sodium Manganese Ferrocyanide Microcubes for Advanced Sodium Ion Battery Cathodes. J. Mater. Chem. A Mater. 2019, 7, 22248–22256. [Google Scholar] [CrossRef]
- Duan, C.; Meng, Y.; Wang, Y.; Zhang, Z.; Ge, Y.; Li, X.; Guo, Y.; Xiao, D. High-Crystallinity and High-Rate Prussian Blue Analogues Synthesized at the Oil–Water Interface. Inorg. Chem. Front. 2021, 8, 2008–2016. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Wang, X.; Bahlawane, N.; Pan, H.; Yan, M.; Jiang, Y. Prussian Blue Analogs for Rechargeable Batteries. iScience 2018, 3, 110–133. [Google Scholar] [CrossRef]
- Jung, S.M.; Lim, Y.J.; Kwon, J.; Wei, K.; Lee, J.; Kim, K.S.; Kang, S.G.; Kim, Y.T. Low-Hysteresis Manganese Hexacyanoferrate (MnHCF) Aqueous Battery for Low-Grade Thermal Energy Harvesting. J. Power Sources 2022, 524, 231080. [Google Scholar] [CrossRef]
- Ju, H.; Lang, H.; Yi, T.; Tian, K.; Yue, J.; Hu, L.; Zhao, L.; Liu, S.; Kong, D. Solid-State Synthesis of Diminutively Granular and Highly Crystalline Manganese Hexacyanoferrate for a Supercapacitor Electrode. CrystEngComm 2024, 26, 1133–1140. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B.; Zhou, F.C.; Sun, Y.H.; Li, J.Q.; Nan, J.M.; Wang, X.J.; Krumeich, F.; et al. Prussian Blue: A New Framework of Electrode Materials for Sodium Batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Krumeich, F.; Nesper, R. Nanocomposite of Manganese Ferrocyanide and Graphene: A Promising Cathode Material for Rechargeable Lithium Ion Batteries. Electrochem. Commun 2013, 34, 246–249. [Google Scholar] [CrossRef]
- Zhou, F.C.; Sun, Y.H.; Li, J.Q.; Nan, J.M. K 1−x Mn 1+x/2 [Fe(CN) 6]·yH 2 O Prussian Blue Analogues as an Anode Material for Lithium-Ion Batteries. Appl. Surf. Sci. 2018, 444, 650–660. [Google Scholar] [CrossRef]
- Heo, J.W.; Chae, M.S.; Hyoung, J.; Hong, S.T. Rhombohedral Potassium-Zinc Hexacyanoferrate as a Cathode Material for Nonaqueous Potassium-Ion Batteries. Inorg. Chem. 2019, 58, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards High-Voltage Aqueous Metal-Ion Batteries beyond 1.5 V: The Zinc/Zinc Hexacyanoferrate System. Adv. Energy Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Morphology-Dependent Electrochemical Performance of Zinc Hexacyanoferrate Cathode for Zinc-Ion Battery. Sci. Rep. 2015, 5, 18263. [Google Scholar] [CrossRef]
- Lee, S.H.; Huh, Y.D. Preferential Evolution of Prussian Blue’s Morphology from Cube to Hexapod. Bull. Korean Chem. Soc. 2012, 33, 1078–1080. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2000; ISBN 9780470027318. [Google Scholar]
- Lejeune, J.; Brubach, J.B.; Roy, P.; Bleuzen, A. Application of the Infrared Spectroscopy to the Structural Study of Prussian Blue Analogues. Comptes Rendus Chim. 2014, 17, 534–540. [Google Scholar] [CrossRef]
- Fang, Z.; Yin, Y.; Qiu, X.; Zhu, L.; Dong, X.; Wang, Y.; Xia, Y. Prussian Blue Cathode with Intercalation Pseudocapacitive Behavior for Low-Temperature Batteries. Adv. Energy Sustain. Res. 2021, 2, 2100105. [Google Scholar] [CrossRef]
- Gerber, S.J.; Erasmus, E. Electronic Effects of Metal Hexacyanoferrates: An XPS and FTIR Study. Mater. Chem. Phys. 2018, 203, 73–81. [Google Scholar] [CrossRef]
- Ojwang, D.O.; Grins, J.; Wardecki, D.; Valvo, M.; Renman, V.; Häggström, L.; Ericsson, T.; Gustafsson, T.; Mahmoud, A.; Hermann, R.P.; et al. Structure Characterization and Properties of K-Containing Copper Hexacyanoferrate. Inorg. Chem. 2016, 55, 5924–5934. [Google Scholar] [CrossRef] [PubMed]
- Sato, O.; Einaga, Y.; Fujishima, A.; Hashimoto, K. Photoinduced Long-Range Magnetic Ordering of a Cobalt—Iron Cyanide. Inorg. Chem. 1999, 38, 4405–4412. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Tang, Y.; Xu, B.B.; Liang, C.; Yan, M.; Jiang, Y. Manganese Hexacyanoferrate Reinforced by PEDOT Coating towards High-Rate and Long-Life Sodium-Ion Battery Cathode. J. Mater. Chem. A Mater. 2020, 8, 3222–3227. [Google Scholar] [CrossRef]
- Mandal, M.; Chattopadhyay, K.; Bhattacharya, S.K. Sunlight Mediated Photocatalytic Reduction of Aqueous Cr(Vl) Using Metal Hexacyanoferrate (M = Mn, Ni, Cu and Zn). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1080, 012047. [Google Scholar] [CrossRef]
- Deng, L.; Qu, J.; Niu, X.; Liu, J.; Zhang, J.; Hong, Y.; Feng, M.; Wang, J.; Hu, M.; Zeng, L.; et al. Defect-Free Potassium Manganese Hexacyanoferrate Cathode Material for High-Performance Potassium-Ion Batteries. Nat. Commun. 2021, 12, 2167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, L.; Feng, M.; Zeng, L.; Hu, M.; Zhu, Y. Low-Defect K2Mn[Fe(CN)6]-Reduced Graphene Oxide Composite for High-Performance Potassium-Ion Batteries. Chem. Commun. 2021, 57, 8632–8635. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Reguera, E.; Lima, E.; Balmaseda, J.; Martínez-García, R.; Yee-Madeira, H. An Atypical Coordination in Hexacyanometallates: Structure and Properties of Hexagonal Zinc Phases. J. Phys. Chem. Solids 2007, 68, 1630–1642. [Google Scholar] [CrossRef]
- Balmaseda, J.; Reguera, E.; Rodríguez-Hernández, J.; Reguera, L.; Autie, M. Behavior of Transition Metals Ferricyanides as Microporous Materials. Microporous Mesoporous Mater. 2006, 96, 222–236. [Google Scholar] [CrossRef]
- Silva, M.N.T.; Ardisson, J.D.; Fabrisa, J.D.; Nossol, E. Zinc Hexacyanoferrate/Multi-Walled Carbon Nanotubes Films for Rechargeable Aqueous Batteries. J. Braz. Chem. Soc. 2020, 31, 1787–1795. [Google Scholar] [CrossRef]
- Sciacca, R.; Zamponi, S.; Berrettoni, M.; Giorgetti, M. Stable Films of Zinc-Hexacyanoferrate: Electrochemistry and Ion Insertion Capabilities. J. Solid. State Electrochem. 2022, 26, 63–72. [Google Scholar] [CrossRef]
- Duan, J.; Min, L.; Yang, T.; Chen, M.; Wang, C. High-Performance and Dendrite-Free Zn/KZnHCF Batteries Boosted by Aqueous Electrolyte Mixed with Ethanol. J. Alloys Compd. 2022, 918, 165619. [Google Scholar] [CrossRef]
- González-Meza, O.A.; Larios-Durán, E.R.; Gutiérrez-Becerra, A.; Casillas, N.; Escalante, J.I.; Bárcena-Soto, M. Development of a Randles-Ševčík-like Equation to Predict the Peak Current of Cyclic Voltammetry for Solid Metal Hexacyanoferrates. J. Solid. State Electrochem. 2019, 23, 3123–3133. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Zhang, X.; Xia, M.; Yang, Z.; Yan, H.; Yu, H.; Zhang, L.; Shu, W.; Shu, J. Sodium Manganese Hexacyanoferrate as Zn Ion Host toward Aqueous Energy Storage. J. Electroanal. Chem. 2021, 881, 114968. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhang, R.; Zhong, Y.; Wu, Z.; Wang, X.; Zhang, Z. Recent Progress of Manganese-Based Prussian Blue Analogue Cathode Materials for Sodium-Ion Batteries. Ionics 2024, 30, 39–59. [Google Scholar] [CrossRef]
- Li, M.; Sciacca, R.; Maisuradze, M.; Aquilanti, G.; Plaisier, J.; Berrettoni, M.; Giorgetti, M. Electrochemical Performance of Manganese Hexacyanoferrate Cathode Material in Aqueous Zn-Ion Battery. Electrochim. Acta 2021, 400, 139414. [Google Scholar] [CrossRef]
- Maisuradze, M.; Li, M.; Gaboardi, M.; Aquilanti, G.; Rikkert Plaisier, J.; Giorgetti, M. Structural Modification of Partially Ni-Substituted MnHCF Cathode Material for Aqueous Zn-Ion Batteries. Adv. Mater. Sci. Technol. 2024, 6, 0629615. [Google Scholar] [CrossRef]
- Kim, J.; Yi, S.H.; Li, L.; Chun, S.E. Enhanced Stability and Rate Performance of Zinc-Doped Cobalt Hexacyanoferrate (CoZnHCF) by the Limited Crystal Growth and Reduced Distortion. J. Energy Chem. 2022, 69, 649–658. [Google Scholar] [CrossRef]
- Trócoli, R.; La Mantia, F. An Aqueous Zinc-Ion Battery Based on Copper Hexacyanoferrate. ChemSusChem 2015, 8, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, Z.; Ye, Y.; Zhou, Z.; Li, Y.; Zhang, M.; Yuan, X.; Hu, J.; Zhao, W.; Huang, Z.; et al. Zn2+ Induced Phase Transformation of K2MnFe(CN)6 Boosts Highly Stable Zinc-Ion Storage. Adv. Energy Mater. 2021, 11, 2003639. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, H.; Miao, C.; Zhang, Y.; Chen, D.; Li, G.; Han, W. Hydroxylation Strategy Unlocking Multi-Redox Reaction of Manganese Hexacyanoferrate for Aqueous Zinc-Ion Battery. Chem. Eng. J. 2023, 457, 141323. [Google Scholar] [CrossRef]
- Ni, G.; Xu, X.; Hao, Z.; Wang, W.; Li, C.; Yang, Y.; Zhou, C.; Qin, L.; Chen, W.; Yao, X.; et al. Tuning the Electrochemical Stability of Zinc Hexacyanoferrate through Manganese Substitution for Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 602–610. [Google Scholar] [CrossRef]
- Li, G.; Sun, L.; Zhang, S.; Zhang, C.; Jin, H.; Davey, K.; Liang, G.; Liu, S.; Mao, J.; Guo, Z. Developing Cathode Materials for Aqueous Zinc Ion Batteries: Challenges and Practical Prospects. Adv. Funct. Mater. 2024, 34, 2301291. [Google Scholar] [CrossRef]






| Sample | ZnSO4 (mol) | Mn(NO3)2 (mol) | K3Fe(CN)6 (mol) | Theoretical Ratio (Zn/Mn) |
|---|---|---|---|---|
| Zn100 | 0.002 | - | 0.002 | 100:0 |
| Zn75Mn25 | 0.0015 | 0.0005 | 0.002 | 75:25 |
| Zn50Mn50 | 0.001 | 0.001 | 0.002 | 50:50 |
| Zn25Mn75 | 0.0005 | 0.0015 | 0.002 | 25:75 |
| Mn100 | - | 0.002 | 0.002 | 0:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beitia, J.; Ahedo, I.; Paredes, J.I.; Goikolea, E.; Ruiz de Larramendi, I. Exploring Zinc-Doped Manganese Hexacyanoferrate as Cathode for Aqueous Zinc-Ion Batteries. Nanomaterials 2024, 14, 1092. https://doi.org/10.3390/nano14131092
Beitia J, Ahedo I, Paredes JI, Goikolea E, Ruiz de Larramendi I. Exploring Zinc-Doped Manganese Hexacyanoferrate as Cathode for Aqueous Zinc-Ion Batteries. Nanomaterials. 2024; 14(13):1092. https://doi.org/10.3390/nano14131092
Chicago/Turabian StyleBeitia, Julen, Isabel Ahedo, Juan Ignacio Paredes, Eider Goikolea, and Idoia Ruiz de Larramendi. 2024. "Exploring Zinc-Doped Manganese Hexacyanoferrate as Cathode for Aqueous Zinc-Ion Batteries" Nanomaterials 14, no. 13: 1092. https://doi.org/10.3390/nano14131092






