Bimetallic PtAu-Decorated SnO2 Nanospheres Exhibiting Enhanced Gas Sensitivity for Ppb-Level Acetone Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Precursors
2.2. Preparation of SnO2 Nanospheres
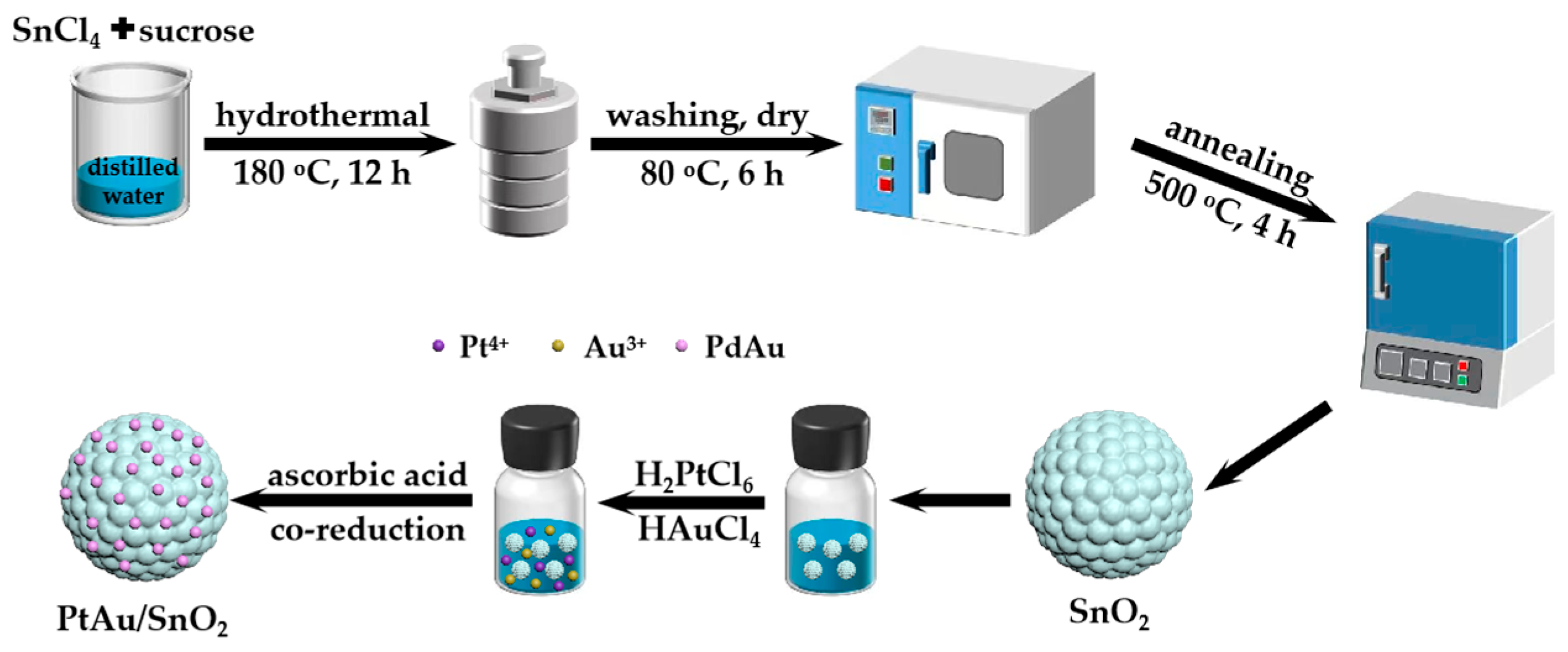
2.3. Synthesis of PtAu/SnO2 Nanospheres
2.4. Material Characterization
2.5. Gas Sensitivity Experiments
3. Results and Discussion
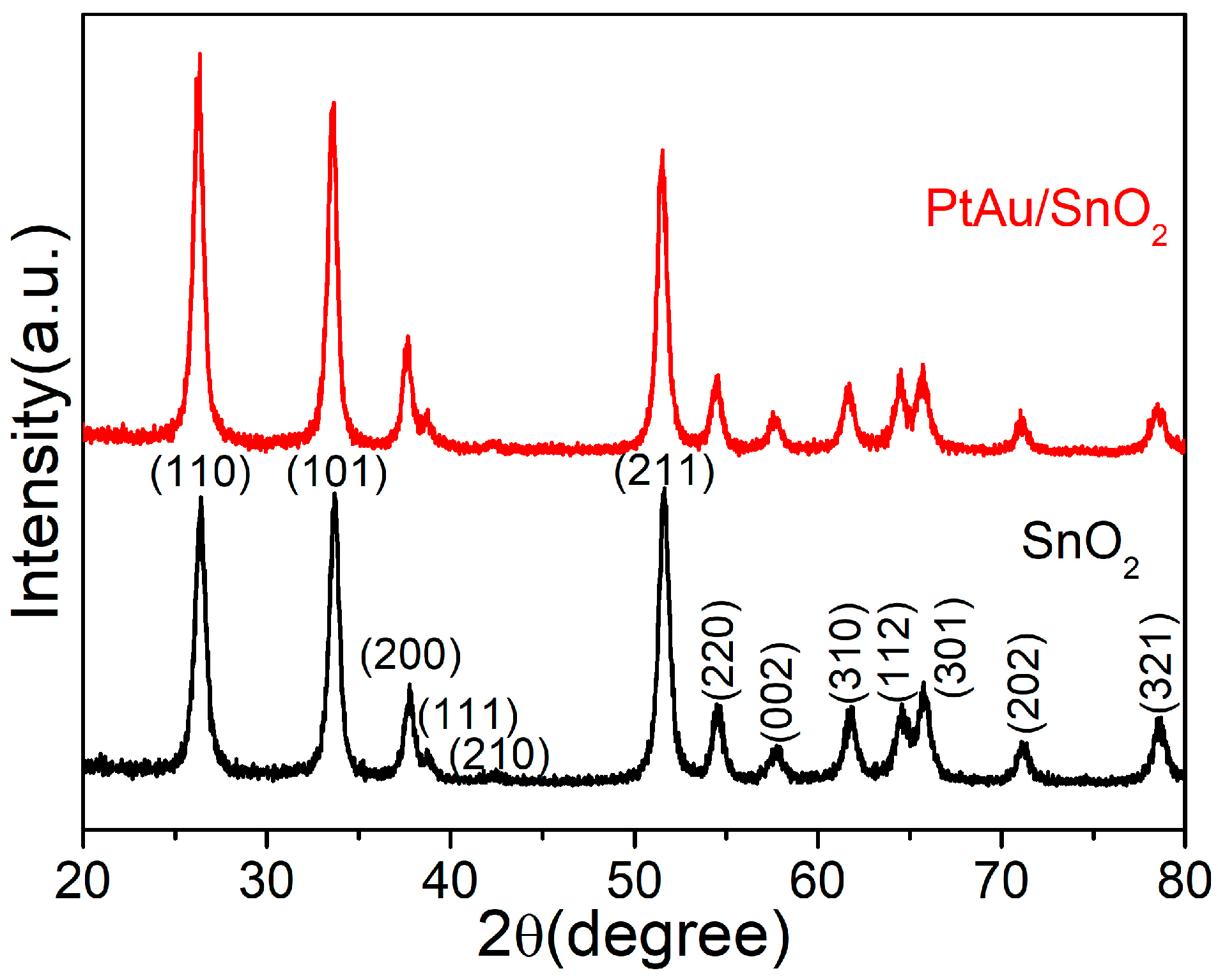
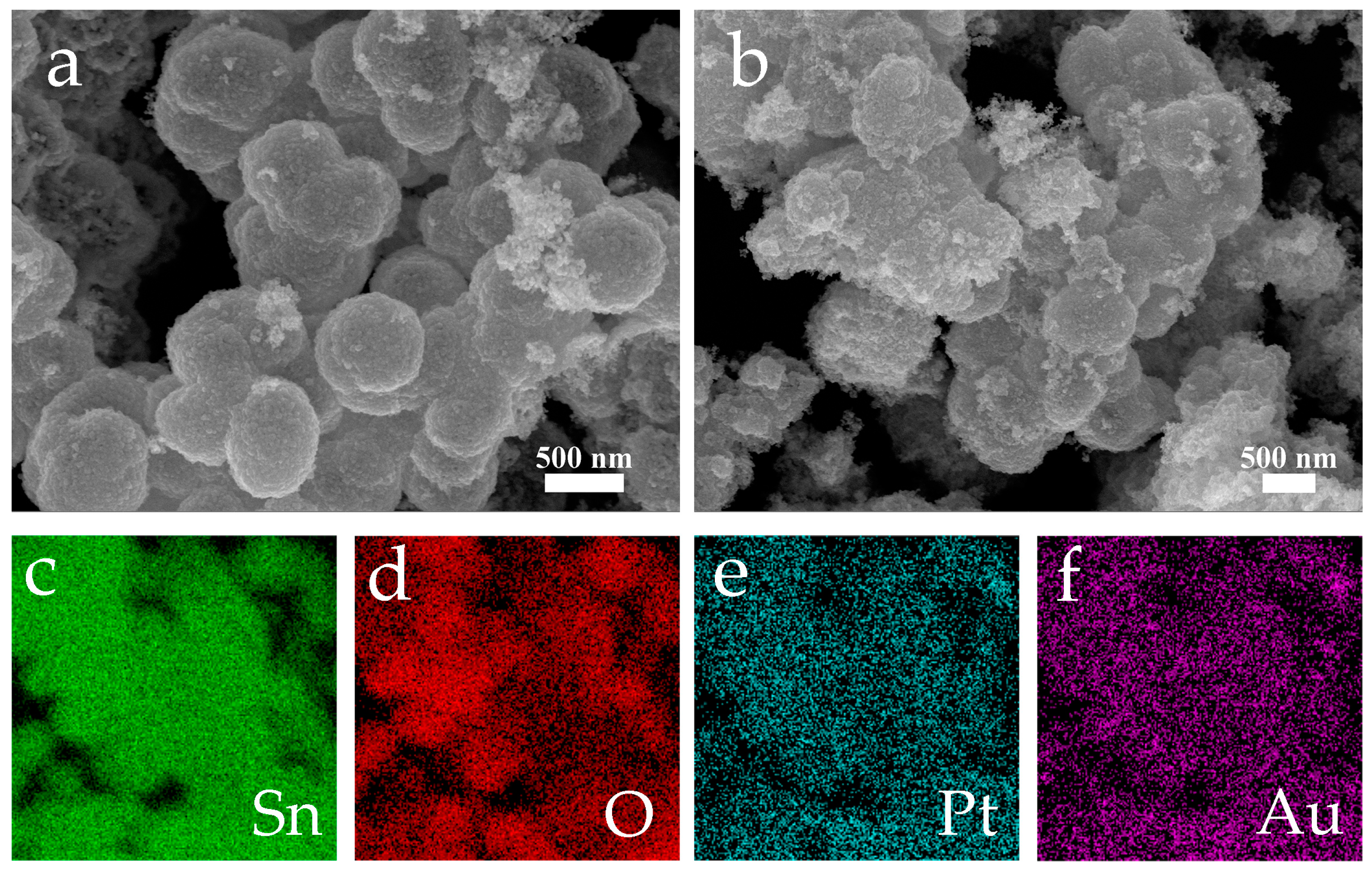
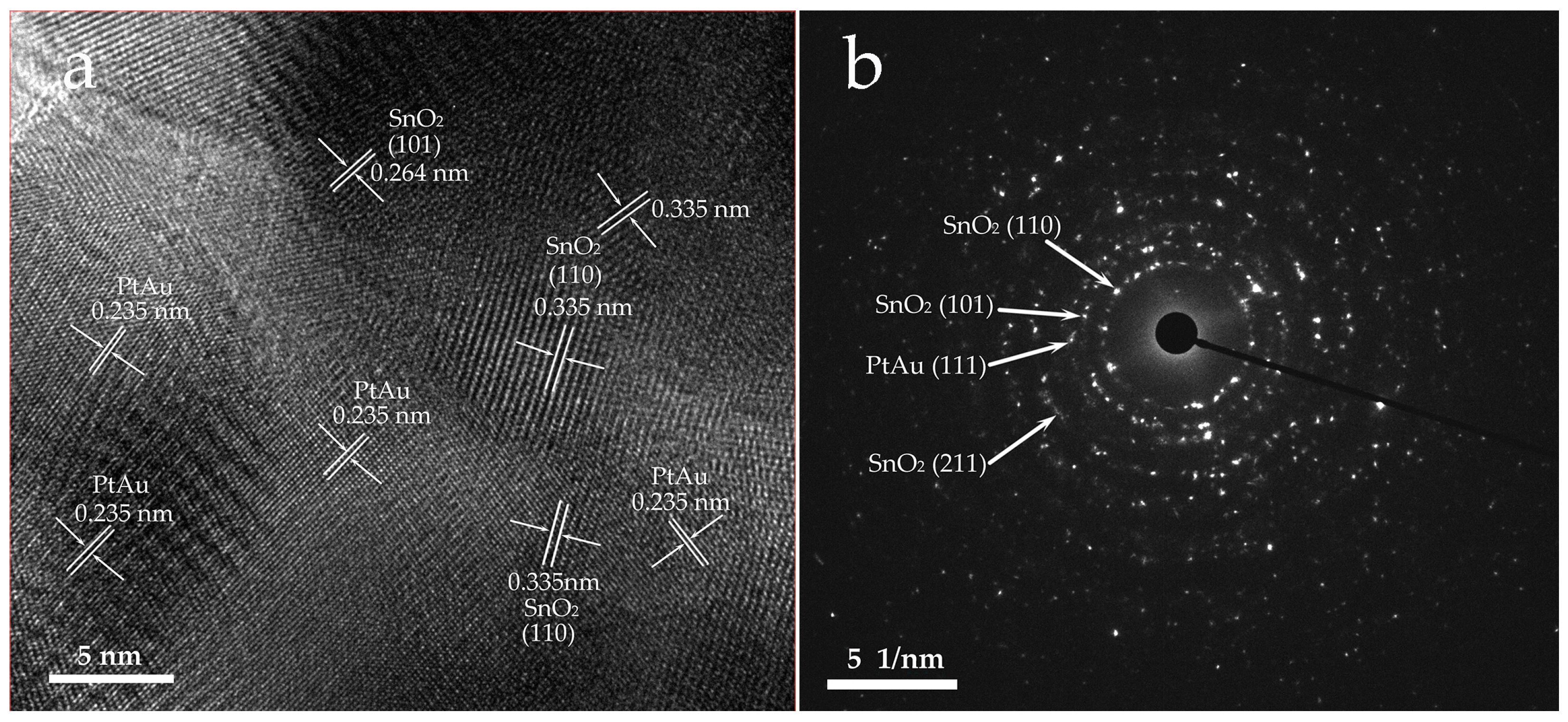
3.1. Material Characterisation
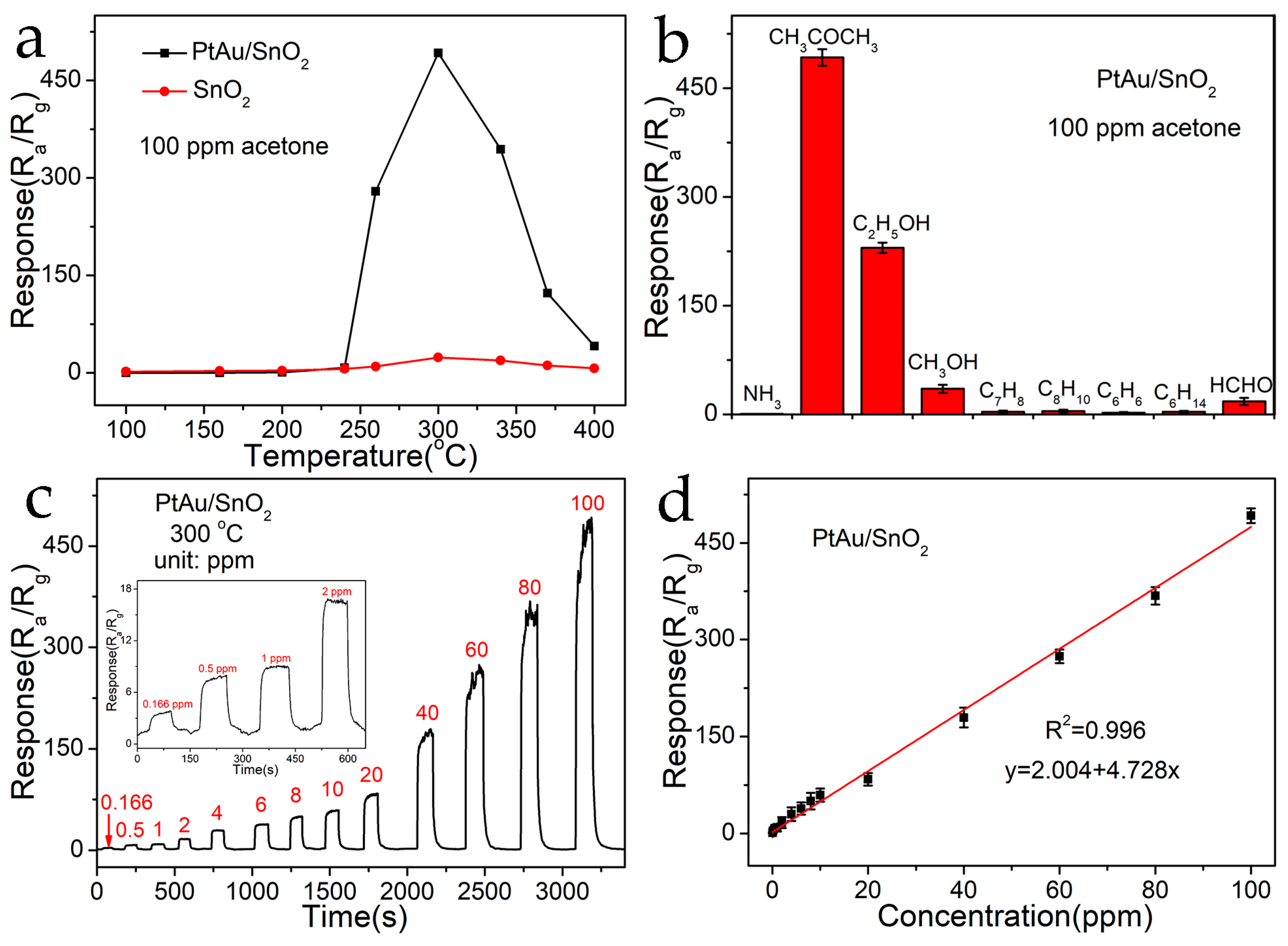
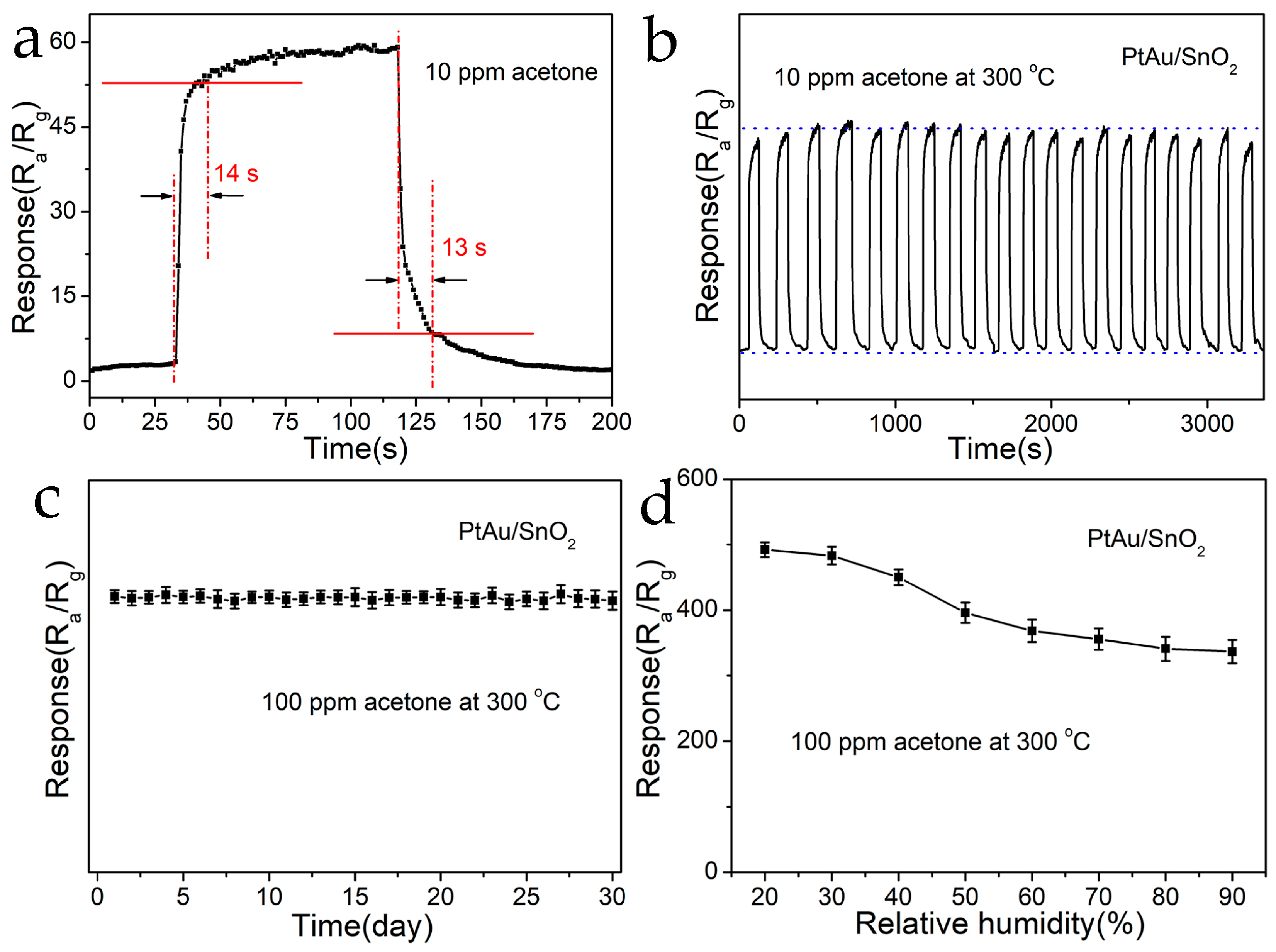
3.2. Gas-Sensing Performance
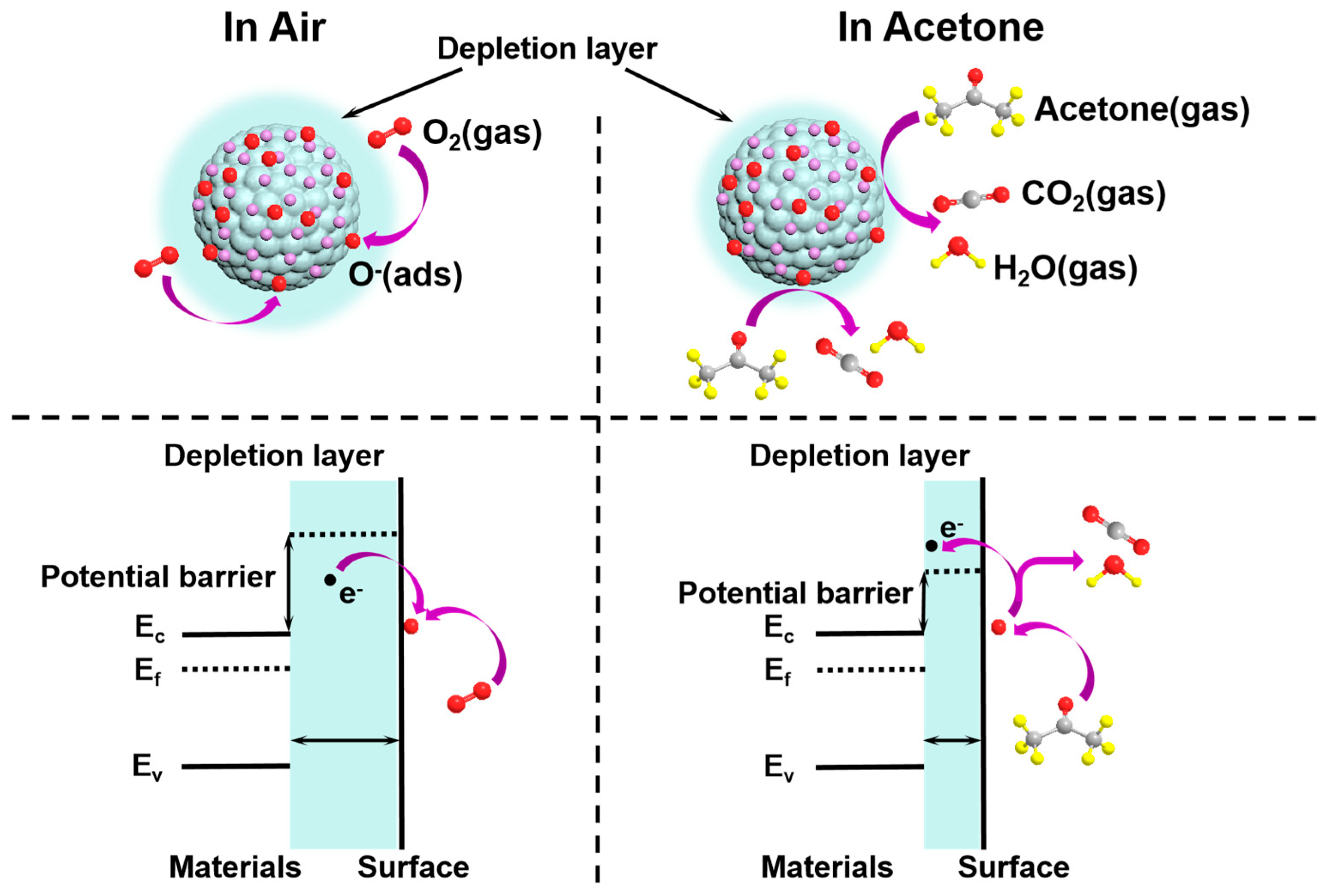
3.3. Gas-Sensing Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Shen, W.; Lv, D.; Yin, J.; Liang, T.; Song, W. Gas-sensing technology for human breath detection. Prog. Chem. 2023, 35, 302–317. [Google Scholar]
- Lee, S.; Choi, S.; Park, S.H.; Cho, S.H.; Sohn, W.; Eom, T.H.; Kim, Y.; Jang, H.W. Synthesis-in-place hydrothermal growth of hematite nanorods on patterned substrate for highly sensitive and rapid acetone detection. Sens. Actuators B Chem. 2023, 395, 134519. [Google Scholar] [CrossRef]
- Alizadeh, N.; Jamalabadi, H.; Tavoli, F. Breath acetone sensors as non-invasive health monitoring systems: A Review. IEEE Sens. J. 2020, 20, 5–31. [Google Scholar] [CrossRef]
- Feng, G.; Che, Y.; Wang, S.; Wang, S.; Hu, J.; Xiao, J.; Song, C.; Jiang, L. Sensitivity enhancement of In2O3/ZrO2 composite based acetone gas sensor: A promising collaborative approach of ZrO2 as the heterojunction and dopant for in-situ grown octahedron–like particles. Sens. Actuators B Chem. 2022, 367, 132087. [Google Scholar] [CrossRef]
- Ma, S.; Xu, J. Nanostructured metal oxide heterojunctions for chemiresistive gas sensors. J. Mater. Chem. A 2023, 11, 23742. [Google Scholar] [CrossRef]
- Li, F.; Jing, J.; Li, J.; Li, S.; Ye, S.; Song, X.; Zhan, Z.; Zhang, Y. Fabrication of ZnO–SnO2 heterojunction inverse opal photonic balls for chemiresistive acetone sensing. Sens. Actuators B Chem. 2024, 400, 134887. [Google Scholar] [CrossRef]
- Mondal, B.; Gogoi, P.K. Nanoscale heterostructured materials based on metal oxides for a chemiresistive gas sensor. ACS Appl. Electron. Mater. 2022, 4, 59–86. [Google Scholar] [CrossRef]
- Walker, J.; Karnati, P.; Akbar, S.A.; Morris, P.A. Selectivity mechanisms in resistive–type metal oxide heterostructural gas sensors. Sens. Actuators B Chem. 2022, 355, 131242. [Google Scholar] [CrossRef]
- Kong, L.; Yuan, Z.; Gao, H.; Meng, F. Recent progress of gas sensors based on metal oxide composites derived from bimetallic metal-organic frameworks. TrAC Trends Anal. Chem. 2023, 166, 117199. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Tang, K.; Wang, H.; Chen, T.; Jiang, K.; Zhou, T.; Quan, H.; Guo, R. Ultralow detection limit and ultrafast response/recovery of the H2 gas sensor based on Pd–doped rGO/ZnO–SnO2 from hydrothermal synthesis. Microsyst. Nanoeng. 2022, 8, 67. [Google Scholar] [CrossRef]
- Xiang, C.; Chen, T.; Zhao, Y.; Sun, J.; Jiang, K.; Li, Y.; Zhu, X.; Zhang, X.; Zhang, N.; Guo, R. Facile hydrothermal synthesis of SnO2 nanoflowers for low–concentration formaldehyde detection. Nanomaterials 2022, 12, 2133. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y. Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuators B Chem. 2022, 364, 131876. [Google Scholar] [CrossRef]
- Zhu, L.; Ou, L.; Mao, L.; Wu, X.; Liu, Y.; Lu, H. Advances in noble metal-decorated metal oxide nanomaterials for chemiresistive gas sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Bahuguna, G.; Singh, S.; Kumari, A.; Ghosh, D.; Haick, H.; Gupta, R. Porous SnO2 nanosheets for room temperature ammonia sensing in extreme humidity. Mater. Horiz. 2024, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Bhardwaj, M.; Kumar, S.; Lawaniya, S.D.; Kumar, M.; Dwivedi, P.K.; Awasthi, K. Synergistic effects of Pd–Ag decoration on SnO/SnO2 nanosheets for enhanced hydrogen sensing. Sens. Actuators B Chem. 2024, 402, 135062. [Google Scholar] [CrossRef]
- Lei, B.; Zhang, H.; Liu, W.; Zhao, Q.; Wei, Y.; Lu, Y.; Yang, X.; Zhang, W.; Xiao, T.; Kong, J.; et al. Micro–heater embedded Ni-SnO2 ordered nanoporous films: On–chip fabrication for fast, sensitive, and selective gas sensing towards multiple VOCs for air quality monitoring. Sens. Actuators B Chem. 2024, 401, 134907. [Google Scholar] [CrossRef]
- Fan, G.; Huo, F.; Guan, J.; Yu, H.; Zhu, Q.; Han, N.; Mo, J.; Chen, Y. MnO2 enhanced low temperature HCHO sensing performance of SnO2. Sens. Actuators B Chem. 2024, 412, 135803. [Google Scholar] [CrossRef]
- Li, G.; Cheng, Z.; Xiang, Q.; Yan, L.; Wang, X.; Xu, J. Bimetal PdAu decorated SnO2 nanosheets based gas sensor with temperature-dependent dual selectivity for detecting formaldehyde and acetone. Sens. Actuators B Chem. 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Zhu, Y.; Meng, X.; Wang, X.; Gao, W. Low detection based on PdPt/In2O3 nanospheres for rapid hydrogen detection. Sens. Actuators B Chem. 2024, 410, 135654. [Google Scholar] [CrossRef]
- Luo, N.; Chen, Y.; Zhang, D.; Guo, M.; Xue, Z.; Wang, X.; Cheng, Z.; Xu, J. High–sensitive MEMS hydrogen sulfide sensor made from PdRh bimetal hollow nanoframe decorated metal oxides and sensitization mechanism study. ACS Appl. Mater. Interfaces 2020, 12, 56203–56215. [Google Scholar] [CrossRef]
- Feng, D.; Zhu, Z.; Du, L.; Xing, X.; Wang, C.; Chen, J.; Tian, Y.; Yang, D. Improved sensing performance of WO3 nanoparticles decorated with Ag and Pt nanoparticles. Rare Met. 2021, 40, 1642–1650. [Google Scholar] [CrossRef]
- Jamnani, S.R.; Moghaddam, H.M.; Leonardi, S.G.; Neri, G.; Ferlazzo, A. VOCs sensing properties of samarium oxide nanorods. Ceram. Int. 2024, 50, 403–411. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, M.; Liu, S.; Hassan, M.; Zhang, X.; Lei, S.; Qiao, G.; Liu, G. One-pot hydrothermal synthesis of self-assembled MoS2/WS2 nanoflowers for chemiresistive room-temperature NO2 sensors. Sens. Actuators B Chem. 2024, 403, 135215. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, W.; Si, X.; Guo, J.; Huo, J.; Zhang, Z.; Cheng, G.; Du, Z. In situ assembly of one–dimensional Pt@ZnO nanofibers driven by a ZIF–8 framework for achieving a high–performance acetone sensor. Nanoscale 2023, 15, 17206. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Kim, S.J.; Choi, S.J.; Kim, N.H.; Hakim, M.; Rothschild, A.; Kim, I.D. Thin–walled SnO2 nanotubes functionalized with Pt and Au catalysts via the protein templating route and their selective detection of acetone and hydrogen sulfide molecules. Nanoscale 2015, 7, 16417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yang, L.; Liu, Z.; Li, Y.; Bai, J.; Liu, F.; Liang, X.; Sun, P.; Lu, G. Fast detection of ppm n–pentanol by PtAu alloy nanocrystals decorated flower-like WO3. Sens. Actuators B Chem. 2022, 371, 132623. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, Y.; Jin, R.; Wang, T.; Liu, F.; Wang, C.; Yan, X.; Sun, P.; Lu, G. Gas sensor based on cobalt–doped 3D inverse opal SnO2 for air quality monitoring. Sens. Actuators B Chem. 2022, 350, 130807. [Google Scholar] [CrossRef]
- Kim, K.; Choi, P.G.; Itoh, T.; Masuda, Y. Catalyst–free highly sensitive SnO2 nanosheet gas sensors for parts per billion–level detection of acetone. ACS Appl. Mater. Interfaces 2020, 12, 51637–51644. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y. Ultrasensitive and low detection limit of acetone gas sensor based on ZnO/SnO2 thick films. RSC Adv. 2020, 10, 35958. [Google Scholar] [CrossRef]
- Wang, W.; Xian, J.; Li, J.; Yu, M.; Duan, Q.; Leung, C.M.; Zeng, M.; Gao, X. Construction of Co3O4/SnO2 yolk–shell nanofibers for acetone gas detection. Sens. Actuators B Chem. 2024, 398, 134724. [Google Scholar] [CrossRef]
- Guo, W.; Luo, R.; Liu, F.; Wang, X. Enhanced acetone gas sensing performance of Zn2SnO4/SnO2 hierarchical stack structure. Mater. Lett. 2023, 352, 135176. [Google Scholar] [CrossRef]
- Bai, J.; Wang, C.; Liu, K.; Wang, H.; Liu, Y.; Liu, F.; Suo, H.; Liang, X.; Zhang, C.; Liu, F.; et al. Ehnahced gas sensing performance based on the PtCu octahedral alloy nanocrystals decorated SnO2 nanoclusters. Sens. Actuators B Chem. 2021, 330, 129375. [Google Scholar] [CrossRef]
- Shao, S.; Chen, X.; Chen, Y.; Lai, M.; Che, L. Ultrasensitive and highly selective detection of acetone based on Au@WO3–SnO2 corrugated nanofibers. Appl. Surf. Sci. 2019, 473, 902–911. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Koo, W.T.; Jang, J.S.; Kim, D.H.; Cho, H.J.; Kim, I.D. Chitosan–templated Pt nanocatalyst loaded mesoporous SnO2 nanofibers: A superior chemiresistor toward acetone molecules. Nanoscale 2018, 10, 13713. [Google Scholar] [CrossRef]
- Sui, N.; Wei, X.; Cao, S.; Zhang, P.; Zhou, T.; Zhang, T. Nanoscale bimetallic AuPt–functionalized metal oxide chemiresistors: Ppb–level and selective detection for ozone and acetone. ACS Sens. 2022, 7, 2178–2187. [Google Scholar] [CrossRef]

| Materials | Morphology | Temp. (°C) | Conc. (ppm) | Res. | Res./Rec. Time (s/s) | DL (ppb) | Ref. |
|---|---|---|---|---|---|---|---|
| SnO2 | Nanosheets | 280 | 1 | 10.4 | 40/610 | 200 | [28] |
| ZnO/SnO2 | 3D inverse opal photonic crystal balls | 260 | 50 | 40.3 | 6/10 | 100 | [6] |
| ZnO/SnO2 | Thick films | 180 | 0.5 | 3.36 | 57/63 | 10 | [29] |
| Co3O4/SnO2 | Yolk-shell nanofibers | 350 | 100 | 217 | 0.62/46.5 | 100 | [30] |
| Zn2SnO4/SnO2 | Hierarchical stack structure | 275 | 30 | 33 | 13/66 | 64.25 | [31] |
| Pt40Cu60/SnO2 | Octahedral alloy nanocrystal-decorated nanoclusters | 240 | 5 | 22.04 | 1.5/58 | 20 | [32] |
| PdAu/SnO2 | 3D nanosheets | 250 | 2 | 6.5 | 5/4 | 45 | [18] |
| Au/WO3-SnO2 | Corrugated nanofibers | 150 | 0.5 | 79.6 | — | — | [33] |
| Chitosan-Pt/SnO2 | Mesoporous nanofibers | 350 | 1 | 38.4 | 12/44 | 5 | [34] |
| PtAu/SnO2 | Nanospheres | 300 | 10 | 59.4 | 14/13 | 158 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Cao, P.; Li, P.; Yu, Y.; Guo, R.; Li, Y.; Yang, H. Bimetallic PtAu-Decorated SnO2 Nanospheres Exhibiting Enhanced Gas Sensitivity for Ppb-Level Acetone Detection. Nanomaterials 2024, 14, 1097. https://doi.org/10.3390/nano14131097
Zhu X, Cao P, Li P, Yu Y, Guo R, Li Y, Yang H. Bimetallic PtAu-Decorated SnO2 Nanospheres Exhibiting Enhanced Gas Sensitivity for Ppb-Level Acetone Detection. Nanomaterials. 2024; 14(13):1097. https://doi.org/10.3390/nano14131097
Chicago/Turabian StyleZhu, Xiaofeng, Pei Cao, Peng Li, Yue Yu, Ruihua Guo, Yongzhen Li, and Hui Yang. 2024. "Bimetallic PtAu-Decorated SnO2 Nanospheres Exhibiting Enhanced Gas Sensitivity for Ppb-Level Acetone Detection" Nanomaterials 14, no. 13: 1097. https://doi.org/10.3390/nano14131097




