Novel Biological-Based Strategy for Synthesis of Green Nanochitosan and Copper-Chitosan Nanocomposites: Promising Antibacterial and Hematological Agents
Abstract
:1. Introduction
2. Experimental Work
2.1. Plant Extract Preparation (Pomegranate Peel Extract)
2.2. Synthesis of Green Chitosan Nanoparticles (PP–Cs–NPs)
2.3. Synthesis of Green Chitosan–Copper Oxide Composite (PP–CS–CuO–NPs)
2.4. Characterization
2.4.1. Structural Assessment
2.4.2. Morphological Characterizations
2.5. Antibacterial Test
2.6. Hematological Test
2.6.1. Chitosan Solution Preparation
2.6.2. Blood Collection
2.6.3. Complete Blood Count (CBC) and Coagulation Time (BCT)
3. Results and Discussion
3.1. Characterization of Chitosan Nanoparticles
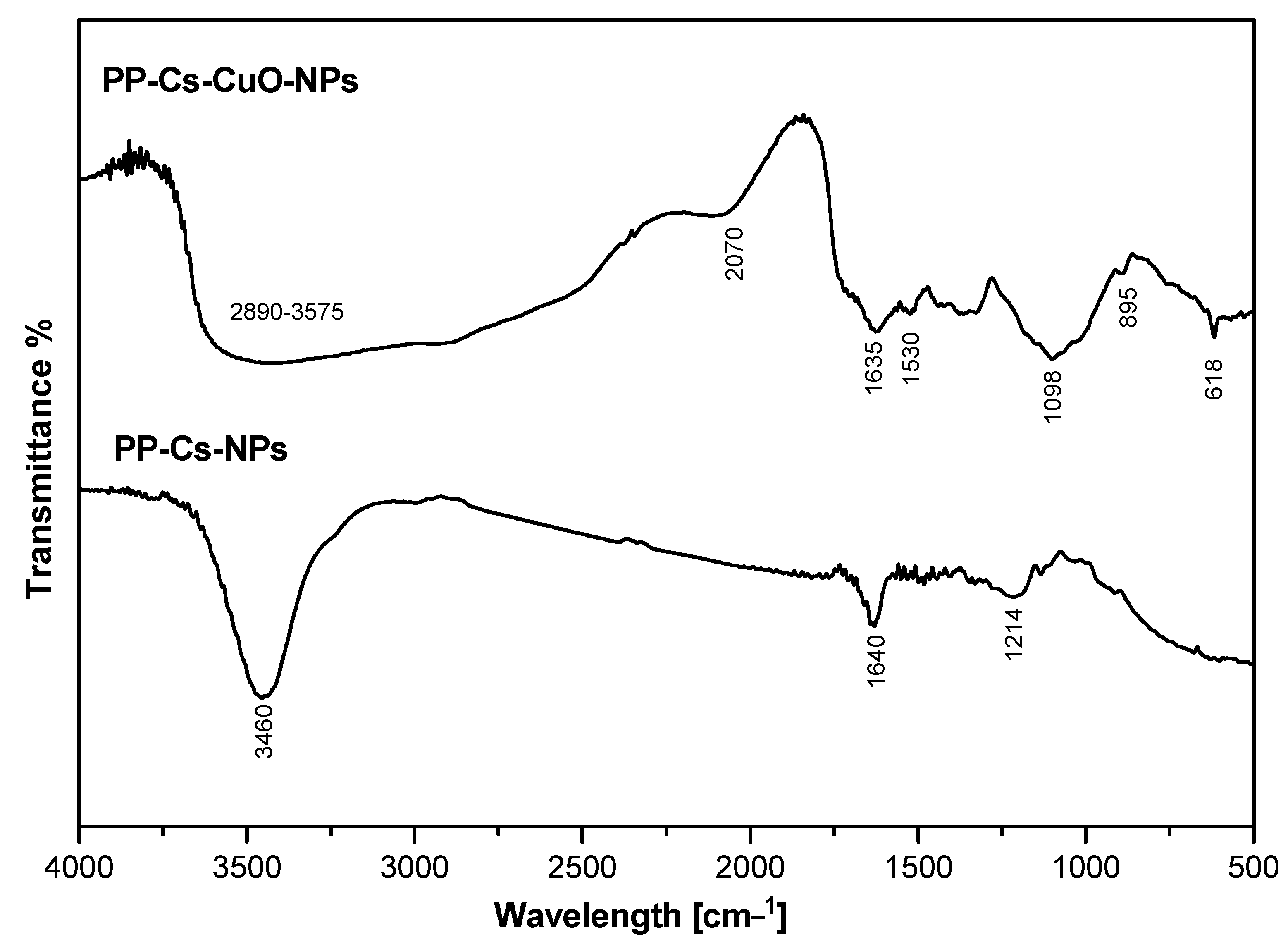
3.1.1. FTIR Analysis
3.1.2. XRD Analysis
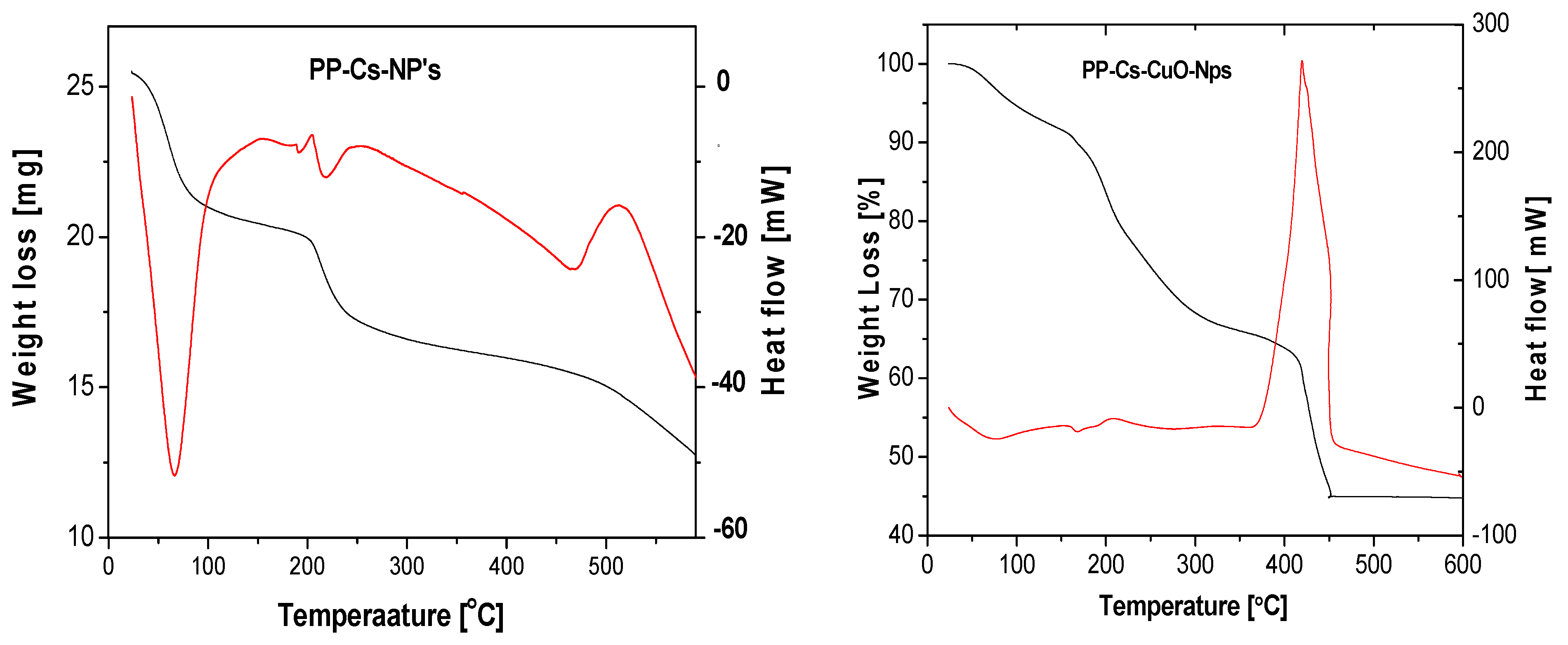
3.1.3. TGA and DTA
| Sample | Degradation Stage | Temperature Range (°C) | Peak Temperature (°C) | % Weight Loss |
|---|---|---|---|---|
| PP–Cs–NPs | First | 25–87 | 68 | 16.7% |
| Second | 87–240 | 218 | 31.5% | |
| Third | 240–600 | 470 | 51.8% | |
| PP–Cs–CuO–NPs | First | 25–117 | 79 | 8.9% |
| Second | 117–314 | 200 | 23.1% | |
| Third | 314–451 | 415 | 54.8% |
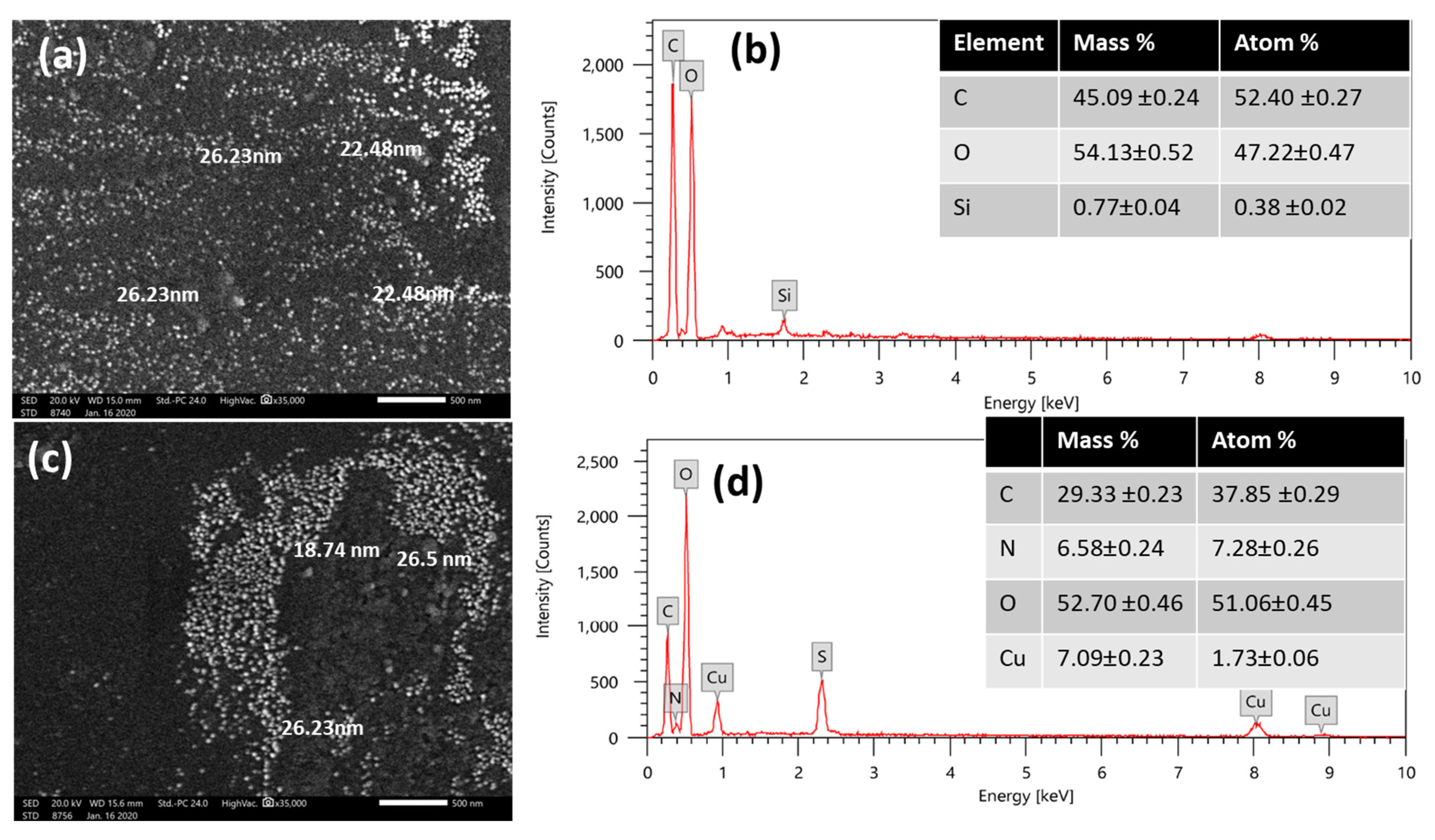
3.1.4. SEM and EDX
3.2. Evaluation of Antimicrobial Activity of Green PP–Cs–NPs and PP–Cs–CuO–NPs
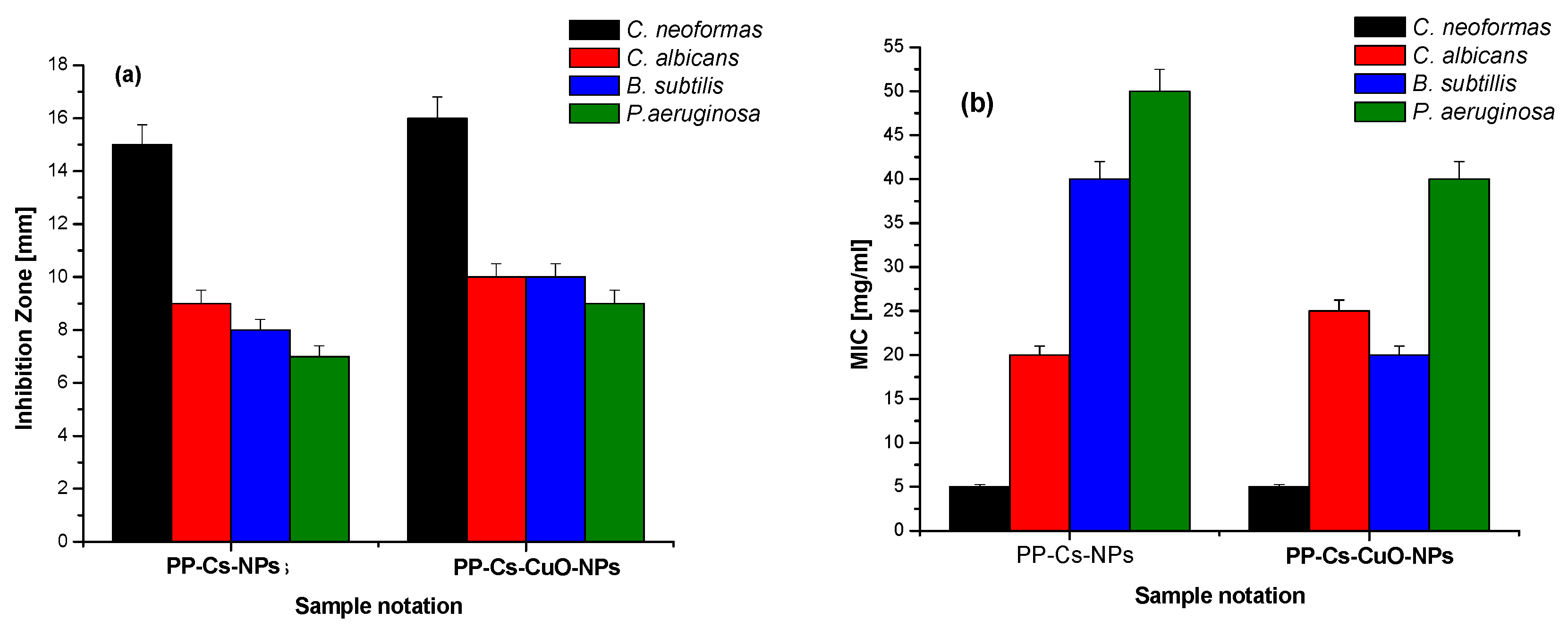
3.3. Hematological Analysis
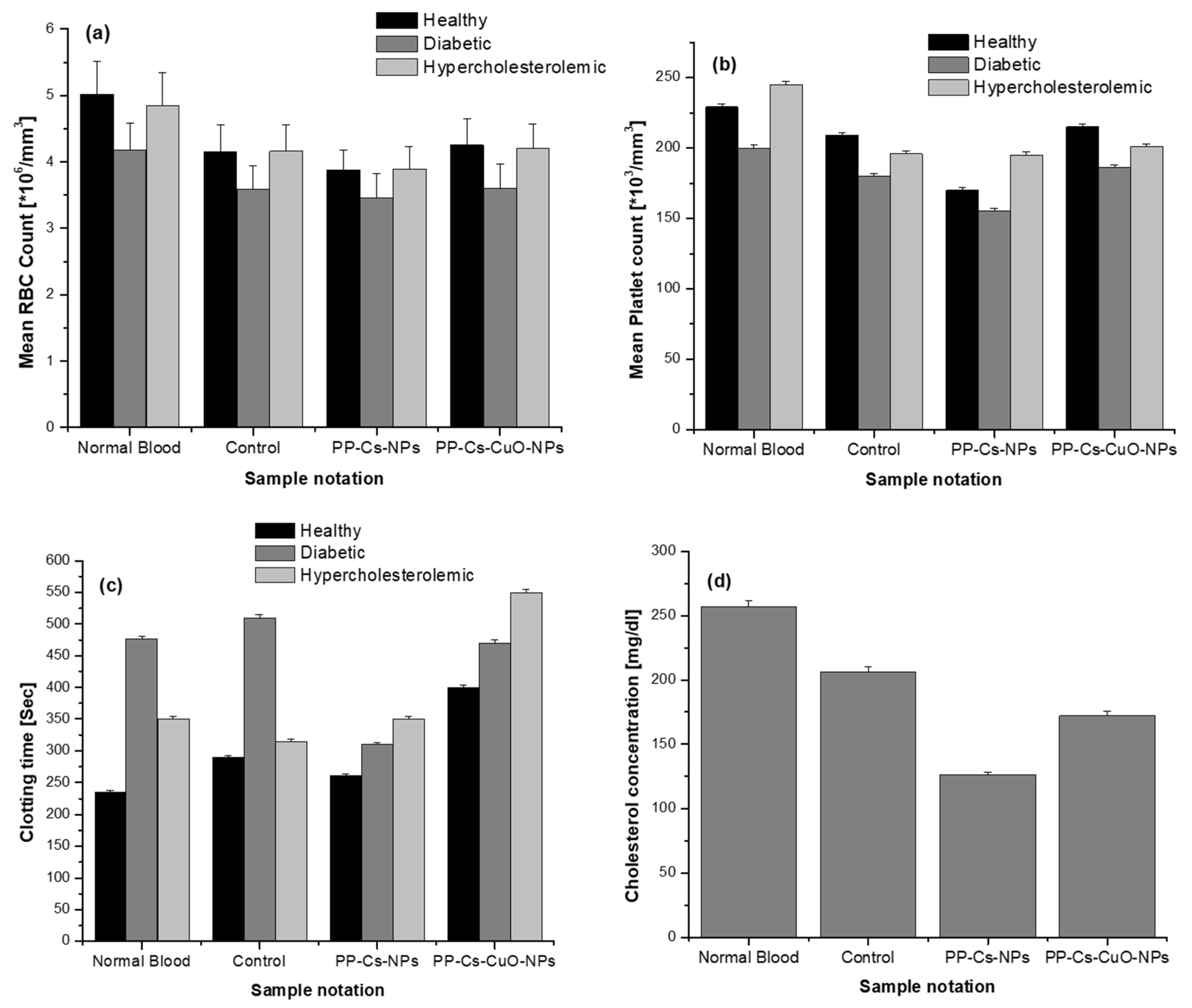
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El-Naggar, N.E.A.; Saber, W.E.I.A.; Zweil, A.M.; Bashir, S.I. An Innovative Green Synthesis Approach of Chitosan Nanoparticles and Their Inhibitory Activity against Phytopathogenic Botrytis Cinerea on Strawberry Leaves. Sci. Rep. 2022, 12, 3515. [Google Scholar] [CrossRef]
- Jogaiah, S.; Satapute, P.; De Britto, S.; Konappa, N.; Udayashankar, A.C. Exogenous Priming of Chitosan Induces Upregulation of Phytohormones and Resistance against Cucumber Powdery Mildew Disease Is Correlated with Localized Biosynthesis of Defense Enzymes. Int. J. Biol. Macromol. 2020, 162, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Boomija, R.V.; Muthukumar, S.; Gandhi, M.; Salma, S.; Prinsha, T.K.; Rengasamy, B. Green Synthesis of Chitosan Nanoparticles Using Tea Extract and Its Antimicrobial Activity against Economically Important Phytopathogens of Rice. Sci. Rep. 2024, 14, 7381. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, D.; Geetha, N.; Khawar, K.M.; Jogaiah, S.; Mujtaba, M. Current Trends and Challenges in the Synthesis and Applications of Chitosan-Based Nanocomposites for Plants: A Review. Carbohydr. Polym. 2021, 261, 117904. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Shiha, A.M.; Mahrous, H.; Mohammed, A.B.A. Green Synthesis of Chitosan Nanoparticles, Optimization, Characterization and Antibacterial Efficacy against Multi Drug Resistant Biofilm-Forming Acinetobacter Baumannii. Sci. Rep. 2022, 12, 19869. [Google Scholar] [CrossRef]
- Sachin, K.; Karn, S.K. Microbial Fabricated Nanosystems: Applications in Drug Delivery and Targeting. Front. Chem. 2021, 9, 617353. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, R.; Daskum, A.; Unyah, Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Singh, S.; Nayik, G.A. Bioactive Compounds from Pomegranate Peels—Biological Properties, Structure–Function Relationships, Health Benefits and Food Applications—A Comprehensive Review. J. Funct. Foods 2024, 116, 106132. [Google Scholar] [CrossRef]
- Elfalleh, W. Total Phenolic Contents and Antioxidant Activities of Pomegranate Peel, Seed, Leaf and Flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.u.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan Nanoparticles as a Promising Tool in Nanomedicine with Particular Emphasis on Oncological Treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Baranwal, A.; Kumar, A.; Priyadharshini, A.; Oggu, G.S.; Bhatnagar, I.; Srivastava, A.; Chandra, P. Chitosan: An Undisputed Bio-Fabrication Material for Tissue Engineering and Bio-Sensing Applications. Int. J. Biol. Macromol. 2018, 110, 110–123. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan–Silver Oxide Nanocomposite Film: Preparation and Antimicrobial Activity. Bull. Mater. Sci. 2011, 34, 29–35. [Google Scholar] [CrossRef]
- Jiang, R.; Shen, T.-T.; Zhu, H.-Y.; Fu, Y.-Q.; Jiang, S.-T.; Li, J.-B.; Wang, J.-L. Magnetic Fe3O4 Embedded Chitosan–Crosslinked-Polyacrylamide Composites with Enhanced Removal of Food Dye: Characterization, Adsorption and Mechanism. Int. J. Biol. Macromol. 2023, 227, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mohamed, H.; Al-Subaie, A.; Al-Ohali, A.; Mahmoud, N. Investigation of the Antimicrobial Activity and Hematological Pattern of Nano-Chitosan and Its Nano-Copper Composite. Sci. Rep. 2021, 11, 9540. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, S.; Ikram, S. Adsorption of Heavy Metal Ions: Role of Chitosan and Cellulose for Water Treatment Saiqa Ikram Jamia Millia Islamia adsorption of heavy metal ions: Role of chitosan and cellulose for water treatment. Int. J. Pharmacogn. 2015, 2, 280–289. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Hashem, A.H.; Al-Askar, A.A.; Haponiuk, J.; Saied, E. A Novel Nanocomposite Based on Mycosynthesized Bimetallic Zinc-Copperoxide Nanoparticles, Nanocellulose and Chitosan: Characterization, Antimicrobial and Photocatalytic Activities. Electron. J. Biotechnol. 2023, 65, 45–55. [Google Scholar] [CrossRef]
- Mescola, A.; Canale, C.; Fragouli, D.; Athanassiou, A. Controlled Formation of Gold Nanostructures on Biopolymer Films upon Electromagnetic Radiation. Nanotechnology 2017, 28, 415601. [Google Scholar] [CrossRef]
- Bejan, A.; Anisiei, A.; Andreica, B.-I.; Rosca, I.; Marin, L. Chitosan Nanofibers Encapsulating Copper Oxide Nanoparticles: A New Approach towards Multifunctional Ecological Membranes with High Antimicrobial and Antioxidant Efficiency. Int. J. Biol. Macromol. 2024, 260, 129377. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Lin, I.H.; Krisnawati, D.I.; Khaerunnisa, S.; Widodo; Hsiao, Y.C.; Kuo, T.R.; Khafid, M. Antibacterial Pathways in Transition Metal-Based Nanocomposites: A Mechanistic Overview. Int. J. Nanomed. 2022, 17, 6821–6842. [Google Scholar] [CrossRef]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper Oxide Nanoparticles: Synthesis, Characterization and Their Antibacterial Activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Sajjad, H.; Sajjad, A.; Haya, R.T.; Khan, M.M.; Zia, M. Copper Oxide Nanoparticles: In Vitro and in Vivo Toxicity, Mechanisms of Action and Factors Influencing Their Toxicology. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109682. [Google Scholar] [CrossRef] [PubMed]
- Syame, S.; Mohamed, W.; Mahmoud, R.; Tawfeeq, S. Synthesis of Copper-Chitosan Nanocomposites and Its Application in Treatment of Local Pathogenic Isolates Bacteria. Orient. J. Chem. 2017, 33, 2959–2969. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liu, C.C.; Cherng, J.H.; Lin, C.S.; Chang, S.J.; Hong, Z.J.; Liu, C.C.; Chiu, Y.K.; Der Hsu, S.; Chang, H. Biological Effects of Chitosan-Based Dressing on Hemostasis Mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef]
- Silva, M.M.P.; de Aguiar, M.I.F.; Rodrigues, A.B.; Miranda, M.D.C.; Araújo, M.Â.M.; Rolim, I.L.T.P.; e Souza, A.M.A. The Use of Nanoparticles in Wound Treatment: A Systematic Review. Rev. da Esc. Enferm. 2017, 51, 1–9. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, X.; Liu, L.; Guan, J.; Liu, M.; Li, Z.; Yang, J.; Huang, S.; Wu, J.; Tian, F.; et al. Blood Coagulation Evaluation of N-Alkylated Chitosan. Carbohydr. Polym. 2017, 173, 259–268. [Google Scholar] [CrossRef]
- Abdel-fattah, W.I.; Eid, M.M.; El-moez, S.I.A.; Mohamed, E.; Ali, G.W. Synthesis of Biogenic Ag @ Pd Core-Shell Nanoparticles Having Anti-Cancer / Anti-Microbial Functions. Life Sci. 2017, 183, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Gabr, D.G. Significance of Fruit and Seed Coat Morphology in Taxonomy and Identification for Some Species of Brassicaceae. Am. J. Plant Sci. 2018, 9, 380–402. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Sivakami, M.S.; Gomathi, T.; Venkatesan, J.; Jeong, H.-S.; Kim, S.-K.; Sudha, P.N. Preparation and Characterization of Nano Chitosan for Treatment Wastewaters. Int. J. Biol. Macromol. 2013, 57, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J.; Cruz-Romero, M.; Collins, T.; Cummins, E.; Kerry, J.P.; Morris, M.A. Synthesis of Monodisperse Chitosan Nanoparticles. Food Hydrocoll. 2018, 83, 355–364. [Google Scholar] [CrossRef]
- Mohamed, E.A. Green Synthesis of Copper & Copper Oxide Nanoparticles Using the Extract of Seedless Dates. Heliyon 2020, 6, e03123. [Google Scholar] [CrossRef]
- Vara, J.A.; Dave, P.N.; Chaturvedi, S. The Catalytic Activity of Transition Metal Oxide Nanoparticles on Thermal Decomposition of Ammonium Perchlorate. Def. Technol. 2019, 15, 629–635. [Google Scholar] [CrossRef]
- Muthukrishnan, A.M. Green Synthesis of Copper-Chitosan Nanoparticles and Study of Its Antibacterial Activity. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Mahmoud, N.; Mohamed, H.I.; Ahmed, S.B.; Akhtar, S. Efficient Biosynthesis of CuO Nanoparticles with Potential Cytotoxic Activity. Chem. Pap. 2020, 74, 2825–2835. [Google Scholar] [CrossRef]
- Veisi, H.; Karmakar, B.; Tamoradi, T.; Hemmati, S.; Hekmati, M.; Hamelian, M. Biosynthesis of CuO Nanoparticles Using Aqueous Extract of Herbal Tea (Stachys Lavandulifolia) Flowers and Evaluation of Its Catalytic Activity. Sci. Rep. 2021, 11, 1983. [Google Scholar] [CrossRef]
- Patil, R.; Patil, U.; Jagdale, A.; Shinde, S.; Patil, S. Ash of Pomegranate Peels (APP): A Bio-Waste Heterogeneous Catalyst for Sustainable Synthesis of α,A′-Bis(Substituted Benzylidine)Cycloalkanones and 2-Arylidene-1-Tetralones. Res. Chem. Intermed. 2020, 46, 3527–3543. [Google Scholar] [CrossRef]
- Hafizi, T.; Shahriari, M.H.; Abdouss, M.; Kahdestani, S.A. Synthesis and Characterization of Vancomycin-Loaded Chitosan Nanoparticles for Drug Delivery. Polym. Bull. 2023, 80, 5607–5621. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thakur, R.; Barnela, M.; Chopra, M.; Manuja, A.; Chaudhury, A. Synthesis, Characterization and in Vitro Evaluation of Cytotoxicity and Antimicrobial Activity of Chitosan–Metal Nanocomposites. J. Chem. Technol. Biotechnol. 2015, 90, 867–873. [Google Scholar] [CrossRef]
- Clark, L.J.; Chan, L.S.; Powars, D.R.; Baker, R.F. Negative Charge Distribution and Density on the Surface of Oxygenated Normal and Sickle Red Cells. Blood 1981, 57, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Howley, P.; Prevost, N.; Condon, B.; Arnold, J.; Diegelmann, R. Positively and Negatively Charged Ionic Modifications to Cellulose Assessed as Cotton-Based Protease-Lowering and Hemostatic Wound Agents. Cellulose 2009, 16, 911–921. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zhou, J.; Li, L. An Investigation of Chitosan and Its Derivatives on Red Blood Cell Agglutination. RSC Adv. 2017, 7, 12247–12254. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the Effects of Molecular Parameters on the Hemostatic Properties of Chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.-J.; Chen, S.-Q. Effect of Chitosan Molecular Weight and Deacetylation Degree on Hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 131–137. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate Juice Consumption Reduces Oxidative Stress, Atherogenic Modifications to LDL, and Platelet Aggregation: Studies in Humans and in Atherosclerotic Apolipoprotein E–Deficient Mice12. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef]
- Marie-Helene, D.; Martine, J.-P.; Jacques, E.; Olivier, B.; Gene, A.H.; Michael, W.M.; Marie-Claude, G. ADP-Induced Platelet Aggregation Depends on the Conformation or Availability of the Terminal Gamma Chain Sequence of Fibrinogen. Study of the Reactivity of Fibrinogen Paris 1. Blood 1987, 70, 558–563. [Google Scholar] [CrossRef]
- Di Cera, E. Thrombin. Mol. Aspects Med. 2008, 29, 203–254. [Google Scholar] [CrossRef] [PubMed]
- Ylitalo, R.; Lehtinen, S.; Wuolijoki, E.; Ylitalo, P.; Lehtimäki, T. Cholesterol-Lowering Properties and Safety of Chitosan. Arzneimittelforschung 2002, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fylling, C.P.; Parnell, L.K.S. Use of Platelet Rich Plasma Gel on Wound Healing: A Systematic Review and Meta-Analysis. Eplasty 2011, 11, e38. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, H.I.; Mahmoud, N.M.R.; Ramadan, A.; Al-Subaie, A.M.; Ahmed, S.B. Novel Biological-Based Strategy for Synthesis of Green Nanochitosan and Copper-Chitosan Nanocomposites: Promising Antibacterial and Hematological Agents. Nanomaterials 2024, 14, 1111. https://doi.org/10.3390/nano14131111
Mohamed HI, Mahmoud NMR, Ramadan A, Al-Subaie AM, Ahmed SB. Novel Biological-Based Strategy for Synthesis of Green Nanochitosan and Copper-Chitosan Nanocomposites: Promising Antibacterial and Hematological Agents. Nanomaterials. 2024; 14(13):1111. https://doi.org/10.3390/nano14131111
Chicago/Turabian StyleMohamed, Hadeer I., Nesrine M. R. Mahmoud, Abeer Ramadan, Abeer M. Al-Subaie, and Somia B. Ahmed. 2024. "Novel Biological-Based Strategy for Synthesis of Green Nanochitosan and Copper-Chitosan Nanocomposites: Promising Antibacterial and Hematological Agents" Nanomaterials 14, no. 13: 1111. https://doi.org/10.3390/nano14131111





