URJC-1: Stable and Efficient Catalyst for O-Arylation Cross-Coupling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Organic Linker
2.3. Synthesis of MOF Materials
2.4. Physicochemical Characterization Techniques
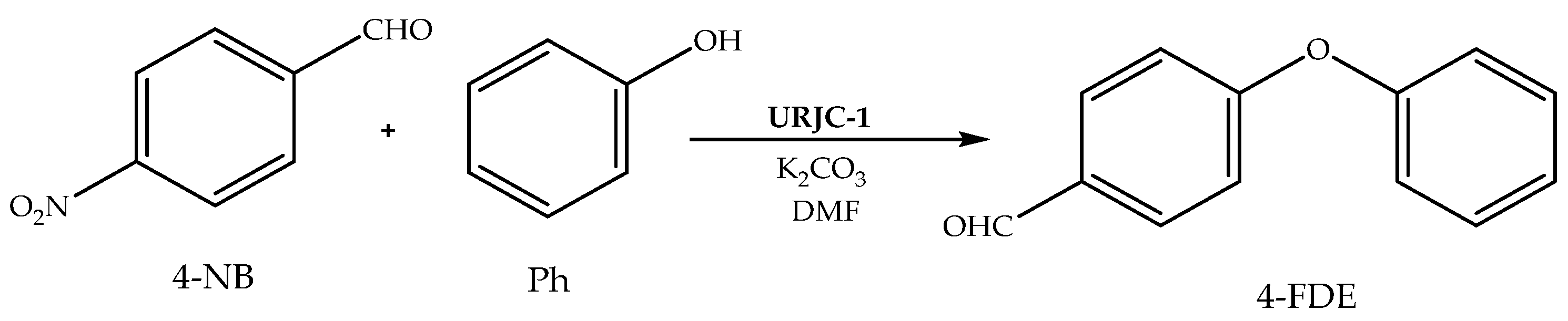
2.5. Reaction Procedure
3. Results and Discussion
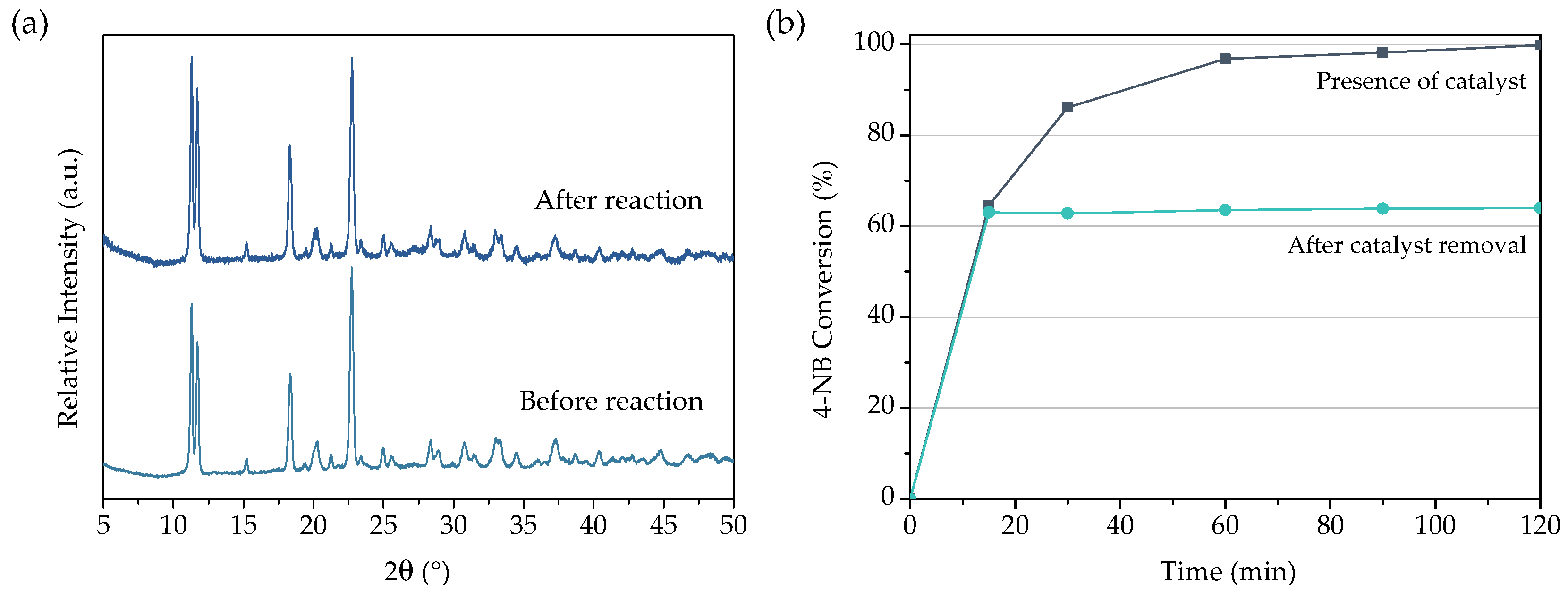
3.1. Characterization of MOF Materials
3.2. Catalytic Study
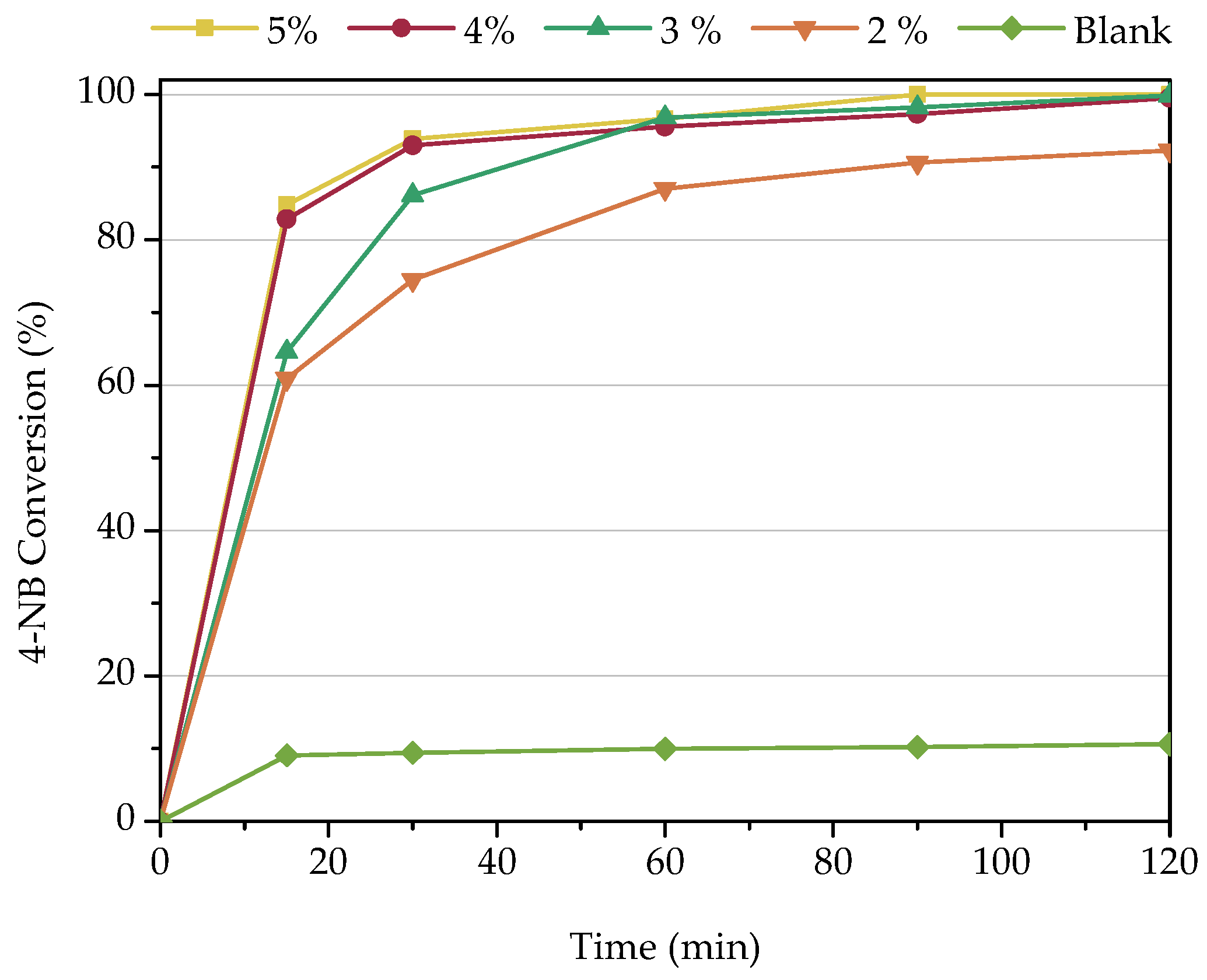
3.2.1. Influence of Catalyst Concentration
3.2.2. Influence of Base Concentration
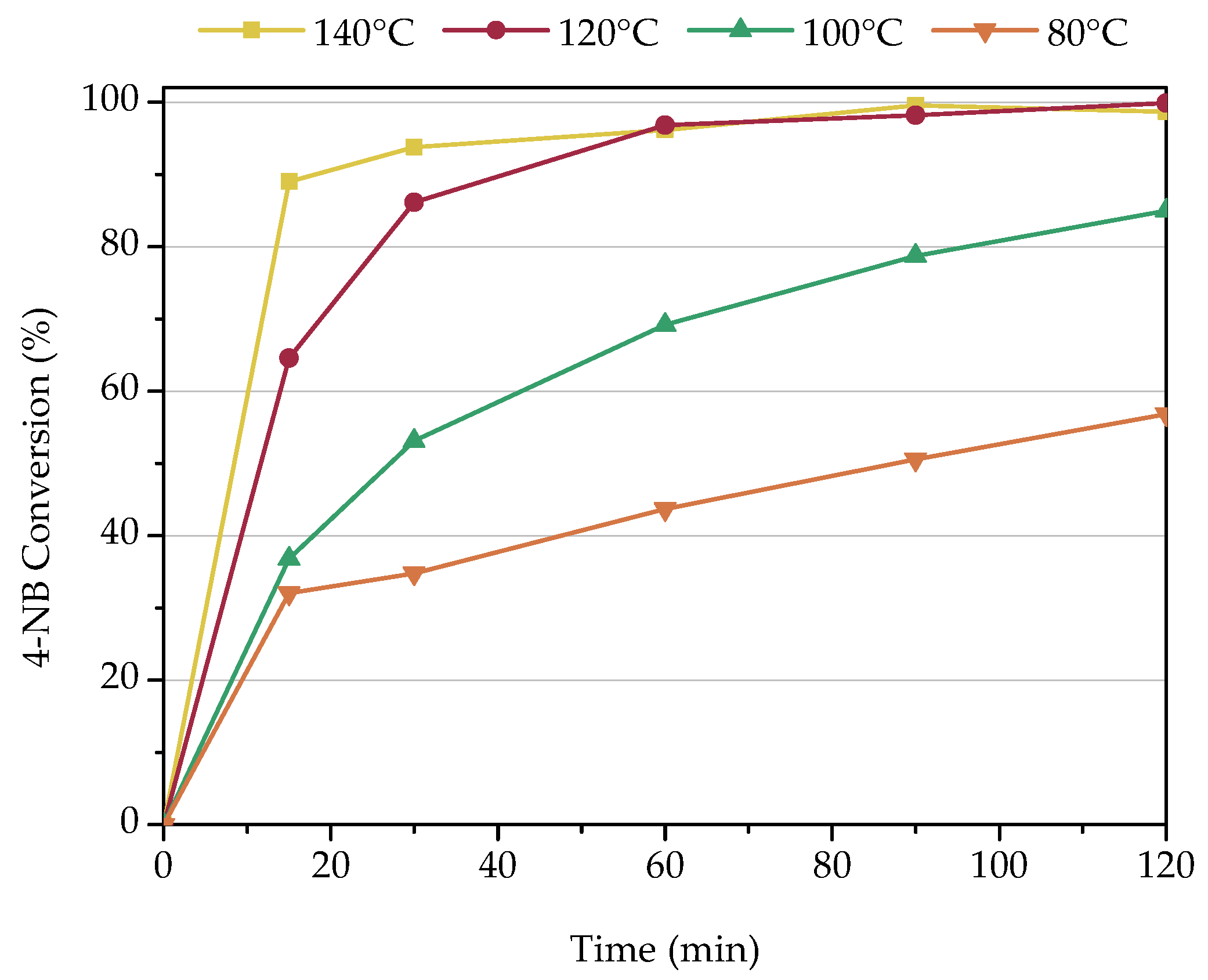
3.2.3. Influence of Temperature
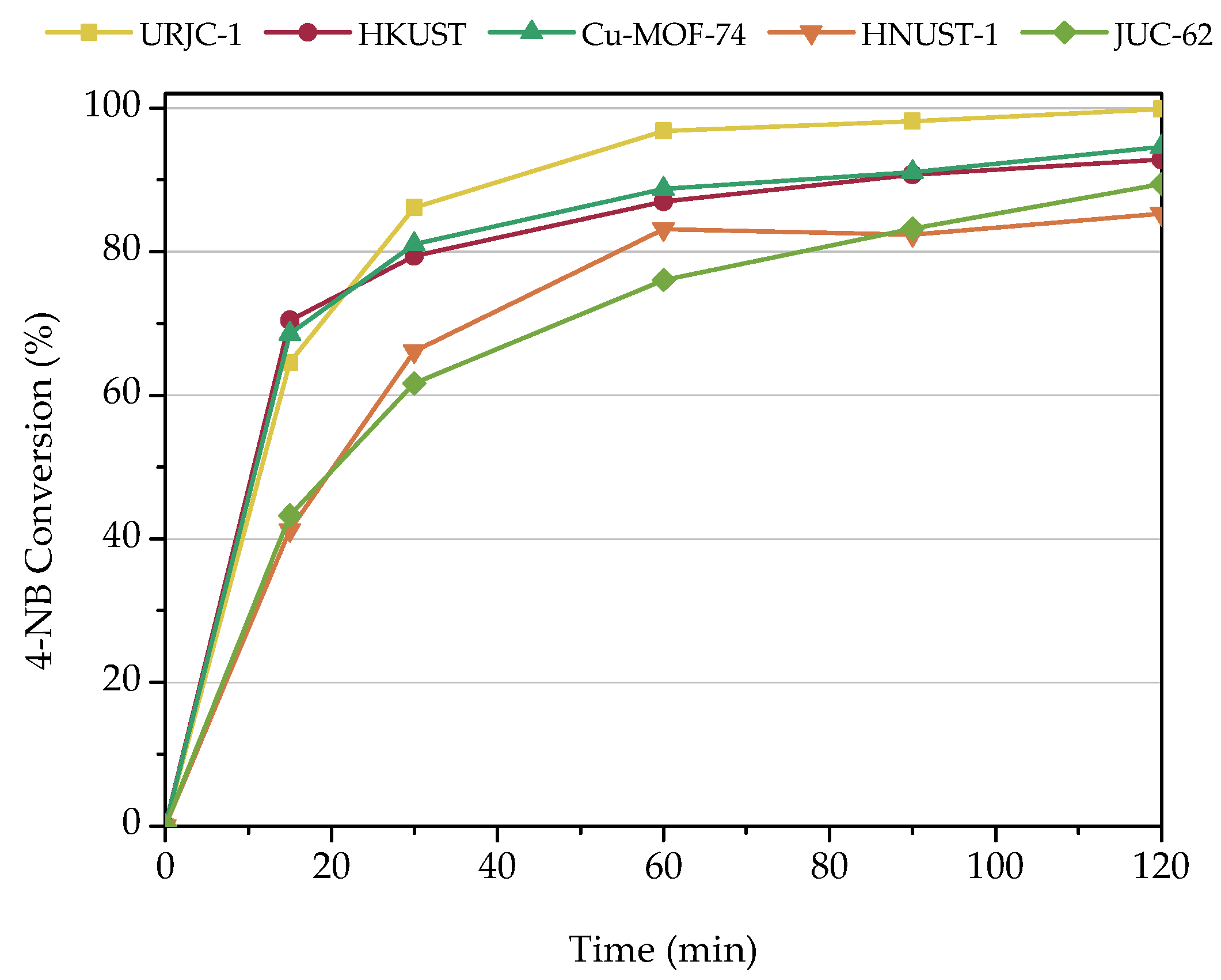
3.2.4. Comparison with Other Catalysts
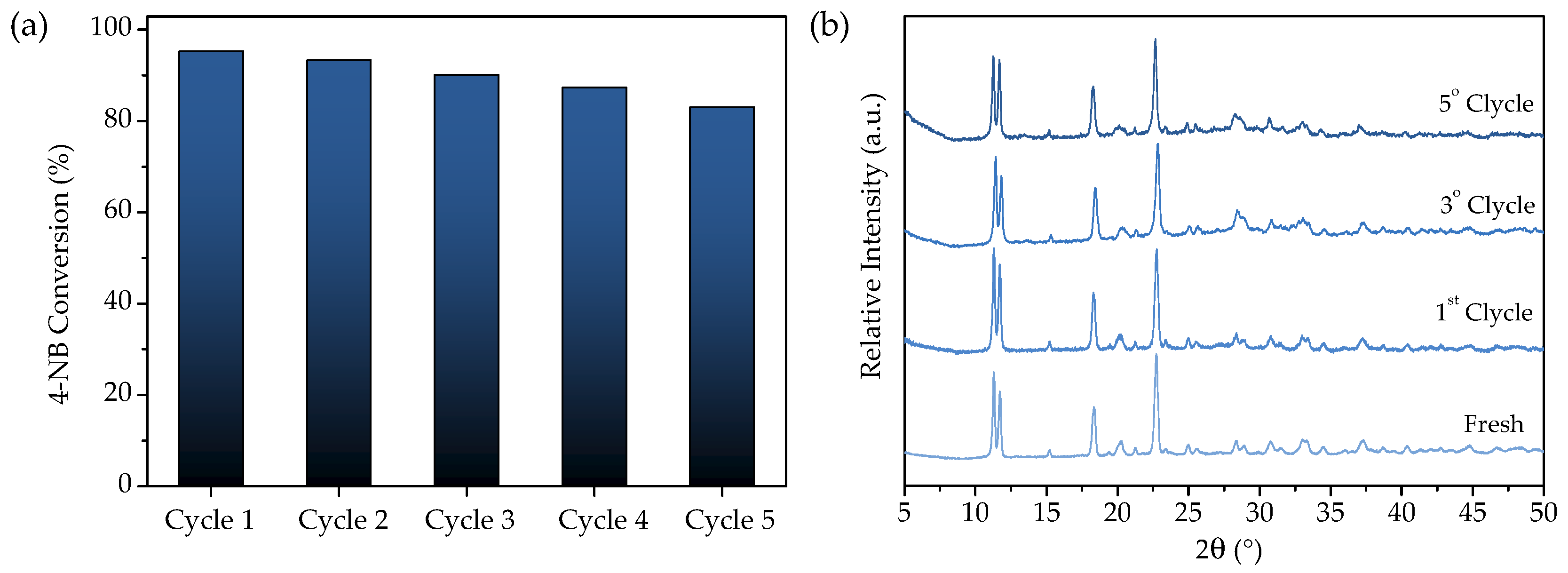
3.2.5. Recyclability of URJC-1
3.2.6. Catalytic Activity of Different Substrates
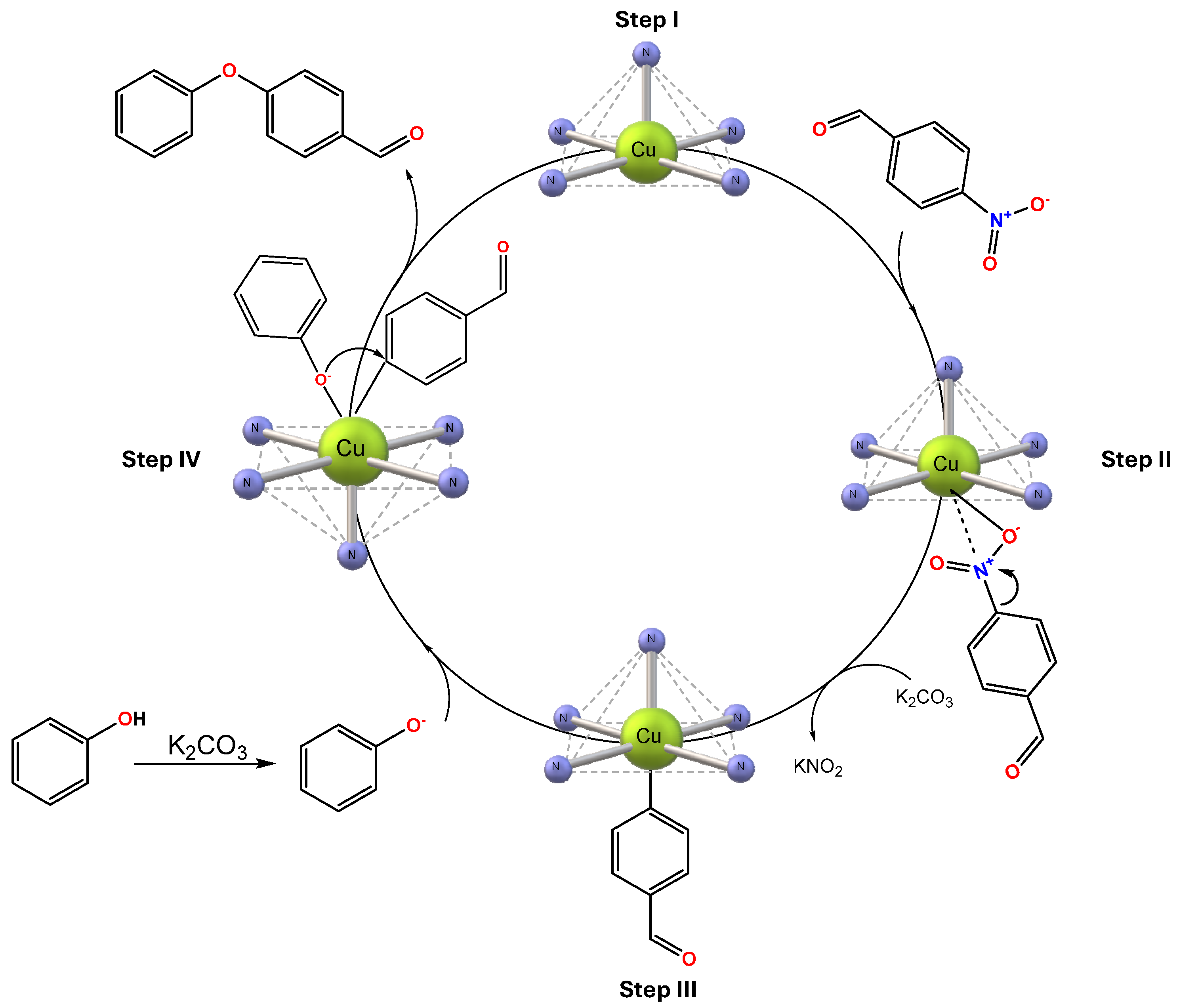
3.2.7. Proposed Mechanism for O-Arylation Cross-Coupling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Zhang, X.; Cheng, X.; Xie, Z.; Kuang, Q.; Zheng, L. The Function of Metal-Organic Frameworks in the Application of MOF-Based Composites. Nanoscale Adv. 2020, 2, 2628–2647. [Google Scholar] [CrossRef] [PubMed]
- Raptopoulou, C.P. Materials Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Alhumaimess, M.S. Metal–Organic Frameworks and Their Catalytic Applications. J. Saudi Chem. Soc. 2020, 24, 461–473. [Google Scholar] [CrossRef]
- Wei, Y.-S.; Zhang, M.; Zou, R.; Xu, Q. Metal−Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [PubMed]
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.G.; Shieh, F.K.; Wu, K.C.W. Metal-Organic Framework (MOF)-Derived Catalysts for Fine Chemical Production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Vásquez-Céspedes, S.; Betori, R.C.; Cismesia, M.A.; Kirsch, J.K.; Yang, Q. Heterogeneous Catalysis for Cross-Coupling Reactions: An Underutilized Powerful and Sustainable Tool in the Fine Chemical Industry? Org. Process Res. Dev. 2021, 25, 740–753. [Google Scholar] [CrossRef]
- Khan, F.; Dlugosch, M.; Liu, X.; Banwell, M.G. The Palladium-Catalyzed Ullmann Cross-Coupling Reaction: A Modern Variant on a Time-Honored Process. Acc. Chem. Res. 2018, 51, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- British Geological Survey. Risk List 2015; British Geological Survey: Nottingham, UK, 2015. [Google Scholar]
- European Medicines Agency. ICH Guideline Q3D (R1) on Elemental Impurities; European Medicines Agency: Amsterdam, The Netherlands, 2019. [Google Scholar]
- U.S. Pharmacopeia (USP). Chemical Tests/232 Elemental Impurities—Limits; U.S. Pharmacopeia (USP): North Bethesda, MD, USA, 2013. [Google Scholar]
- Corbett, J.W.; Rauckhorst, M.R.; Qian, F.; Hoffman, R.L.; Knauer, C.S.; Fitzgerald, L.W. Heteroatom-Linked Indanylpyrazines Are Corticotropin Releasing Factor Type-1 Receptor Antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 6250–6256. [Google Scholar] [CrossRef]
- Pitsinos, E.N.; Vidali, V.P.; Couladouros, E.A. Diaryl Ether Formation in the Synthesis of Natural Products. Eur. J. Org. Chem 2011, 2011, 1207–1222. [Google Scholar] [CrossRef]
- Bedos-Belval, F.; Rouch, A.; Vanucci-Bacqué, C.; Baltas, M. Diaryl Ether Derivatives as Anticancer Agents—A Review. Medchemcomm 2012, 3, 1356. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical Overview of Sorafenib, a Multikinase Inhibitor That Targets Both Raf and VEGF and PDGF Receptor Tyrosine Kinase Signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Peters, W. The Evolution of Tafenoquine—Antimalarial for a New Millennium? J. R. Soc. Med. 1999, 92, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Buskes, M.J.; Blanco, M.-J. Impact of Cross-Coupling Reactions in Drug Discovery and Development. Molecules 2020, 25, 3493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, D.; Wang, X.; Ding, K. (2-Pyridyl)Acetone-Promoted Cu-Catalyzed O-Arylation of Phenols with Aryl Iodides, Bromides, and Chlorides. J. Org. Chem. 2009, 74, 7187–7190. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhou, W.; Jiang, Y.; Ma, D. CuI/Oxalamide Catalyzed Couplings of (Hetero)Aryl Chlorides and Phenols for Diaryl Ether Formation. Angew. Chem. Int. Ed. 2016, 55, 6211–6215. [Google Scholar] [CrossRef] [PubMed]
- Salvi, L.; Davis, N.R.; Ali, S.Z.; Buchwald, S.L. A New Biarylphosphine Ligand for the Pd-Catalyzed Synthesis of Diaryl Ethers under Mild Conditions. Org. Lett. 2011, 14, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Platon, M.; Cui, L.; Mom, S.; Richard, P.; Saeys, M.; Hierso, J.-C. Etherification of Functionalized Phenols with Chloroheteroarenes at Low Palladium Loading: Theoretical Assessment of the Role of Triphosphane Ligands in C-O Reductive Elimination. Adv. Synth. Catal. 2011, 353, 3403–3414. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Unger, J.B.; Taft, B.R. Copper-in-Charcoal (Cu/C) Promoted Diaryl Ether Formation. Org. Lett. 2007, 9, 1089–1092. [Google Scholar] [CrossRef]
- Naidu, A.B.; Jaseer, E.A.; Sekar, G. General, Mild, and Intermolecular Ullmann-Type Synthesis of Diaryl and Alkyl Aryl Ethers Catalyzed by Diol−Copper(I) Complex. J. Org. Chem. 2009, 74, 3675–3679. [Google Scholar] [CrossRef]
- Benyahya, S.; Monnier, F.; Taillefer, M.; Man, M.W.C.; Bied, C.; Ouazzani, F. Efficient and Versatile Sol-Gel Immobilized Copper Catalyst for Ullmann Arylation of Phenols. Adv. Synth. Catal. 2008, 350, 2205–2208. [Google Scholar] [CrossRef]
- Cheng, A.-Y.; Hsieh, J.-C. Highly Efficient Copper Catalytic System for the O-Arylation of Phenol with Iodoarene. Tetrahedron Lett. 2012, 53, 71–75. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Dumbre, D.K.; Yadav, P.N.; Bhargava, S.K. Thermally Decomposed Cu–Fe-Hydrotalcite: A Novel Highly Active Catalyst for o-Arylation of Naphthol and Phenols by Aryl Halides. Catal. Commun. 2012, 29, 132–136. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Wang, S.; Niu, J.; Xia, C.; Sun, W. Magnetic CuFe2O4 Nanoparticles as an Efficient Catalyst for C-O Cross-Coupling of Phenols with Aryl Halides. ChemCatChem 2011, 3, 146–149. [Google Scholar] [CrossRef]
- Takise, R.; Isshiki, R.; Muto, K.; Itami, K.; Yamaguchi, J. Decarbonylative Diaryl Ether Synthesis by Pd and Ni Catalysis. J Am Chem. Soc. 2017, 139, 3340–3343. [Google Scholar] [CrossRef]
- Lin, Y.; Cai, M.; Fang, Z.; Zhao, H. Highly Efficient Heterogeneous Copper-Catalyzed Decarboxylative Cross-Coupling of Potassium Polyfluorobenzoates with Aryl Halides Leading to Polyfluorobiaryls †. RSC Adv. 2017, 7, 34722–34729. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. Copper in Cross-Coupling Reactions. Coord. Chem. Rev. 2004, 248, 2337–2364. [Google Scholar] [CrossRef]
- Thapa, S.; Shrestha, B.; Gurung, S.K.; Giri, R. Copper-Catalysed Cross-Coupling: An Untapped Potential. Org. Biomol. Chem. 2015, 13, 4816–4827. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Heterogeneous Catalysts for the One-Pot Synthesis of Chemicals and Fine Chemicals. Chem. Rev. 2010, 111, 1072–1133. [Google Scholar] [CrossRef]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral Metal–Organic Frameworks for Asymmetric Heterogeneous Catalysis. Chem. Rev. 2012, 112, 1196–1231. [Google Scholar] [CrossRef]
- Diyali, N.; Rasaily, S.; Biswas, B. Metal–Organic Framework: An Emergent Catalyst in C–N Cross-Coupling Reactions. Coord. Chem. Rev. 2022, 469, 214667. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal-Organic Frameworks Catalyzed C-C and C-Heteroatom Coupling Reactions. Chem. Soc. Rev. 2015, 44, 1922–1947. [Google Scholar] [CrossRef] [PubMed]
- Leo, P.; Martínez, F.; Calleja, G.; Briones, D.; Wojtas, L.; Orcajo, G. New URJC-1 Material with Remarkable Stability and Acid-Base Catalytic Properties. Polymers 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Tapiador, J.; García-Rojas, E.; Leo, P.; Martos, C.; Calleja, G.; Orcajo, G. Copper MOFs Performance in the Cycloaddition Reaction of CO2 and Epoxides. Microporous Mesoporous Mater. 2023, 361, 112741. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, H.; Wang, Z.; Yu, X.; Yi, P.; Bai, J. Porous NbO-Type Metal–Organic Framework with Inserted Acylamide Groups Exhibiting Highly Selective CO2 Capture. CrystEngComm 2013, 15, 3517. [Google Scholar] [CrossRef]
- Montes-Andrés, H.; Leo, P.; Orcajo, G.; Rodríguez-Diéguez, A.; Choquesillo-Lazarte, D.; Martos, C.; Botas, J.Á.; Martínez, F.; Calleja, G. Novel and Versatile Cobalt Azobenzene-Based Metal-Organic Framework as Hydrogen Adsorbent. ChemPhysChem 2019, 20, 1334–1339. [Google Scholar] [CrossRef]
- Sanz, R.; Martínez, F.; Orcajo, G.; Wojtas, L.; Briones, D. Synthesis of a Honeycomb-like Cu-Based Metal–Organic Framework and Its Carbon Dioxide Adsorption Behaviour. Dalton Trans. 2013, 42, 2392–2398. [Google Scholar] [CrossRef]
- Xue, M.; Zhu, G.; Li, Y.; Zhao, X.; Jin, Z.; Kang, E.; Qiu, S. Structure, Hydrogen Storage, and Luminescence Properties of Three 3D Metal−Organic Frameworks with NbO and PtS Topologies. Cryst. Growth Des. 2008, 8, 2478–2483. [Google Scholar] [CrossRef]
- Leo, P.; Orcajo, G.; Briones, D.; Calleja, G.; Sánchez-Sánchez, M.; Martínez, F. A Recyclable Cu-MOF-74 Catalyst for the Ligand-Free O-Arylation Reaction of 4-Nitrobenzaldehyde and Phenol. Nanomaterials 2017, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.T.S.; Nguyen, T.T.; Nguyen, V.T.; Nguyen, K.D. Ligand-Free Copper-Catalyzed Coupling of Phenols with Nitroarenes by Using a Metal-Organic Framework as a Robust and Recoverable Catalyst. ChemCatChem 2013, 5, 2374–2381. [Google Scholar] [CrossRef]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A Retrospective-Prospective Review of Suzuki–Miyaura Reaction: From Cross-Coupling Reaction to Pharmaceutical Industry Applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Mullick, K.; Biswas, S.; Kim, C.; Ramprasad, R.; Angeles-Boza, A.M.; Suib, S.L. Ullmann Reaction Catalyzed by Heterogeneous Mesoporous Copper/Manganese Oxide: A Kinetic and Mechanistic Analysis. Inorg. Chem. 2017, 56, 10290–10297. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.; Mishra, N.K.; Bansal, V.; Kumar, A.; Mozumdar, S. Cu-Nanoparticle Catalyzed O-Arylation of Phenols with Aryl Halides via Ullmann Coupling. Tetrahedron Lett 2007, 48, 8883–8887. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Jones, C.W. Highly Accessible Catalytic Sites on Recyclable Organosilane-Functionalized Magnetic Nanoparticles: An Alternative to Functionalized Porous Silica Catalysts. J. Mol. Catal. A Chem. 2006, 253, 123–131. [Google Scholar] [CrossRef]
- Naidu, A.B.; Raghunath, O.R.; Prasad, D.J.C.; Sekar, G. An Efficient BINAM-Copper(II) Catalyzed Ullmann-Type Synthesis of Diaryl Ethers. Tetrahedron Lett. 2008, 49, 1057–1061. [Google Scholar] [CrossRef]
- Ouali, A.; Spindler, J.F.; Cristau, H.J.; Taillefer, M. Mild Conditions for Copper-Catalyzed Coupling Reaction of Phenols and Aryl Iodides and Bromides. Adv. Synth. Catal. 2006, 348, 499–505. [Google Scholar] [CrossRef]
- Jogdand, N.R.; Shingate, B.B.; Shingare, M.S. Tris-(2-Aminoethyl) Amine as a Novel and Efficient Tripod Ligand for a Copper(I)-Catalyzed C-O Coupling Reaction. Tetrahedron Lett. 2009, 50, 4019–4021. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, B.; Ma, T.; Jiang, H.; Li, Y. Ligand-Free Coupling of Phenols and Alcohols with Aryl Halides by a Recyclable Heterogeneous Copper Catalyst. RSC Adv. 2012, 2, 5528–5530. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Jarahiyan, A.; Heidarian Haris, M.; Pourjavadi, A. An Advancement in the Synthesis of Nano Pd@magnetic Amine-Functionalized UiO-66-NH2 Catalyst for Cyanation and O-Arylation Reactions. Sci. Rep. 2021, 11, 11387. [Google Scholar] [CrossRef]
- Ling, P.; Li, D.; Wang, X. Supported CuO/γ-Al2O3 as Heterogeneous Catalyst for Synthesis of Diaryl Ether under Ligand-Free Conditions. J Mol Catal A Chem 2012, 357, 112–116. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Liu, M.; Zheng, X.; Ding, J.; Wu, H. Ligand-Free Copper-Catalyzed Coupling of Nitroarenes with Arylboronic Acids. Green Chem. 2012, 14, 912–916. [Google Scholar] [CrossRef]
- Santhoshkumar, P.; Rajalakshmi, C.; Mathews, L.E.; Sulay, R.; Thomas, V.I. Mechanistic Views on First-Row Earth-Abundant Transition Metal Catalyzed Ullmann-Type O-Arylation Reactions. Chimia 2023, 77, 246. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, C.; Radhakrishnan, A.; Ujwaldev, S.M.; Anilkumar, G.; Thomas, V.I. Unravelling the Mechanism of Cobalt (II) Catalyzed O-Arylation Reaction between Aryl Halides and Phenols: A DFT Study. J. Organomet. Chem. 2022, 972, 122385. [Google Scholar] [CrossRef]




| Material | SBET a (m2/g) | VP b (cm3/g) | DP c (Å) |
|---|---|---|---|
| URJC-1 | 408 | 0.24 | 14.8 |
| Cu-MOF-74 | 1126 | 0.55 | 12.0 |
| HKUST-1 | 1455 | 0.55 | 10.0 |
| JUC-62 | 2037 | 0.87 | 9.6 |
| HNUST-1 | 232 | 0.15 | 11–16 |
| Entry | Catalyst | Reaction Conditions | Yield (%) | Ref. |
|---|---|---|---|---|
| 1 | MOF-253·0.5CuI | 0.4 mmol of iodobenzene, 0.6 mmol of phenol, 0.8 mmol Cs2CO3, 20% mol of catalyst, 2 mL of DMSO, 80 °C, 24 h | 97 | [51] |
| 2 | Cu2(BDC)2(DABCO) | 4-nitrobenzaldehyde/phenol molar ratio of 1:2, 2 eq. of K2CO3, 5 mol% of catalyst at 100 °C, 120 min | ≈100 | [43] |
| 3 | Pd0@magnetic amine-FunctionalizedUiO-66-NH2 | 1 mmol of iodobencene, 1 mmol of phenol, 1.2 mmol of KOH, 2 mL of H2O and 2 mg of catalyst, 3 h, 80 °C | 95 | [52] |
| 4 | Cu-MOF-74 | 4-nitrobenzaldehyde/phenol molar ratio of 1:2, 2 eq. of K2CO3, 5 mol% of catalyst, 10 mL of DMF, 120 min, 120 °C | ≈100 | [42] |
| 5 | CuO/γ-Al2O3 | 2 mmol of m-cresol, 3 mmol of iodophenol, 4 mmol of K3PO4, 32 mg of catalysts, 4 mL of DMSO, 10 h, 150 °C | 76 | [53] |
| 6 | Cu/C | 1 mmol of para-bromoanisole, 2.0 mmol of para-t-butylphenol, 2.0 mmol of Cs2CO3, 0.5 mmol of 1,10-phenanthroline, 60 mg of Cu/C, 2.0 mL of dioxane, 1 h, 200 °C | 94 | [22] |
| 7 | Nano CuO | 0.6 mmol of 4-nitrobenzaldehyde, 0.3 mmol of arylboronic acid, 5 mol% of catalyst, 3 eq. of Cs2CO3, 1 eq. of oxone, 3 mL of DMF, 48 h, 100 °C | 95 | [54] |
| 8 | URJC-1 | 4-nitrobenzaldehyde/phenol molar ratio of 1:2, 2 eq. of K2CO3, 3 mol% of catalyst, 10 mL of DMF, 60 min, 120 °C | ≈100 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rojas, E.; Leo, P.; Tapiador, J.; Martos, C.; Orcajo, G. URJC-1: Stable and Efficient Catalyst for O-Arylation Cross-Coupling. Nanomaterials 2024, 14, 1103. https://doi.org/10.3390/nano14131103
García-Rojas E, Leo P, Tapiador J, Martos C, Orcajo G. URJC-1: Stable and Efficient Catalyst for O-Arylation Cross-Coupling. Nanomaterials. 2024; 14(13):1103. https://doi.org/10.3390/nano14131103
Chicago/Turabian StyleGarcía-Rojas, Elena, Pedro Leo, Jesús Tapiador, Carmen Martos, and Gisela Orcajo. 2024. "URJC-1: Stable and Efficient Catalyst for O-Arylation Cross-Coupling" Nanomaterials 14, no. 13: 1103. https://doi.org/10.3390/nano14131103
APA StyleGarcía-Rojas, E., Leo, P., Tapiador, J., Martos, C., & Orcajo, G. (2024). URJC-1: Stable and Efficient Catalyst for O-Arylation Cross-Coupling. Nanomaterials, 14(13), 1103. https://doi.org/10.3390/nano14131103






