The Hydrothermal-Assisted Approach Improves the Photocatalytic and Energy Storage Performance of Novel CuSe-TiO2-GO Composite
Abstract
1. Introduction
2. Sample Preparation
2.1. Materials
2.2. CuSe Preparation
2.3. TiO2 Preparation
2.4. Fabrication of GO
2.5. Preparation of CuSe-TiO2-GO Ternary Composites
2.6. Characterization
2.7. Photocatalytic Experiments
3. Results and Discussion
4. Conclusions
- The synergy between different nanomaterials in the composite and the highly conductive skeleton promotes the effective interaction of the GO with the CuSe-TiO2 composite;
- The ternary CuSe-TiO2-GO composites showed enhanced photocatalytic properties due to the good optical properties of CuSe and the high efficiency of GO to upgrade the photoexcited electron–hole pairs for the catalyst under visible light irradiation;
- The composites with the relative ratios of CuSe, TiO2, and GO (CuSe-TiO2 and CuSe-TiO2-GO) outperformed their pure CuSe and GO counterparts in photocatalytic activity. The ternary CuSe-TiO2-GO composite demonstrated remarkable performance in this study;
- For supercapacitor application, the ternary CuSe-TiO2-GO composite exhibits enhanced energy storage performance, including capacitance, low charge transfer resistance, and rapid charge transport kinetics facilitated by its high conductive matrix, thereby achieving superior performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghaffar, S.; Abbas, A.; Naeem-ul-Hassan, M.; Assad, N.; Sher, M.; Ullah, S.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Al Bratty, M.; et al. Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles. Antioxidants 2023, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Khan, A.U.; Li, B.; Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Tahir, K.; Khan, S.; Nazir, S. A Facile Fabrication of Silver/Copper Oxide Nanocomposite: An Innovative Entry in Photocatalytic and Biomedical Materials. Photodiagnosis Photodyn. Ther. 2020, 31, 101814. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Khan, A.U.; Li, B.; Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Alqarni, A.O.; Khan, Z.U.H.; Ullah, S.; Wasim, M.; et al. Biosynthesis of Silver Capped Magnesium Oxide Nanocomposite Using Olea Cuspidata Leaf Extract and Their Photocatalytic, Antioxidant and Antibacterial Activity. Photodiagnosis Photodyn. Ther. 2021, 33, 102153. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.B.; Amr, D.; Abbas, A.; Zohra, L.; Irfan, M.I.; Alhoshani, A.; Ashraf, S.; Amin, H.M.A. Synthesis of Hydroxyethylcellulose Phthalate-Modified Silver Nanoparticles and Their Multifunctional Applications as an Efficient Antibacterial, Photocatalytic and Mercury-Selective Sensing Agent. Int. J. Biol. Macromol. 2024, 256, 128009. [Google Scholar] [CrossRef] [PubMed]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Muneer, M.; Khalid, R.; Amin, H.M.A. ZnO-Bi2O3 Heterostructured Composite for the Photocatalytic Degradation of Orange 16 Reactive Dye: Synergistic Effect of UV Irradiation and Hydrogen Peroxide. Catalysts 2023, 13, 1328. [Google Scholar] [CrossRef]
- Alkherraz, A.M.; Ali, A.K.; Elsherif, K.M. Removal of Pb (II), Zn (II), Cu (II) and Cd (II) from Aqueous Solutions by Adsorption onto Olive Branches Activated Carbon: Equilibrium and Thermodynamic Studies. Chem. Int. 2020, 6, 11–20. [Google Scholar]
- Tariq, F.; Hussain, R.; Noreen, Z.; Javed, A.; Shah, A.; Mahmood, A.; Sajjad, M.; Bokhari, H.; Rahman, S. ur Enhanced Antibacterial Activity of Visible Light Activated Sulfur-Doped TiO2 Nanoparticles against Vibrio Cholerae. Mater. Sci. Semicond. Process. 2022, 147, 106731. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, Y.; Guan, L.; Muhammad, S.; Jiang, Y.; Zhang, S.; Yu, C.; Jiao, Y.; Zhang, S.; Ren, Y.; et al. Effect of Impurity in Cu2O Nanowires on the Degradation of Methyl Orange. J. Mater. Sci. Mater. Electron. 2020, 31, 3817–3824. [Google Scholar] [CrossRef]
- Daij, K.B.; Bellebia, S.; Bengharez, Z. Comparative Experimental Study on the COD Removal in Aqueous Solution of Pesticides by the Electrocoagulation Process Using Monopolar Iron Electrodes. Chem. Int. 2017, 3, 420–427. [Google Scholar]
- Djehaf, K.; Bouyakoub, A.Z.; Ouhib, R.; Benmansour, H.; Bentouaf, A.; Mahdad, A.; Moulay, N.; Bensaid, D.; Ameri, M. Textile Wastewater in Tlemcen (Western Algeria): Impact, Treatment by Combined Process. Chem. Int. 2018, 3, 414–419. [Google Scholar]
- Morales, A.M.; Lieber, C.M. A Laser Ablation Method for the Synthesis of Crystalline Semiconductor Nanowires. Science 1998, 279, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Ullah Shah, M.Z.; Javed, M.S.; Sajjad, M.; Shah, A.; Shah, M.S.; Rahman, S.U.; Mahmood, A.; Ahmad, M.A.; Assiri, M.A.; Hou, H. A Novel TiO2/CuSe Based Nanocomposite for High-Voltage Asymmetric Supercapacitors. J. Sci. Adv. Mater. Devices 2022, 7, 100418. [Google Scholar] [CrossRef]
- Yan, X.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is Methylene Blue an Appropriate Substrate for a Photocatalytic Activity Test? A Study with Visible-Light Responsive Titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef]
- Serra-Pérez, E.; Dražić, G.; Takashima, M.; Ohtani, B.; Kovačič, S.; Žerjav, G.; Tušar, N.N. Influence of the Surface Structure of the TiO2 Support on the Properties of the Au/TiO2 Photocatalyst for Water Treatment under Visible Light. Catal. Today 2024, 437, 114764. [Google Scholar] [CrossRef]
- Kageshima, Y.; Inuzuka, H.; Kumagai, H.; Ohtani, B.; Teshima, K.; Nishikiori, H. Photothermal Boosting of Photocatalytic Hydrogen Evolution Induced by Defects and Cocatalysts on TiO2. J. Phys. Chem. C 2023, 127, 18327–18339. [Google Scholar] [CrossRef]
- Dudziak, S.; Kowalska, E.; Wang, K.; Karczewski, J.; Sawczak, M.; Ohtani, B.; Zielińska-Jurek, A. The Interplay between Dopant and a Surface Structure of the Photocatalyst—The Case Study of Nb-Doped Faceted TiO2. Appl. Catal. B Environ. 2023, 328, 122448. [Google Scholar] [CrossRef]
- Di Paola, A.; García-López, E.; Ikeda, S.; Marcì, G.; Ohtani, B.; Palmisano, L. Photocatalytic Degradation of Organic Compounds in Aqueous Systems by Transition Metal Doped Polycrystalline TiO2. Environ. Catal. Step Forw. 2002, 75, 87–93. [Google Scholar] [CrossRef]
- Zhang, L.; Chuaicham, C.; Balakumar, V.; Sekar, K.; Ohtani, B.; Sasaki, K. Determination of the Roles of FeIII in the Interface between Titanium Dioxide and Montmorillonite in FeIII-Doped Montmorillonite/Titanium Dioxide Composites as Photocatalysts. Appl. Clay Sci. 2022, 227, 106577. [Google Scholar] [CrossRef]
- Shah, M.Z.U.; Sajjad, M.; Hou, H.; Rahman, S.u.; Mahmood, A.; Aziz, U.; Shah, A. A New CuO/TiO2 Nanocomposite: An Emerging and High Energy Efficient Electrode Material for Aqueous Asymmetric Supercapacitors. J. Energy Storage 2022, 55, 105492. [Google Scholar] [CrossRef]
- Gu, Y.; Su, Y.; Chen, D.; Geng, H.; Li, Z.; Zhang, L.; Zhang, Y. Hydrothermal Synthesis of Hexagonal CuSe Nanoflakes with Excellent Sunlight-Driven Photocatalytic Activity. CrystEngComm 2014, 16, 9185–9190. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Ayeshamariam, A.; Manikandan, E.; Kennedy, J.; Ladchumananandasivam, R.; Umbelino Gomes, U.; Jayachandran, M.; Maaza, M. Solution Processing of CuSe Quantum Dots: Photocatalytic Activity under RhB for UV and Visible-Light Solar Irradiation. Mater. Sci. Eng. B 2016, 210, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Yang, L.; Liu, C.; Chen, Y.; Meng, D. Photo-Assisted Synthesis of Rosalike CuSe Hierarchical Nanostructures on TiO2 Nanotubes with Remarkable Photocatalytic Performance. Electrochimica Acta 2012, 83, 394–401. [Google Scholar] [CrossRef]
- Qureshi, K.; Ahmad, M.Z.; Bhatti, I.A.; Zahid, M.; Nisar, J.; Iqbal, M. Graphene Oxide Decorated ZnWO4 Architecture Synthesis, Characterization and Photocatalytic Activity Evaluation. J. Mol. Liq. 2019, 285, 778–789. [Google Scholar] [CrossRef]
- Aamir, M.; Bibi, I.; Ata, S.; Majid, F.; Kamal, S.; Alwadai, N.; Sultan, M.; Iqbal, S.; Aadil, M.; Iqbal, M. Graphene Oxide Nanocomposite with Co and Fe Doped LaCrO3 Perovskite Active under Solar Light Irradiation for the Enhanced Degradation of Crystal Violet Dye. J. Mol. Liq. 2021, 322, 114895. [Google Scholar] [CrossRef]
- Ata, S.; Shaheen, I.; Qurat-ul-Ayne; Ghafoor, S.; Sultan, M.; Majid, F.; Bibi, I.; Iqbal, M. Graphene and Silver Decorated ZnO Composite Synthesis, Characterization and Photocatalytic Activity Evaluation. Diam. Relat. Mater. 2018, 90, 26–31. [Google Scholar] [CrossRef]
- Li, J.-C.; Gong, J.; Zhang, X.; Lu, L.; Liu, F.; Dai, Z.; Wang, Q.; Hong, X.; Pang, H.; Han, M. Alternate Integration of Vertically Oriented CuSe@FeOOH and CuSe@MnOOH Hybrid Nanosheets Frameworks for Flexible In-Plane Asymmetric Micro-Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 3692–3703. [Google Scholar] [CrossRef]
- Yu, F.; Wang, C.; Wang, R.; Li, Y.; Ohtani, B.; Fujishima, A.; Zhang, X. Solution Plasma Engineering the Surface of Nitrogen Doped TiO2 for Photothermal Catalysis. Appl. Surf. Sci. 2023, 624, 157119. [Google Scholar] [CrossRef]
- Amin, S.; Sher, M.; Ali, A.; Rehman, M.F.; Hayat, A.; Ikram, M.; Abbas, A.; Amin, H.M.A. Sulfonamide-Functionalized Silver Nanoparticles as an Analytical Nanoprobe for Selective Ni(II) Sensing with Synergistic Antimicrobial Activity. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100735. [Google Scholar] [CrossRef]
- Sajjad, M.; Shah, M.Z.U.; Javed, M.S.; Shah, M.S.; Shah, A.; Lu, W.; Mao, Z. A Novel High-Performance All-Solid-State Asymmetric Supercapacitor Based on CuSe Nanoflakes Wrapped on Vertically Aligned TiO2 Nanoplates Nanocomposite Synthesized via a Wet-Chemical Method. J. Energy Storage 2022, 55, 105304. [Google Scholar] [CrossRef]
- Khan, A.U.; Tahir, K.; Shah, M.Z.U.; Khalil, M.Y.; Almarhoon, Z.M.; Hassan, H.M.A.; Abdulaziz, F.; Alanazi, A.A.; El-Zahhar, A.A.; Munshi, A.M. A New Cadmium Oxide (CdO) and Copper Selenide (CuSe) Nanocomposite: An Energy-Efficient Electrode for Wide-Voltage Hybrid Supercapacitors. Colloids Surf. Physicochem. Eng. Asp. 2023, 656, 130327. [Google Scholar] [CrossRef]
- Sajjad, M.; Tao, R.; Qiu, L. Phosphine Based Covalent Organic Framework as an Advanced Electrode Material for Electrochemical Energy Storage. J. Mater. Sci. Mater. Electron. 2021, 32, 1602–1615. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.U.; Tahir, K.; Liu, J.; Zaki, M.E.A.; Almarhoon, Z.M.; Alanazi, A.A.; Althagafi, T.M.; Al-Saeedi, S.I.; Mohamedbakr, H.G. FeSe2/TiO2 Heterostructure as an Efficient Photocatalyst and Their Electrochemical Energy Storage Applications. Mater. Chem. Phys. 2023, 303, 127793. [Google Scholar] [CrossRef]
- Sajjad, M.; Xu, C.; Guan, L.; Zhang, S.; Jiao, Y.; Zhang, S.; Lin, Y.; Ren, Y.; Zhou, X.; Liu, Z. Influence of Stirring Time on the Electrochemical Properties of NiCo2S4 Hexagonal Plates and NiCo−OH Nanoparticles as High-Performance Pseudocapacitor Electrode Materials. ChemistrySelect 2020, 5, 2634–2642. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.u.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Özuğur Uysal, B.; Pekcan, Ö.; Erim, F.B. Efficient Photocatalytic Degradation of Methylene Blue Dye from Aqueous Solution with Cerium Oxide Nanoparticles and Graphene Oxide-Doped Polyacrylamide. ACS Omega 2023, 8, 13004–13015. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Thang, T.Q.; An, H.; Hai, N.D.; Duy, P.H.A.; Duyen, D.T.M.; Oanh, D.T.Y.; Van Tuyen, N.; Nam, H.M.; Phong, M.T.; et al. Synthesis of TiO2-Doped Carbon Aerogel from Sugarcane Bagasse for High Efficiency of Photodegradation of Methylene Blue in the Water. Vietnam J. Chem. 2023, 61, 227–237. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, F.; Zhang, P.; An, Z.; Zhao, Y.; Chen, L. The Photodegradation of Methylene Blue in Water with PVDF/GO/ZnO Composite Membrane. Mater. Sci. Eng. C 2019, 96, 684–692. [Google Scholar] [CrossRef]
- Sultana, S.; Rafiuddin; Khan, M.Z.; Umar, K.; Ahmed, A.S.; Shahadat, M. SnO2–SrO Based Nanocomposites and Their Photocatalytic Activity for the Treatment of Organic Pollutants. J. Mol. Struct. 2015, 1098, 393–399. [Google Scholar] [CrossRef]
- Sajjad, M.; Jiang, Y.; Guan, L.; Chen, X.; Iqbal, A.; Zhang, S.; Ren, Y.; Zhou, X.; Liu, Z. NiCo2S4 Nanosheet Grafted SiO2@C Core-Shelled Spheres as a Novel Electrode for High Performance Supercapacitors. Nanotechnology 2019, 31, 045403. [Google Scholar] [CrossRef]
- Sajjad, M.; Tao, R.; Kang, K.; Luo, S.; Qiu, L. Phosphine-Based Porous Organic Polymer/rGO Aerogel Composites for High-Performance Asymmetric Supercapacitor. ACS Appl. Energy Mater. 2021, 4, 828–838. [Google Scholar] [CrossRef]
- Khan, A.U.; Tahir, K.; Shah, M.Z.U.; Zaki, M.E.A.; Saleh, E.A.M.; Hou, H.; Alabbad, E.A.; Althagafi, T.M.; El-Zahhar, A.A.; Alqarni, S.; et al. Polypyrrole Decorated on Irregular SnSe Particles: A High Energy Density and Stable Durability for Asymmetric Supercapacitor Applications. J. Energy Storage 2023, 73, 108801. [Google Scholar] [CrossRef]


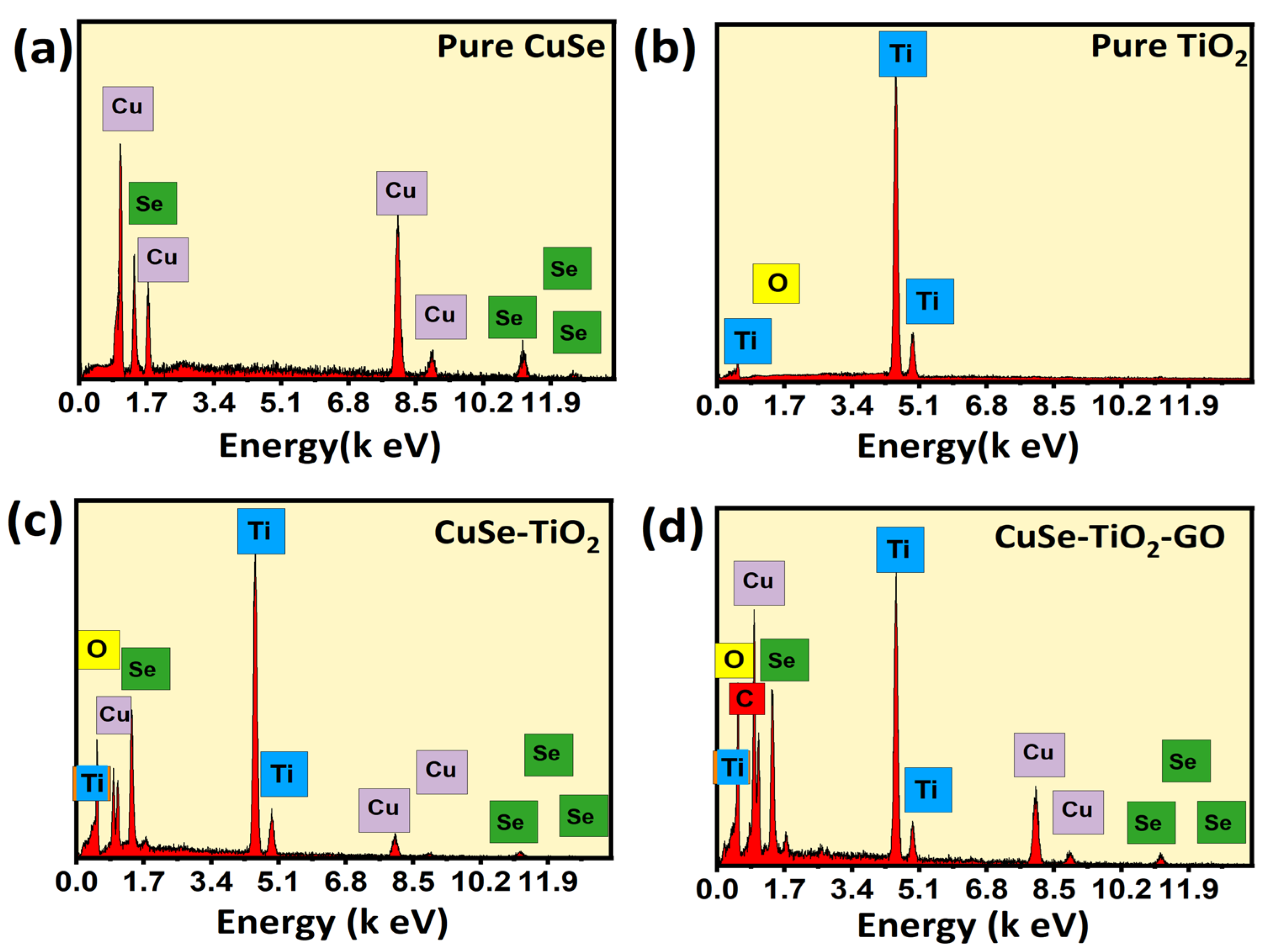

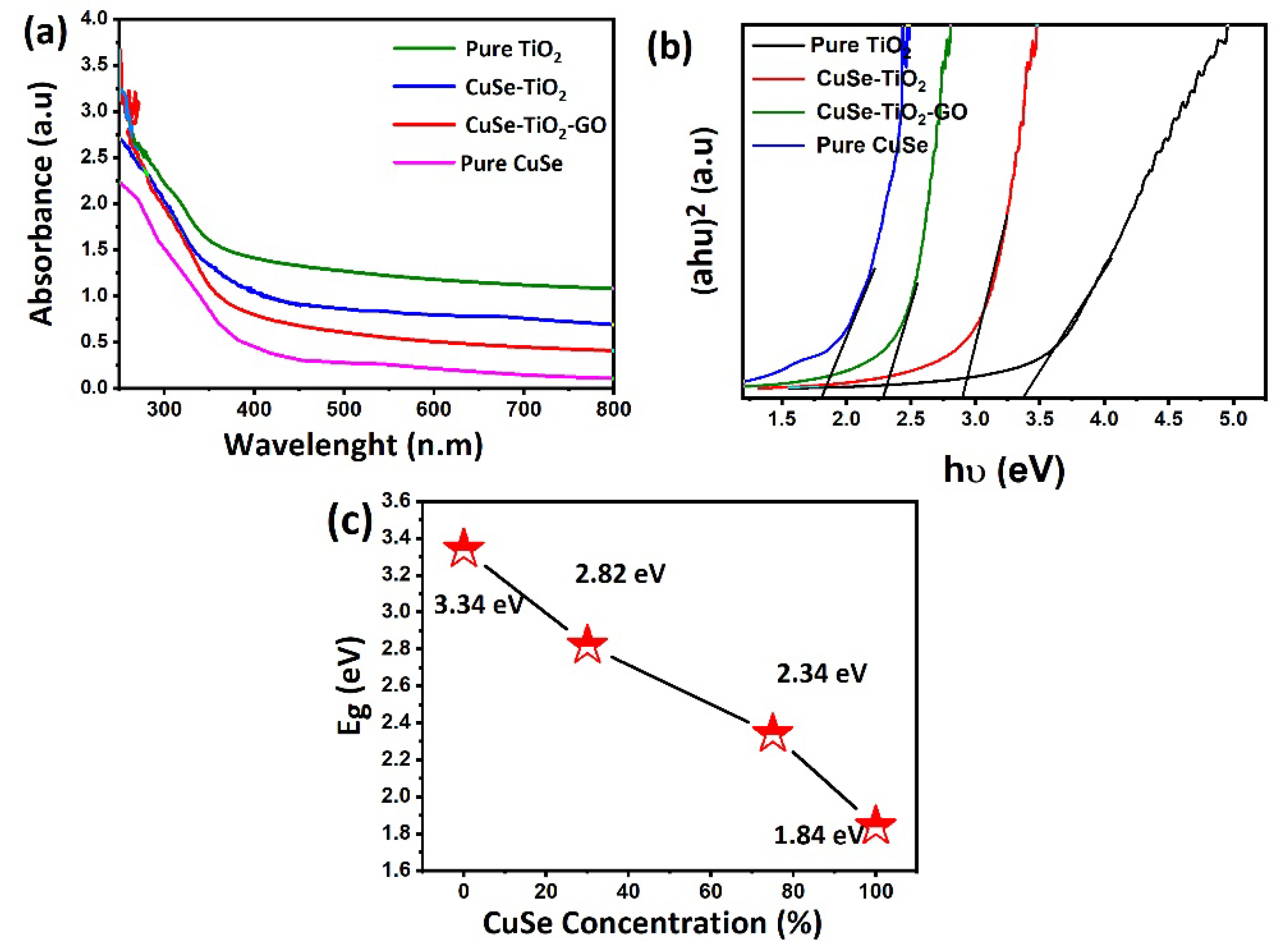
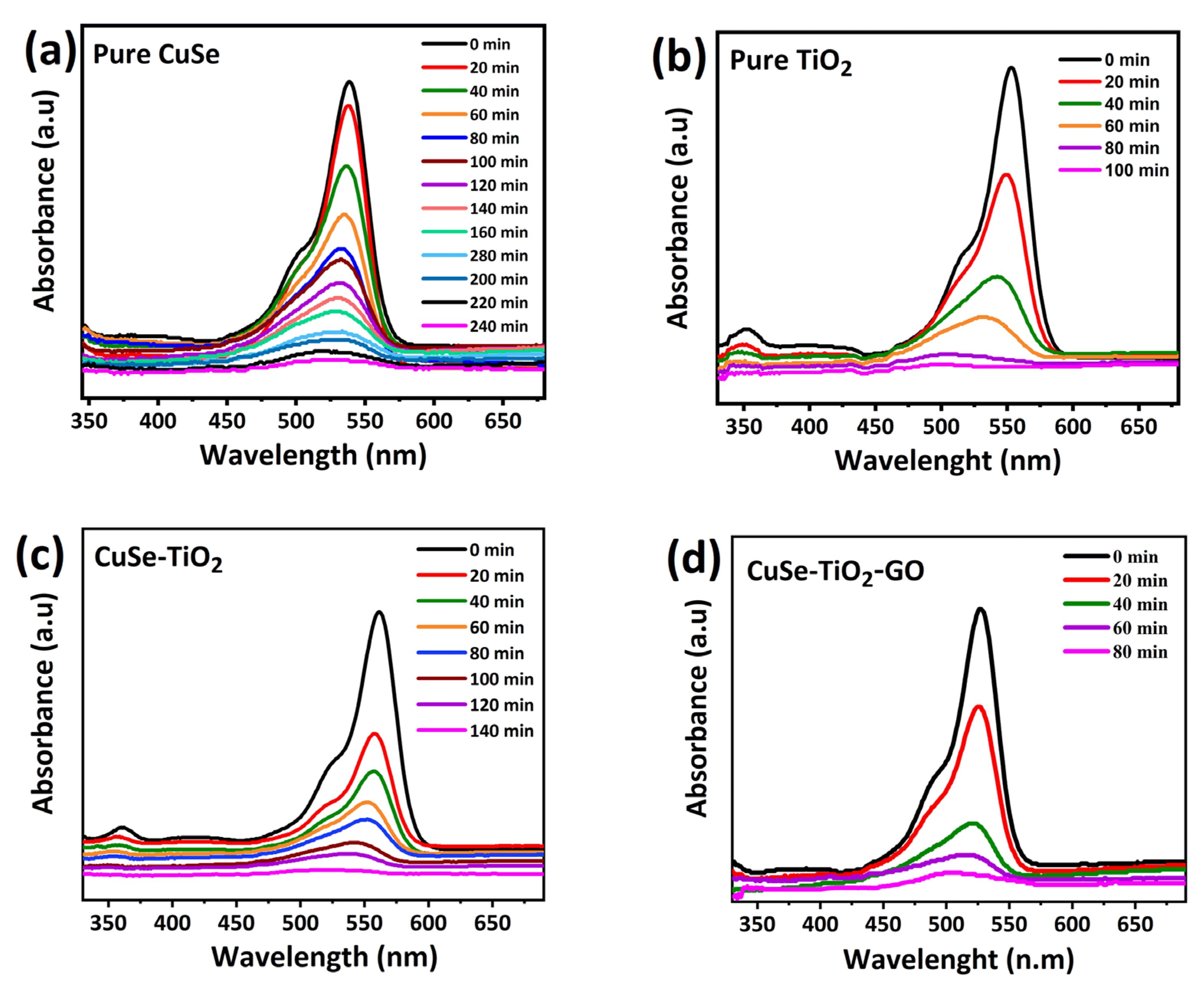


| Sample Name | Average Crystallite Sizes (nm) |
|---|---|
| CuSe | 23.42 |
| TiO2 | 17.4 |
| CuSe-TiO2 | 21.52 |
| CuSe-TiO2-GO | 14.18 |
| Catalyst | Dye | Light Source | %Dye Degradation | Time (min) | References |
|---|---|---|---|---|---|
| Ni-Cr/TiO2 | MB | Sunlight | 95.6% | 90 | [35] |
| CeO2-NPs/GO/PAM | MB | UV | 90% | 90 | [36] |
| ZnO@OFE | MB | sunlight | 75% | 180 | [1] |
| TiO2/CA | MB | UV | 93.86% | 80 | [37] |
| GO/ZnO | MB | 300 w Xe lamp | 86.86% | 100 | [38] |
| CuSe-TiO2-GO | MB | 400 w Xe lamp | 98% | 80 | This work |
| Current Density (A g−1) | TiO2 (F g−1) | CuSe-TiO2 (F g−1) | CuSe-TiO2-GO (F g−1) |
|---|---|---|---|
| 1 | 156 | 184 | 310.6 |
| 3 | 153 | 168 | 285.3 |
| 5 | 148 | 156 | 225.1 |
| 7 | 140 | 150 | 178 |
| 9 | 133 | 147 | 160.5 |
| 12 | 126 | 135 | 135.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.U.; Tahir, K.; Shah, M.Z.U.; Albaqawi, H.S.; Almarhoon, Z.M.; Alanazi, A.A.; Alkudaisi, N.A.; Althagafi, T.M.; Badi, N.; Zaki, M.E.A. The Hydrothermal-Assisted Approach Improves the Photocatalytic and Energy Storage Performance of Novel CuSe-TiO2-GO Composite. Nanomaterials 2024, 14, 1136. https://doi.org/10.3390/nano14131136
Khan AU, Tahir K, Shah MZU, Albaqawi HS, Almarhoon ZM, Alanazi AA, Alkudaisi NA, Althagafi TM, Badi N, Zaki MEA. The Hydrothermal-Assisted Approach Improves the Photocatalytic and Energy Storage Performance of Novel CuSe-TiO2-GO Composite. Nanomaterials. 2024; 14(13):1136. https://doi.org/10.3390/nano14131136
Chicago/Turabian StyleKhan, Afaq Ullah, Kamran Tahir, Muhammad Zia Ullah Shah, Hissah Saedoon Albaqawi, Zainab M. Almarhoon, Abdulaziz A. Alanazi, Nora Awad Alkudaisi, Talal M. Althagafi, Nacer Badi, and Magdi E. A. Zaki. 2024. "The Hydrothermal-Assisted Approach Improves the Photocatalytic and Energy Storage Performance of Novel CuSe-TiO2-GO Composite" Nanomaterials 14, no. 13: 1136. https://doi.org/10.3390/nano14131136
APA StyleKhan, A. U., Tahir, K., Shah, M. Z. U., Albaqawi, H. S., Almarhoon, Z. M., Alanazi, A. A., Alkudaisi, N. A., Althagafi, T. M., Badi, N., & Zaki, M. E. A. (2024). The Hydrothermal-Assisted Approach Improves the Photocatalytic and Energy Storage Performance of Novel CuSe-TiO2-GO Composite. Nanomaterials, 14(13), 1136. https://doi.org/10.3390/nano14131136








